Abstract
The development of type 2 diabetes (T2D) is strongly associated with obesity. In humans, T2D increases the risk for end organ complications. Among these, heart disease has been ranked as the leading cause of death. We used a proteomic methodology to test the hypothesis that a pre-diabetic state generated by high-fat diet leads to changes in proteins related to heart function and structure. Over 300 proteins spots were resolved by 2-DE. Fifteen protein spots were found to be altered (7 decreased and 8 increased) in pre-diabetic hearts. The protein spots were then identified by mass spectrometry and immunoblots. Among the decreased proteins, 3 are involved in heart structure (one isoform of desmin, troponin T2 and α-cardiac actin), 3 are involved in energy metabolism (mitochondrial ATP synthase β subunit, adenylate kinase and creatine kinase) and one is a component of the citric acid cycle (isocitrate dehydrogenase 3). In contrast, proteins involved in fatty acid oxidation (two isoforms of peroxisomal enoyl-CoA hydratase) and the citric acid cycle (three isoforms of malate dehydrogenase) were increased in pre-diabetic hearts. The results suggest that changes in the levels of several heart proteins may have implications in the development of the cardiac phenotype associated to T2D.
Keywords: Type 2 diabetes, obesity, murine models of obesity-type 2 diabetes, heart proteome, heart dysfunction
1. Introduction
Over the past 30 years, obesity rates have increased at an alarming rate in the United States and worldwide [1, 2]. About a third of the USA adult population is obese (body mass index (BMI) ≥ 30 kg/ml) [3]. Obesity is accompanied by an increased risk for the development of hypertension, heart disease, type 2 diabetes (T2D) and cancer. Of particular interest is the association between obesity and type 2 diabetes, a complex disorder affecting multiple organs [4, 5]. Heart disease is a major health issue and is the leading cause of death associated with obesity and T2D [6–9]. It is estimated that two out of three diabetes patients develop heart failure, and eventually die from myocardial infarction or stroke [9, 10]. Therefore, the use of animal models that develop T2D for the study of proteins altered in heart with this disease may be of value in helping to determine the molecular events associated with progression of cardiac end organ damage associated with T2D.
Several murine models of obesity and T2D have provided important insight into the molecular mechanism underlying the pathological consequences of the disease [11–16]. However, the obesity and T2D observed in many of these models results from single gene mutations (e.g. leptin receptor deficient mice (db/db) or leptin deficient (ob/ob) mice). Since obesity and T2D are largely polygenic, the use of monogenic models is limited to the specific effects of the single altered gene [17, 18]. Furthermore, obesity, T2D and heart disease are strongly associated with poor nutrition, further suggesting that the use of single gene mutation models might be limiting as opposed to environmental influences such as high-fat feeding [18, 19].
Several studies have shown that high-fat feeding in rodents mimic the characteristics of the T2D progression in humans, including cardiac and arterial dysfunction [18–20]. The C57BL/6J strain of mice has been shown to significantly increase body weight, develop hyperinsulinemia, insulin resistance and ultimately hyperglycemia when fed a high-fat diet [17, 21–24]. In addition, vascular dysfunction and cardiac dysfunction are observed in C57BL/6J mice when fed a high-fat diet [25, 26]. In the present study, we explored the use of proteomics to identify proteins involved in cardiac dysfunction associated with high-fat diet induced obesity and T2D. The goal of our study is to reveal changes in the heart proteome associated with obesity and the development T2D. The results will identify potential therapeutic targets for the treatments and/or prevention of cardiac complications before the onset of diabetes induced end organ damage.
2. Materials and methods
2.1 Animals
Three week old male C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME). Two weeks after arrival, the mice were separated in two groups. One group was fed a high-fat diet (#F1850, Bioserve, Frenchtown, NJ) in which 17 % of the calories were provided by protein, 27 % by carbohydrates, and 56% by fat. A second group remained on a standard rodent chow diet (Prolab RMH 3000, PMI Nutrition International, St. Louis, MO) in which 26 % of the calories were provided by protein, 60 % by carbohydrates, and 14 % by fat. Mice were housed in cages that were kept on a 14-h light, 10-h dark cycle with water and food provided ad libitum. High-fat fed and control animals were weighed once every two weeks to track the progression of obesity. All animal protocols used in this study were consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association, and were approved by the Ohio University Institutional Animal Care and Use Committee and conform to local, state, and federal laws.
2.2 Glucose and insulin levels
For glucose and insulin measurements, mice were fasted for 8 hours. Blood samples were obtained by tail bleeding at 2, 4, 8 and 16 weeks. For glucose and insulin measurements, mice were fasted for 8 hours. The first drop of blood was used to determine glucose levels employing the OneTouch glucometer from Lifescan (Milpitas, CA). To determine plasma insulin levels, approximately 100 μl of blood was collected in heparinized capillary tubes. Insulin concentrations were determined using the rat insulin ELISA kit and rat insulin standards (ALPCO, Windham, NH) following the manufacturer’s instructions. Values were adjusted according to the manufacturer’s instructions, by a factor of 1.23 to correct for the species difference in cross reactivity with the antibody [23, 27].
2.3 Tissue collection and processing
Mice were sacrificed by cervical dislocation after 8 weeks on high-fat or control diet. Hearts were removed and immediately frozen in liquid nitrogen, transferred to a freezer, and kept at −80 ºC until sample processing. Cardiac tissues were processed as previously described by List et al. 2007 [23]. Briefly, cardiac tissues were thawed on ice, weighed and then freeze fracture homogenized employing a biopulverizer (Fisher Scientific, Pittsburgh, PA). The pulverized hearts were then transferred to a 1.5 ml eppendorf tube containing the solubilization buffer (7M Urea; 2M Thiourea; 1% SB 3–10; 3% CHAPS; 40 % Biolytes (BioRad, Hercules, CA). Samples were then treated by sonication (Sonicator Dismembrator, model 100, Fisher Scientific) and centrifugation for 45 min at 150,000 x g. Supernatants were transferred to new tubes and protein concentrations determined using the Bradford assay (BioRad) following the manufacturer’s instructions.
2.4 Two-dimensional gel electrophoresis (2-DE)/imaging/protein identification
Proteins were separated by 2-DE following a procedure previously described [22–24, 28–31]. For 2-DE, 0.15 mg of protein was dissolved in 400 μl of solubilization buffer containing 2mM tributylphosphine (TBP) and 1.5% of protease inhibitor cocktail (Sigma, St. Lewis, MO). The samples were then incubated at room temperature for 2 hours. Following disulfide bonds reduction with TBP, 6 μl of freshly prepared 16% (g/ml) iodoacetamide (BioRad) solution was added to the samples to alkylated reduced disulfide bonds. Proteins were then separated by isoelectric focusing (IEF) at 10000 V for 60000 V.h in a PROTEAN IEF cell (BioRad, Hercules). Proteins were then resolved by gel electrophoresis at 270 V.h. Following electrophoresis, the gels were stained by SYPRO orange fluorescent stain (Molecular Probes, Eugene, OR) using a modified protocol by Malone [32]. Images of SYPRO orange stained gels were obtained using a Pharos FX Plus Imaging System (BioRad). Protein spot detection and density were determined using the PDQuest Advanced v. 8.0 (BioRad, Hercules) image analysis software. Proteins whose levels were altered in T2D hearts compared to controls (P<0.05) were excised from the gels and sent to ProteaBioscience, Inc (Morgantown, WV) for identification by mass spectrometry (MS) and tandem-MS (MS/MS) as described below.
2.5 MS analysis (performed at ProteaBioscience)
All proteins were analyzed by Proteabioscience, Inc. All procedures have been previously described [15, 24, 29–31]. Briefly, acrylamide gel spots were dehydrated and then rehydrated with acetonitrile and 50 mM ammonium bicarbonate, respectively. Proteins were then reduced with 250mM DTT for 60 min at 55 ºC, followed by alkylation with 650mM iodoacetamide for 60 min at room temperature in the dark. Digestion was performed with 500ng trypsin in 50 mM ammonium bicarbonate buffer overnight. Extraction of peptides was performed using 5% formic acid in 50% acetonitrile (dehydration), followed by rehydration with 50 mM ammonium bicarbonate. The recovered peptides were then lyophilized, reconstituted in 10 mM acetic acid, and relyophilized to yield a purified, protein digest extract. For MS and MS/MS analyses, the protein digest solution was loaded onto a C18 ProteaTip by aspirating and expelling the sample 5–10 times within the sample vial. The bound sample was washed twice with the 0.1%TFA/2% acetonitrile solution by aspirating and expelling 20 ml of the wash solution 5–10 times. The sample was spotted directly onto a MALDI target that was prespotted with 0.6μL MALDI matrix (CHCA) using 1μl of an elution solution (0.1%TFA/90% acetonitrile). Mass spectra were acquired on an ABI 4800 MALDI TOF/TOF analyzer. MS spectra were acquired in Reflector Positive Ion mode. Peptide masses were acquired for the range from 850–4000 Da. MS spectra were summed from 400 laser shots. Internal calibration was performed using a minimum of three trypsin autolysis peaks. For MS/MS, spectra were acquired until at least 4 peaks in the MS/MS spectra achieved a S/N (signal-to-noise ratio) equal to 70.
2.6 Manual protein database searching with MS and MS/MS-generated peak lists (Performed at Ohio University)
Database searching for protein identification from combined MS and MS/MS was performed using Applied Biosystems GPS Explorer v3.6 with Mascot (Matrix Science). These procedures have been described previously [23, 24, 28]. Search parameters included the following: MS: database: NCBInr; taxonomy: Mus musculus; enzyme: trypsin; missed cleavages allowed: 1; fixed modifications: Carbamidomethyl (C); protein mass: not specified; peptide tolerance: ±1.2 Da; mass values: MH+; monoisotopic/average: monoisotopic. Tandem MS: database: NCBInr; taxonomy: Mus musculus; enzyme: trypsin; missed cleavages allowed: 1; fixed modifications: none; quantitation: none; peptide tolerance: ±1.2 Da; MS/MS tolerance: ±0.6 Da; peptide charge: 1+; monoisotopic/average: monoisotopic; precursor m/z: not specified; instrument: MALDI–TOF–TOF. Variable modifications that were included in separate and combined submissions for both MS and MS/MS were Acetyl (K), Carbamidomethyl (C), Deamidated (NQ), Oxidation (M), Phospho (ST), Phospho (Y), Sulfo (S), Sulfo (T), Sulfo (Y). The general criteria used for assessment of protein identity were a minimum match of two MS/MS fragments with significant scores (MOWSE score ≥ 64).
2.7 Immunoblots analysis
Western blot analyses were performed to confirm the altered levels of creatine kinase-M, αB-crystallin, troponin T-C and desmin. For 1-dimension (D) immunoblots, 50 μg of mouse heart extract was resolved by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA). For 2-D Western blots, 150 μg of mouse heart extract was subjected to 2-DE and transferred to PVDF membranes. Membranes were then blocked in 5% non-fat dry milk, and probed for 2 hours with primary antibody. Goat polyclonal antibodies against creatine kinase-M (1:400 dilution), troponin T-C (1:500) and desmin (1:500) were obtained from Santa Cruz Biotechnology, Santa Cruz, CA. For αB-crystallin detection, a rabbit polyclonal antibody against αB-crystallin was used (1:400) (Santa Cruz Biotechnology, CA). Protein bands and protein isoforms were identified employing horseradish peroxidase-conjugate secondary antibodies (1:5000) and the Pierce ECL western blotting substrate (Thermo scientific, Rockford, IL). The resulting blots were scanned using a Pharos FX Plus Imaging System (BioRad) and normalized against a loading control, Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH); (Santa Cruz Biotechnology). The images of the 1-D and 2-D blots were subject to densitometry analyses using Quantity One Quantification Program Software (Bio-Rad, Hercules, CA) and PDQuest, software version 8.0 (Bio-Rad).
2.8 Statistical analysis
Physiological parameters were analyzed by single factor ANOVA and Student’s t-test. Protein expression data were analyzed by Student’s t-test. Mouse physiological data are presented as mean ± SEM. All statistical analyses were performed using SPS software v. 14.0. (P values lower than 0.05 were considered significant). For MS analysis, a probability-based MOWSE score of 64 or higher was considered statistically significant. For MS/MS MASCOT peptide mass fingerprint searches, MOWSE scores of 64 or greater were considered significant for each individual fragment (For specific details on MS and MS/MS analysis, see supporting materials).
3. Results
3.1 Physiological measurements (body weight, glucose and insulin levels)
Male mice were fed a high-fat diet or standard chow for a period of 16 weeks. High-fat feeding resulted in increased body weight, insulin and glucose compared to those fed standard chow (Figure 1(A–C)). Based on the high insulin levels observed in the high-fat fed mice, we have classified them as pre-diabetic. For our proteomic studies, we selected 10 mice (5 high-fat fed mice and 5 standard chow control mice) at the 8 weeks time point. To ensure only pre-diabetic mice were studied in the experimental group, each of the 5 mice had significantly elevated body weight, insulin and glucose. Physiological measurements of the mice used for proteomics are shown in Figure 1(D–F).
Figure 1.
Effects on high-fat diet on body weight, glucose and insulin levels. (A–C) Solid lines (−) represent controls fed on standard chow diet (n=20). Dashed lines (--) represent mice fed on high-fat diet (n=48). A) Body weight was measured every 2 weeks. The zero time-point represents 21 days of age, when the high-fat diet was initiated. The 2, 4, 8, and 16 weeks time points represent 5, 7, 11 and 19 week of age, respectively. B) Blood glucose levels were taken at 2, 4, 8, and 16 weeks on the diet. Glucose was significantly increased after 2 weeks on high-fat diet as compared to controls, and remained significantly elevated throughout the study. C) Plasma insulin levels significantly increased over time with significant differences at 8 and 16 weeks. Body weight, glucose and insulin levels in the C57BL/6J mice used for heart proteomic profiling. (D–F) White bars represent control mice fed standard chow (n=5); black bars represent mice fed on high-fat diet (n=5). This group was designated as pre-diabetic. D) Body weight. E) Blood glucose levels F) Plasma insulin levels. Errors bars represent the SEM. Statistical analysis was performed using one way ANOVA and Student’s t-test * P<0.05.
3.2 Heart proteome patterns
The heart protein patterns observed for each sample were similar and reproducible from animal to animal and gel to gel. A representative 2-D gel is shown in Figure 2(I). Over 300 spots per gel were resolved using the PDQuest Advanced v. 8.0 program. Of the 300 resolved protein spots, 15 were found to be significantly altered between pre-diabetic compared to control mice. Of these 15 protein spots, 8 increased while 7 decreased in pre-diabetic heart tissue (Figure 2(I)).
Figure 2.
I) Representative 2-D gel of heart proteins. Proteins whose level was altered were labeled A–O and indicated by arrows. II) Representative 3D view of protein spots displaying altered levels in pre-diabetic mice as compared to controls. A) Protein spots displaying lower levels in pre-diabetic hearts (A, B, D–G and I). B) Protein spots displaying higher levels in pre-diabetic hearts (C, H, J–O). The identities of these protein spots are listed in Table 1 and Table 2. The images were generated using PDQuest software version 8.0. The 3D view images were generated based on one representative gel, but they do not represent the average spot intensity change of all the samples. The spot intensity data was converted to topographical peaks and valleys.
3.3 Protein identity
In order to identify the altered proteins, the protein spots were excise from the gels and analyzed by two methods; MALDI-TOF and MS/MS analysis. The 7 protein spots that decreased in pre-diabetic mice (Figure 2(II)) are listed in Table 1. These include proteins related to oxidative phosphorylation, energy metabolism, and cardiac contractile function. Spot A was identified as ATP synthase, H+ transporting F1 complex β subunit (P= 0.002, −33.39%). Protein spots B, D and E were identified as desmin (P= 0.01, −35.5%), troponin T2 cardiac isoform f (P= 0.01, −57.99%) and alpha-cardiac actin (P= 0.02, −62.85%), respectively. Spots F, G and I were identified as isocitrate dehydrogenase 3 (NAD+) alpha (P= 0.02, −66.91%), adenylate kinase isoenzyme 1 (P= 0.01, −82.3%) and creatine kinase (P= 0.01, −100%). Table 2 and Figure 2(II) displays eight protein spots that were found to be significantly increased in pre-diabetic hearts compared to controls. These proteins are active in cardiac architecture, energy metabolism, citric acid cycle, fatty acid oxidation and signal transduction. Among these proteins, spot C was identified as desmin (P= 0.003, 74%). Spots H, J, K were identified as isoforms of cytosolic malate dehydrogenase 1 (P= 0.01, 80%; P= 0.01, 55.42%; P=0.01, 40.23%). Spots L and M were identified as two isoforms of peroxisomal enoyl-Co A hydratase 1 (P= 0.007, 36.85%; P=0.04, 36.86%). And spots N and O were identified as nucleoside diphosphate kinase (P=0.01, 100%) and αB-crystallin (P=0.01, 65.9%), respectively.
Table 1.
Proteins which were decreased in pre-diabetic mice when compared to control hearts
| Spot | Identity a | Accession no.b | Function | Gel pI/MW | Pc | Mascot scores/Matched peptides (% coverage)/MS-MS ions (score) |
|---|---|---|---|---|---|---|
| A | Mitochondrial ATP synthase, H+ transporting F1 complex β subunit | P56480 | Energy Metabolism | 5.6/50 | 0.002 | 81/16 (55)/R.FTQAGSEVSALLGR.I (39); K.VLDSGAPIKIPVGPETLGR.I (38) |
| B | Desmin | P31001 | Class-III intermediate filaments found in muscle cells | 5.8/50 | 0.01 | 42/5 (18)/R.INLPIQTFSALNFR.E (71); R.TFGGAPGFSLGSPLSSPVFPR.A(48) |
| D | Troponin T2, cardiac isoform f | P50752 | Cardiac contractile function | 5.8/40 | 0.01 | 81/10 (30)/K.DLNELQTLIEAHFENR.K (108); K.ALAIDHLNEDQLR.E (62); K.YEINVLR.N (40) |
| E | Alpha-cardiac actin | Q61274 | Cardiac contractile function | 6.2/37 | 0.02 | 129/14 (56)/K.SYELPDGQVITIGNER.F (114); K.IWHHTFYNELR.V (56) |
| F | Isocitrate dehydrogenase 3 (NAD+) alpha | Q9D6R2 | Krebs Cycle | 6.3/38 | 0.02 | 67/9 (39)/R.IAEFAFEYAR.N (58); R.ENTEGEYSGIEHVIVDGVVQSIK.L (42) |
| G | Adenylate kinase isoenzyme 1d | Q9R0Y5 | Energy Metabolism | 6.3/22 | 0.01 | 79/9(52)/K.IGQPTLLLYVDAGAETMTQR.L (35) |
| I | Creatine kinase d | P07310 | Energy metabolism | 6.8/43 | 0.01 | 82/18(29)/K.SFLVWVNEEDHLR.V (52) |
A minimum of two significant MS/MS peptide fragments was considered to assign an ID for a spot.
Accession numbers: http://www.uniprot.org
Only differences that were statistically significant P<0.05 as determined by t-test are reported.
One significant MS/MS peptide fragment and a MS score> 64 was considered sufficient to assign a protein ID
Table 2.
Proteins which were increased in pre-diabetic hearts when compared to control hearts
| Spot | Identity a | Accession no. | Function | Gel pI/MW | Pc | Mascot scores/Matched peptides (%coverage)/MS-MS ions (score) |
|---|---|---|---|---|---|---|
| C | Desmin | P31001 | Class-III intermediate filaments found in muscle cells | 5.9/50 | 0.003 | 41/5(19)/R.INLPIQTFSALNFR.E (72); R.TFGGAPGFSLGSPLSSPVFPR.A (48) |
| H | Cytosolic malate dehydrogenase 1 | P14152 | Krebs Cycle | 6.8/35 | 0.01 | 77/6(27)/K.DLDVAVLVGSMPR.R (36); K.FVEGLPINDFSR.E (51) |
| J | Cytosolic malate dehydrogenase 1 | P14152 | Krebs Cycle | 7.1/38 | 0.01 | 32/8(26)/K.DLDVAVLVGSMPR.R (48); K.FVEGLPINDFSR.E (71) |
| K | Cytosolic malate dehydrogenase 1d | P14152 | Krebs Cycle | 7.2/35 | 0.01 | 72/6(27)/K.FVEGLPINDFSR.E (44) |
| L | Peroxisomal enoyl-Co A hydratase 1d | Q91W49 | Fatty Acid Oxidation | 7.3/29 | 0.007 | 76/11(49)/K.VIGNQSLVNELTFSAR.K (110) |
| M | Peroxisomal enoyl-Co A hydratase 1d | Q91W49 | Fatty Acid Oxidation | 7.3/26 | 0.04 | 104/12(49)/K.VIGNQSLVNELTFSAR.K (78) |
| N | Nucleoside Diphosphate Kinase B | Q01768 | Signal Transduction | 7.5/15 | 0.01 | 70/11(67)/K.DRPFFPGLVK.Y (43); R.TFIAIKPDGVQR.G (45) |
| O | Alpha-crystallin B chaine | P23927 | Heat shock protein | 7.0/23 | 0.01 | 91/12(60) |
A minimum of two significant MS/MS peptide fragments was considered to assign an ID for a spot.
Accession numbers: http://www.uniprot.org
Only differences that were statistically significant p<0.05 as determined by t-test are reported.
One significant MS/MS peptide fragment and a MS score> 64 was considered sufficient to assign a protein ID.
Identity of this protein was validated by Western blots.
3.4 Western blot analysis
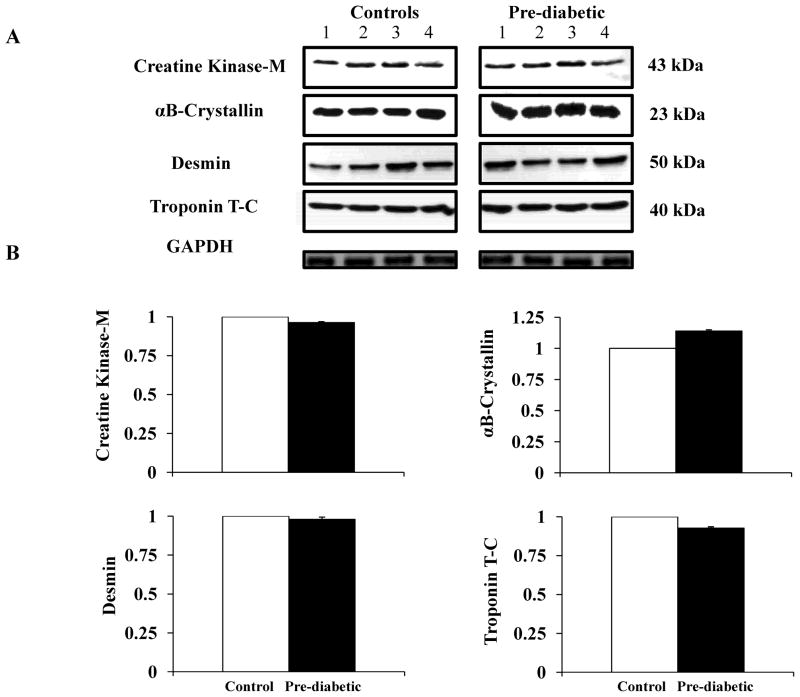
Western blotting analysis showed no significant differences in total creatine kinase levels between the groups (Figure 3A–B). Also, total levels of αB-crystallin, desmin and troponin were not significantly different when comparing pre-diabetic and control hearts (Figure 3A–B).
Figure 3.
Western blot analysis of the levels of creatine kinase and structural proteins in heart tissues. A) Western blots to detect creatine kinase, αB-crystallin, desmin and troponin T-C in whole heart homogenates from controls (n=4) (left panel) and pre-diabetic mice (n=4) (right panel). Equal amounts of total protein were loaded (50 μg) and resolved by SDS-PAGE. B) Quantification of immunoblots was obtained using Quantity One Program Software (BioRad). Protein intensity is expressed as arbitrary units relative to controls. Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) was used as loading control. The error bars indicate ± SEM.
2-D western analysis showed the existence of 8 isoforms of creatine kinase (Figure 4A(I)). Isoform 1 (Figure 4B(I)) corresponds to protein spot I (Table 1, Figure 2(I)) that was identified as creatine kinase by MS. Compared to control hearts, creatine kinase isoform 1 was significantly decreased in pre-diabetic hearts (Figure 4C(I)). Four isoforms of αB-crystallin (Figure 4A(II)) were identified. Isoform 1 (Figure 4B(II)) corresponds to protein spot O (Figure 2(I), Table 2) confirming the MS identification. Analyses of the 2-D Western images also show that αB-crystallin isoform 1 levels (Figure 4C(II) are increased in pre-diabetic as compared to control hearts.
Figure 4.
Isoforms observable in 2-D western blots. 150 μg of total protein from heart extracts was resolved in the 1st dimension in a linear pH-range of 3–17 and in 15% acrylamide in the 2nd dimension. A) Representative Western blot images showing the location (~MWs and ~pIs) of the different isoforms of (I) creatine kinase (1–8), (II) αB-crystallin (1–4), (III) desmin and (IV) troponin T-C (1–5). The approximate MWs and pIs are shown in italics and bold numbers. Molecular weight markers are indicated to the left of the panels and pH gradient markers are indicated above. The dotted grids were added to facilitate the localization of the protein isoforms. B) The protein isoforms that were detected in our proteomic analysis are indicated by dashed squares. (I) Creatine kinase isoforms (1–8); (II) αB-crystallin isoforms; (III) desmin isoforms (1–13); (IV) troponin isoforms (1–5). C) Intensity values (mean ± SEM) for each protein isoform of creatine kinase(I), αB-crystallin (II), desmin (III) and troponin (IV). Quantification of immunoblots was obtained using PDQuest software version 8.0 (BioRad). White bars represent control hearts (n=4); black bars represent pre-diabetic hearts (n=4). Errors bars represent the SEM. Statistical analysis was performed using Student’s t-test * P<0.05.
Thirteen isoforms of desmin were identified in the 2-D Western blots (Figure 4A(III)). Isoforms 1 and 2 (Figure 4B(III)) correspond to protein spots B (Table 1, Figure 2(I)) and C (Table 2, Figure 2(I)), and were identified by MS as desmin. Figure 4C(III) shows that isoform 1 of desmin is less prominent in pre-diabetic hearts. In contrast, isoform 2 of desmin is significantly increased in pre-diabetic versus control heart tissues (Figure 4C(III)).
Five troponin isoforms (Figure 4A–B (IV)) were identified by Western blots. One of these isoforms corresponds to protein spot D (Table 1, Figure 2(I)). Troponin (isoform 2) was found to be less prominent in pre-diabetic hearts relative to controls (Figure 4C(IV)).
4. Discussion and Conclusions
Heart disease is the leading causes of mortality associated with obesity and T2D [1, 6, 8]. 2-DE is a powerful technique that provides a record of intact proteins and can reveal changes in the levels of protein between samples. Additionally, the presence of different isoforms or post-translation modifications of a given protein can be determined [24, 33, 34]. Human heart proteome databases have been used to identify cardiac protein composition and levels [34–36]. However, as one might imagine, it is very difficult to obtain suitable samples for studies, due to limited tissue availability, genetic variability of the subjects, and few controlled experiments [34]. Therefore, the use of animal models provides a tool to investigate global changes in protein levels associated to heart disease relative to wild-type controls [26, 34]. Few 2-D gel-based datasets have been established for the murine heart [34]. In addition, limited studies have investigated the changes in protein levels in the heart associated with disease states such as T2D [26, 34]. The goal of the present study was to study the early effects of high-fat feeding induced obesity/diabetes on the heart proteomic profile.
In this study C57BL/6J mice were fed on a high-fat or control diet (described in the materials and methods section). As previously reported [17, 21–24], this treatment results in significant increases in body weight after two weeks. While glucose was elevated at all time points, it was not until the 8 week time point when insulin levels were significantly elevated representing a pre-diabetic state (obesity, hyperglycemia and hyperinsulinemia). Since we wanted to observe early changes to cardiac tissue during T2D, we chose the 8 week time point for our analysis. We hypothesized that employing the 2-DE proteomic technique would allow for the examination of slight alterations in protein levels which may provide new insights into cellular mechanisms involved in the cardiac dysfunction associated to T2D in at the pre-diabetic stage of the disease. This information may ultimately lead to new therapeutic targets to treat and prevent the deleterious effects of T2D in cardiovascular health.
4.1 2-DE profiling
For this study, we compared the proteomic profile of pre-diabetic and control mouse hearts. A recent study by Bousette N. et al. [37] identified 4906 proteins in cardiac tissue of wild-type mice using a shotgun approach. Although, this study provided a good estimation of the relative protein abundances in the murine heart, one potential problem of this technique is that as the highly abundant proteins are digested, their intensities can ‘swamp out’ or mask the identities of other tryptic digested fractions [38]. Moreover, using this technique it is not possible to visualize protein isoforms that result from post-translational modifications (PMTs) that lead to shifts in isoelectric point (pI) or molecular weight (MW) [28]. On the other hand, 2-DE is a powerful tool that provides a reference map of intact proteins and shows changes in protein levels, and the presence of different protein isoforms or PTMs [34, 38–40] that lead to shifts in isoelectric point (pI) or molecular weight (MW). Therefore, in the present study cardiac proteins were separated by 2-DE.
A total of 300 proteins spots were resolved by 2-DE. We identified 15 proteins whose levels were altered in the pre-diabetic heart compared to control. Interestingly, several spots were identified as protein isoforms. The existence of several isoforms of the same protein was further confirmed by immunobloting techniques. 2-D Western blotting revealed that only specific isoforms of the same protein are differentially expressed in pre-diabetic hearts. Additional studies are needed to reveal the biological implications of these findings.
Briefly, we will discuss the effects of the high-fat feeding in the proteomic changes observed in the heart of pre-diabetic mice as compared to controls.
4.2 Effects of high-fat diet in the murine heart
During normal physiological states, the heart uses fatty acids as its main source of energy. However, oversupply of lipids lead to imbalances in cardiac ATP generation from fatty acid oxidation, which may result in cardiac hypertrophy [41, 42]. Chronic increases in glucose and insulin as a result of high-fat feeding, have been associated to altered expression of important regulatory proteins of the fatty acid pathway and energy metabolism [14–16, 43]. An increased in fatty acid oxidative capacity has been observed in skeletal muscle of C57BL/6J mice fed a high-fat diet for 5–20 weeks [43]. Similar findings have been obtained in other murine models of obesity/T2D [15, 16]. In addition, energy balance studies suggested that perfused livers from rats fed a high-fat diet showed a decrease in mitochondrial/cytosolic pH difference (ΔpHm) and a significant increase in mitochondrial membrane potential (Δψm), which leads to imbalances in ATP synthesis and in the distribution of several metabolites critical for mitochondrial function [44].
Recent proteomic studies have also shown that imbalances in several proteins such as, ATP synthase, ubiquinol cytochrome-C reductase, among others, may also contribute to down-regulation in ATP production in the obese animals [15]. In agreement with these reports, our results suggest that most of the cardiac proteins that differ between the pre-diabetic and control groups included proteins related to energy metabolism, citric acid cycle and oxidative phosphorylation. Therefore, alterations in the cardiac metabolic network due to fatty acid overload leads to impaired cardiac ATP generation, which may result in structural changes due to alterations in heart efficiency [16, 45]. Interestingly, we also found changes in the levels of proteins associated to heart architecture and contractile function.
Overall, our proteomic results indicate that high-fat feeding has prominent effects on cardiac energy homeostasis. Further studies are required to define the precise role of the identified proteins in heart function in an obese/pre-diabetic state, and what their function might be in propagating the deleterious effects associated with T2D.
4.3 Proteins identified by MS and MS/MS
4.3.1 Proteins involved in energy metabolism
Previous studies have showed that impairments in the levels of mitochondrial proteins in diabetic hearts involved in fatty acid oxidation are consistent with a reduction in oxygen utilization and ATP synthesis [15, 16, 46, 47]. The ATP-synthase complex is composed of two major units: F0 (inner membrane), and F1 (the catalytic core) [48]. The α/β-subunits belong to the ATPase complex catalytic core that synthesizes/hydrolyses ATP [48]. Studies have shown that the ATP-synthase system is altered during normal aging and in several pathophysiological conditions, such as type 1 and 2 diabetes [15, 16, 45, 46, 49–51]. In agreement with these findings, our results suggest that ATP synthesis is decreased. We found that the level of the mitochondrial ATP synthase complex β-subunit (spot A; ~MW 50 kDa, ~pI 5.6) was significantly decreased in pre-diabetic hearts. This finding suggests that reductions in ATP formation in heart tissue is an early event associated to impairments in glucose metabolism [15, 16]. Interestingly, this decrease was accompanied by a significant decrease of adenylate kinase isoenzyme 1 (spot G; ~MW 22 kDa, ~pI 6.3), which is a phosphotransfer enzyme that catalyzes adenine nucleotide exchange and the transfer of both β- and γ-phosphoryls in ATP [52, 53].
Adenylate kinase 1 is the major isoform (theoretical MW 21.64 kDa, pI 5.67) found in the mitonchondria, cytosol, and cellular membranes [54, 55]. Decreased levels of adenylate kinase 1 has been observed in the metabolically compromised hearts [54, 55]. Our results showed significant reduction in the level of Adenylate kinase 1 in pre-diabetic hearts suggesting compromised ATP formation. This finding is consistent with reductions in isocitrate dehydrogenase 3 (NAD+) alpha (spot F; ~MW 38 kDa, ~pI 6.3), which plays an important role in the citric acid cycle. Isocitrate dehydrogenase 3 (NAD+) alpha is an enzyme responsible for converting pyruvic acid into hydrogen ion, carbon dioxide and electrons and finally producing ATP. Proteomic studies have showed that the levels isocitrate dehydrogenase 3 (NAD+) alpha are also significant altered in heart tissues of db/db mice [15]. These findings support the results obtained in our model.
Thus, decreased levels of isocitrate dehydrogenase 3 (NAD+) alpha and adenylate kinase isoenzyme 1 in pre-diabetic hearts correlates with reductions in mitochondrial ATP synthase complex β-subunit levels, and perhaps ATP synthesis.
These results are further supported by the decreased level of one isoform of creatine kinase (spot I; ~MW 43 kDa, ~pI 6.8) in pre-diabetic hearts. Previous proteomic studies that have shown that creatine kinase levels are compromised in the hearts of streptozotocinin induced diabetic rats (type 1 diabetes animal model) [46, 56]. In addition, imbalances in creatine kinase levels are associated with reductions in ATP synthesis [57]. In the clinical setting, creatine kinase is often measured in patients with chest pain and acute renal failure [58]. Elevation of creatine kinase is usually an indication of skeletal and cardiac muscle damage, and has been associated with acute myocardial infarction in humans [59]. Interestingly, our 2-D western analyses showed the existence of at least eight isoforms of creatine kinase (~MW 43 kDa, ~pI 6.8–7.2). Proteomic studies of the skin of C57BL/6J diabetic mice performed in our laboratory also have shown alterations in the levels of several isoforms of creatine kinase [23]. In that study, a similar pattern of creatine kinase isoforms were found, including the two different sized charge trains [23]. Additionally, one of the creatine kinase isoforms identified in that report corresponds to protein spot I, which was also identified as creatine kinase in our study. Therefore, the creatine kinase isoform identified in this study represents a specific isoform of this protein associated T2D.
In addition to these results, we found increases in nucleoside diphosphate kinase (NDPK) (spot N, ~MW 15, ~pI 7.5). Increases in NDPK activity has been observed in failing hearts [60]. When cardiomyocytes are stimulated to contract, ATP-synthase activity is up-regulated in line with the increased energy demand. It has been hypothesized that NDPK has an inhibitory effect on cAMP signaling [60]. Imbalances in cAMP signaling may lead to negative effects on the cardiac contraction components, such as Ca+ channels and myofilament proteins [61]. Therefore, up-regulation of NDPK might correlate with imbalances in the expression of proteins associated with heart structure and contractile function. This idea is further supported by the proteomic changes observed in proteins involved in heart architecture, as discussed below.
4.3.2 Proteins involved in cardiac contraction function and architecture
We found changes in the levels of two of the major proteins involved in cardiac structure and contractile function: troponin and desmin.
Protein spot D was identified as troponin T2 isoform f. We found significantly decreased levels of troponin T2 isoform f (~MW 40 kDa, ~pI 5.8) in pre-diabetic hearts. Troponin is a complex of three regulatory proteins (Troponin I, Troponin T and Troponin C) which mediates muscle contraction in cardiac and skeletal muscle [62]. Interestingly, altered troponin T isoforms expression has been associated to cardiac hypertrophy and diabetes [63, 64]. As showed in the results section, 2-D Westerns revealed 5 different troponin isoforms (~MW 40 kDa, ~pI 5.6–6.0). These findings suggest that different isoforms of Troponin may respond differently to imbalances in glucose metabolism. Further studies are needed to identify the PTMs associated to these isoforms.
Another protein that is essential for the maintenance of muscle architecture and contractile function is desmin [65]. We found imbalances in the levels of two desmin isoforms. While one desmin isoform (spot B; ~MW 50, ~pI 5.8) was decreased, the level of a second isoform (spot C; ~MW 50, ~pI 5.9) was increased in pre-diabetic hearts. This finding is consistent with previous reports, where imbalances in desmin protein levels were associated with cardiac hypertrophy and failure [66, 67]. Moreover, Western blot analyses revealed fifteen isoforms of desmin (~MW 50 kDa, ~pI 5.8–7.2). As stated in the results section, two of the identified isoforms (isoforms 1 and 2) correspond to protein spots B and C. Isoform 1 is less prominent in pre-diabetic hearts, while the density of isoform 2 is increased in the pre-diabetic state. These results are of particular interest, because they clearly show that levels of different isoforms of the same protein may not display the same profile relative to each other. Therefore, assessment of total protein levels will not provide information about these changes. As discussed above, further studies are needed to investigate the role of these isoforms in heart disease.
In addition to these findings, protein spot E was identified as alpha-cardiac actin (~MW 37 kDa, ~pI 6.2), which is also involved in cell structure and muscle contraction [68]. A recent proteomic study showed down-regulation in the levels of one isoform of alpha-cardiac actin (~MW 42.33 kDa, ~pI 5.23) in mitochondria isolated from heart tissues of db/db mice [16]. Conversely, we found reduced levels of an isoform alpha-cardiac actin in pre-diabetic hearts. This finding may be associated with loss of cell integrity and myocyte contractile function associated to the diabetic phenotype.
Supporting these findings, changes in the levels of αB-crystallin were found in our proteomic analysis, and confirmed by 2-D Western blots. αB-crystallin (spot O; ~MW 23 kDa, ~pI 7.0 ), which is a predominant protein of the ocular lens, is the most abundant small heat shock protein (HSP) in the heart [69]. Heat shock proteins (HSP) expression is usually increased when cells are exposed to stress. Impairments in troponin and desmin levels might lead to cardiac injury promoting the expression of HSP. Previous studies have shown that αB-crystallin mRNA and protein expression are elevated in heart tissue of streptozotocin (STZ)-induced diabetic rats [70], which correlates with our findings.
Together, these results indicate that imbalances in the levels of key proteins needed to maintain normal heart morphology and function may explain in part the increased risk to develop cardiovascular complications in T2D.
4.3.3 Proteins involved in fatty acid oxidation and the citric acid cycle
Alterations in proteins of the citric acid cycle and fatty acid oxidation were also found. Three isoforms of cytosolic malate dehydrogenase 1 (spots H, J and K; ~MW 35–38, ~pI 6.8–7.2) were increased in pre-diabetic hearts. In addition the levels of two isoforms of peroxisomal enoyl-Co A hydratase 1 (spots M and L; ~MW 26 and 29, ~pI 7.3) was elevated in the pre-diabetic hearts.
Previous studies have shown that up-regulation in the protein levels of fatty acid oxidation enzymes increase the risk of developing congestive heart failure and acute myocardial infarction associated to diabetes [9, 14, 71]. In addition, proteomic studies on diabetic hearts have shown significant imbalances in the levels of several enzymes involved in fatty acid oxidation and in the citric acid cycle [15]. Therefore, it is not surprising that we identified alterations in the levels of cytosolic malate dehydrogenase 1 and peroxisomal enoyl-Co A hydratase 1 in pre-diabetic hearts.
Recent studies have shown that p53 expression in the heart has been associated to changes in the cardiac levels of metabolic enzymes, such as cytosolic malate dehydrogenase 1 [72]. Cytosolic malate dehydrogenase 1 has been shown to regulate p53-dependent cell apoptosis in response to metabolic stress, such as glucose deprivation [72]. Thus, our results raise the possibility that p53 levels may be up-regulated in pre-diabetic hearts in response to changes in cytosolic malate dehydrogenase 1 isoforms levels in the heart. However, we found no differences in p53 levels by Western blotting (data not shown). A possible explanation is that the levels of only three isoforms of cytosolic malate dehydrogenase 1 were altered, which may not reflect changes in the total levels of this protein. Moreover, it is possible that the identified isoforms are associated to PTMs that lead to protein conformational changes, which prevent cytosolic malate dehydrogenase 1 interacting with p53. Further studies are needed to examine the association between this enzyme and p53 expression, in heart disease associate to metabolic disorders as T2D.
4.4 Conclusions
In summary, our results suggest that alterations in the levels of proteins involved in energy production, cardiac architecture and contractility occurred early in response to imbalances in glucose metabolism. Of particular interest is that only specific isoforms of the identified proteins were altered in pre-diabetic hearts, but not differences in total protein levels were detected by conventional Western blots.
It is important to acknowledge that the 300 protein spots resolved in this study do not represent the entire proteome of the heart and that 2-DE has its limitations, such as poor resolution of proteins at high and low MWs and pIs, and the amount of sample that can be loaded on the gel [38]. However, as previously discussed, 2-DE offers a particular advantage by visualizing isoforms of a given protein (shifts in pI and MW) [38–40]. Further studies are needed to unravel the chemical nature of the PTMs associated to those isoforms, and their impact in energy production in the diabetic heart.
In addition, future studies are needed to investigate if the same isoforms are present in plasma. If so, and if the results are extrapolated to humans, then these protein isoforms could be early biomarkers of heart disease in diabetic patients, similar to other heart specific proteins that are found in blood as an indicative of cardiac impairment.
Finally, it remains to be investigated if any of the proteins describe herein could be used as therapeutic targets for obesity/T2D related heart disease.
Supplementary Material
Acknowledgments
JJK supported by National Institutes of Health Grants DK075436, AG019899, and AG031736; DoD:W81XWH-08-PCRP-IDA; and by the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Jama. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–50. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 3.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29:1263–8. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 4.Resnick HE, Harris MI, Brock DB, Harris TB. American Diabetes Association diabetes diagnostic criteria, advancing age, and cardiovascular disease risk profiles: results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2000;23:176–80. doi: 10.2337/diacare.23.2.176. [DOI] [PubMed] [Google Scholar]
- 5.Conarello SL, Li Z, Ronan J, Roy RS, Zhu L, Jiang G, et al. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100:6825–30. doi: 10.1073/pnas.0631828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 7.Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–13. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- 8.McNulty PH. Metabolic responsiveness to insulin in the diabetic heart. Am J Physiol Heart Circ Physiol. 2006;290:H1749–51. doi: 10.1152/ajpheart.00037.2006. [DOI] [PubMed] [Google Scholar]
- 9.Lopaschuk GD. Metabolic abnormalities in the diabetic heart. Heart Fail Rev. 2002;7:149–59. doi: 10.1023/a:1015328625394. [DOI] [PubMed] [Google Scholar]
- 10.Maier C, Clodi M, Neuhold S, Resl M, Elhenicky M, Prager R, et al. Endothelial markers may link kidney function to cardiovascular events in type 2 diabetes. Diabetes Care. 2009;32:1890–5. doi: 10.2337/dc08-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll R, Carley AN, Dyck JR, Severson DL. Metabolic effects of insulin on cardiomyocytes from control and diabetic db/db mouse hearts. Am J Physiol Endocrinol Metab. 2005;288:E900–6. doi: 10.1152/ajpendo.00491.2004. [DOI] [PubMed] [Google Scholar]
- 12.Hafstad AD, Solevag GH, Severson DL, Larsen TS, Aasum E. Perfused hearts from Type 2 diabetic (db/db) mice show metabolic responsiveness to insulin. Am J Physiol Heart Circ Physiol. 2006;290:H1763–9. doi: 10.1152/ajpheart.01063.2005. [DOI] [PubMed] [Google Scholar]
- 13.Greer JJ, Ware DP, Lefer DJ. Myocardial infarction and heart failure in the db/db diabetic mouse. Am J Physiol Heart Circ Physiol. 2006;290:H146–53. doi: 10.1152/ajpheart.00583.2005. [DOI] [PubMed] [Google Scholar]
- 14.Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, et al. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–74. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 15.Dabkowski ER, Baseler WA, Williamson CL, Powell M, Razunguzwa TT, Frisbee JC, et al. Mitochondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially distinct mitochondrial proteomes. Am J Physiol Heart Circ Physiol. 299:H529–40. doi: 10.1152/ajpheart.00267.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Essop MF, Chan WY, Hattingh S. Proteomic analysis of mitochondrial proteins in a mouse model of type 2 diabetes. Cardiovasc J Afr. 21:1–4. doi: 10.5830/CVJA-2010-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53 (Suppl 3):S215–9. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 18.Marsh SA, Dell’italia LJ, Chatham JC. Interaction of diet and diabetes on cardiovascular function in rats. Am J Physiol Heart Circ Physiol. 2009;296:H282–92. doi: 10.1152/ajpheart.00421.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cefalu WT. Animal models of type 2 diabetes: clinical presentation and pathophysiological relevance to the human condition. Ilar J. 2006;47:186–98. doi: 10.1093/ilar.47.3.186. [DOI] [PubMed] [Google Scholar]
- 20.Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, et al. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology. 2003;144:3483–90. doi: 10.1210/en.2003-0242. [DOI] [PubMed] [Google Scholar]
- 21.Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, et al. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44:645–51. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 22.Qiu L, List EO, Kopchick JJ. Differentially expressed proteins in the pancreas of diet-induced diabetic mice. Mol Cell Proteomics. 2005;4:1311–8. doi: 10.1074/mcp.M500016-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.List EO, Berryman DE, Palmer AJ, Qiu L, Sankaran S, Kohn DT, et al. Analysis of mouse skin reveals proteins that are altered in a diet-induced diabetic state: a new method for detection of type 2 diabetes. Proteomics. 2007;7:1140–9. doi: 10.1002/pmic.200600641. [DOI] [PubMed] [Google Scholar]
- 24.Okada S, List EO, Sankaran S, Kopchick JJ. Plasma Protein Biomarkers Correlated with the Development of Diet-Induced Type 2 Diabetes in Mice. Clin Proteomics. 6:6–17. doi: 10.1007/s12014-009-9040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Q, Larson DF, Slayback D, Lundeen TF, Baxter JH, Watson RR. Characterization of high-salt and high-fat diets on cardiac and vascular function in mice. Cardiovasc Toxicol. 2004;4:37–46. doi: 10.1385/ct:4:1:37. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez JC, Chiappe D, Converset V, Hoogland C, Binz PA, Paesano S, et al. The mouse SWISS-2D PAGE database: a tool for proteomics study of diabetes and obesity. Proteomics. 2001;1:136–63. doi: 10.1002/1615-9861(200101)1:1<136::AID-PROT136>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Kelder B, Berryman DE, Clark R, Li A, List EO, Kopchick JJ. CIDE-A gene expression is decreased in white adipose tissue of growth hormone receptor/binding protein gene disrupted mice and with high-fat feeding of normal mice. Growth Horm IGF Res. 2007;17:346–51. doi: 10.1016/j.ghir.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Sackmann-Sala L, Ding J, Frohman LA, Kopchick JJ. Activation of the GH/IGF-1 axis by CJC-1295, a long-acting GHRH analog, results in serum protein profile changes in normal adult subjects. Growth Horm IGF Res. 2009;19:471–7. doi: 10.1016/j.ghir.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding J, Kopchick JJ. Age (Dordr) Plasma biomarkers of mouse aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruz-Topete D, Christensen B, Sackmann-Sala L, Okada S, Jorgensen JO, Kopchick JJ. Eur J Endocrinol. Serum Proteome Changes in Acromegalic Patients following Transsphenoidal Surgery: Novel Biomarkers of Disease Activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen B, Sackmann-Sala L, Cruz-Topete D, Jorgensen JO, Jessen N, Lundby C, et al. Novel serum biomarkers for erythropoietin use in humans: A proteomic approach. J Appl Physiol. doi: 10.1152/japplphysiol.00665.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malone JP, Radabaugh MR, Leimgruber RM, Gerstenecker GS. Practical aspects of fluorescent staining for proteomic applications. Electrophoresis. 2001;22:919–32. doi: 10.1002/1522-2683()22:5<919::AID-ELPS919>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 33.McGregor E, Dunn MJ. Proteomics of the heart: unraveling disease. Circ Res. 2006;98:309–21. doi: 10.1161/01.RES.0000201280.20709.26. [DOI] [PubMed] [Google Scholar]
- 34.Raddatz K, Albrecht D, Hochgrafe F, Hecker M, Gotthardt M. A proteome map of murine heart and skeletal muscle. Proteomics. 2008;8:1885–97. doi: 10.1002/pmic.200700902. [DOI] [PubMed] [Google Scholar]
- 35.Evans G, Wheeler CH, Corbett JM, Dunn MJ. Construction of HSC-2DPAGE: a two-dimensional gel electrophoresis database of heart proteins. Electrophoresis. 1997;18:471–9. doi: 10.1002/elps.1150180322. [DOI] [PubMed] [Google Scholar]
- 36.Westbrook JA, Wheeler JX, Wait R, Welson SY, Dunn MJ. The human heart proteome: Two-dimensional maps using narrow-range immobilised pH gradients. Electrophoresis. 2006;27:1547–55. doi: 10.1002/elps.200500777. [DOI] [PubMed] [Google Scholar]
- 37.Bousette N, Kislinger T, Fong V, Isserlin R, Hewel JA, Emil A, et al. Large-scale characterization and analysis of the murine cardiac proteome. J Proteome Res. 2009;8:1887–901. doi: 10.1021/pr800845a. [DOI] [PubMed] [Google Scholar]
- 38.Gucek M, Murphy E. What can we learn about cardioprotection from the cardiac mitochondrial proteome? Cardiovasc Res. 88:211–8. doi: 10.1093/cvr/cvq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayr M, Mayr U, Chung YL, Yin X, Griffiths JR, Xu Q. Vascular proteomics: linking proteomic and metabolomic changes. Proteomics. 2004;4:3751–61. doi: 10.1002/pmic.200400947. [DOI] [PubMed] [Google Scholar]
- 40.Mayr M, Mandal K, Xu Q. Pitfalls of proteomics. Circulation. 2004;110:e316. doi: 10.1161/01.CIR.0000142204.34987.09. author replye. [DOI] [PubMed] [Google Scholar]
- 41.Liao R, Jain M, Cui L, D’Agostino J, Aiello F, Luptak I, et al. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–31. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- 42.Yan J, Young ME, Cui L, Lopaschuk GD, Liao R, Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation. 2009;119:2818–28. doi: 10.1161/CIRCULATIONAHA.108.832915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, et al. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 2007;56:2085–92. doi: 10.2337/db07-0093. [DOI] [PubMed] [Google Scholar]
- 44.Mollica MP, Iossa S, Liverini G, Soboll S. Steady state changes in mitochondrial electrical potential and proton gradient in perfused liver from rats fed a high fat diet. Mol Cell Biochem. 1998;178:213–7. doi: 10.1023/a:1006899632413. [DOI] [PubMed] [Google Scholar]
- 45.Feuvray D, Darmellah A. Diabetes-related metabolic perturbations in cardiac myocyte. Diabetes Metab. 2008;34 (Suppl 1):S3–9. doi: 10.1016/S1262-3636(08)70096-X. [DOI] [PubMed] [Google Scholar]
- 46.Turko IV, Murad F. Quantitative protein profiling in heart mitochondria from diabetic rats. J Biol Chem. 2003;278:35844–9. doi: 10.1074/jbc.M303139200. [DOI] [PubMed] [Google Scholar]
- 47.Harmancey R, Taegtmeyer H. The complexities of diabetic cardiomyopathy: lessons from patients and animal models. Curr Diab Rep. 2008;8:243–8. doi: 10.1007/s11892-008-0042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noji H, Yoshida M. The rotary machine in the cell, ATP synthase. J Biol Chem. 2001;276:1665–8. doi: 10.1074/jbc.R000021200. [DOI] [PubMed] [Google Scholar]
- 49.Grant JE, Bradshaw AD, Schwacke JH, Baicu CF, Zile MR, Schey KL. Quantification of protein expression changes in the aging left ventricle of Rattus norvegicus. J Proteome Res. 2009;8:4252–63. doi: 10.1021/pr900297f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Preston CC, Oberlin AS, Holmuhamedov EL, Gupta A, Sagar S, Syed RH, et al. Aging-induced alterations in gene transcripts and functional activity of mitochondrial oxidative phosphorylation complexes in the heart. Mech Ageing Dev. 2008;129:304–12. doi: 10.1016/j.mad.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim N, Lee Y, Kim H, Joo H, Youm JB, Park WS, et al. Potential biomarkers for ischemic heart damage identified in mitochondrial proteins by comparative proteomics. Proteomics. 2006;6:1237–49. doi: 10.1002/pmic.200500291. [DOI] [PubMed] [Google Scholar]
- 52.Dzeja P, Kalvenas A, Toleikis A, Praskevicius A. The effect of adenylate kinase activity on the rate and efficiency of energy transport from mitochondria to hexokinase. Biochem Int. 1985;10:259–65. [PubMed] [Google Scholar]
- 53.Dzeja PP, Zeleznikar RJ, Goldberg ND. Adenylate kinase: kinetic behavior in intact cells indicates it is integral to multiple cellular processes. Mol Cell Biochem. 1998;184:169–82. [PubMed] [Google Scholar]
- 54.Pucar D, Dzeja PP, Bast P, Juranic N, Macura S, Terzic A. Cellular energetics in the preconditioned state: protective role for phosphotransfer reactions captured by 18O-assisted 31P NMR. J Biol Chem. 2001;276:44812–9. doi: 10.1074/jbc.M104425200. [DOI] [PubMed] [Google Scholar]
- 55.Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, et al. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci U S A. 2001;98:7623–8. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turko IV, Li L, Aulak KS, Stuehr DJ, Chang JY, Murad F. Protein tyrosine nitration in the mitochondria from diabetic mouse heart. Implications to dysfunctional mitochondria in diabetes. J Biol Chem. 2003;278:33972–7. doi: 10.1074/jbc.M303734200. [DOI] [PubMed] [Google Scholar]
- 57.Mahajan VB, Pai KS, Lau A, Cunningham DD. Creatine kinase, an ATP-generating enzyme, is required for thrombin receptor signaling to the cytoskeleton. Proc Natl Acad Sci U S A. 2000;97:12062–7. doi: 10.1073/pnas.97.22.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong Q, Li Q, Mansoor A, Jameel MN, Du F, Chen W, et al. Novel strategy for measuring creatine kinase reaction rate in the in vivo heart. Am J Physiol Heart Circ Physiol. 2009;297:H1010–9. doi: 10.1152/ajpheart.01195.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A. 2005;102:808–13. doi: 10.1073/pnas.0408962102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Y, Artman M. Nucleoside diphosphate kinase: a new player in heart failure? Cardiovasc Res. 2001;49:7–10. doi: 10.1016/s0008-6363(00)00273-x. [DOI] [PubMed] [Google Scholar]
- 61.Lutz S, Mura R, Baltus D, Movsesian M, Kubler W, Niroomand F. Increased activity of membrane-associated nucleoside diphosphate kinase and inhibition of cAMP synthesis in failing human myocardium. Cardiovasc Res. 2001;49:48–55. doi: 10.1016/s0008-6363(00)00222-4. [DOI] [PubMed] [Google Scholar]
- 62.Wu AH. The role of cardiac troponin in the recent redefinition of acute myocardial infarction. Clin Lab Sci. 2004;17:50–2. [PubMed] [Google Scholar]
- 63.Akella AB, Ding XL, Cheng R, Gulati J. Diminished Ca2+ sensitivity of skinned cardiac muscle contractility coincident with troponin T-band shifts in the diabetic rat. Circ Res. 1995;76:600–6. doi: 10.1161/01.res.76.4.600. [DOI] [PubMed] [Google Scholar]
- 64.Du CK, Morimoto S, Nishii K, Minakami R, Ohta M, Tadano N, et al. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res. 2007;101:185–94. doi: 10.1161/CIRCRESAHA.106.146670. [DOI] [PubMed] [Google Scholar]
- 65.Paulin D, Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res. 2004;301:1–7. doi: 10.1016/j.yexcr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Lam L, Tsoutsman T, Arthur J, Semsarian C. Differential protein expression profiling of myocardial tissue in a mouse model of hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, et al. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res. 2000;86:846–53. doi: 10.1161/01.res.86.8.846. [DOI] [PubMed] [Google Scholar]
- 68.Chaponnier C, Gabbiani G. Pathological situations characterized by altered actin isoform expression. J Pathol. 2004;204:386–95. doi: 10.1002/path.1635. [DOI] [PubMed] [Google Scholar]
- 69.Wang X, Klevitsky R, Huang W, Glasford J, Li F, Robbins J. AlphaB-crystallin modulates protein aggregation of abnormal desmin. Circ Res. 2003;93:998–1005. doi: 10.1161/01.RES.0000102401.77712.ED. [DOI] [PubMed] [Google Scholar]
- 70.Kumar PA, Haseeb A, Suryanarayana P, Ehtesham NZ, Reddy GB. Elevated expression of alphaA- and alphaB-crystallins in streptozotocin-induced diabetic rat. Arch Biochem Biophys. 2005;444:77–83. doi: 10.1016/j.abb.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 71.Heart E, Cline GW, Collis LP, Pongratz RL, Gray JP, Smith PJ. Role for malic enzyme, pyruvate carboxylation, and mitochondrial malate import in glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2009;296:E1354–62. doi: 10.1152/ajpendo.90836.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee SM, Kim JH, Cho EJ, Youn HD. A nucleocytoplasmic malate dehydrogenase regulates p53 transcriptional activity in response to metabolic stress. Cell Death Differ. 2009;16:738–48. doi: 10.1038/cdd.2009.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.