Abstract
The accessibility of large substrates to buried enzymatic active sites is dependent upon the utilization of proteinaceous channels. The necessity of these channels in the case of small substrates is questionable as diffusion through the protein matrix is often assumed. Copper amine oxidases (CAOs) contain a buried protein-derived quinone cofactor and a mononuclear copper center that catalyze the conversion of two substrates, primary amines and molecular oxygen, to aldehydes and hydrogen peroxide respectively. The nature of molecular oxygen migration to the active site in the enzyme from Hansenula polymorpha2 (HPAO) is explored using a combination of kinetic, X-ray crystallographic and computational approaches. A crystal structure of HPAO in complex with xenon gas, which serves as an experimental probe for molecular oxygen binding sites, reveals buried regions of the enzyme suitable for transient molecular oxygen occupation. Calculated O2 free energy maps using CAO crystal structures in the absence of xenon, correspond well with later experimentally observed xenon sites in these systems, and allow the visualization of O2 migration routes of differing probabilities within the protein matrix. Site-directed mutagenesis designed to block individual routes has little effect on overall kcat/Km[O2], supporting multiple dynamic pathways for molecular oxygen to reach the active site.
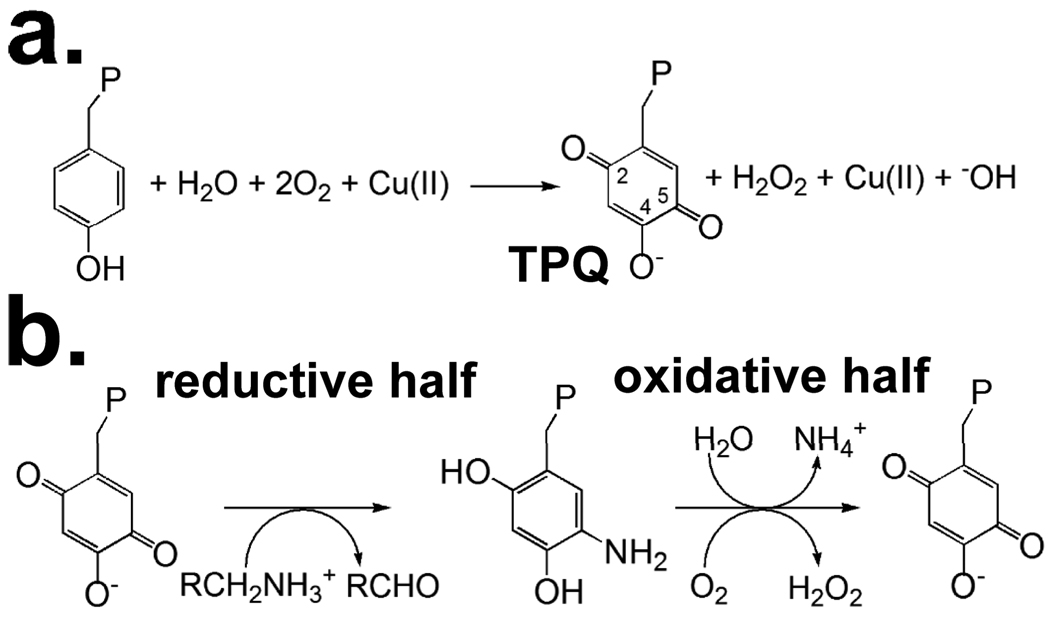
Copper amine oxidases are ubiquitous copper containing enzymes that oxidize primary amines to aldehydes through the reduction of molecular oxygen to hydrogen peroxide. CAO catalysis is dependent upon the protein derived cofactor 2,4,5-trihydroxyphenylalaninequinone (TPQ). The TPQ is derived from an endogenous tyrosine through a self-catalytic process requiring only molecular oxygen and Cu(II) (Fig. 1a) (1).
Figure 1.
Reactions catalyzed by copper amine oxidases are biogenesis (a) and catalysis (b). (a) The protein derived cofactor, 2,4,5-trihydroxyphenylalaninequinone (TPQ) is the product of biogenesis and generates the mature enzyme. P represents the rest of the polypeptide chain. (b) Catalysis is divided into two half-reactions, reductive and oxidative. R represents the moiety of the substrate amine, which varies from a hydrogen atom to a polypeptide.
Hansenula polymorpha amine oxidase is the eukaryotic CAO that has been kinetically characterized in the most detail (2–7). HPAO follows a Bi Bi ping-pong reaction mechanism that can be expressed as two half-reactions, reductive and oxidative (Fig. 1b). In the reductive half-reaction the enzyme oxidizes a primary amine to an aldehyde, generating the 2e− reduced aminoquinol form of the cofactor. In the subsequent oxidative half-reaction molecular oxygen is reduced to hydrogen peroxide via cofactor reoxidation to TPQ. Biochemical studies from several different laboratories have led to mechanistic proposals for the catalytic cycle of CAOs (8,9). These studies have given significant insight into the mechanism for the reductive half-reaction (10). However, the details surrounding the activation of molecular oxygen, both in terms of the biogenesis of the TPQ (11,12) and of the catalytic oxidative half-reaction, remain the subject of intense study (13–16). The utilization of copper as a redox center has been the focus of recent controversy. As CAOs contain a copper ion in their active site, chemical intuition suggests Cu(I) as the O2 activating species to give Cu(II)-superoxide (17). Upon anaerobic amine reduction of CAOs an equilibrium between Cu(II)-aminoquinol and Cu(I)-semiquinone is observed, with Cu(I)-semiquinone yields varying from 0–40 % depending on the enzyme source (18,19). Plant CAOs have high yields of Cu(I)-semiquinone, bacterial CAOs are in the middle of the range, while non-plant eukaryotic CAOs have minimal or undetectable amounts of Cu(I) following amine reduction. It has been postulated that despite the unobservable amount of Cu(I)-semiquinone in many non-plant eukaryotic CAOs, there must still be enough Cu(I) present for this to be the O2 activating species during catalysis. However, Co(II)-substituted HPAO has a kcat almost identical to Cu-HPAO at pH 7 (20,21). The reduction potential for Co(II)/Co(I) (e.g. −0.4 to −0.5V vs. SHE in methionine synthase) (22) makes Co(I) an unlikely intermediate in catalysis, and effectively rules out the requirement for a copper redox change during HPAO catalysis. Additionally, kinetic isotope effects, steady state kinetics, viscosogen and stopped-flow experiments have shown that the reduction of molecular oxygen contributes 29 % to the overall kcat (3,23). This is a surprising result as the reduction of O2 by Cu(I) to give superoxide is likely to be extremely fast (17). Based on these results a new mechanism was proposed for both HPAO and bovine serum amine oxidase (BSAO) that invoked an off-metal molecular oxygen binding pocket for the first electron transfer to O2 directly from the aminoquinol (2). The off-metal binding site at which O2 undergoes the initial 1-electron reduction was proposed to be adjacent to the metal, bounded by the side-chains of M634, L425 and Y407 (24). Site-directed mutagenesis of residue M634 found a size dependence on kcat/Km[O2] in support of this proposal.
Xenon complexation can be used to probe the interior of protein structures for sites that favor molecular oxygen gas binding. Due to its analogous properties in size and hydrophobicity, any region that binds xenon is proposed to also be favorable for O2 (25). In myoglobin, for instance, xenon binding cavities observed crystallographically were also shown to bind photolyzed CO, another dioxygen mimic (26–30). X-ray crystal structures of CAOs bound to Xe are available from bacterial (Arthrobacter globiformis, AGAO), yeast (Pichia pastoris, PPLO), plant (Pisum sativum, PSAO) (31) and mammalian sources (BSAO) (32). An additional solution phase NMR study found that Xe also bound to lentil seedling CAO (Lens esculenta, LSAO), and the data implicated a Xe induced conformational change at the active site during amine reduction (33). In the four available crystal structures there is one consistent xenon site that is ~7 Å from the copper and ~7.5 Å from the TPQ. We term this site the “anteroom” and the residues bordering this pocket in HPAO are Leu425, Tyr407, Phe460, Ile423, Ile622, and Ile639 (Fig. 2). The anteroom is likely too far from either the copper or aminoquinol to be directly involved in the reduction of molecular oxygen to superoxide, but could act as a holding pocket for O2 close to the active site, and is consistent with results which indicate O2 is pre-bound to the enzyme prior to reduction (3,24). The off-metal hydrophobic pocket proposed through mutagenesis studies at Met634 in HPAO is adjacent to the anteroom, and the two pockets share a common residue, Leu425 (Fig. 2) (2). However, a L425A mutant had surprisingly little effect on kcat/Km[O2] (2).
Figure 2.
Modeled regions of xenon binding in HPAO. The orange sphere represents a putative site suggested in HPAO by mutational and kinetic analysis (2). The cyan sphere represents the consensus Xe binding site found in AGAO, PSAO, PPLO and BSAO surrounded by the anteroom residues colored in green (31,32). Waters shown in small red spheres and connected by dashed black lines indicate path from the active site to the inland lake. Figure generated using Pymol (66).
The shortest distance from the anteroom to the protein-solvent boundary in CAOs (~10 Å long) follows a chain of ordered waters from the equatorial water ligand of the copper ion to an area of solvent termed the “inland lake” (Fig. 2) (34,35). The inland lake is conserved at the dimer interface of CAOs, and has been suggested as the entry point for O2 (35). However, as a polar channel often only one water molecule wide, it is hard to reconcile this with the hydrophobic nature of molecular oxygen. To help resolve all these data, we wanted to delineate more clearly molecular oxygen holding pockets in HPAO, as well as define other potential molecular oxygen pathways into the buried active site.
Here we report a crystal structure of HPAO in complex with xenon which offers new insights into CAO-molecular oxygen interactions. Bound xenon in the dense hydrophobic interior of the catalytic domain suggests a putative channel within CAOs accessible to molecular oxygen. Free energy maps of molecular oxygen, computed from dynamics simulations of several CAOs in the absence of xenon, correlate with experimental observations and suggest new pathways that molecular oxygen might take to the buried active site.
Experimental Procedures
HPAO Purification
wtHPAO for crystallization was heterologously expressed in Saccharomyces cerevisiae and purified as described previously (7,36) with modifications. In brief, single colonies from URA− plates incubated at 30 °C were streaked and used to inoculate URA− liquid medium supplemented with 10 µM CuSO4 and a sterile filtered nucleotide/amino acid mixture (5.0 g/l adenine, 5.0 g/l histidine, 7.5 g/l leucine, and 5.0 g/l tryptophan). The preparation of the URA− media contained 1.7 g/l yeast nitrogen base (without amino acids) to minimize the Zn2+ concentration in the media. Previous protocols called for 6.7 g/l yeast nitrogen base (36). Once they had reached an OD600 of 5~6 the cells were collected by centrifugation and ruptured by Bead-Beater (Biospec). The soluble fraction of the freshly lysed cells was not dialyzed as previously reported, but was instead equilibrated into 5 mM potassium phosphate buffer by 10-fold dilution. The diluted soluble fraction was then immediately loaded onto the DEAE anion exchange column. This was followed by size exclusion chromatography on a Sephadex S-300 column. The protein was buffer exchanged into 50 mM HEPES pH 7.0 and concentrated to 13 mg/ml before crystallization.
HPAO Crystallization
Crystallization was performed as previously reported (13 mg/ml HPAO in 8.0–9.5 % PEG 8000, 0.28–0.30 M phosphate at pH 6.0 at 293 K) with the exception of the use of hanging drop crystallization rather than sitting drop (34). The ratio of protein to well solution in the hanging drops was 1:1, with volumes of 2.5 µl, giving a total volume of 5.0 µl. The best diffracting crystals were cube-shaped with dimensions of 40 × 40 × 30 µm and grew within 3 days. The space group of the crystals used in this study was P21, although crystals also grew in the space groups C2 and P212121 from the same crystallization conditions. Cryoprotection was performed by soaking crystals for 10 s in 25 % high purity glycerol (Hampton Research) mixed with mother liquor directly from the crystallization well.
Xenon Complexation
Crystals of HPAO in cryoprotected mother liquor were complexed with xenon using a pressure cell (Rigaku) at a pressure of 10 atm for 10 min. To prevent dehydration of the crystal during the pressurization, a small knot of water-saturated Kimwipe was placed at the bottom of the chamber near where the loop containing the crystal would rest during the pressurization. Before pressurization the crystals were soaked in cryoprotectant. Within 5–10 seconds of depressurization the crystals were flash frozen in liquid nitrogen.
Single Crystal Microspectrophotometry
Single crystal spectra were collected at 100 K using the 4DX Systems AB microspectrophotometer equipped with an MS125™ 1/8 m spectrograph (Thermo Oriel), CCD detector (Andor Technology) and xenon lamp (Zeiss) emitting from 300–800 nm. Spectra were generated from the integration of ten 19 ms exposures (37).
Structure Determination and Refinement
Each X-ray data set was collected from a single crystal at 100 K at the Advanced Photon Source, Argonne National Laboratory (Beamline ID-19, SBC-CAT). High resolution diffraction data were measured at λ = 0.979 Å (Table 1). For the xenon complex, data were collected at two detector distances; one for the optimal collection of high resolution data, and another for the minimization of overlaps at lower resolution. These two data sets were merged for optimal completeness. From the same crystal, an additional lower resolution data set was collected at λ = 1.72 Å to optimally collect anomalous scattering associated with the bound xenon (for Xe f” = 9.0 electrons) (Supplementary Table S1). Data were processed with HKL2000 and SCALEPACK (38). Molecular replacement was performed with MOLREP, part of the CCP4 suite (39) using the previously deposited HPAO model, PDB ID 1a2v. Calculation of the anomalous map was performed using the programs of the CCP4 suite (39). Model building was performed using COOT (40). Refinement of the model was performed using REFMAC5 (41). There was no use of non-crystallographic symmetry restraints during refinement. Waters were placed in the model at peaks > 3.0 σ in the 2Fo−Fc map using ARP waters, part of REFMAC5 (39). Atomic coordinates for the position of xenon atoms were located by 6-fold averaging of the anomalous map. Peaks above 6.5 σ were assigned to xenon in the xenon complex provided that they did not overlap with other anomalous scatterers at this wavelength, including copper and sulfur, and were in agreement with expected hydrophobic backbone and side-chain interactions. Individual Xe occupancies were set at the occupancy that equated Xe B-factors to those of the surrounding protein during refinement, and absence of Fo−Fc electron density.
TABLE 1.
Data collection and refinement statistics
| Native HPAO | Xe-complexed HPAO | |
|---|---|---|
| Data collection | ||
| Detector type | MAR-CCD | MAR-CCD |
| Source | APS | APS |
| Space group | P21 | P21 |
| Unit Cell (Å) | 104.1 × 223.1 × 104.3 | 103.4 × 222.8 × 103.7 |
| β = 95.9° | β = 95.8° | |
| Wavelength (Å) | 0.9785 | 0.9785 |
| Resolution (Å)a | 50-1.70(1.74-1.70) | 50-1.60(1.64-1.60) |
| Measured reflections | 2,007,440 | 1,612,831 |
| Unique reflections | 437,335 | 587,360 |
| Completeness (%)a | 85.0(32.1)b | 96.3(72.9) |
| Rsym (%)a,c | 0.085(0.550) | 0.079(0.373) |
| I/σIa | 14.5(1.4) | 21.4(1.4) |
| Redundancya | 4.6(2.8) | 2.7(1.6) |
| Refinement | ||
| Resolution (Å) | 38.0-1.70 (1.74-1.70) | 47.0-1.60(1.64-1.60) |
| Number of reflections; working set/test set | 437,276/21,965 (12,226/606) | 587,353/29,665 (29,592/1,585) |
| R-factord,a | 14.6(22.6) | 16.3(27.8) |
| R-freee,a | 17.7(27.6) | 18.9(31.9) |
| Protein atoms | 31,432 | 31,594 |
| Water molecules | 5,054 | 3,803f |
| Other atoms | 177 | 293 |
| r.m.s.d. | ||
| Bond lengths (Å) | 0.011 | 0.010 |
| Bond angles (°) | 1.33 | 1.34 |
| Average B-factor (Å2) | 22.7 | 20.2 |
| DPI (Å)g | 0.093 | 0.076 |
Numbers in parentheses represent values in the highest resolution shell.
The data had excellent completeness to 1.91 Å resolution, where overall completeness was 94.2 %, and the data were 94.2 % complete in the 2.02-1.91 Å resolution shell. However, as there are 6 monomers in the asymmetric unit, 2Fo−Fc electron density showed improved atomicity and clarity out to 1.7 Å resolution despite reduced completeness. Thus, data out to 1.7 Å resolution were used in the refinement.
Rsym = Σ|Ihkl − <I>|/ΣIhkl, where Ihkl = observed intensity, and <I> = average intensity obtained from multiple measurements.
R-factor = Σ||Fo| − |Fc||/ Σ|Fo|, where |Fo| = observed structure factor amplitude and |Fc| = calculated structure factor amplitude.
R-free, R factor based on 5% of the data excluded from refinement.
The lower number of waters in this model compared to the native HPAO model is due to disordering of 2nd and 3rd sphere waters at the HPAO surface as a consequence of Xe pressurization, and ~100 waters being displaced by 10 additional ordered glycerols (cryoprotectant) due to the longer time this crystal was soaked in cryoprotectant before freezing (10 mins c.f. 10 s).
Diffraction-component precision indicator based on R-factor (67).
Mutagenesis
Mutations were made to the pDB20-HPAO plasmid (7) using the Stratagene Quick Change kit. Primers were purchased HPLC purified from Operon. The forward primers are given below, the reverse primers were complementary to these. The mutated codon is given in bold and the changed bases are underlined. Sequences were confirmed by automated DNA sequencing (UC Berkeley). The mutated plasmids were transformed into the Saccharomyces cerevisiae cell line CG379 (ATCC) by lithium acetate chemical transformation.
L425F 5′-CAGACTCGACATCAGATTCACCGGTATTCTGAAC-3′
I622Y 5′-CTTCCATACTTTCGGTTACACCCATTTCCCAGCTC-3′
L643F 5′-CCTATCACCTTGATGTTTAGACCTCGGCAC-3′
I639F 5′-GATGCCTGCCGAGCCTTTCACCTTGATGCTTAG-3′
Mutant Characterization
TPQ content was determined by titration against phenylhydrazine in 100 mM KPi pH 7.2 at 30 °C and measuring the change in absorbance at λ = 448 nm using ε = 40,500 M−1 cm−1. TPQ per subunit concentrations were then calculated using a total protein concentration obtained from a Bio-rad protein assay using a Bovine Serum Albumin standard and a MW of 75,700 Da for HPAO. Metal content was determined by ICP-AES using a Perkin-Elmer Optima 3000DV spectrometer, analyzing the Cu wavelengths 327.4 nm, 324.8 nm, 224.7 nm and the Zn wavelengths 206.2 nm, 213.9 nm, 202.5 nm.
Electron Paramagnetic Resonance
Electron paramagnetic resonance (EPR) spectra were collected at 15K using a Varian E9 spectrometer with a scan range of 1790–4790 G, microwave power 5 mW, microwave 9.2450 GHz, modulation amplitude 20 G and receiver gain 32000. A buffer blank spectra was subtracted from sample spectra before further processing.
Steady State Kinetics
Steady state kinetic measurements were carried out by monitoring oxygen consumption using a Clark electrode and a YSI-5300 biological oxygen monitor. Standard conditions were as follows; a final volume of 1 mL, 2 5 °C and reactions were initiated by addition of HPAO. For determinations of kcat/Km[O2] the methylamine (MeA) concentration was kept constant at 5 mM, while for determinations of kcat/Km[MeA] the oxygen concentration was kept constant at 258 µM. Solutions were equilibrated to atmospheric conditions by stirring at 1000 rpm for 5 min just prior to initiation. For reactions at different oxygen concentrations two flow meters were used to regulate the flow of O2 and N2, and the equilibration time was extended to 10 min. For assays in the pH range 6–8 and 8–9, 100 mM KPi and 25 mM pyrophosphate buffers were used respectively. The ionic strength of all buffers was kept constant at 0.3 M by addition of an appropriate amount of KCl. Data were fit directly to the Michaelis-Menten equation and kcat was calculated using the active protein concentration as determined by phenylhydrazine titration. The variation of kcat/Km[O2] with pH best fit to an increase with 2 pKa model using the following equation:
Where x = kcat/Km[O2].
O2 Free Energy Maps
Computational analysis was used to predict the location of preferred pathways taken by O2 to reach the active sites of HPAO, AGAO, PPLO and PSAO. The starting co-ordinates for each simulation were the native oxidized structures, and thus contained no information regarding experimentally determined Xe binding sites. The protein Protein Data Bank (PDB) coordinates (HPAO, 2oov; AGAO, 1w6g; PPLO, 1w7c; PSAO, 1ksi) were first solvated in a 50 mM NaCl water box. If the TPQ was in the inactive “copper-on” conformation (HPAO and PPLO), it was modeled into the active “copper-off” conformation prior to molecular dynamics. Keeping everything else fixed, the solution was then equilibrated for 30 ps, followed by a combination of the solution and protein side-chains for 50 ps, and finally the entire system for 950 ps using the NAMD simulation program (42). Trajectories were then recorded for 10 ns simulations performed at constant pressure (1 atm, Langevin piston) and temperature (300 K, Langevin dynamics), using long-range PME electrostatics, and the CHARMM22 force-field (43) with custom parameters, built by analogy, for the TPQ residue and histidine-copper bonds. The trajectories were used as input for an implicit ligand sampling analysis (44) contained in the VMD software package (45), resulting in detailed 3D free energy profiles for O2 placement inside the protein, based on the assumption that the presence of gas molecules can be treated as a weak perturbation to a protein’s natural thermal motion (46).
RESULTS
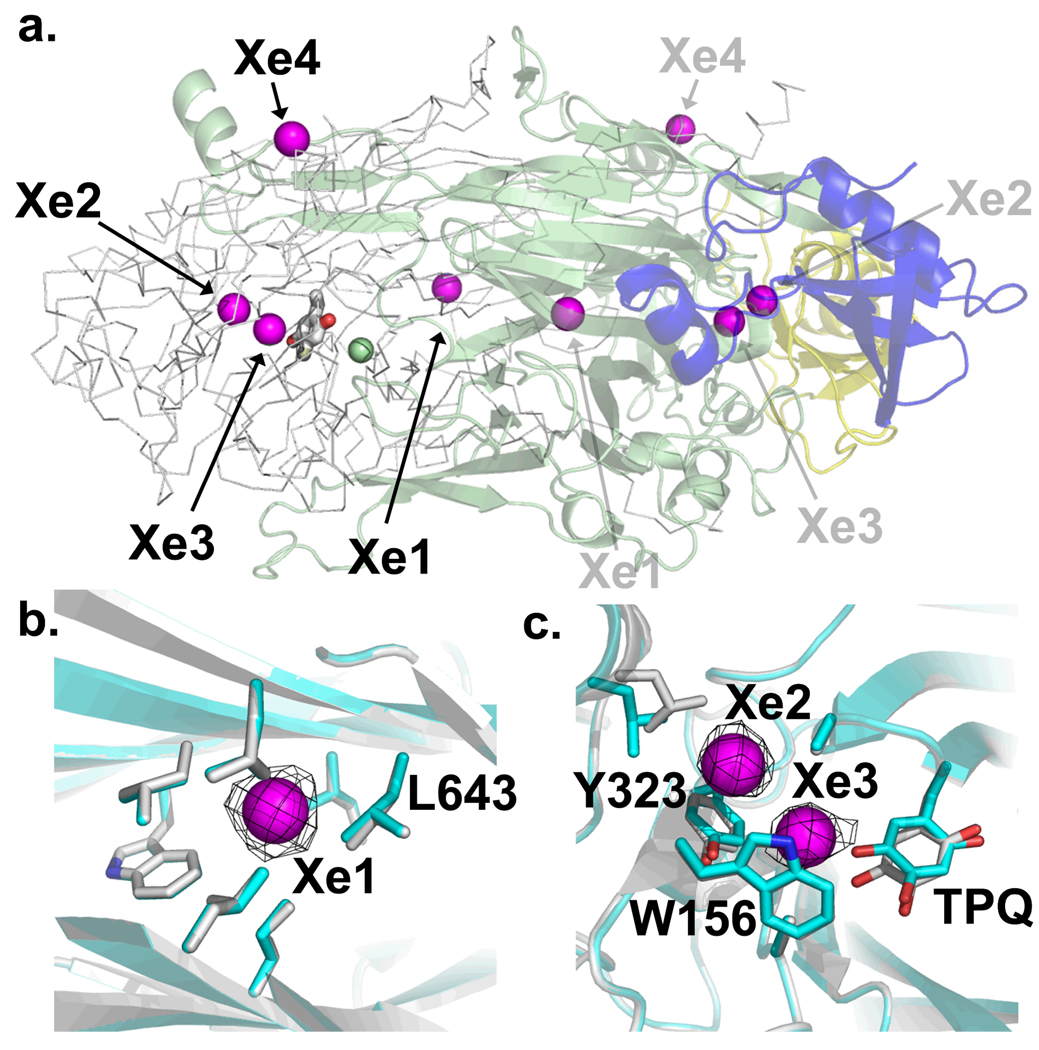
Diffraction data were recorded to 1.60 Å for the complex between xenon and oxidized HPAO (Table 1). Both unaveraged and 6-fold averaged anomalous electron density maps revealed four major xenon binding sites per HPAO monomer (Fig. 3a). Xe1 is the strongest anomalous peak and lies within the center of the large β-sandwich of the catalytic domain (Fig. 3b) (34). Xe2 and Xe3 (Fig. 3c) lie in the amine substrate entry channel that leads from the surface of the molecule to the reactive O5 position of the TPQ (Fig. 1) (47). The weakest anomalous peak, Xe4, was detected near Pro225 at the surface of HPAO. Aside from minor side-chain movements, there are no gross changes in the overall structure due to Xe binding (Figs. 3b and 3c blue/grey comparison). Measurement of the absorbance spectrum from the crystal following X-ray data collection revealed a characteristic 480 nm absorbance peak ensuring that it remained oxidized throughout exposure to the X-irradiation (Supplementary Fig. S2). We have also solved the oxidized structure alone to 1.70 Å resolution in space group P21 (Table 1) for control purposes. Notable structural features include an active “copper-off” TPQ orientation (Fig. 2) in one of the three dimers present in the asymmetric unit, while the other two dimers have mixed TPQ orientations comprised of both active “copper-off” and inactive “copper-on” (TPQ axially ligated to Cu via O4) conformations, and were modeled as such. The copper-off active sites also shows the presence of an oxidized methionine (Met634) near the Cu(II) ion, with no evidence of oxidation at any other methionines in the structure. This site is probably particularly sensitive to radiation damage due to its proximity to the redox active site of the enzyme. It is difficult to judge whether Met634 is oxidized in the subunits with both copper-on and copper-off TPQ conformations because the copper-on form obscures the electron density of that position.
Figure 3.
Xenon binding sites in HPAO. (a) Overall view of the HPAO dimer. One monomer is displayed in cartoon and colored by domain: D1, blue; D2, yellow; and D3, green. The monomer displayed in Cα trace has the TPQ in stick and the copper as a green sphere. Xenons are displayed as magenta spheres and the numbering corresponds to peak size in the anomalous map. (b) Xe1 within the D3 β-sandwich, and (c) Xe2 and Xe3 within the substrate amine channel. The black mesh represents the anomalous map contoured at 9.5 σ. Residues with side-chains within van der Waals contact are displayed. Residues in grey belong to the HPAO + Xe complex, those in blue belong to oxidized HPAO. Figure generated using Pymol (66).
To further investigate the relevance of xenon binding pockets in CAOs in influencing reactivity with molecular oxygen, we designed several point mutations at residues around these sites (Table 2). The residue Leu425 which divides the two proposed O2 binding pockets (Fig. 2), was mutated to phenylalanine to complement the previously studied L425A mutant (2), and investigate the effects of increasing residue size and hydrophobicity at this position. Ile622 is a possible candidate residue for controlling entry of gaseous molecules into the anteroom from the inland lake, and so this was mutated to a tyrosine in an effort to block entry to this site (Fig. 2). The side-chain of Ile639 sits in the anteroom and is comparatively remote from the copper, so this residue was mutated to a phenylalanine in an attempt to restrict gas binding (Fig. 2). Finally a L643F mutation was made at the Xe1 site in the hydrophobic core of the catalytic domain in an attempt to disrupt gas conduction through this region (Fig. 3b).
TABLE 2.
Steady state kinetic measurements on wild-type HPAO and mutants.a
| Enzyme Form |
kcat (s−1) | kcat/Km[MeA] (µM−1 s−1) | kcat/Km[O2](µM−1 s−1) |
|---|---|---|---|
| Wt | 6.2 ± 0.5 | 0.18 ± 0.04 | 0.80 ± 0.10 |
| L425F | 4.9 ± 0.1 | 0.15 ± 0.02 | 0.077 ± 0.007 |
| I622Y | 5.3 ± 0.1 | 0.091 ± 0.01 | 0.69 ± 0.10 |
| I639F | 5.3 ± 0.1 | 0.17 ± 0.02 | 1.2 ± 0.2 |
| L643F | 4.5 ± 0.4 | 0.13 ± 0.02 | 0.76 ± 0.12 |
All measurements carried out at pH 8 and 25°C.
All four mutants had approximately 1 Cu and 0.5 TPQ per subunit as for wtHPAO, except I622Y which had 1 Cu and 0.1 TPQ per subunit (Supplementary Table S3). The Cu(II) EPR spectra of L425F, I639F and I622Y were all similar to wtHPAO, indicating that the primary coordination environment of the copper had not been perturbed (Supplementary Table S4). The limiting rate constant, kcat, for all the mutants were reasonably similar to that of wild-type (wt), with the slowest being down not more than 2-fold. The value of kcat/Km[MeA] was similar to that of wtHPAO for all four mutants, indicating little change in the reductive half-reaction. This is to be expected as these mutations are remote from the site where the chemistry for this step occurs. When kcat/Km[O2] was measured at pH 8, I639F, I622Y and L643F gave very similar values to wtHPAO, but that for L425F was down by an order of magnitude (Table 2). To see if the I639F and L425F mutations were affecting the active site or the anteroom, kcat/Km[O2] was measured as a function of pH (Supplementary Fig. S5). Previously, the variation of kcat/Km[O2] with pH for wtHPAO was found to fit a 2 pKa model, with both deprotonations increasing the rate (3). The pKa values of 6.8 ± 0.1 and 7.9 ± 0.1 have been assigned to the protonated form of the aminoquinol cofactor and the Cu-OH2 respectively. For both L425F and I639F, the data fit well to a 2pKa model. For I639F the pKa’s (6.8 ± 0.3 and 7.7 ± 0.2) were essentially the same as wtHPAO while for L425F they were both up by 0.5 pH units (7.3 ± 0.2 and 8.4 ± 0.1). The altered pKa values for L425F indicate that this mutation is directly influencing the active site while that of I639F is not.
The migration of molecular oxygen through CAOs from a protein-wide perspective was explored using a computational method called implicit ligand sampling (44), developed for finding gas migration pathways inside proteins. This method takes advantage of the fact that O2 travels along transient cavities in the protein that continuously form and disappear over relatively short timescales (1 ns). By monitoring these transient cavities as they appear over a 10ns molecular dynamics window in the protein, even in the absence of O2, the potential of mean force (PMF) corresponding to the placement of O2 everywhere inside the protein can be computed (44). The result is a complete three-dimensional map of the free energy of placing an O2 molecule anywhere inside a protein. Networks of potential O2 entry and exit pathways inside the protein are inferred by connecting favorable areas for O2 that are in close proximity to one another forming gas migration pathways. Since the thermal fluctuations responsible for the migration of gas molecules such as O2 inside proteins are well sampled during the simulation time used (10 ns), the computed O2 pathways are highly probable (with the caveat that possible O2 pathways which are caused by rare events may not be identified by this method).
This approach has been applied here to HPAO, AGAO, PPLO and PSAO to correlate the experimentally observed xenon sites with calculated pathways (Fig. 4 and Supplementary Data Files pmf-xxxx-o2-10ns.dx [O2 free energy maps] and rotated-xxxx-equilibrated.pdb [rotated co-ordinates to match maps], where xxxx = hpao, agao, pplo, psao). Although the starting models in each case had TPQ modeled in the active copper-off conformation, where TPQ hydrogen bonds via its O2 position to the axial water ligand of the copper (Fig. 4c), in AGAO and PPLO the TPQ migrated onto the copper during the 1 ns equilibration that is part of the free energy calculation (Fig. 4d, e). This copper-on form, in which the TPQ displaces the axial water ligand to directly ligate to the copper via its O4 position, is a common conformation observed in CAO crystal structures (including the HPAO structures presented here), and is required for the biogenesis of TPQ (35,48). None of the calculated pathways were affected by this change in TPQ conformation. The location of experimental Xe binding correlates well with the locations of minimum free energy computed from implicit ligand sampling on a 10 ns molecular dynamics simulation in each CAO (Supplementary Figure S6). The only exceptions are surface bound Xe sites (Xe4 in HPAO and Xe903 in AGAO). In addition, implicit ligand sampling provides a complete three-dimensional map for the migration of O2 between favorable regions. This map is created by contouring energy isosurfaces representing elevated PMF values (1.8 and 3.0 kcal/mol, Fig. 4). In HPAO, we identify two major regions that contain the most probable pathways from protein solvent boundary to the buried active site according to the implicit ligand sampling calculation (Fig. 4b). One starts from the amine entry channel close to the 5 position of the TPQ cofactor where the reductive half-reaction chemistry is known to take place (left pathway). Another leads through the hydrophobic core of the β-sandwich toward the active site (right pathway). The two pathways merge near the active site and there is an energetically favorable O2 docking site which could play a role in pre-binding O2 in HPAO prior to its entering the active site for activation (Fig. 4c). This is distinct from the previously described anteroom, and is consistent with the lack of an anteroom Xe binding site in HPAO. The PMF maps of AGAO, PPLO and PSAO have a larger (less favorable) PMF value in the area of the HPAO docking site, and in these enzymes the anteroom is the most favorable area close to the active site for pre-binding O2. The AGAO PMF maps suggest that the most favorable migration route is from the D3 β-sandwich hydrophobic core through the anteroom to the active site, which is similar to that suggested by the HPAO PMF maps (Fig 4d). In PPLO and PSAO passage via this route appears much less favorable (Fig 4e, f). In all cases the short polar channel that exists between the inland lake and the active site, which had previously been proposed as the probable entry site for molecular oxygen, is not the only pathway between these two areas (35). Rather, there are connections from the inland lake to the anteroom, particularly in PPLO and PSAO (Fig. 4e, f) where an equally favorable route appears to occur near, but distinct from, the previously described polar channel.
Figure 4.
Implicit ligand sampling performed in CAOs with a molecular oxygen probe. (a) A ribbon representation of HPAO that is indicative of the orientation and slice depth of the subsequent PMF maps. Regions discussed in the text are indicated. PMF maps of (b) HPAO, (d) AGAO (e) PPLO and (f) PSAO. Surfaces: red opaque represents a 0 kcal/mol isosurface; binding regions for molecular oxygen. Blue transparent represents a 1.8 kcal/mol isosurface; regions of molecular oxygen occupancy more favorable than occupation in solvent. Black mesh represents a 3.0 kcal/mol isosurface; lowest energy pathways between regions of binding. The grey/black surface represents the monomer surfaces. Most favorable routes for O2 from the surface are marked by green lines. Residues shown include the TPQ and histidine ligands of the Cu(II) (shown as a green sphere). The box in the HPAO map (b) is enlarged (c) to show a close up of the HPAO active site with PMF map displayed. Surrounding residues are colored in purple and green corresponding to the energetically favorable HPAO O2 binding site and the anteroom, respectively. Figure (a) and (c) generated using Pymol (66). Figures (b, d, e, f) generated using VMD (45).
DISCUSSION
The location of Xe1 in the HPAO + Xe complex has revealed the presence of a binding region that is not accessed by a traditionally defined channel. Xe1 represents ligand binding in a solvent inaccessible void. The puzzling nature of this singular site in HPAO provoked us to look for similar sites in other CAOs. In examining a composite overlay of all available xenon complex data, totaling four different species, we were immediately struck by the chain of Xe atoms that occupied the hydrophobic core of the catalytic β-sandwich (Fig. 5). The overlaid sites are not coincident, yet they appear to mark a highly favorable area for a small hydrophobic molecule, such as molecular oxygen, to reside. This chain of Xe atoms also appears to indicate a pathway whereby molecular oxygen might enter at either end of the catalytic β-sandwich and then proceed toward the active site, passing through or close by the anteroom along the way (arrows, Fig. 5b). Indeed, the lining of the catalytic β-sandwich with larger hydrophobic residues well suits it for the favorable accommodation and conduction of molecular oxygen (Fig. 3b). Large hydrophobic side-chains, like those found in this region, are highly flexible and mobile, offering a higher propensity for packing defects that allow the passage of small hydrophobic molecules. The sequence conservation in this region for the CAOs is similar to that seen for any large protein hydrophobic core, suggesting there is nothing special about the CAO catalytic β-sandwich in terms of O2 migration.
Figure 5.
Comparison of CAO xenon binding sites. (a) Overlay of CAO xenon sites deposited in the PDB with a monomer of the HPAO/Xe complex. The backbone of HPAO is displayed and colored by domain: D1, blue stick; D2, yellow stick, D3 green ribbon. Xenon sites from individual complexes are coded by color: in red, PSAO [1w2z]; yellow, PPLO [1rky]; blue, AGAO [1rjo]; magenta, HPAO [2oqe], except surface bound xenon sites, which are displayed as grey spheres (31). The top inset shows the amine channel. The bottom inset shows a proposed molecular oxygen pathway identified in this study. The active site is shown in stick and the copper is shown as a green sphere. (b) The HPAO D3 β-sandwich domain viewed from the dimer interface (90° rotation c.f. view in (a)). Only the xenon sites in the internal D3 β-sandwich and close to the anteroom are shown. Colors are the same as in (a) and arrows indicate the direction of molecular oxygen movement to the active site using the proposed pathway. Figure generated using Pymol (66).
An additional striking feature of the CAO + Xe composite overlay was the presence of Xe atoms in the amine channel. In the HPAO + Xe complex the amine channel supports the binding of two atoms of xenon in place of ordered waters normally found in the oxidized structure (Fig. 3c & 5a, upper inset). AGAO provides an additional site, nearer the surface of the enzyme. As part of the amine channel, these observed sites are on the opposite side of the cofactor from the location of dioxygen chemistry (49). In addition to marking a region suitable for O2 binding, the closest peak to the TPQ in HPAO, Xe3, probably indicates a favorable binding site for the aliphatic portion of the small amine substrates favored by this CAO, and helps orient the substrate for correct nucleophilic attack at the C5 of TPQ. In HPAO, orientation of substrate would be mediated through a favorable hydrophobic interaction with Trp156 (Fig. 3c). By structural overlay, the position of Trp156 aligns it with gating residues found in other CAOs (35). CAOs generally have a tyrosine or a phenylalanine at this position that is mobile and acts like a “gate” between the amine channel and the TPQ (32,35,50–55). In the case of ECAO, Tyr381 is known to stack on the aromatic portion of its preferred aromatic monoamine substrates, and has a stabilizing and orienting effect (54). Since no side-chain movement is involved in the binding of Xe2 in HPAO, Trp156 does not seem to act as a gate per se, being already in the open conformation (Fig 3c).
The analysis of putative molecular oxygen pathways was taken one step further by the generation of potential of mean force (PMF) maps through implicit ligand sampling analysis. PMF maps reveal two important features present in protein structures. The first feature is the location of hydrophobic binding sites in CAOs that are favorable for small hydrophobic molecules and which may or may not correspond to preexisting static cavities. This is accomplished by generating surfaces at the 0 kcal/mol energy level, which correspond to areas of the protein where the free energy of placing O2 is more favorable than in a vacuum of the same volume. The second feature is the lowest energy route available between determined binding sites by generating isosurfaces at elevated PMF levels. The free energy map calculation in HPAO confirms with excellent fidelity the experimentally observed xenon binding sites across CAOs within the core of the catalytic β-sandwich (Supplementary Figure S6). By elucidating several regions within the catalytic β-sandwich (D3 domain) that are favorable and accessible to molecular oxygen binding we can visualize a series of binding sites potentially used en route to the active site (Fig. 4b). Similar binding possibilities in the β-sandwich are seen in map calculations for AGAO, PPLO and PSAO, with a route being most apparent in AGAO (Fig. 4d). The molecular oxygen pathways vary slightly between CAOs, especially as they near the active site, and suggest that use of the anteroom is not an absolute requirement. However in PPLO, PSAO and AGAO, where utilization of the anteroom has been suggested by Xe complexation, the free energy maps suggest a high probability for O2 binding in that region (31).
The HPAO PMF maps revealed an equally energetically favored O2 pathway to the buried active site through the amine channel. In the case of a partially polar molecule, such as the amine, this pathway terminates near the O5 position of the cofactor. However, when considering O2, PMF maps show that the path instead continues past the backside of the TPQ and merges with the β-sandwich route near an HPAO specific binding site (Fig. 4c). Due probably to HPAO’s narrow amine channel structure and the presence of some hydrophobic residues, this channel is equally suited to conduct O2 as well as amine substrate. This again differs from AGAO, PSAO and PPLO whose amine channel PMF maps do not indicate such a high binding potential for O2 (Fig. 4d, e, f) and relates well to the fact that HPAO is the only characterized CAO with a specific preference for small aliphatic primary amines (ethylamine > methylamine >> benzylamine) (51,56). Modeling of methylamine and ethylamine Schiff base intermediates from the reductive half-reaction did not interfere with the proposed O2 pathway from the amine channel, although the Schiff base with benzylamine did (54,57). However, since benzylamine is a poor substrate for the enzyme, with a 100-fold decrease in kcat compared to ethylamine, it is not thought to be a physiologically relevant substrate (58). Most of the decrease in kcat comes from the reductive half-reaction (Fig. 1b), with the benzylamine kcat/Km[O2] only down 5-fold compared to ethylamine (58). So for the preferred small aliphatic substrates of HPAO, there appears to be no step of the catalytic cycle when the amine channel would not be available for O2 migration.
Originally, the entry point for molecular oxygen to the active site of CAOs was proposed to be from the inland lake (32,34,59–61). Since the PMF maps confirm this region as a prime reservoir for molecular oxygen, it seems likely it could act as a source of O2. The previously proposed narrow polar channel that has been described between the active site and the inland lake does appear to have some probability of acting as an entry point for O2 in AGAO, PSAO and PPLO based on the implicit ligand sampling analysis, although other connections from the inland lake are also evident (Fig. 4e, f). In the case of HPAO, however, this path is energetically unfavorable for O2 (Fig. 4b, purple marking). As noted by Duff et al., if molecular oxygen accesses the anteroom from the polar channel linking the inland lake to the active site, it would need to first pass through the active site and by the probable site of O2 activation. This route seems at odds with one meant to deliver O2 to a site of pre-binding prior to activation at the active site (31). These factors cause us to favor describing the previous proposal primarily as an exit channel for the product H2O2, benefiting both from its polar nature and short length, which reduces the possibility for oxidative damage to the enzyme during exit of this reactive molecule (35).
In this study, site directed mutagenesis of anteroom residues I639F and I622F in HPAO that were designed to minimize or eliminate space for the binding or passage of molecular oxygen through the anteroom lacked any difference in kcat/Km[O2] values. Taken together, the I639F and I622Y mutations, the PMF maps and the lack of xenon in this region imply that the anteroom is not a significant region for O2 pre-binding in HPAO. This is different from AGAO, PSAO and PPLO where xenon complex data and implicit ligand sampling support a role for the anteroom (31). The L643F mutant of this study, which was designed to alter the side-chain size and flexibility within the hydrophobic core of the catalytic β-sandwich at the HPAO Xe1 site also had no effect on O2 kinetics. This is consistent with the probability that there are multiple paths to the active site, including in HPAO via the substrate amine channel. Further studies are required to assess how important the favorable area within the D3 β-sandwich is for O2 channeling in HPAO, but our results indicate that single or even double point mutants may do little to significantly affect oxygen kinetics. The L425F mutant, whose bulk may affect the ability of O2 to migrate to the site of activation, is the only one in this study to have a significant impact on kcat/Km[O2]. However, in light of the fact that this residue raises key pKa’s within the active site by ~ 0.5 pH units, its effect may be complex in terms of the active site chemistry.
Kinetic data support O2 being pre-bound to the enzyme prior to activation (3,24) which could involve one or more favorable holding areas for molecular oxygen distinct from the site of O2 activation. We propose that the catalytic β-sandwich and amine channel, in addition to the inland lake, serve as reservoirs of molecular oxygen, containing multiple pre-binding sites en route to the active site. In this way, multiple regions of the protein act as internal reservoirs for small hydrophobic gases, continuously supplying the active site with molecular oxygen. The apparent flexibility in approach routes for molecular oxygen in the different CAOs suggests that initial entry may not be at a single location. Instead, it is better viewed as pathways of differing probabilities where O2 is transiently held prior to movement to the pocket containing Met634, which still remains the best candidate site for off-copper activation.
The accumulated evidence from crystallography, computational and biochemical studies on several different protein systems supports the notion that there are specific pathways for gas migration through the protein matrix (25,62–65). The combination of xenon complexation and implicit ligand sampling calculations on CAOs agrees with this premise, revealing that molecular oxygen migration appears to follow preferred routes. There is some conservation in the pathways for molecular oxygen migration in CAOs, although their importance varies from one species to another. In particular, the hydrophobic core of the D3 catalytic β-sandwich can act as a reservoir for molecular oxygen, with O2 then moving into the vicinity of the active site for eventual activation. The presence of routes is largely dictated by the overall architecture of the protein, where certain secondary and tertiary structural folds, such as the hydrophobic core of the β-sandwich found in CAOs, can create favorable reservoirs for small hydrophobic molecules such as molecular oxygen. Although the overall fold is conserved in the catalytic β-sandwich, differences in the primary amino acid sequence probably account for the species specific differences in the pathways. Implicit ligand sampling analysis, by monitoring transient level of cavity formation, allows the accurate prediction of hydrophobic binding regions that match with high fidelity the experimentally observed xenon binding sites. Because there appear to be multiple routes in any given CAO, it is unlikely that a single site-directed mutant is going to impact molecular oxygen access, and this fits with the data in this study. Molecular oxygen pathways, unlike many other substrate channels, seem to rely on a delicate balance of tertiary structure, side-chain hydrophobicity, and conformational flexibility to allow the efficient transport of this small hydrophobic molecule.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Teresa De la Mora-Rey for help during X-ray data collection, as well as the staff of SBC-CAT, especially Steve Ginell. We would also like to thank Patton Fast of the University of Minnesota Supercomputing Institute for computer support.
The abbreviations used are
- CAO
copper amine oxidase
- TPQ
2,4,5-trihydroxyphenylalaninequinone
- HPAO
Hansenula polymorpha amine oxidase
- AGAO
Arthrobacter globiformis amine oxidase
- PPLO
Pichia pastoris lysyl oxidase
- PSAO
pea seedling amine oxidase
- Xe
xenon
- EPR
electron paramagnetic resonance
- PDB
Protein Data Bank
- wt
wild-type
- PMF
potential of mean force
- MeA
methylamine
Footnotes
This work was support by NIH Training Grant GM-008700 (B.J.J), and by NIH grants GM-66569 (C.M.W.), GM-25765 (J.P.K.) and P41-RR05969 (K.S.), and by Minnesota Medical Foundation grant 3714-9221-06 (C.M.W.), and by NSF supercomputer time grant NRAC MCA93S028. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under Contract No. W-31-109-Eng-38. In-house X-ray data collection was supported by a Minnesota Partnership for Biotechnology and Medical Genomics Grant (SPAP-05-0013-PFY06). Computer resources were provided by the Basic Sciences Computing Laboratory of the University of Minnesota Supercomputing Institute.
The atomic coordinates and structure factors for the xenon complex with oxidized HPAO and oxidized HPAO alone (PDB # 2oqe and PDB # 2oov respectively) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
Hansenula polymorpha has been reclassified as Pichia angusta. The names Hansenula polymorpha and HPAO are used to correspond with past literature.
REFERENCES
- 1.Janes SM, Mu D, Wemmer D, Smith AJ, Kaur S, Maltby D, Burlingame AL, Klinman JP. Science. 1990;248(4958):981–987. doi: 10.1126/science.2111581. [DOI] [PubMed] [Google Scholar]
- 2.Goto Y, Klinman JP. Biochemistry. 2002;41(46):13637–13643. doi: 10.1021/bi0204591. [DOI] [PubMed] [Google Scholar]
- 3.Mills SA, Goto Y, Su Q, Plastino J, Klinman JP. Biochemistry. 2002;41(34):10577–10584. doi: 10.1021/bi0200864. [DOI] [PubMed] [Google Scholar]
- 4.Plastino J, Green EL, Sanders-Loehr J, Klinman JP. Biochemistry. 1999;38(26):8204–8216. doi: 10.1021/bi9826660. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz B, Olgin AK, Klinman JP. Biochemistry. 2001;40(9):2954–2963. doi: 10.1021/bi0021378. [DOI] [PubMed] [Google Scholar]
- 6.Mills SA, Klinman JP. J. Am. Chem. Soc. 2000;122(41):9897–9904. [Google Scholar]
- 7.Cai D, Klinman JP. Biochemistry. 1994;33(24):7647–7653. doi: 10.1021/bi00190a019. [DOI] [PubMed] [Google Scholar]
- 8.Mure M, Mills SA, Klinman JP. Biochemistry. 2002;41(30):9269–9278. doi: 10.1021/bi020246b. [DOI] [PubMed] [Google Scholar]
- 9.Dooley DM. J Biol Inorg Chem. 1999;4(1):1–11. doi: 10.1007/s007750050283. [DOI] [PubMed] [Google Scholar]
- 10.Klinman JP. Chem Rev. 1996;96(7):2541–2562. doi: 10.1021/cr950047g. [DOI] [PubMed] [Google Scholar]
- 11.Okajima T, Kishishita S, Chiu YC, Murakawa T, Kim M, Yamaguchi H, Hirota S, Kuroda S, Tanizawa K. Biochemistry. 2005;44(36):12041–12048. doi: 10.1021/bi051070r. [DOI] [PubMed] [Google Scholar]
- 12.Samuels NM, Klinman JP. Biochemistry. 2005;44(43):14308–14317. doi: 10.1021/bi051176m. [DOI] [PubMed] [Google Scholar]
- 13.Dove JE, Klinman JP. Adv Protein Chem. 2001;58:141–174. doi: 10.1016/s0065-3233(01)58004-3. [DOI] [PubMed] [Google Scholar]
- 14.Dooley DM, Scott RA, Knowles PF, Colangelo CM, McGuirl MA, Brown DE. J. Am. Chem. Soc. 1998;120(11):2599–2605. [Google Scholar]
- 15.Hirota S, Iwamoto T, Kishishita S, Okajima T, Yamauchi O, Tanizawa K. Biochemistry. 2001;40(51):15789–15796. doi: 10.1021/bi011631o. [DOI] [PubMed] [Google Scholar]
- 16.Padiglia A, Medda R, Lorrai A, Paci M, Pedersen JZ, Boffi A, Bellelli A, Agro AF, Floris G. Eur J Biochem. 2001;268(17):4686–4697. doi: 10.1046/j.1432-1327.2001.02390.x. [DOI] [PubMed] [Google Scholar]
- 17.Lewis EA, Tolman WB. Chem Rev. 2004;104(2):1047–1076. doi: 10.1021/cr020633r. [DOI] [PubMed] [Google Scholar]
- 18.Dooley DM, McGuirl MA, Brown DE, Turowski PN, McIntire WS, Knowles PF. Nature. 1991;349(6306):262–264. doi: 10.1038/349262a0. [DOI] [PubMed] [Google Scholar]
- 19.Medda R, Padiglia A, Bellelli A, Pedersen JZ, Agro AF, Floris G. Febs Letters. 1999;453(1–2):1–5. doi: 10.1016/s0014-5793(99)00675-4. [DOI] [PubMed] [Google Scholar]
- 20.Mills SA, Klinman JP. Journal of the American Chemical Society. 2000;122(41):9897–9904. [Google Scholar]
- 21.Mills SA, Goto Y, Su QJ, Plastino J, Klinman JP. Biochemistry. 2002;41(34):10577–10584. doi: 10.1021/bi0200864. [DOI] [PubMed] [Google Scholar]
- 22.Drummond JT, Matthews RG. Biochemistry. 1994;33(12):3732–3741. doi: 10.1021/bi00178a033. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Klinman JP. Biochemistry. 2006;45(14):4683–4694. doi: 10.1021/bi0521893. [DOI] [PubMed] [Google Scholar]
- 24.Su Q, Klinman JP. Biochemistry. 1998;37(36):12513–12525. doi: 10.1021/bi981103l. [DOI] [PubMed] [Google Scholar]
- 25.Scott EE, Gibson QH, Olson JS. J Biol Chem. 2001;276(7):5177–5188. doi: 10.1074/jbc.M008282200. [DOI] [PubMed] [Google Scholar]
- 26.Bourgeois D, Vallone B, Schotte F, Arcovito A, Miele AE, Sciara G, Wulff M, Anfinrud P, Brunori M. Proc Natl Acad Sci U S A. 2003;100(15):8704–8709. doi: 10.1073/pnas.1430900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt M, Nienhaus K, Pahl R, Krasselt A, Anderson S, Parak F, Nienhaus GU, Srajer V. Proc Natl Acad Sci U S A. 2005;102(33):11704–11709. doi: 10.1073/pnas.0504932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schotte F, Lim M, Jackson TA, Smirnov AV, Soman J, Olson JS, Phillips GN, Jr, Wulff M, Anfinrud PA. Science. 2003;300(5627):1944–1947. doi: 10.1126/science.1078797. [DOI] [PubMed] [Google Scholar]
- 29.Srajer V, Ren Z, Teng TY, Schmidt M, Ursby T, Bourgeois D, Pradervand C, Schildkamp W, Wulff M, Moffat K. Biochemistry. 2001;40(46):13802–13815. doi: 10.1021/bi010715u. [DOI] [PubMed] [Google Scholar]
- 30.Srajer V, Teng T, Ursby T, Pradervand C, Ren Z, Adachi S, Schildkamp W, Bourgeois D, Wulff M, Moffat K. Science. 1996;274(5293):1726–1729. doi: 10.1126/science.274.5293.1726. [DOI] [PubMed] [Google Scholar]
- 31.Duff AP, Trambaiolo DM, Cohen AE, Ellis PJ, Juda GA, Shepard EM, Langley DB, Dooley DM, Freeman HC, Guss JM. J Mol Biol. 2004;344(3):599–607. doi: 10.1016/j.jmb.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 32.Lunelli M, Di Paolo ML, Biadene M, Calderone V, Battistutta R, Scarpa M, Rigo A, Zanotti G. J Mol Biol. 2005;346(4):991–1004. doi: 10.1016/j.jmb.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 33.Medda R, Mura A, Longu S, Anedda R, Padiglia A, Casu M, Floris G. Biochimie. 2006;88(7):827–835. doi: 10.1016/j.biochi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Li R, Klinman JP, Mathews FS. Structure. 1998;6(3):293–307. doi: 10.1016/s0969-2126(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 35.Wilce MC, Dooley DM, Freeman HC, Guss JM, Matsunami H, McIntire WS, Ruggiero CE, Tanizawa K, Yamaguchi H. Biochemistry. 1997;36(51):16116–16133. doi: 10.1021/bi971797i. [DOI] [PubMed] [Google Scholar]
- 36.DuBois JL, Klinman JP. Methods Enzymol. 2004;378:17–31. doi: 10.1016/S0076-6879(04)78002-7. [DOI] [PubMed] [Google Scholar]
- 37.Hadfield A, Hajdu J. J. Appl. Crystallogr. 1993;26:839–842. [Google Scholar]
- 38.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 39.Acta Crystallogr D Biol Crystallogr. 1994;50(Pt 5):760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 40.Emsley P, Cowtan K. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 41.Murshudov GN, Vagin AA, Dodson EJ. Acta Crystallogr D Biol Crystallogr. 1997;53(Pt 3):240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 42.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. J Comput Chem. 2005;26(16):1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M. J. Phys. Chem. B. 1998;102(18):3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 44.Cohen J, Arkhipov A, Braun R, Schulten K. Biophys J. 2006;91(5):1844–1857. doi: 10.1529/biophysj.106.085746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Humphrey W, Dalke A, Schulten K. J Mol Graph. 1996;14(1):33–38. 27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 46.Cohen J, Kim K, King P, Seibert M, Schulten K. Structure. 2005;13(9):1321–1329. doi: 10.1016/j.str.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Mure M, Klinman JP. J Am Chem Soc. 1993;115(16):7117–7127. [Google Scholar]
- 48.Kim M, Okajima T, Kishishita S, Yoshimura M, Kawamori A, Tanizawa K, Yamaguchi H. Nat Struct Biol. 2002;9(8):591–596. doi: 10.1038/nsb824. [DOI] [PubMed] [Google Scholar]
- 49.Wilmot CM, Hajdu J, McPherson MJ, Knowles PF, Phillips SE. Science. 1999;286(5445):1724–1728. doi: 10.1126/science.286.5445.1724. [DOI] [PubMed] [Google Scholar]
- 50.Airenne TT, Nymalm Y, Kidron H, Smith DJ, Pihlavisto M, Salmi M, Jalkanen S, Johnson MS, Salminen TA. Protein Sci. 2005;14(8):1964–1974. doi: 10.1110/ps.051438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duff AP, Cohen AE, Ellis PJ, Kuchar JA, Langley DB, Shepard EM, Dooley DM, Freeman HC, Guss JM. Biochemistry. 2003;42(51):15148–15157. doi: 10.1021/bi035338v. [DOI] [PubMed] [Google Scholar]
- 52.Kumar V, Dooley DM, Freeman HC, Guss JM, Harvey I, McGuirl MA, Wilce MC, Zubak VM. Structure. 1996;4(8):943–955. doi: 10.1016/s0969-2126(96)00101-3. [DOI] [PubMed] [Google Scholar]
- 53.Parsons MR, Convery MA, Wilmot CM, Yadav KD, Blakeley V, Corner AS, Phillips SE, McPherson MJ, Knowles PF. Structure. 1995;3(11):1171–1184. doi: 10.1016/s0969-2126(01)00253-2. [DOI] [PubMed] [Google Scholar]
- 54.Wilmot CM, Murray JM, Alton G, Parsons MR, Convery MA, Blakeley V, Corner AS, Palcic MM, Knowles PF, McPherson MJ, Phillips SE. Biochemistry. 1997;36(7):1608–1620. doi: 10.1021/bi962205j. [DOI] [PubMed] [Google Scholar]
- 55.Jakobsson E, Nilsson J, Ogg D, Kleywegt GJ. Acta Crystallogr D Biol Crystallogr. 2005;61(Pt 11):1550–1562. doi: 10.1107/S0907444905028805. [DOI] [PubMed] [Google Scholar]
- 56.Duff AP, Cohen AE, Ellis PJ, Hilmer K, Langley DB, Dooley DM, Freeman HC, Guss JM. Acta Crystallogr D Biol Crystallogr. 2006;62(Pt 9):1073–1084. doi: 10.1107/S0907444906026333. [DOI] [PubMed] [Google Scholar]
- 57.Chiu YC, Okajima T, Murakawa T, Uchida M, Taki M, Hirota S, Kim M, Yamaguchi H, Kawano Y, Kamiya N, Kuroda S, Hayashi H, Yamamoto Y, Tanizawa K. Biochemistry. 2006;45(13):4105–4120. doi: 10.1021/bi052464l. [DOI] [PubMed] [Google Scholar]
- 58.Hevel JM, Mills SA, Klinman JP. Biochemistry. 1999;38(12):3683–3693. doi: 10.1021/bi982199m. [DOI] [PubMed] [Google Scholar]
- 59.Wilce MCJ, Dooley DM, Freeman HC, Guss JM, Matsunami H, McIntire WS, Ruggiero CE, Tanizawa K, Yamaguchi H. Biochemistry. 1997;36(51):16116–16133. doi: 10.1021/bi971797i. [DOI] [PubMed] [Google Scholar]
- 60.Li RB, Klinman JP, Mathews FS. Structure. 1998;6(3):293–307. doi: 10.1016/s0969-2126(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 61.Duff AP, Cohen AE, Ellis PJ, Kuchar JA, Langley DB, Shepard EM, Dooley DM, Freeman HC, Guss JM. Biochemistry. 2003;42(51):15148–15157. doi: 10.1021/bi035338v. [DOI] [PubMed] [Google Scholar]
- 62.Knapp MJ, Klinman JP. Biochemistry. 2003;42(39):11466–11475. doi: 10.1021/bi0300884. [DOI] [PubMed] [Google Scholar]
- 63.Darnault C, Volbeda A, Kim EJ, Legrand P, Vernede X, Lindahl PA, Fontecilla-Camps JC. Nat Struct Biol. 2003;10(4):271–279. doi: 10.1038/nsb912. [DOI] [PubMed] [Google Scholar]
- 64.Scott EE, Gibson QH. Biochemistry. 1997;36(39):11909–11917. doi: 10.1021/bi970719s. [DOI] [PubMed] [Google Scholar]
- 65.Whittington DA, Rosenzweig AC, Frederick CA, Lippard SJ. Biochemistry. 2001;40(12):3476–3482. doi: 10.1021/bi0022487. [DOI] [PubMed] [Google Scholar]
- 66.DeLano WL. The Pymol User's Manual. San Carlos, CA: 2002. [Google Scholar]
- 67.Cruikshank DWJ. Co-ordinate uncertainty. In: Rossman MG, Arnold E, editors. International Tables for Crystallography. Springer; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.