Abstract
Mesenteric liposarcoma is a rare neoplasm. Here, we report the case of a 73-year-old Japanese man with a well-differentiated (WD) liposarcoma of the mesentery. Due to rapid growth of the abdominal mass and abdominal insufficiency, a tumorectomy was performed. The excised tumor was 12.4 × 9.6 cm in size and weighed 548 g. Cut sections showed a lobulated yellow and/or grayish-colored appearance. The histological features were predominantly those of the sclerotic and lipoma-like variants of WD liposarcoma. The cytoplasm of most spindle cells was diffusely immunoreactive for CD34, while fat cells were positive for S-100 protein. Some spindle cell nuclei were positive for CDK4, and a few were positive for MDM2. The average Ki-67 proliferation index in tumor cells was 10%, and androgen receptor expression was detected in tumor cell nuclei. The present case and 11 cases identified from a literature search were reviewed. The WD mesenteric liposarcomas developed in patients in the fourth to seventh decades of life (mean age 57.9 years). The patients consisted of 7 men and 5 women. All tumors were larger than 10 cm in diameter at the time of surgery. Complete resection might be the only curative therapy for WD liposarcomas of the mesentery, but long-term follow-up is needed because of the possibility of a local recurrence of the tumor.
Key Words: Well-differentiated liposarcoma, Atypical lipomatous tumor, Mesentery, CD34, CDK4, MDM2, Androgen receptor, Immunohistochemistry
Introduction
Liposarcoma is one of the most common types of soft tissue tumors in adults. The incidence of liposarcoma peaks between 40 and 60 years of age. Liposarcomas are histologically divided into five subtypes: myxoid, pleomorphic, dedifferentiated, round cell, and well-differentiated (WD) liposarcoma (atypical lipomatous tumor) [1]. WD liposarcoma is the most common histological subgroup. WD liposarcomas are locally aggressive but incapable of metastasis. Approximately 75% develop in the deep soft tissue of the limbs, followed by 20% in the retroperitoneum and a much smaller percentage in the inguinal region [2]. Other sites are uncommon; in particular, primary WD liposarcoma in the mesentery is rare. WD liposarcoma is considered to be a low-grade malignancy. However, tumors that occur in deep anatomic sites tend to recur. Here, a case of WD liposarcoma in the duodenal mesentery is presented, and the literature is reviewed.
Case Report
The patient was a 73-year-old Japanese man who received follow-up care for viral hepatitis, liver cirrhosis, and hepatocellular carcinoma by a gastroenterologist at the Takii Hospital of the Kansai Medical University. The patient had diabetes mellitus that was controlled with antidiabetic drugs. He had noticed the presence of an upper abdominal mass for approximately 1 year, but did not seek treatment due to the lack of symptoms. Finally, he consulted a gastrointestinal surgeon because of the rapid growth of the abdominal mass and upper abdominal insufficiency. Physical examination revealed a large and movable mass palpated in the left upper abdomen, and computed tomography showed a well-circumscribed tumor mass with no metastases. Tumor markers, including carcinoembryonic antigen, CA19-9, and α-fetoprotein, were within normal limits. A laparotomy was performed. The upper abdominal tumor was located in the duodenal mesentery close to the ligament of Treitz with no adhesion to the surrounding organs, such as the transverse colon and liver. The tumor was completely excised. No chemotherapy or radiotherapy was performed. The patient remained healthy without any evidence of recurrence for 6 months after the surgery.
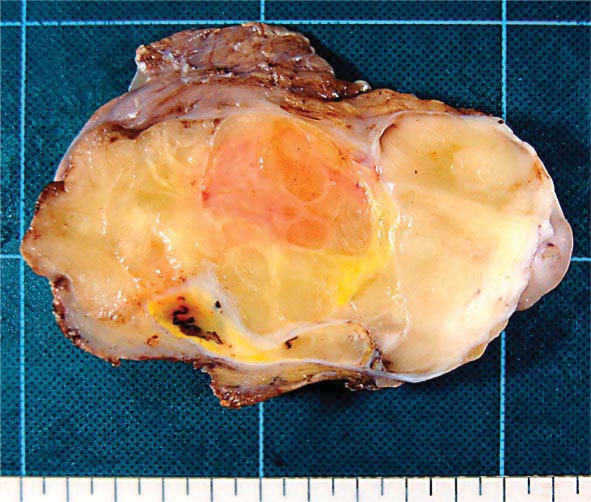
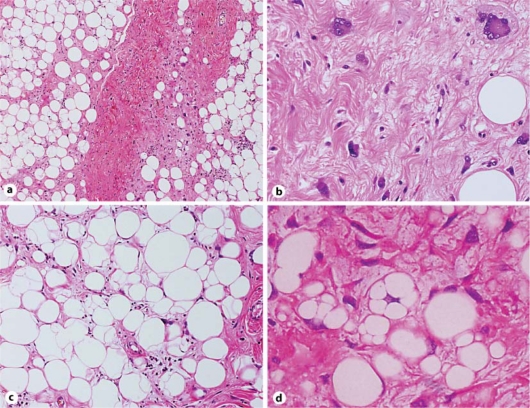
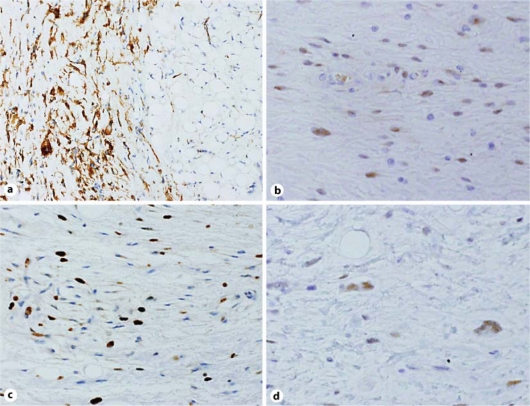
The excised tumor was a well-circumscribed mass that was 12.4 × 9.6 cm in size and weighed 548 g. Cut sections of the tumor showed a lobulated yellow and/or grayish-colored appearance with no necrotic areas (fig. 1). The histologic appearance of the tumor was variable. Different histologic areas were separated by broad fibrous septa containing cells with enlarged hyperchromatic and atypical nuclei (fig. 2a). Some areas contained collagen fibrils of varying density embedded with spindle cells, including multinucleated giant cells and some cells exhibiting a floret-like pattern (fig. 2b). Other areas consisted predominantly of mature fat cells that were variable in size; the nuclei of these cells were slightly pleomorphic and hyperchromatic (fig. 2c). Additionally, there were areas of dense lymphocytic infiltration and/or myxoid change. Within the tumor, a few multivacuolated spidery lipoblasts were scattered (fig. 2d). The labeled streptavidin biotin (LSAB) technique was performed with an LSAB staining kit (Dako, Carpinteria, Denmark) and the antibodies listed in table 1. Immunohistochemically, the cytoplasm of most spindle cells was positive for CD34 (fig. 3a), and a few cells were positive for α-smooth muscle actin but negative for desmin. Some spindle cell nuclei were positive for CDK4 (fig. 3b), and a few nuclei were positive for MDM2. Fat cells were S-100 positive, whereas spindle cells were S-100 negative. In tumor cells (including both spindle cells and fat cells), the average Ki-67 proliferation index was 10% (fig. 3c), and androgen receptor expression was detected (fig. 3d). Based on these results, the tumor was determined to be a WD liposarcoma (atypical lipomatous tumor) that primary originated from the duodenal mesentery.
Fig. 1.
Cut surface of the well-differentiated liposarcoma originating in the mesentery. The circumscribed lobulated tumor was yellow and/or gray in color.
Fig. 2.
Histology of the well-differentiated mesenteric liposarcoma. a Broad fibrous septa containing atypical and bizarre cells separated by various histologic areas (HE, ×40). b Spindle cells and bizarre and floret-like giant cells are embedded in a sclerotic background (sclerosing variant, HE, ×200). c The presence of fat cells with pleomorphic and hyperchromatic nuclei is evident (lipoma-like variant, HE, ×100). d A few multivacuolated lipoblasts are present (HE, ×400).
Table 1.
Antibodies used in the present study
| Antibody | Clone | Dilution | Source |
|---|---|---|---|
| AR | AR441 | 1:150 | Diagnostic BioSystems, Pleasanton, Calif., USA |
| CD34 | Nu-4A1 | Prediluted | Nichirei Biosciences, Tokyo, Japan |
| CDK4 | 97/Cdk4 | 1:50 | BD Biosciences, Franklin Lakes, N.J., USA |
| Desmin | D33 | Prediluted | Dako Corporation, Glostrup, Denmark |
| Ki-67 | MIB-1 | Prediluted | Dako Corporation, Glostrup, Denmark |
| MDM2 | SMP14 | 1:50 | BioCarta, San Diego, Calif., USA |
| S-100 | Polyclonal | Prediluted | Nichirei Biosciences, Tokyo, Japan |
| α-SMA | 1A4 | Prediluted | Thermo Fisher Scientific, Waltham, Mass., USA |
AR = Androgen receptor; α-SMA = a-smooth muscle actin.
Fig. 3.
Immunohistochemical analysis of the well-differentiated mesenteric liposarcoma. a The cytoplasm of most spindle cells is positive for CD34 (CD34, ×100). b Few spindle cell nuclei are positive for CDK4 (CDK4, ×200). c The average Ki-67 index was 10% (Ki-67, ×200). d Some tumor cell nuclei were androgen receptor positive (androgen receptor, ×200).
Discussion
We report a case of primary WD liposarcoma originating in the mesentery of a 73-year-old man. The histological variants of primary mesenteric liposarcoma are either WD or myxoid form [3]. The most common differential diagnostic problem of WD liposarcoma is its distinction from spindle cell/pleomorphic lipoma. Lipoblasts are the hallmark of any liposarcoma subtype [1]. However, only a few lipoblasts may be present. Lipoma-like WD liposarcomas can be identified by the population of various-sized fat cells, including atypical nuclei and immature fat cells. A characteristic feature of spindle cell/pleomorphic lipomas is the presence of floret-type giant cells [4]. However, floret-type giant cells are rarely seen in WD liposarcomas, and then only in small numbers [5]. In the present case, floret-like multinucleated cells were observed in sclerotic regions. Similar to myxoid liposarcomas, WD liposarcomas occasionally may have a predominantly myxoid appearance [5].
Immunohistochemistry may be useful in the identification of WD liposarcomas. Spindle cells in spindle cell/pleomorphic lipomas are strongly positive for CD34 [4]. However, it is not definite that widespread CD34 immunoreactivity can exclude other lipomatous neoplasms; diffuse CD34 staining in a lipomatous tumor may simply reflect the presence of spindle cells [6, 7].
In the present case, CD34 showed diffuse and strong positivity in spindle-shaped and multinucleated giant cells embedded in the sclerotic stroma. Immunohistochemical detection of CDK4 and MDM2 as well as gene amplification of CDK4 and MDM2 status has been shown to be a sensitive and specific means of identifying WD liposarcoma from other benign lipomatous tumors [8]. In our study, some tumor cell nuclei were CDK4 positive, and a smaller number of cell nuclei were MDM2 positive. Moreover, in agreement with a previous report, the Ki-67 index was highly suggestive of WD liposarcoma in the present case [9]. The cytogenetic pattern of WD liposarcoma is characterized by the consistent presence of circular (ring) and giant rod chromosomes [1, 5]. However, in the present case, fresh material was not available for a cytogenetic study.
Primary WD mesenteric liposarcomas are rare with only 12 well-documented cases (including the present case) in the English literature (table 2) [10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20].
Table 2.
Clinicopathological features of 12 cases of well-differentiated liposarcoma of the mesentery
| Case | Age | Sex | Size, cm | Weight, g | Histological variant | Follow-up | Origin | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 54 | M | 16 × 9.5 × 9 | 560 | ND | NED, 3 yr | jejunum | [10] |
| 2 | 52 | M | 10 × 6 × 5 | ND | lipoma-like/sclerosing | Rec, 4.5 mo | jejunum | [11] |
| 3 | 46 | F | ND | ND | lipoma-like/sclerosing | ND | ND | [12] |
| 4 | 69 | F | 15 × 10 | ND | inflammatory | NED, 8 mo | ND | [13] |
| 5 | 63 | M | 40 × 29 × 15 | 7,700 | lipoma-like | NED, 1 yr | small bowel | [14] |
| 6 | 75 | F | ND | ND | lipoma-like | NED, 2 yr | sigmoid | [15] |
| 7 | 43 | F | 20 × 16 | ND | lipoma-like | NED, 33 mo | small bowel | [16] |
| 8 | 55 | M | 19/14 | 8,500 | sclerosing/myxoid | ND | ND | [17] |
| 9 | 55 | M | 40 | 9,000 | sclerosing | NED, 1 yr | ND | [18] |
| 10 | 65 | F | 16 × 13 × 9 | 700 | lipoma-like | ND | jejunum | [19] |
| 11 | 45 | M | 28 × 28 × 3.5 | ND | lipoma-like/sclerosing | Rec, 5 yr | colon | [20] |
| 12 | 73 | M | 12.4 × 9.6 | 548 | sclerosing/lipoma-like | NED, 6 mo | duodenum | Present case |
ND = Not described; NED = no evidence of disease; yr = year; Rec = recurrence; mo = month.
WD mesenteric liposarcoma tends to occur in the fourth to seventh decades of life (mean age 57.9 years) with no difference in frequency based on the patients’ sex (men/women: 7/5). Androgen receptor is frequently detected in spindle cell lipoma with marked male predilection [21]. Although immunoreactivity for androgen receptor has not been systematically studied in WD liposarcomas, the expression of androgen receptor was seen in the tumor cells in the present case. Endogenous and/or exogenous steroids via the androgen receptor may be involved in the growth of this tumor. WD liposarcomas are relatively large in size, and most tumors are over 10 cm in diameter at the time of surgery. WD liposarcomas can be subdivided into the following groups: lipoma-like, sclerosing, inflammatory, and spindle cell [1]. The presence of more than one histologic variant is common in single tumors, and subclassification does not indicate any prognostic significance. In our survey, we found that mixed variants are frequently described in WD liposarcomas. Among the 12 cases, local recurrence occurred in 2 cases. Surgical margin may be the most important prognostic factor in that incomplete resection may lead to recurrence [11], and the risk of dedifferentiation may also be considered [22].
In conclusion, complete resection and long-term follow-up are necessary for WD mesenteric liposarcoma due to the risk of local recurrence of the tumor.
Disclosure Statement
The authors have no conflicts of interest to declare.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Dei Tos AP, Pedeutour F. Atypical lipomatous tumor/well-differentiated liposarcoma. In: Fletcher CDM, Unni K, Mertens F, editors. Pathology and Genetics of Tumours of Soft Tissue and Bone, WHO Classification of Tumours. Lyon: IARC Press; 2002. pp. 35–46. [Google Scholar]
- 2.Weiss SW, Rao VK. Well-differentiated liposarcoma (atypical lipoma) of deep soft tissue of the extremities, retroperitoneum, and miscellaneous sites. A follow-up study of 92 cases with analysis of the incidence of 'dedifferentiation'. Am J Surg Pathol. 1992;16:1051–1058. doi: 10.1097/00000478-199211000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Moyana TN. Primary mesenteric liposarcoma. Am J Gastroenterol. 1988;83:89–92. [PubMed] [Google Scholar]
- 4.Miettinen MM, Mandahl N. Spindle cell lipoma/pleomorphic lipoma. In: Fletcher CDM, Unni K, Mertens F, editors. Pathology and Genetics of Tumours of Soft Tissue and Bone, WHO Classification of Tumours. Lyon: IARC Press; 2002. pp. 31–32. [Google Scholar]
- 5.Enzinger FM, Weiss SW. Liposarcoma. In: Weiss SW, Goldblum JR, editors. Soft Tissue Tumors. Philadelphia: Mosby Elsevier; 2008. pp. 477–516. [Google Scholar]
- 6.Austin CD, Tiessen JR, Gopalan A, Williams JM, Jr, Bangs CD, Cherry AM, Lehnert BA, Rouse RV. Spindle cell lipoma of the foot and the application of CD34 immunohistochemistry to atypical lipomatous tumors in unusual locations. Appl Immunohistochem Mol Morphol. 2000;8:222–227. doi: 10.1097/00129039-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Mentzel T, Toennissen J, Rütten A, Schaller J. Palmar atypical lipomatous tumour with spindle cell features (well-differentiated spindle cell liposarcoma): a rare neoplasm arising in an unusual anatomical location. Virchows Arch. 2005;446:300–304. doi: 10.1007/s00428-004-1138-6. [DOI] [PubMed] [Google Scholar]
- 8.Binh MB, Sastre-Garau X, Guillou L, de Pinieux G, Terrier P, Lagacé R, Aurias A, Hostein I, Coindre JM. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol. 2005;29:1340–1347. doi: 10.1097/01.pas.0000170343.09562.39. [DOI] [PubMed] [Google Scholar]
- 9.Jakobiec FA, Nguyen J, Bhat P, Fay A. MDM2-positive atypical lipomatous neoplasm/well-differentiated liposarcoma versus spindle cell lipoma of the orbit. Ophthal Plast Reconstr Surg. 2010;26:413–415. doi: 10.1097/IOP.0b013e3181cd62eb. [DOI] [PubMed] [Google Scholar]
- 10.Takagi H, Kato K, Yamada E, Suchi T. Six recent liposarcomas including largest to date. J Surg Oncol. 1984;26:260–267. doi: 10.1002/jso.2930260412. [DOI] [PubMed] [Google Scholar]
- 11.Papadopoulos T, Kirchner T, Bergmann M, Müller-Hermelink HK. Primary liposarcoma of the jejunum. Pathol Res Pract. 1990;186:803–806. doi: 10.1016/S0344-0338(11)80276-1. [DOI] [PubMed] [Google Scholar]
- 12.Kim T, Murakami T, Oi H, Tsuda K, Matsushita M, Tomoda K, Fukuda H, Nakamura H. CT and MR imaging of abdominal liposarcoma. AJR Am J Roentgenol. 1996;166:829–833. doi: 10.2214/ajr.166.4.8610559. [DOI] [PubMed] [Google Scholar]
- 13.Argani P, Facchetti F, Inghirami G, Rosai J. Lymphocyte-rich well-differentiated liposarcoma: report of nine cases. Am J Surg Pathol. 1997;21:884–895. doi: 10.1097/00000478-199708000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Takekawa Y, Yoshikawa T, Shibaji T, Asao Y, Nakano H. Mesenteric liposarcoma: a case report. J Nara Med Ass. 1997;48:246–250. [Google Scholar]
- 15.Amato G, Martella A, Ferraraccio F, Di Martino N, Maffettone V, Landolfi V, Fei L, Del Genio A. Well differentiated ‘lipoma-like’ liposarcoma of the sigmoid mesocolon and multiple lipomatosis of the rectosigmoid colon. Report of a case. Hepatogastroenterology. 1998;45:2151–2156. [PubMed] [Google Scholar]
- 16.Calò PG, Farris S, Tatti A, Tuveri M, Catani G, Nicolosi A. Primary mesenteric liposarcoma. Report of a case. G Chir. 2007;28:318–320. [PubMed] [Google Scholar]
- 17.Khan N, Afroz N, Fatima U, Raza MH, Rab AZ. Giant primary mesenteric liposarcoma: a rare case report. Indian J Pathol Microbiol. 2007;50:787–789. [PubMed] [Google Scholar]
- 18.Cerullo G, Marrelli D, Rampone B, Perrotta E, Caruso S, Roviello F. Giant liposarcoma of the mesentery. Report of a case. Ann Ital Chir. 2007;78:443–445. [PubMed] [Google Scholar]
- 19.Hirakoba M, Kume K, Yamasaki M, Kanda K, Yoshikawa I, Otsuki M. Primary mesenteric liposarcoma successfully diagnosed by preoperative imaging studies. Intern Med. 2007;46:373–375. doi: 10.2169/internalmedicine.46.6045. [DOI] [PubMed] [Google Scholar]
- 20.Gupta R, Sharma A, Arora R, Kulkarni MP, Chattopadhaya TK, Singh MK. Well-differentiated mesenteric liposarcoma with osseous metaplasia: a potential diagnostic dilemma for the pathologist. J Gastrointest Cancer. 2010;41:79–83. doi: 10.1007/s12029-009-9119-2. [DOI] [PubMed] [Google Scholar]
- 21.Syed S, Martin AM, Haupt H, Podolski V, Brooks JJ. Frequent detection of androgen receptors in spindle cell lipomas: an explanation for this lesion's male predominance? Arch Pathol Lab Med. 2008;132:81–83. doi: 10.5858/2008-132-81-FDOARI. [DOI] [PubMed] [Google Scholar]
- 22.Lucas DR, Nascimento AG, Sanjay BK, Rock MG. Well-differentiated liposarcoma. The Mayo Clinic experience with 58 cases. Am J Clin Pathol. 1994;102:677–683. doi: 10.1093/ajcp/102.5.677. [DOI] [PubMed] [Google Scholar]