Abstract
Rationale
A variety of behavioral procedures have been developed to assess cannabinoid activity in mice; however, the feasibility of establishing Δ9-THC as a discriminative stimulus in mice has not been documented.
Objective
One goal was to establish Δ9-THC as a discriminative stimulus in mice; after having done so, another goal was to examine the in vivo mechanism of action of Δ9-THC with other cannabinoids and noncannabinoids.
Materials and methods
C57BL/6J mice (n=8) were trained to discriminate Δ9-THC (10 mg/kg i.p.) from vehicle while responding under a fixed ratio 30 schedule of food presentation.
Results
Mice satisfied the discrimination criteria in 18–98 (median=67) sessions and the discriminative stimulus effects of Δ9-THC were dose-dependent (ED50=2.6 mg/kg). CP 55940 and WIN 55212-2 dose-dependently increased Δ9-THC-appropriate responding to 100% (ED50=0.032 and 0.45 mg/kg, respectively), whereas methanandamide and a variety of noncannabinoids (cocaine, ethanol, and ketamine) produced a maximum of 34% Δ9-THC-appropriate responding. The cannabinoid CB1 antagonist SR 141716A (rimonabant) surmountably antagonized the discriminative effects of Δ9-THC, CP 55940, and WIN 55212-2; methanandamide did not significantly modify the Δ9-THC discriminative stimulus.
Conclusions
The discriminative stimulus effects of Δ9-THC, CP 55940, and WIN 55212-2 are mediated by the same (i.e., CB1) receptors, whereas the effects of methanandamide or a metabolite of methanandamide are mediated at least in part by non-CB1 receptors. The discriminative stimulus effects of Δ9-THC in mice could be used to evaluate mechanisms of cannabinoid activity with approaches (e.g., inducible knockouts) currently unavailable in nonmurine species.
Keywords: Antagonist, Cannabinoid, Δ9-THC, Drug discrimination, Efficacy, Mouse, Rimonabant, SR 141716A
Introduction
Drug discrimination can be a highly sensitive, quantitative, and pharmacologically selective approach for evaluating the mechanism of action of drugs in vivo, and this approach has well-documented utility for examining the in vivo effects of cannabinoids. For example, cannabinoid CB1 agonists can have discriminative stimulus effects at doses smaller than doses producing some other in vivo effects (e.g., De Vry et al. 2004) and CB1 receptor agonists and not other drugs typically substitute for the discriminative stimulus effects of Δ9-THC (Balster and Prescott 1992; McMahon 2006a). Cannabinoid agonist discriminations appear to be selectively mediated by CB1 and not CB2 receptors insofar as the discriminative stimulus effects of nonselective agonists (e.g., Δ9-THC) are blocked by selective CB1 antagonists (SR 141716A or rimonabant; Wiley et al. 1995; Järbe et al. 2001; McMahon 2006b) and not by selective CB2 antagonists (Järbe et al. 2006; McMahon 2006a). In addition to providing a selective measure of CB1 receptor activity in vivo, the discriminative stimulus effects of cannabinoid agonists in nonhumans are highly predictive of cannabis-like effects in humans (Balster and Prescott 1992 for review).
Cannabinoid agonists have been trained as discriminative stimuli in a variety of species including rats, pigeons, and primates (Järbe et al. 1977; Chait et al. 1988; Balster and Prescott 1992; Gold et al. 1992). However, it does not appear that cannabinoid discriminations have been established in mice. There are a variety of indices that have been used to evaluate cannabinoid agonist activity in mice including hypothermia, catalepsy, antinociception, and hypoactivity (Martin et al. 1991). While efficient, these measures are not necessarily selective for cannabinoid agonists (Wiley and Martin 2003) and drug discrimination in mice could provide greater pharmacologic selectivity relative to other behavioral procedures. Mice also provide the opportunity to examine the molecular determinants of behavioral effects with genetic manipulations (i.e., knockouts) that are currently unavailable in rats, pigeons, or primates. In particular, temporal control over the expression of a gene and its products with inducible knockouts (Morozov et al. 2003) might be particularly useful for drug discrimination studies in which the presence of the gene is desired under one condition (drug discrimination training) and not another (drug discrimination testing). The first goal of this study was to establish Δ9-THC as a discriminative stimulus in C57BL/6J mice; after having done so, the pharmacologic mechanisms responsible for the Δ9-THC discriminative stimulus were examined with a variety of cannabinoids (CP 55940, WIN 55212-2, methanandamide, and rimonabant). To examine pharmacologic specificity, cocaine, ketamine, and ethanol were chosen for study because their behavioral activity has been indexed with operant procedures in mice (Shelton 2004) and these and related compounds (i.e., phencyclidine) were shown to not substitute for the discriminative stimulus effects of Δ9-THC in rats (Järbe and Henriksson 1974). The C57BL/6J strain of mouse was chosen because this strain is frequently used for genetic manipulation.
Materials and methods
Subjects
Eight male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were purchased at 6 weeks of age (approximately 15 g) and were housed individually on a 12/12-h light/dark cycle. Mice received approximately 1.5 cc of 50% condensed milk during experimental sessions (Monday through Friday) and 2.5 g of food (Dustless Precision Pellets 500 mg, Rodent Grain-Based Diet, Bio-Serv, Frenchtown, NJ) per day after sessions and on the weekend; water was available ad libitum in the home cage. Mice were habituated to the experimental room for 7 days before the first experimental session, and testing was conducted during the light period. Mice were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio and with the “Principles of Laboratory Animal Care” and the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003).
Apparatus
Discrimination experiments were conducted in commercially available mouse operant conditioning chambers (MedAssociates, St. Albans, VT) that were placed in ventilated, sound-attenuating enclosures. The ceiling of each operant conditioning chamber contained a light (i.e., house light), and the side of one wall contained two recessed holes (2.2-cm diameter) spaced 12.7 cm apart from center to center; the center of each hole was positioned 1.6 cm from the floor. Each hole contained a photobeam, a light, and a dipper to which 0.01 cc of condensed milk could be delivered from a tray positioned outside the operant conditioning chamber. An interface (MedAssociates) connected the operant conditioning chambers to a computer, and experimental events were controlled and recorded with Med-PC software.
Discrimination training
Sessions were conducted once daily Monday through Friday. Vehicle (a mixture of 1 part absolute ethanol, 1 part Emulphor-620, and 18 parts of physiologic saline) was administered in the home cage, and 30 min later, mice were placed inside the operant conditioning chambers. During 30-min sessions, each recessed hole was illuminated and mice could insert their snouts to disrupt a photobeam. A single disruption of the photobeam (continuous reinforcement) in either hole resulted in 10-s access to 0.01 cc of condensed milk diluted to 50% with tap water, during which time the lights in each hole were turned off and the house light was illuminated. Disruptions of a photobeam during the 10-s period of milk availability had no programmed consequence. Training under continuous reinforcement continued until a minimum of 100 reinforcers was obtained in three consecutive sessions. Thereafter, the number of photobeam disruptions required to obtain a reinforcer (i.e., fixed ratio or FR) was increased every two sessions (i.e., to an FR3, FR10, and FR30). To minimize odor traces between sessions, holes were wiped with absolute ethanol and milk dippers and trays were washed with a dilute soap solution and then rinsed with tap water.
Once responding was maintained at an FR30, mice received injections (vehicle or Δ9-THC) and were placed into the darkened operant chamber without schedule contingencies for 30 min (i.e., timeout). Thereafter, during a 30-min response period, lights in each hole were illuminated and mice could respond under the FR30 schedule of milk presentation described above except that responding in only one of the holes (i.e., correct hole) was reinforced depending on whether vehicle or Δ9-THC was administered before the session. Determination of correct holes (e.g., left, vehicle; right, Δ9-THC) varied among mice and remained the same for that mouse for the duration of the study. Training with vehicle was alternated daily with Δ9-THC and dose was increased from 1 to 10 mg/kg in 1/2 log unit. A training dose of 10 mg/kg of Δ9-THC was used for the remainder of the study and, thereafter, two consecutive vehicle training sessions were alternated with two consecutive Δ9-THC training sessions (i.e., double-alternation sequence). The first test was conducted when, for 5 consecutive or for 6 out of 7 days, at least 80% of the total responses occurred on the correct hole and fewer than 30 responses occurred in the incorrect hole before completion of the first FR on the correct hole (i.e., fewer than 59 total responses were made before delivery of the first reinforcer).
Discrimination testing
Test sessions were identical to training sessions except that 30 responses in either hole resulted in milk presentation and mice received vehicle or a dose of Δ9-THC or another drug. Test sessions were conducted Wednesday, Thursday, or Friday, and a maximum of two tests was conducted per week. Tests subsequent to the first test were conducted only after mice satisfied the criteria specified above during consecutive vehicle and Δ9-THC training sessions earlier in the week, and a second test in the same week was conducted only after mice satisfied the test criteria during either a vehicle or Δ9-THC training session on an intervening day. Training conditions (vehicle or Δ9-THC) preceding test sessions were nonsystematic.
Δ9-THC, CP 55940, WIN 55212-2, and methanandamide were studied by administering a dose of drug at the beginning of a 1-h session (beginning with a 30-min timeout followed by a 30-min response period). Rimonabant and methanandamide were studied in combination with other cannabinoid agonists by administering a dose of rimonabant or methanandamide immediately before a dose of cannabinoid agonist. For tests with drugs requiring pretreatment intervals shorter than 30 min, mice were placed in the chamber without injection at the beginning of the 30-min timeout. A dose of ketamine was administered 10 min before the response period, and a dose of cocaine or ethanol was administered 5 min before the response period; the response period was 10 min for each of these tests. The order of testing among drugs was nonsystematic except that tests with different doses of Δ9-THC were conducted at the beginning of the study and again with the same doses of Δ9-THC at end of the study, and tests involving combination of rimonabant or methanandamide with cannabinoid agonists were conducted after establishing the individual dose–response functions for methanandamide and other cannabinoid agonists.
Drugs
The levo enantiomer of Δ9-THC (100 mg/ml in absolute ethanol; The Research Technology Branch, National Institute on Drug Abuse, Rockville, MD) was prepared as a concentration of 5 mg/ml in a mixture of 1:1:18 of absolute ethanol, Emulphor-620 (Rhone-Poulenc, Princeton, NJ), and physiologic saline; smaller concentrations (1 mg/ml used for the training dose of 10 mg/kg) of Δ9-THC were diluted with physiologic saline. Rimonabant base (The Research Technology Branch, National Institute on Drug Abuse), WIN 55212-2 (Sigma, St. Louis, MO), and CP 55940 (Tocris, Ellisville, MO) were dissolved in a 1:1:18 mixture of Tween 80 (Sigma), propylene glycol (Sigma), and physiologic saline. (R)-methanandamide (in Tocrisolve vehicle; Tocris) and ketamine hydrochloride (100 mg/ml in physiologic saline; Fort Dodge Laboratories, Fort Dodge, IA) were diluted with physiologic saline. Cocaine hydrochloride (The Research Technology Branch, National Institute on Drug Abuse) was dissolved in physiologic saline. Drugs were administered i.p. in a volume of 0.1 ml/kg at doses (milligrams per kilogram) expressed as the weight of the forms listed above. Ethanol was administered in concentrations of 5–20% (w/v).
Data analyses
Discrimination data were expressed as an average (±S.E.M.) percentage of Δ9-THC-appropriate responding (i.e., number of Δ9-THC-appropriate responses divided by the total responses made during the duration of the test) and were plotted as a function of dose. All data for a particular drug treatment were collected in six to eight mice. For each mouse contributing to a particular drug treatment, the dose–effect curve for that mouse was defined by one ineffective dose and larger doses up to a dose producing greater than 80% Δ9-THC-appropriate responding or decreasing rate of responding to less than 20% of the control response rate. The potencies of cannabinoid agonists for producing discriminative stimulus effects were estimated by analyzing the dose–effect curves by simultaneously fitting straight lines to the individual dose–response data by means of GraphPad Prism version 4.03 for Windows (San Diego, CA), using the following equation: effect = slope × log(dose) + intercept. Straight lines were fitted to the linear portion of dose–effect curves, defined by doses producing 20–80% of the maximum effect, including not more than one dose producing less than 20% of the maximum effect. Other doses were excluded from the analyses. The same approach was used to establish the potency of an agonist alone and in combination with various doses of an antagonist.
The slopes of dose–effect curves were compared with an F-ratio test using GraphPad. If the slopes were not significantly different, then a common, best-fitting slope was used for further analyses (for detailed examples of this approach, see Kenakin 1997). Doses corresponding to the 50% level of the effect (ED50 value), potency ratios, and their 95% confidence limits (CL) were calculated by parallel line analyses of data from individual subjects (Tallarida 2000). The potencies of different agonists were considered significantly different when the 95% confidence limits of their potency ratio did not include 1.
Response rate was averaged (±S.E.M.) among mice and plotted as a function of dose. The effects of a drug on response rate were considered significant when the mean rate of responding was not contained within the 95% confidence limits of the mean control response rate, defined in individual mice as mean response rate calculated from ten consecutive vehicle training sessions in which the criteria for testing were satisfied and that were conducted after the first test. Discrimination data were not included for analysis when response rate for an individual mouse was less than 20% of the vehicle control rate for that mouse; however, all response rate data were included in the group average.
Results
Discriminative stimulus and rate effects of Δ9-THC and other cannabinoid agonists
The criteria for testing were satisfied in 18–98 sessions (median=67 sessions) including both vehicle and Δ9-THC training sessions. During tests, Δ9-THC dose-dependently increased drug-appropriate responding with the training dose (10 mg/kg) producing a mean (±S.E.M.) of 97 (±1)% of Δ9-THC-appropriate responses (Fig. 1, top left, circles). Administration of vehicle produced 1% Δ9-THC-appropriate responding (Fig. 1, top left, VEH). CP 55940 and WIN 55212-2 also dose-dependently increased Δ9-THC-appropriate responding to a mean of 100% at doses of 0.1 and 3.2 mg/kg, respectively (Fig. 1, top left, triangles and squares, respectively). The dose–effect curves for the discriminative stimulus effects of CP 55940, WIN 55212-2, and Δ9-THC did not deviate from parallelism; ED50 values (95% confidence limits) were 0.032 (0.018–0.056) mg/kg for CP 55940, 0.45 (0.27–0.74) mg/kg for WIN 55212-2, and 2.6 (1.8–3.7) mg/kg for Δ9-THC. CP 55940 was significantly more potent than WIN 55212-2 and Δ9-THC (14- and 82-fold, respectively; Table 1, top), and WIN 55212-2 was significantly more potent than Δ9-THC (5.8-fold; Table 1, top). The mean control response rate (95% confidence limits) calculated from ten vehicle training sessions was 4.4 (3.2–5.6) responses per second. Some doses of CP 55940 and WIN 55212-2 increased rate of responding, whereas rate of responding was not significantly modified by Δ9-THC up to a dose of 10 mg/kg (Fig. 1, bottom left).
Fig. 1.
Discriminative stimulus effects of cannabinoid agonists and noncannabinoids in C57BL/6J mice discriminating Δ9-THC. Abscissae: dose in milligram per kilogram body weight; VEH vehicle. Ordinates: mean (±S.E.M.) percentage of Δ9-THC-appropriate responding (top) and mean (±S.E.M.) response rate expressed in responses per second. Dashed lines show the 95% confidence limits of the vehicle control response rate determined from ten vehicle training sessions; symbols outside the confidence limits are considered significantly different from control
Table 1.
ED50 values, potency ratios (expressed as most divided by least potent), and 95% confidence limits (95% CL) for drugs that produced Δ9-THC-appropriate responding (top), for Δ9-THC in combination with rimonabant or methanandamide (middle), and for CP 55940 or WIN 55212-2 in combination with rimonabant (bottom)
| Drug(s) | ED50 (95% CL) in mg/kg | Dose ratio (95% CL) versus Δ9-THC | Dose ratio (95% CL) versus CP 55940 | Dose ratio (95% CL) versus control |
|---|---|---|---|---|
| Cannabinoid agonists alone | ||||
| Δ9-THC | 2.6 (1.8–3.7) | – | 82 (45–150)a | |
| CP 55940 | 0.032 (0.018–0.056) | 82 (45–150)a | – | |
| WIN 55212-2 | 0.45 (0.27–0.74) | 5.8 (3.2–10)a | 14 (7.0–29)a | |
| Δ9-THC alone and in combination with rimonabant or methanandamide | ||||
| Δ9-THC | 2.6 (1.8–3.7) | – | ||
| + 0.32 Rimonabant | 5.3 (3.1–8.9) | 2.0 (1.1–3.6)a | ||
| + 1 Rimonabant | 10 (5.2–20) | 3.9 (2.0–7.6)a | ||
| + 10 Methanandamide | 4.5 (2.4–8.6) | 1.7 (0.9–3.3) | ||
| CP 55940 or WIN 55212-2 alone and in combination with rimonabant | ||||
| CP 55940 | 0.031 (0.017–0.058) | – | ||
| +0.32 Rimonabant | 0.091 (0.053–0.16) | 2.9 (1.4–6.2)a | ||
| WIN 55212-2 | 0.35 (0.22–0.56) | – | ||
| +0.32 Rimonabant | 1.0 (0.59–1.8) | 3.0 (1.5–5.7)a | ||
Dose ratio significantly different from 1, i.e., significant difference in potency
Methanandamide, in contrast to the other cannabinoid agonists, produced limited Δ9-THC-appropriate responding up to doses that significantly decreased rate of responding. For example, maximum Δ9-THC-appropriate responding was a mean (±S.E.M.) of 25 (±12)% at a dose of 10 mg/kg of methanandamide, and larger doses (32 and 56 mg/kg) significantly decreased rate of responding (Fig. 1, second from left). Rate of responding at 56 mg/kg of methanandamide was 24% of the vehicle control rate, and six out of eight mice responded at rates below 20% of the vehicle control rate. A variety of noncannabinoids also produced limited Δ9-THC-appropriate responding. For example, maximum Δ9-THC-appropriate responding was a mean (±S.E.M.) of 34 (±18)% at a dose of 10 mg/kg of ketamine, and larger doses (17.8 and 32 mg/kg) of ketamine significantly decreased rate of responding (Fig. 1, second from right, closed squares). A dose of 17.8 mg/kg of cocaine produced a mean (±S.E.M.) of 9 (±8)% of Δ9-THC-appropriate responses and larger doses (32 and 56 mg/kg) significantly decreased response rate (Fig. 1, second from right, open diamonds). Ethanol produced a maximum average (±S.E.M.) of 25 (±12)% of Δ9-THC-appropriate responses and response rate was markedly decreased at larger doses (1,000 and 3,200 mg/kg; Fig. 1, right, closed diamonds).
Re-determination of the dose–response curve for Δ9-THC at the end of the study (data not shown) demonstrated the potency of Δ9-THC (ED50 value of 3.4 mg/kg; 95% confidence limits of 1.8–6.4 mg/kg) to be not significantly different from its potency established at the beginning of the study.
Antagonism of the discriminative stimulus effects of Δ9-THC by rimonabant and not methanandamide
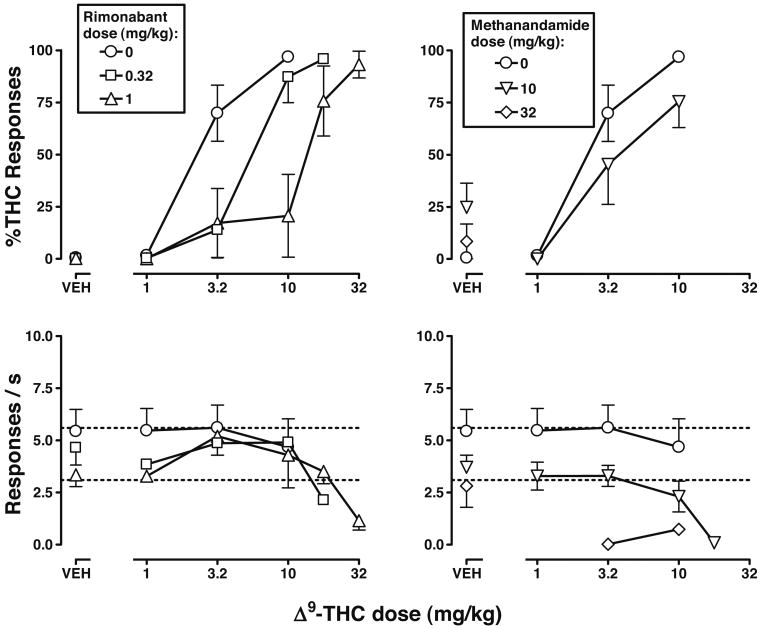
Rimonabant (0.32 and 1 mg/kg) produced a mean of 0% Δ9-THC-appropriate responses and surmountably and dose-dependently antagonized the discriminative stimulus effects of Δ9-THC (Fig. 2, top left). For example, doses of 0.32 and 1 mg/kg of rimonabant significantly increased the ED50 of Δ9-THC by 2.0- and 3.9-fold, respectively (Table 1, middle). Rimonabant alone, at doses of 0.32 and 1 mg/kg, did not significantly decrease rate of responding (Fig. 2, bottom left, VEH) and did not appear to attenuate decreases in response rate produced by larger doses (greater than 10 mg/kg) of Δ9-THC (Fig. 2, bottom left).
Fig. 2.
Effects of Δ9-THC, alone (circles) and in combination with rimonabant (left; squares and triangles) or methanandamide (right; triangles), in C57BL/6J mice discriminating Δ9-THC. Circles, control dose–effect functions for Δ9-THC were re-plotted from Fig. 1. See legend of Fig. 1 for further details
Methanandamide, in contrast to rimonabant, did not significantly modify the discriminative stimulus effects of Δ9-THC (Fig. 2, top right). For example, a dose of 10 mg/kg of methanandamide did not significantly modify the ED50 of Δ9-THC (Table 1, middle). The combined effects of methanandamide and Δ9-THC on rate of responding appeared to be additive. For example, response rate was significantly decreased when methanandamide and Δ9-THC were combined at doses (10 mg/kg for both) that separately did not significantly modify response rate (Fig. 2, bottom right). Moreover, methanandamide (32 mg/kg) slightly decreased response rate when administered alone yet markedly decreased response rate when combined with Δ9-THC at doses (3.2 and 10 mg/kg) that by themselves did not significantly modify response rate.
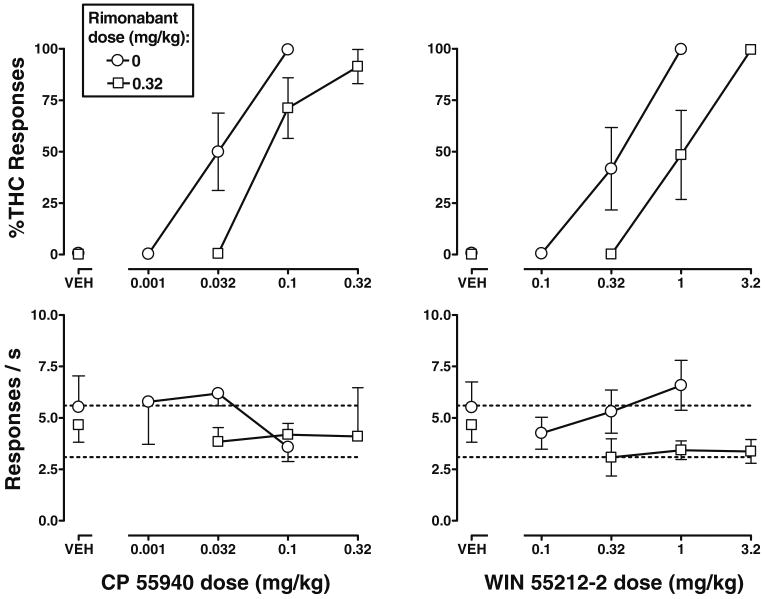
Antagonism of the discriminative stimulus effects of CP 55940 and WIN 55212-2 by rimonabant
Rimonabant (0.32 mg/kg) surmountably antagonized the Δ9-THC-like discriminative stimulus effects of CP 55940 and WIN 55212-2 (Fig. 3, top left and right, respectively), as evidenced by significant 2.9- and 3.0-fold increases in their ED50 values, respectively. Rate of responding was not significantly altered by rimonabant (0.32 mg/kg) in combination with CP 55940 and WIN 55212-2 up to doses of 0.32 and 3.2 mg/kg, respectively (Fig. 3, bottom left and right, respectively).
Fig. 3.
Effects of CP 55940 (left) and WIN 55212-2 (right), alone (circles) and in combination with rimonabant (squares), in C57BL/6J mice discriminating Δ9-THC. See legend to Fig. 1 for details
The potency of rimonabant as an antagonist of discriminative stimulus effects did not vary as a function of cannabinoid agonist. Thus, a dose of 0.32 mg/kg of rimonabant produced the same magnitude of increase in the ED50 values of Δ9-THC, CP 55940 and WIN 55212-2 (2.0-, 2.9-, and 3.0-fold, respectively), as evidenced by overlap in the 95% confidence limits for each of these potency ratios.
Discussion
The behavioral effects of many cannabinoid agonists (e.g., Δ9-THC, CP 55940, and WIN 55212-2) are strikingly similar across different species and procedures including drug discrimination (Wiley 1999 for review; McMahon 2006a); the current study extends these observations to discriminative stimulus effects in C57BL/6J mice discriminating Δ9-THC. CP 55940 and WIN 55212-2 substituted for the discriminative stimulus effects of Δ9-THC, whereas noncannabinoids (cocaine, ethanol, and ketamine) did not substitute for Δ9-THC. The CB1 receptor-selective antagonist rimonabant surmountably antagonized the discriminative stimulus effects of not only Δ9-THC but also the Δ9-THC-like discriminative stimulus effects of CP 55940 and WIN 55212-2, whereas methanandamide did not substitute for or otherwise modify the discriminative stimulus effects of Δ9-THC. Thus, CB1 receptors appear to mediate the discriminative stimulus effects of Δ9-THC, CP 55940, and WIN 55212-2, whereas CB1 and other receptors appear to mediate the effects of methanandamide in mice.
CP 55940 and WIN 55212-2 substituted for the discriminative stimulus effects of Δ9-THC in mice, consistent with previous studies in rats and rhesus monkeys (Gold et al. 1992; Compton et al. 1992; Wiley et al. 1995; McMahon 2006a). The relative potency of cannabinoids to produce discriminative stimulus effects is similar in rats and mice, with Δ9-THC being approximately 100-fold and sixfold less potent than CP 55940 and WIN 55212-2, respectively. Differences in relative potency are smaller in rhesus monkeys, with Δ9-THC being somewhat more potent than WIN 55212-2, perhaps reflecting differences in metabolism of these cannabinoids as a function of species. The qualitatively similar discriminative stimulus effects of Δ9-THC, CP 55940, and WIN 55212-2 across species suggests that their shared mechanism of action (i.e., CB1 receptor agonism; Howlett et al. 2002) is conserved across species. An advantage of drug discrimination is its pharmacologic selectivity relative to other behavioral assays, and the discriminative stimulus effects of Δ9-THC in rats are highly selective for cannabinoid agonist activity (Balster and Prescott 1992). The discriminative stimulus effects of Δ9-THC in mice appear also to be pharmacologically selective insofar as noncannabinoids (cocaine, ethanol, and ketamine) failed to substitute for the Δ9-THC discriminative stimulus up to doses producing other behavioral effects (i.e., decreases in rate of fixed ratio responding).
Rimonabant binds reversibly to cannabinoid CB1 receptors and has little or no efficacy in vitro (Childers 2006), and the effects of rimonabant in mice discriminating Δ9-THC are consistent with this pharmacologic profile. Rimonabant dose-dependently and surmountably antagonized the discriminative stimulus effects of Δ9-THC and also surmountably antagonized the Δ9-THC-like discriminative stimulus effects of CP 55940 and WIN 55212-2. The potency of rimonabant as an antagonist was similar among the agonists, suggesting that the same receptors mediated the discriminative stimulus effects of Δ9-THC, CP 55940, and WIN 55212-2. Rimonabant also has similar potency as an antagonist of the hypothermic and cataleptic effects of Δ9-THC and WIN 55212-2 in C57BL/6J mice (McMahon and Koek 2007). Collectively, the cannabinoid antagonist activity of rimonabant in mice suggests that rimonabant-sensitive receptors have a similar role in mediating the various in vivo effects of Δ9-THC, CP 55940, and WIN 55212-2.
The endogenous cannabinoid CB1 agonist anandamide does not always substitute for the discriminative stimulus effects of Δ9-THC (Wiley et al. 1997; 1998; Järbe et al. 2001). Anandamide is rapidly metabolized by fatty acid amide hydrolase and appears to have limited activity in vivo (Deutsch and Chin 1993). Metabolism of anandamide can be decreased with fatty acid amide hydrolase inhibitors (e.g., URB 597; Fegley et al. 2005) and URB 597 was reported to increase the potency of anandamide to substitute for a Δ9-THC discriminative stimulus in rats (Solinas et al. 2007). Modification of the chemical structure of anandamide has yielded high affinity CB1 agonists with greater metabolic stability than anandamide (e.g., methanandamide; Abadji et al. 1994), and methanandamide has been shown to substitute for the discriminative stimulus effects of Δ9-THC in rats and monkeys (Burkey and Nation 1997; Järbe et al. 1998; McMahon 2006a). Nevertheless, substitution of methanandamide for the discriminative stimulus effects of Δ9-THC is not unanimous (present results).
Intrinsic activity (agonist efficacy) can determine the extent to which agonists acting at the same receptors share behavioral effects and, under conditions requiring relatively high efficacy to achieve a particular behavioral effect, a low-efficacy agonist is less likely to produce the effect obtained with a high efficacy agonist. According to receptor theory, a low-efficacy agonist that does not mimic the effect of a high efficacy agonist can block or antagonize the effect of the high-efficacy agonist (Bergman et al. 2000 for review). The efficacy required for agonist activity in drug discrimination assays apparently can be increased by increasing the training dose of an agonist and a previous study showed that substitution of methanandamide for a Δ9-THC discriminative stimulus decreases as a function of increasing the training dose of Δ9-THC (Järbe et al. 1998). Failure of methanandamide to substitute for Δ9-THC might indicate that methanandamide has lower agonist efficacy than Δ9-THC at CB1 receptors. However, methanandamide did not block the discriminative stimulus effects of Δ9-THC in the current study, indicating that differences in CB1 agonist efficacy were not responsible for the qualitatively different effects of methanandamide and Δ9-THC in mice.
The mechanism by which methanandamide exerts behavioral activity in C57BL/6J mice appears to involve non-CB1 receptors in both the present and previous studies. For example, Δ9-THC was shown to decrease rate of responding for food in wildtype C57BL/6J mice and not transgenic mice lacking CB1 receptors, whereas methanandamide produced similar decreases in rate of responding in both wildtype and CB1 knockout mice (Baskfield et al. 2004). These results do not appear to reflect inability of methanandamide to bind to CB1 receptors in mouse brain inasmuch as a previous study (Petitet et al. 1999) reported that methanandamide binds to cannabinoid receptors in mouse brain after i.p. administration at the doses and interval of pretreatment chosen for the present study. Aside from CB1 receptors, vanilloid VR1 receptors appear to mediate some of the effects of methanandamide (Ross et al. 2001); however, a VR1 receptor antagonist did not attenuate the Δ9-THC-like discriminative stimulus effects of methanandamide in rats (Solinas et al. 2007). Differences in the effects of methanandamide in rats and mice might be due to metabolic factors. For example, methanandamide might be converted to a metabolite that exerts behavioral activity at non-CB1 receptors in mice and the same metabolite might be absent or have less prevalent activity in rats.
In summary, this study shows that Δ9-THC can be readily trained as a discriminative stimulus in mice, and further shows the pharmacologic profile of this discrimination to be similar to that obtained in other mammalian species inasmuch as other cannabinoid agonists (CP 55940 and WIN 55212-2) substitute for the Δ9-THC discriminative stimulus and noncannabinoids do not. The same receptors appear to mediate the discriminative stimulus effects of Δ9-THC, CP 55940, and WIN 55212-2 in mice, as evidenced by a similar potency of rimonabant to antagonize the effects of these agonists. Methanandamide or a behaviorally active metabolite of methanandamide, on the other hand, appears to act at receptors in addition to or instead of CB1 receptors to produce behavioral effects in mice, and it is not clear whether non-CB1 receptors also contribute to the behavioral effects of methanandamide in other mammalian species (e.g., primates). The Δ9-THC discriminative stimulus in mice appears to have utility not only for predicting cannabis-like activity in humans but also for evaluating molecular mechanisms of cannabinoid activity with genetic manipulations such as inducible knockouts.
Acknowledgments
The authors thank G. Martinez for providing technical assistance.
Supported by U.S. Public Health Service Grants DA15468, DA19222, and AA012337.
Contributor Information
Lance R. McMahon, Email: mcmahonl@uthscsa.edu, Department of Pharmacology, The University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900, USA.
Brett C. Ginsburg, Department of Psychiatry, The University of Texas Health Science Center at San Antonio, San Antonio, TX, USA
R. J. Lamb, Department of Pharmacology, The University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900, USA; Department of Psychiatry, The University of Texas Health Science Center at San Antonio, San Antonio, TX, USA
References
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. (R)-methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Δ9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Baskfield CY, Martin BR, Wiley JL. Differential effects of Δ9-tetrahydrocannabinol and methanandamide in CB1 knockout and wild-type mice. J Pharmacol Exp Ther. 2004;309:86–91. doi: 10.1124/jpet.103.055376. [DOI] [PubMed] [Google Scholar]
- Bergman J, France CP, Holtzman SG, Katz JL, Koek W, Stephens DN. Agonist efficacy, drug dependence, and medications development: preclinical evaluation of opioid, dopaminergic, and GABAA-ergic ligands. Psychopharmacology. 2000;153:67–84. doi: 10.1007/s002130000567. [DOI] [PubMed] [Google Scholar]
- Burkey RT, Nation JR. (R)-methanandamide, but not anandamide, substitutes for Δ9-THC in a drug-discrimination procedure. Exp Clin Psychopharmacol. 1997;5:195–202. doi: 10.1037//1064-1297.5.3.195. [DOI] [PubMed] [Google Scholar]
- Chait LD, Evans SM, Grant KA, Kamien JB, Johanson CE, Schuster CR. Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology. 1988;94:206–212. doi: 10.1007/BF00176846. [DOI] [PubMed] [Google Scholar]
- Childers SR. Activation of G-proteins in brain by endogenous and exogenous cannabinoids. AAPS J. 2006;8:E112–E117. doi: 10.1208/aapsj080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1992;263:1118–1126. [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR, Kuhl E, Eckel G. Behavioral effects of cannabinoids show differential sensitivity to cannabinoid receptor blockade and tolerance development. Behav Pharmacol. 2004;15:1–12. doi: 10.1097/00008877-200402000-00001. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Gold LH, Balster RL, Barrett RL, Britt DT, Martin BR. A comparison of the discriminative stimulus properties of delta Δ9-tetrahydrocannabinol and CP 55,940 in rats and rhesus monkeys. J Pharmacol Exp Ther. 1992;262:479–486. [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Henriksson BG. Discriminative response control produced with hashish, tetrahydrocannabinols (Δ8-THC and Δ9-THC), and other drugs. Psychopharmacologia. 1974;40:1–16. doi: 10.1007/BF00429443. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Henriksson BG, Ohlin GC. Δ9-THC as a discriminative cue in pigeons: effects of delta8-THC, CBD, and CBN. Arch Int Pharmacodyn Ther. 1977;228:68–72. [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Makriyannis A, Lin S, Goutopoulos A. Δ9-THC training dose as a determinant for (R)-methanandamide generalization in rats. Psychopharmacology. 1998;140:519–522. doi: 10.1007/s002130050797. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Lin S, Makriyannis A. (R)-methanandamide and Δ9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology. 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Liu Q, Makriyannis A. Antagonism of discriminative stimulus effects of delta(9)-THC and (R)-methanandamide in rats. Psychopharmacology. 2006;184:36–45. doi: 10.1007/s00213-005-0225-y. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Pharmacologic analysis of drug-receptor interaction. Lippincott-Raven; Philadelphia, PA: 1997. [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2006a;319:1211–1218. doi: 10.1124/jpet.106.107110. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Discriminative stimulus effects of the cannabinoid CB1 antagonist SR 141716A in rhesus monkeys pretreated with Δ9-tetrahydrocannabinol. Psychopharmacology. 2006b;188:306–314. doi: 10.1007/s00213-006-0500-6. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Koek W. Differences in the relative potency of SR 141716A and AM 251 as antagonists of various in vivo effects of cannabinoid agonists in C57BL/6J mice. Eur J Pharmacol. 2007 doi: 10.1016/j.ejphar.2007.04.054. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov A, Kellendonk C, Simpson E, Tronche F. Using conditional mutagenesis to study the brain. Biol Psychiatry. 2003;54:1125–1133. doi: 10.1016/s0006-3223(03)00467-0. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- Petitet F, Jeantaud B, Bertrand P, Imperato A. Cannabinoid penetration into mouse brain as determined by ex vivo binding. Eur J Pharmacol. 1999;374:417–421. doi: 10.1016/s0014-2999(99)00189-2. [DOI] [PubMed] [Google Scholar]
- Ross RA, Gibson TM, Brockie HC, Leslie M, Pashmi G, Craib SJ, Di Marzo V, Pertwee RG. Structure–activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br J Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KL. Substitution profiles of N-methyl-D-aspartate antagonists in ethanol-discriminating inbred mice. Alcohol. 2004;34:165–175. doi: 10.1016/j.alcohol.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism and dose–effect data analysis. CRC; Boca Raton, FL: 2000. [Google Scholar]
- Wiley JL. Cannabis: discrimination of “internal bliss”? Pharmacol Biochem Behav. 1999;64:257–260. doi: 10.1016/s0091-3057(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol. 2003;471:185–193. doi: 10.1016/s0014-2999(03)01856-9. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Huffman JW, Balster RL, Martin BR. Pharmacological specificity of the discriminative stimulus effects of Δ9-tetrahydrocannabinol in rhesus monkeys. Drug Alcohol Depend. 1995;40:81–86. doi: 10.1016/0376-8716(95)01193-5. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Golden KM, Ryan WJ, Balster RL, Razdan RK, Martin BR. Evaluation of cannabimimetic discriminative stimulus effects of anandamide and methylated fluoroanandamide in rhesus monkeys. Pharmacol Biochem Behav. 1997;58:1139–1143. doi: 10.1016/s0091-3057(97)00327-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Ryan WJ, Razdan RK, Martin BR. Evaluation of cannabimimetic effects of structural analogs of anandamide in rats. Eur J Pharmacol. 1998;355:113–118. doi: 10.1016/s0014-2999(98)00502-0. [DOI] [PubMed] [Google Scholar]