Abstract
Children and women of reproductive age are increasingly surviving cancer diagnoses, and therefore long-term quality-of-life issues are of greater importance at the time of diagnosis. Cancer therapies including radiation and chemotherapy can be detrimental to fertility, and therefore many patients are motivated to preserve fertility prior to cancer treatment. The only highly successful method in preserving fertility to date is embryo cryopreservation, which may not be appropriate for some patients due to age, delay in treatment, cancer type and stage, as well as availability of an acceptable sperm donor. Alternative methods including oocyte cryopreservation and ovarian tissue banking may also preserve fertility while providing additional flexibility to patients. In vitro ovarian follicle maturation following tissue banking is one potential approach that would not require a delay in cancer therapy for ovarian stimulation, would not require an immediate sperm donor, and does not carry the risk of reintroducing malignant cells following tissue transplantation. In vitro follicle culture systems have resulted in successful live births in the mouse. However, many challenges must be addressed in translating the system to the human. This review summarizes current approaches to fertility preservation and discusses recent developments and future challenges in developing a human in vitro follicle culture system.
Keywords: fertility preservation, follicle, oncofertility
INTRODUCTION
Motivation
Recent developments in techniques to preserve female fertility following cancer treatment have improved the outlook for patients facing premature infertility due to cancer therapies [1]. Approaches to fertility preservation include embryo cryopreservation, oocyte cryopreservation, and tissue banking followed by tissue transplant or in vitro follicle culture. While embryo cryopreservation has been largely successful in the past, alternative approaches are emerging. Oocyte cryopreservation has become an increasingly successful method, now accounting for over 200 live births [2], and transplantation of cryopreserved tissue has resulted in two live births [3,4]. The in vitro culture of follicles in “in follicle maturation” or IFM systems has recently been shown to support follicle development and maintain oocyte health during long-term culture. Although IFM systems are in the early stages of development, this approach offers great promise for fertility preservation method to patients limited by age, marital status, or cancer diagnosis and treatment options. This review highlights recent developments in fertility preservation technologies and discusses the successes and future directions for investigation in developing an in vitro culture system for human follicles.
Aggressive chemotherapy and radiotherapy treatments have greatly increased the survivorship of young patients with cancer diagnoses; however these treatments are often detrimental to fertility. Women in developed countries are increasingly choosing to delay childbearing until later in life for social or financial reasons so that increasing numbers of reproductive age women are interested in preserving fertility at the time of cancer diagnosis [5–7]. An important quality-of-life issue to these patients and their families is the ability to bear healthy children [8]. While sperm cryopreservation has been shown to be an effective method to preserve fertility for men and adolescent males [9,10], few methods are available for women. If no action is taken prior to cancer treatment, women can adopt or use donor oocytes to conceive. Many women wish to conceive using their own oocytes, which has led to an increased focus on preserving female fertility through cryopreservation and assisted reproduction [11].
Toxicity of Cancer Therapies
Current cancer therapies, including both radiation and chemotherapy, can lead to premature infertility in women, which is attributed to decreased ovarian reserve. The type of cancer, the prescribed treatment, and the age of the woman all contribute to the risk of infertility following cancer therapy. Radiotherapy is known to adversely affect gonadal function, and its impact on fertility is dependent upon the dose and field of therapy, and the patient’s age at the time of treatment [12]. Radiation was shown to deplete primordial follicles in mouse ovaries in a dose-dependent manner [13], and both total body radiation [14] and pelvic radiation [15] have been shown to cause premature ovarian failure in women. The ovarian reserve decreases with age, and therefore older women (women over 38–40 years of age) are more sensitive to damage by radiotherapy [12,16,17]. Chemotherapy also causes damage to the ovary, and the risk of infertility is also dependent upon the patient age, as well as the type(s) of chemotherapeutic drugs used and the total dosage administered [18]. A variety of cytotoxic drugs and their effects on fertility have been reviewed in detail [19–21], and many, most notably alkylating agents, can induce ovarian failure [22]. Due to the variety of treatment combinations and the variability among patients, no comprehensive data exist associating the exact rates of fertility impairment with courses of treatment [23]. Current findings, however, clearly demonstrate that the fertility of many long-term survivors will be severely reduced following treatment [12,24].
METHODS TO PRESERVE FEMALE FERTILITY
To preserve fertility for these young women, many techniques are currently under investigation including various prevention techniques, embryo cryopreservation, oocyte cryopreservation, and ovarian tissue banking [19,20,25]. To date, embryo cryopreservation is the only method widely used for fertility preservation; however both oocyte cryopreservation and ovarian tissue banking followed by tissue transplant have also been successful in producing a limited number of live human births. These and other methods, including in vitro culture of cryopreserved ovarian tissue, are currently in development. Each approach has associated advantages and disadvantages relating to current success rate, required delay in cancer treatment, sperm requirement, and risk of reintroducing cancer cells.
Prevention/Minimizing Impact/Ovarian Protection
A variety of methods can be employed to reduce the risk of fertility damage during treatment. Depending on the malignancy, chemotherapeutic agents and doses can be selected which are known to posses a decreased risk of ovarian damage. Radiation doses during total body irradiation can be fractionated and the pelvic organs can be shielded to reduce risk. In cases where pelvic radiotherapy is recommended, oophoeropexy or surgical transposition of the ovaries, away from the radiation field has been shown to reduce, although not eliminate, ovarian damage [26,27]. Administration of GnRH agonists prior to and during treatment has also been demonstrated to convey some ovarian protection against chemotherapy, although results are contradictory and further investigation is underway [20,28].
Embryo Cryopreservation
Embryo cryopreservation is currently the only highly successful method of preserving fertility for female cancer patients. Embryo cryopreservation is a routine procedure in IVF facilities, and reported survival rates per thawed embryo range from 35% to 90%, implantation rates from 8% to 30%, and cumulative pregnancy rates of 30–40% [11,29]. This procedure requires ovarian stimulation to collect mature oocytes, which are then fertilized in vitro and cryopreserved for use following cancer treatment. In addition, a male partner or donor sperm is required for fertilization. This procedure may not be an option for women with highly aggressive malignancies; including leukemia, some lymphomas, and sarcomas, which warrant immediate cancer treatment [30]. Successful ovarian stimulation requires several weeks to months depending on the response of the patient to the stimulation protocol, which may compromise the patient’s long-term outcome. Many cancer patients do not respond well to the standard IVF stimulation protocols, which may result in decreased quality and/or quantity of oocytes collected during the cycle [31,32]. In addition, ovarian stimulation is associated with high-serum estrogen concentrations, and should therefore be avoided when patients have hormone-sensitive tumors, including breast and endometrial cancers [33,34]. Alternative stimulation protocols, including the use of tamoxifen or letrizole, have been investigated and have shown some success in breast cancer patients [35,36]. However, this is still an investigational procedure. Finally, ovarian stimulation is often not considered ethically appropriate for pubertal females, and the requirement for a partner or sperm donor makes embryo cryopreservation undesirable for single women who prefer not to use a sperm donor.
Oocyte Cryopreservation
Oocyte cryopreservation is an alternative fertility preservation option that would obviate the need for a sperm donor. As of 2005, more than 200 children had been born using cryopreserved oocytes. However, the overall live birth-rate per cryopreserved oocyte is approximately 2%, which is much lower than the rate using fresh oocytes [2]. Oocyte cryopreservation is therefore still in the developmental stages, and much higher success rates must be obtained for this to be widely offered to cancer patients. Oocytes are much more sensitive to freezing and thawing than embryos due to their large volume, high-lipid content, and temperature-sensitive spindle, to which the chromosomes are attached in the mature oocyte [30,37]. The cryopreservation procedure can therefore lead to oocyte death as well as aneuploidy [38]. Oocyte cryopreservation also requires ovarian hyperstimulation, and therefore carries with it the associated issues described above for embryo cryopreservation including non-feasibility for prepubertal girls, delay in treatment, and concerns regarding hormone-responsive cancers. Due to the relatively low success rate of oocyte cryopreservation, greater numbers of oocytes (relative to embryos) must be cryopreserved to have a reasonable chance of success. Thus, this methodology is associated with longer delays in treatment for multiple cycles and a decreased overall chance of success.
Tissue Banking
Cryopreservation of ovarian tissue is a promising option for fertility preservation that precludes the need for ovarian stimulation and a sperm donor. Ovarian tissue banking may be the only acceptable method to preserve fertility for prepubertal girls [7], and may be a preferred option for single women, women who cannot delay cancer treatment for stimulation, and women with hormone-sensitive malignancies [19]. In this procedure, ovarian tissue is surgically removed and processed into cortical strips for cryopreservation. By this method, hundreds of primordial follicles are preserved in a single procedure that can be performed immediately and does not require a delay in cancer treatment [19]. Studies have demonstrated that follicle viability and developmental potential is preserved following cryopreservation and thawing [39,40]. Most follicles that survive cryopreservation are the small primordial follicles [41], which are much more tolerant of cryopreservation than mature oocytes due to their size, decreased metabolism, and absence of a metaphase spindle, zona pellucida, and cortical granules [42,43]. The challenge in this approach therefore lies in developing methods to support the maturation of these primordial follicles to produce mature, fertilizable oocytes. Several approaches have been investigated, including autotransplantation and in vitro maturation (IVM).
Autotransplantation
Autotransplantation of cryopreserved ovarian tissue is the only tissue banking approach that has, to date, produced human births [3,4]. In this procedure, cryopreserved and thawed cortical strips are transplanted back into the patient, to either an orthotopic or hererotopic site. Previous studies in multiple animal species demonstrated successful pregnancies and live births using this method [25,44–46], leading researchers to attempt this in humans. The first live birth in humans was reported in a patient previously treated for Hodgkin lymphoma, in which thawed tissue was implanted into an intraperitoneal location adjacent to the atrophic ovary [4]. Due to the presence of the ovary, although atrophic, it could not be proven that the oocyte was released from the transplanted tissue. A subsequent and more convincing report of a successful live birth following ovarian transplant involved autografting of cryopreserved tissue to the remaining ovary in a 28-year-old patient who demonstrated several years of ovarian failure following treatment for non-Hodgkin lymphoma [3]. This patient spontaneously menstruated 2 months after surgery, and underwent in vitro fertilization, resulting in delivery of a term infant.
Heterologous transplantation offers the advantages of accessibility for implantation and removal (if necessary), ease of monitoring, and does not require general anesthesia for transplantation. A recent study reported transplantation of fresh monkey tissue to heterologous sites, producing multiple oocytes that were fertilized and transferred resulting in a live monkey birth [47]. In the human, multiple reports of heterotopic transplantation to various sites including the abdominal wall and forearm [48,49] have demonstrated that transplanted cortical strips lead to resumption of menstrual cycling and follicle development, and has resulted in the production of a four-cell embryo [48]. Embryo transfer has been attempted in one patient, but has not resulted in pregnancy [48]. Spontaneous menstrual cycles resumed in this patient 6 months following the transplant, and 2 months later, she conceived naturally.
One limitation common to many tissue transplants is the potential for ischemia within the tissue before it can become revascularized. Such ischemia may result in follicle atresia, thereby compromising the long-term function of the transplanted ovarian tissue [50]. Reports have indicated that most follicles survive the freeze–thaw cycle. However, up to two-thirds are lost following transplantation [51]. Approaches to prevent such ischemia may include varying the size of tissue transplants or selecting a site of implantation known to be responsive to angiogenesis. An alternative approach may be to deliver angiogenesis-promoting factors, such as VEGF, to the implantation site at the time of surgery. Research in the area of regenerative medicine has demonstrated the ability to improve neovascularization into tissues using such techniques [52], and applying these techniques to cortical strip transplantation may reduce follicle loss due to ischemia.
The main concern regarding ovarian transplantation is the risk of reintroducing malignant cells to the patient. Studies in the mouse have demonstrated that such tumor recurrences may occur following reimplantation [53]. However, this risk varies with cancer type, activity, and stage, as well as the mass of malignant cells transferred [54]. With the exception of some hematological malignancies and some advanced stage solid tumors, most malignant tumors that occur in reproductive age women do not metastasize to the ovaries [55]. Xenotransplantation is a theoretical concept that has yet to be tested. Alternative animal species, such as the nude mouse, would serve as a non-human “incubator” for human follicle development with the intent of developing a mature gamete that could be recovered and fertilized in vitro.
In Vitro Culture
In vitro follicle maturation is an alternative approach to the maturation of immature follicles that precludes the need for tissue transplant and therefore eliminates the risk of reintroducing cancer cells. As is true for all tissue banking approaches, in vitro culture would not require a delay in cancer treatment for ovarian stimulation. It would allow for a delay in sperm selection, which may be desirable for children or single women. Furthermore, tissue banking, with the storage of gametes rather than embryos, may be more acceptable to patients who oppose embryo cryopreservation due to social or religious factors. A successful in vitro culture system would support the development of follicles to produce mature oocytes that could be fertilized using well-established in vitro fertilization techniques. Two approaches to in vitro follicle maturation have been investigated to date [56]. The first is organ culture, in which whole slices of ovarian cortical tissue are cultured intact. The second approach is isolated follicle culture, in which individual follicles are removed from the surrounding cortical tissue prior to culture. Both approaches have demonstrated an ability to support human follicle growth, although results differ between various systems.
Organ Culture
Organ culture, or the culture of intact ovarian cortical strips, retains the organizational structure of the ovarian tissue and maintains the interactions between the follicle and surrounding stromal cells. This method has supported growth of human primordial follicles to the secondary follicle stage [17,57–62], and has supported follicle survival for up to 4 weeks [60]. Approaches have included both culture on a two-dimensional surface as well as cultures in three-dimensional gels [60]. The main challenge with in vitro organ culture, as in transplantation, is preventing atresia due to ischemia in the interior of the tissue, since there is no possibility of revascularization in the in vitro environment. Varying the size and geometry of the cultured tissue pieces or slices has been shown to improve follicle survival [58], although ischemia is difficult to eliminate entirely by this approach. A second disadvantage to organ culture is the inability to observe and monitor follicles during culture by light microscopy due to the thickness of the tissue. For this reason, it is also impossible to know the follicle content of each tissue piece until the tissue is removed and dissected or sectioned, and therefore carries the risk of culturing empty tissue pieces [56], particularly in the case of patients with diminished ovarian reserve due to age, disease, or previous cancer therapy.
In Follicle Maturation
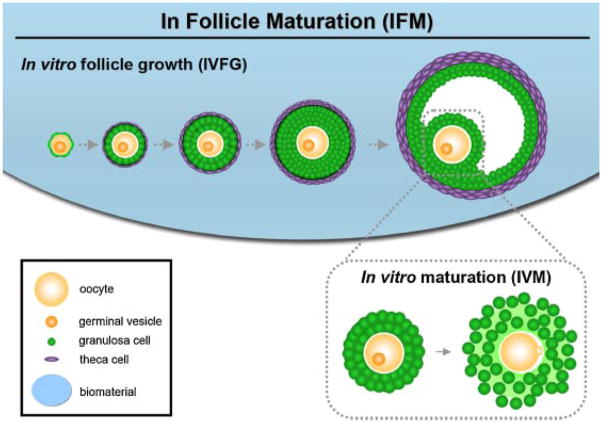
Culture of isolated follicles allows for the individual monitoring and tracking of each follicle to assess its developmental state. The IFM approach involves in vitro follicle growth (IVFG), or the in vitro culture of immature follicles, followed by IVM of the oocyte within the follicle (Fig. 1). In IFM systems, follicles are isolated from the ovary using a mechanical or enzymatic approach. Follicles are then cultured for a period of time during which the growth, steroid production, and morphology of each follicle can be monitored. Several IFM systems have produced live mouse pups following culture of immature follicles [63–66]. Systems have also been demonstrated in humans and non-human primates to support follicle growth and development [67,68]. The development of culture systems for human follicles is in the early stages, however, and to date, no meiotically competent oocytes have been produced.
Fig. 1.
In follicle maturation (IFM): In follicle maturation describes the process of the in vitro growth of an immature follicle to produce a mature oocyte. Immature follicles are encapsulated in a biomaterial and grown to maturity in a process termed in vitro follicle growth (IVFG). The mature follicle is then taken through the in vitro maturation process (IVM) during which the administration of hCG and EGF result in cumulus expansion and the maturation of the oocyte to the MII stage.
Two basic approaches have been utilized in developing IFM systems [69]. In the first approach, generally referred to as an attached follicle approach, isolated follicles attach and spread onto a two-dimensional surface. As the follicle develops, proliferating granulosa cells attach and migrate onto the two-dimensional culture substrate and away from the oocyte [70]. In the second approach, referred to as a three-dimensional intact follicle approach, follicles do not attach to a substrate and therefore are able to maintain their three-dimensional architecture. As the follicle grows, the growth occurs radially from the center of the follicle as it would occur in the ovary [70]. Both approaches have produced live murine offspring. However, three-dimensional approaches maintain the cell–cell and cell–matrix interactions, which are known to be important in regulating follicle development [70–72]. In addition, while successful in the mouse, two-dimensional systems have not been able to support normal follicle development in bovine [73], ovine [74], or human systems [75,76]. Three-dimensional approaches have been proposed to be increasingly beneficial in overcoming the difficulty of maintaining follicle architecture during culture for larger species [56,77], and in one human follicle culture study, follicles grew in a three-dimensional collagen gel, while follicles on a two-dimensional collagen coated surface only maintained their original size [68].
Several approaches to three-dimensional IFM have been investigated [70], of which the encapsulated follicle approach has produced the only live mouse births to date [66]. This system utilizes alginate, a hydrogel derived from brown algae, as a three-dimensional encapsulation matrix. Alginate cross-links in the presence of divalent cations such as Ca2+ and can be degraded for follicle recovery using an alginate-specific enzyme, alginate lyase. In addition, alginate does not interact biochemically with mammalian cells, therefore allowing for the creation of a well-defined culture environment by adding specific biomolecules to the culture system. These and other properties make alginate a good candidate for three-dimensional IFM [70]. In the alginate culture system, follicles have been able to grow [78], produce fluid-filled antral cavities [79], and produce meiotically competent oocytes [80,81], which were successfully fertilized and implanted to yield multiple live births of healthy mouse pups [66]. The three-dimensional encapsulated follicle approach to IFM therefore demonstrates potential in supporting human follicle development.
CONCLUSIONS AND FUTURE DIRECTIONS
The increasing numbers of children and reproductive-age women surviving cancer diagnoses has resulted in an increased focus on preserving fertility prior to cancer treatment. While embryo cryopreservation is the only established method used to preserve fertility, many alternative techniques are currently being developed. Options such as oocyte cryopreservation and ovarian tissue banking followed by tissue transplantation have produced a limited number of live births in humans. Methods for the in vitro culture of cryopreserved tissue or follicles are in relatively early stages of development. In order to successfully translate the system to the human, the optimal techniques for culture must be identified for isolation techniques, matrix conditions, media composition, assessment of the developmental state of the follicle and oocyte, and for determination of the timing of oocyte IVM. A successful in vitro follicle culture system may therefore be beneficial to many young girls and women. The final outcome for success of such systems will be a healthy human birth following in vitro culture, maturation, and fertilization.
Acknowledgments
Grant sponsor: Oncofertility Consortium (NIH); Grant numbers: UL1 RR024926, RL1-HD058295, PL1 EB008542, PL1 CA133835, RL1 HD058293, RL1 HD058294, KL1 CA133839.
References
- 1.Woodruff T, Snyder K. Oncofertility: Fertility preservation for cancer survivors. New York: Springer; 2007. [Google Scholar]
- 2.Gosden RG. Prospects for oocyte banking and in vitro maturation. J Natl Cancer Inst Monogr. 2005;34:60–63. doi: 10.1093/jncimonographs/lgi007. [DOI] [PubMed] [Google Scholar]
- 3.Meirow D, Levron J, Eldar-Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 4.Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Schover LR, Partridge AH, et al. American society of clinical oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 6.Lobo RA. Potential options for preservation of fertility in women. N Engl J Med. 2005;353:64–73. doi: 10.1056/NEJMra043475. [DOI] [PubMed] [Google Scholar]
- 7.Simon B, Lee SJ, Partridge AH, et al. Preserving fertility after cancer. CA Cancer J Clin. 2005;55:211–228. doi: 10.3322/canjclin.55.4.211. quiz 263–214. [DOI] [PubMed] [Google Scholar]
- 8.Nieman CL, Kinahan KE, Yount SE, et al. Fertility preservation and adolescent cancer patients: Lessons from adult survivors of childhood cancer and their parents. Cancer Treat Res. 2007;138:201–217. doi: 10.1007/978-0-387-72293-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kliesch S, Behre HM, Jurgens H, et al. Cryopreservation of semen from adolescent patients with malignancies. Med Pediatr Oncol. 1996;26:20–27. doi: 10.1002/(SICI)1096-911X(199601)26:1<20::AID-MPO3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Revel A, Haimov-Kochman R, Porat A, et al. In vitro fertilization-intracytoplasmic sperm injection success rates with cryopreserved sperm from patients with malignant disease. Fertil Steril. 2005;84:118–122. doi: 10.1016/j.fertnstert.2005.01.121. [DOI] [PubMed] [Google Scholar]
- 11.Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10:251–266. doi: 10.1093/humupd/dmh021. [DOI] [PubMed] [Google Scholar]
- 12.Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7:535–543. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 13.Gosden RG, Wade JC, Fraser HM, et al. Impact of congenital or experimental hypogonadotrophism on the radiation sensitivity of the mouse ovary. Hum Reprod. 1997;12:2483–2488. doi: 10.1093/humrep/12.11.2483. [DOI] [PubMed] [Google Scholar]
- 14.Thibaud E, Rodriguez-Macias K, Trivin C, et al. Ovarian function after bone marrow transplantation during childhood. Bone Marrow Transplant. 1998;21:287–290. doi: 10.1038/sj.bmt.1701075. [DOI] [PubMed] [Google Scholar]
- 15.Chiarelli AM, Marrett LD, Darlington G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964–1988 in Ontario, Canada. Am J Epidemiol. 1999;150:245–254. doi: 10.1093/oxfordjournals.aje.a009995. [DOI] [PubMed] [Google Scholar]
- 16.Lushbaugh CC, Casarett GW. The effects of gonadal irradiation in clinical radiation therapy: A review. Cancer. 1976;37:1111–1125. doi: 10.1002/1097-0142(197602)37:2+<1111::aid-cncr2820370821>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Behringer K, Breuer K, Reineke T, et al. Secondary amenorrhea after Hodgkin’s lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: A report from the German Hodgkin’s lymphoma study group. J Clin Oncol. 2005;23:7555–7564. doi: 10.1200/JCO.2005.08.138. [DOI] [PubMed] [Google Scholar]
- 18.Minton SE, Munster PN. Chemotherapy-induced amenorrhea and fertility in women undergoing adjuvant treatment for breast cancer. Cancer Control. 2002;9:466–472. doi: 10.1177/107327480200900603. [DOI] [PubMed] [Google Scholar]
- 19.Marhhom E, Cohen I. Fertility preservation options for women with malignancies. Obstet Gynecol Surv. 2007;62:58–72. doi: 10.1097/01.ogx.0000251029.93792.5d. [DOI] [PubMed] [Google Scholar]
- 20.Maltaris T, Koelbl H, Seufert R, et al. Gonadal damage and options for fertility preservation in female and male cancer survivors. Asian J Androl. 2006;8:515–533. doi: 10.1111/j.1745-7262.2006.00206.x. [DOI] [PubMed] [Google Scholar]
- 21.Maltaris T, Seufert R, Fischl F, et al. The effect of cancer treatment on female fertility and strategies for preserving fertility. Eur J Obstet Gynecol Reprod Biol. 2007;130:148–155. doi: 10.1016/j.ejogrb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Meirow D. Ovarian injury and modern options to preserve fertility in female cancer patients treated with high-dose radio-chemotherapy for hemato-oncological neoplasias and other cancers. Leuk Lymphoma. 1999;33:65–76. doi: 10.3109/10428199909093726. [DOI] [PubMed] [Google Scholar]
- 23.Weintraub M, Gross E, Kadari A, et al. Should ovarian cryopreservation be offered to girls with cancer. Pediatr Blood Cancer. 2007;48:4–9. doi: 10.1002/pbc.20946. [DOI] [PubMed] [Google Scholar]
- 24.Waring AB, Wallace WH. Subfertility following treatment for childhood cancer. Hosp Med. 2000;61:550–557. doi: 10.12968/hosp.2000.61.8.1398. [DOI] [PubMed] [Google Scholar]
- 25.Abir R, Fisch B, Raz A, et al. Preservation of fertility in women undergoing chemotherapy: Current approach and future prospects. J Assist Reprod Genet. 1998;15:469–477. doi: 10.1023/A:1022578303272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feeney DD, Moore DH, Look KY, et al. The fate of the ovaries after radical hysterectomy and ovarian transposition. Gynecol Oncol. 1995;56:3–7. doi: 10.1006/gyno.1995.1002. [DOI] [PubMed] [Google Scholar]
- 27.Morice P, Castaigne D, Haie-Meder C, et al. Laparoscopic ovarian transposition for pelvic malignancies: Indications and functional outcomes. Fertil Steril. 1998;70:956–960. doi: 10.1016/s0015-0282(98)00284-2. [DOI] [PubMed] [Google Scholar]
- 28.Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12:1044–1054. doi: 10.1634/theoncologist.12-9-1044. [DOI] [PubMed] [Google Scholar]
- 29.Son WY, Yoon SH, Yoon HJ, et al. Pregnancy outcome following transfer of human blastocysts vitrified on electron microscopy grids after induced collapse of the blastocoele. Hum Reprod. 2003;18:137–139. doi: 10.1093/humrep/deg029. [DOI] [PubMed] [Google Scholar]
- 30.Stern CJ, Toledo MG, Gook DA, et al. Fertility preservation in female oncology patients. Aust NZ J Obstet Gynaecol. 2006;46:15–23. doi: 10.1111/j.1479-828X.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- 31.Ginsburg ES, Yanushpolsky EH, Jackson KV. In vitro fertilization for cancer patients and survivors. Fertil Steril. 2001;75:705–710. doi: 10.1016/s0015-0282(00)01802-1. [DOI] [PubMed] [Google Scholar]
- 32.Dolmans MM, Demylle D, Martinez-Madrid B, et al. Efficacy of in vitro fertilization after chemotherapy. Fertil Steril. 2005;83:897–901. doi: 10.1016/j.fertnstert.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 33.Pena JE, Chang PL, Chan LK, et al. Supraphysiological estradiol levels do not affect oocyte and embryo quality in oocyte donation cycles. Hum Reprod. 2002;17:83–87. doi: 10.1093/humrep/17.1.83. [DOI] [PubMed] [Google Scholar]
- 34.Chen CH, Zhang X, Barnes R, et al. Relationship between peak serum estradiol levels and treatment outcome in in vitro fertilization cycles after embryo transfer on day 3 or day 5. Fertil Steril. 2003;80:75–79. doi: 10.1016/s0015-0282(03)00504-1. [DOI] [PubMed] [Google Scholar]
- 35.Oktay K, Buyuk E, Libertella N, et al. Fertility preservation in breast cancer patients: A prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 36.Oktay K, Buyuk E, Davis O, et al. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod. 2003;18:90–95. doi: 10.1093/humrep/deg045. [DOI] [PubMed] [Google Scholar]
- 37.Baka SG, Toth TL, Veeck LL, et al. Evaluation of the spindle apparatus of in-vitro matured human oocytes following cryopreservation. Hum Reprod. 1995;10:1816–1820. doi: 10.1093/oxfordjournals.humrep.a136182. [DOI] [PubMed] [Google Scholar]
- 38.Pickering SJ, Braude PR, Johnson MH, et al. Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the human oocyte. Fertil Steril. 1990;54:102–108. doi: 10.1016/s0015-0282(16)53644-9. [DOI] [PubMed] [Google Scholar]
- 39.Gook DA, Edgar DH, Borg J, et al. Diagnostic assessment of the developmental potential of human cryopreserved ovarian tissue from multiple patients using xenografting. Hum Reprod. 2005;20:72–78. doi: 10.1093/humrep/deh550. [DOI] [PubMed] [Google Scholar]
- 40.Maltaris T, Dimmler A, Muller A, et al. Comparison of two freezing protocols in an open freezing system for cryopreservation of rat ovarian tissue. J Obstet Gynaecol Res. 2006;32:273–279. doi: 10.1111/j.1447-0756.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 41.Newton H, Aubard Y, Rutherford A, et al. Low temperature storage and grafting of human ovarian tissue. Hum Reprod. 1996;11:1487–1491. doi: 10.1093/oxfordjournals.humrep.a019423. [DOI] [PubMed] [Google Scholar]
- 42.Oktay K, Newton H, Aubard Y, et al. Cryopreservation of immature human oocytes and ovarian tissue: An emerging technology? Fertil Steril. 1998;69:1–7. doi: 10.1016/s0015-0282(97)00207-0. [DOI] [PubMed] [Google Scholar]
- 43.Seli E, Tangir J. Fertility preservation options for female patients with malignancies. Curr Opin Obstet Gynecol. 2005;17:299–308. doi: 10.1097/01.gco.0000169108.15623.34. [DOI] [PubMed] [Google Scholar]
- 44.Aubard Y, Piver P, Cogni Y, et al. Orthotopic and heterotopic autografts of frozen-thawed ovarian cortex in sheep. Hum Reprod. 1999;14:2149–2154. doi: 10.1093/humrep/14.8.2149. [DOI] [PubMed] [Google Scholar]
- 45.Baird DT, Webb R, Campbell BK, et al. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196°C. Endocrinology. 1999;140:462–471. doi: 10.1210/endo.140.1.6453. [DOI] [PubMed] [Google Scholar]
- 46.Candy CJ, Wood MJ, Whittingham DG. Restoration of a normal reproductive lifespan after grafting of cryopreserved mouse ovaries. Hum Reprod. 2000;15:1300–1304. doi: 10.1093/humrep/15.6.1300. [DOI] [PubMed] [Google Scholar]
- 47.Lee DM, Yeoman RR, Battaglia DE, et al. Live birth after ovarian tissue transplant. Nature. 2004;428:137–138. doi: 10.1038/428137a. [DOI] [PubMed] [Google Scholar]
- 48.Oktay K, Buyuk E, Veeck L, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363:837–840. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- 49.Wolner-Hanssen P, Hagglund L, Ploman F, et al. Autotransplantation of cryopreserved ovarian tissue to the right forearm 4(1/2) years after autologous stem cell transplantation. Acta Obstet Gynecol Scand. 2005;84:695–698. doi: 10.1111/j.0001-6349.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- 50.Silber SJ, Lenahan KM, Levine DJ, et al. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med. 2005;353:58–63. doi: 10.1056/NEJMoa043157. [DOI] [PubMed] [Google Scholar]
- 51.Oktay K, Nugent D, Newton H, et al. Isolation and characterization of primordial follicles from fresh and cryopreserved human ovarian tissue. Fertil Steril. 1997;67:481–486. doi: 10.1016/s0015-0282(97)80073-8. [DOI] [PubMed] [Google Scholar]
- 52.Nomi M, Atala A, Coppi PD, et al. Principals of neovascularization for tissue engineering. Mol Aspects Med. 2002;23:463–483. doi: 10.1016/s0098-2997(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 53.Shaw JM, Bowles J, Koopman P, et al. Fresh and cryopreserved ovarian tissue samples from donors with lymphoma transmit the cancer to graft recipients. Hum Reprod. 1996;11:1668–1673. doi: 10.1093/oxfordjournals.humrep.a019467. [DOI] [PubMed] [Google Scholar]
- 54.Donnez J, Dolmans MM, Martinez-Madrid B, et al. The role of cryopreservation for women prior to treatment of malignancy. Curr Opin Obstet Gynecol. 2005;17:333–338. doi: 10.1097/01.gco.0000175348.72566.47. [DOI] [PubMed] [Google Scholar]
- 55.Yada-Hashimoto N, Yamamoto T, Kamiura S, et al. Metastatic ovarian tumors: A review of 64 cases. Gynecol Oncol. 2003;89:314–317. doi: 10.1016/s0090-8258(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 56.Abir R, Nitke S, Ben-Haroush A, et al. In vitro maturation of human primordial ovarian follicles: Clinical significance, progress in mammals, and methods for growth evaluation. Histol Histopathol. 2006;21:887–898. doi: 10.14670/HH-21.887. [DOI] [PubMed] [Google Scholar]
- 57.Scott JE, Zhang P, Hovatta O. Benefits of 8-bromo-guanosine 3′,5′-cyclic monophosphate (8-br-cGMP) in human ovarian cortical tissue culture. Reprod Biomed Online. 2004;8:319–324. doi: 10.1016/s1472-6483(10)60912-1. [DOI] [PubMed] [Google Scholar]
- 58.Scott JE, Carlsson IB, Bavister BD, et al. Human ovarian tissue cultures: Extracellular matrix composition, coating density, and tissue dimensions. Reprod Biomed Online. 2004;9:287–293. doi: 10.1016/s1472-6483(10)62143-8. [DOI] [PubMed] [Google Scholar]
- 59.Wright CS, Hovatta O, Margara R, et al. Effects of follicle-stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Hum Reprod. 1999;14:1555–1562. doi: 10.1093/humrep/14.6.1555. [DOI] [PubMed] [Google Scholar]
- 60.Hovatta O, Silye R, Abir R, et al. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12:1032–1036. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- 61.Louhio H, Hovatta O, Sjoberg J, et al. The effects of insulin, and insulin-like growth factors I and II on human ovarian follicles in long-term culture. Mol Hum Reprod. 2000;6:694–698. doi: 10.1093/molehr/6.8.694. [DOI] [PubMed] [Google Scholar]
- 62.Zhang P, Louhio H, Tuuri T, et al. In vitro effect of cyclic adenosine 3′,5′-monophosphate (cAMP) on early human ovarian follicles. J Assist Reprod Genet. 2004;21:301–306. doi: 10.1023/B:JARG.0000043704.10845.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod. 1989;41:268–276. doi: 10.1095/biolreprod41.2.268. [DOI] [PubMed] [Google Scholar]
- 64.Spears N, Boland NI, Murray AA, et al. Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum Reprod. 1994;9:527–532. doi: 10.1093/oxfordjournals.humrep.a138539. [DOI] [PubMed] [Google Scholar]
- 65.O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 66.Xu M, Kreeger PK, Shea LD, et al. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abir R, Roizman P, Fisch B, et al. Pilot study of isolated early human follicles cultured in collagen gels for 24 hr. Hum Reprod. 1999;14:1299–1301. doi: 10.1093/humrep/14.5.1299. [DOI] [PubMed] [Google Scholar]
- 68.Abir R, Fisch B, Nitke S, et al. Morphological study of fully and partially isolated early human follicles. Fertil Steril. 2001;75:141–146. doi: 10.1016/s0015-0282(00)01668-x. [DOI] [PubMed] [Google Scholar]
- 69.Nayudu PL, Fehrenbach A, Kiesel P, et al. Progress toward understanding follicle development in vitro: Appearances are not deceiving. Arch Med Res. 2001;32:587–594. doi: 10.1016/s0188-4409(01)00339-3. [DOI] [PubMed] [Google Scholar]
- 70.West ER, Shea LD, Woodruff TK. Engineering the follicle microenvironment. Semin Reprod Med. 2007;25:287–299. doi: 10.1055/s-2007-980222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Albertini DF, Combelles CM, Benecchi E, et al. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121:647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- 72.Woodruff TK, Shea LD. The role of the extracellular matrix in ovarian follicle development. Reprod Sci. 2007;14:6–10. doi: 10.1177/1933719107309818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gutierrez CG, Ralph JH, Telfer EE, et al. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod. 2000;62:1322–1328. doi: 10.1095/biolreprod62.5.1322. [DOI] [PubMed] [Google Scholar]
- 74.Tambe SS, Nandedkar TD. Steroidogenesis in sheep ovarian antral follicles in culture: Time course study and supplementation with a precursor. Steroids. 1993;58:379–383. doi: 10.1016/0039-128x(93)90041-k. [DOI] [PubMed] [Google Scholar]
- 75.Roy SK, Treacy BJ. Isolation and long-term culture of human preantral follicles. Fertil Steril. 1993;59:783–790. [PubMed] [Google Scholar]
- 76.Abir R, Franks S, Mobberley MA, et al. Mechanical isolation and in vitro growth of preantral and small antral human follicles. Fertil Steril. 1997;68:682–688. doi: 10.1016/s0015-0282(97)00264-1. [DOI] [PubMed] [Google Scholar]
- 77.Ksiazkiewicz LK. Recent achievements in in vitro culture and preservation of ovarian follicles in mammals. Reprod Biol. 2006;6:3–16. [PubMed] [Google Scholar]
- 78.Pangas SA, Saudye H, Shea LD, et al. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9:1013–1021. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 79.Xu M, West E, Shea LD, et al. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- 80.Kreeger PK, Fernandes NN, Woodruff TK, et al. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73:942–950. doi: 10.1095/biolreprod.105.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kreeger PK, Deck JW, Woodruff TK, et al. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–723. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]