Abstract
A ‘critical window of opportunity’ has been proposed for efficacy of ovarian hormone intervention in peri- and postmenopausal women. We sought to address this hypothesis using a long-term ovariectomized non-human primate (NHP) model, the cynomolgus macaque (Macaca fascicularis). In these studies, we assessed the ability of 17β-estradiol and equol to regulate markers of hippocampal bioenergetic capacity. Results indicated that 17β-estradiol treatment significantly increased expression of mitochondrial respiratory chain proteins complex-I and –III in hippocampus when compared to non-hormone-treated animals. Expression of the TCA cycle protein succinate dehydrogenase α was decreased in animals treated with equol compared to those treated with 17β-estradiol. There were no significant effects of either 17β-estradiol or equol treatment on glycolytic protein expression in hippocampus, nor were there significant effects of treatment on expression levels of antioxidant enzymes. Similarly, 17β-estradiol and equol treatment had no effect on mitochondrial fission and fusion protein expression. In summary, findings indicate that while 17β-estradiol induced a significant increase in several proteins, the overall profile of bioenergetic system proteins was neutral to slightly positively responsive. The profile of responses with the ERβ-preferring molecule equol was consistent with overall nonresponsiveness. Collectively, the data indicate that long-term ovariectomy is associated with a decline in response to estrogens and estrogen-like compounds. By extension, the data are consistent with a primary tenet of the critical window hypothesis, i.e., that the brains of postmenopausal women ultimately lose their ability to respond positively to estrogenic stimulation.
Keywords: 17β-estradiol, equol, hippocampus, hormone therapy, menopause, mitochondria
Introduction
The brain accounts for 25 percent of the body’s total glucose utilization and 10 percent of the body’s total energy expenditure. Neurons are the most metabolically active cells in the brain (Irwin et al, 2008), and they require high substrate bioavailability (for review, see Simpkins et al, 2008) and proper mitochondrial enzymatic function to produce the substantial amounts of ATP needed for action potentials and synaptic plasticity (Kostanyan and Nazaryan, 1992). As mitochondria are the organelles responsible for the production of 90 percent of the total cellular ATP, optimal mitochondrial functioning is thus critical to neuronal function (for review, see Cadenas and Davies, 2000). Brain hypometabolism is a well-known antecedent to several neurodegenerative conditions, including Huntington’s disease (Ciarmiello et al, 2006), Parkinson’s disease (Eberling et al, 1994), and Alzheimer’s disease (Reimen et al, 2004). This provides strong evidence that decreased mitochondrial function is associated with and can lead to development of neurodegenerative disease.
It is estimated that 5.3 million Americans are currently suffering from Alzheimer’s disease (AD) (Alzheimer’s Association, 2010); of these, approximately 68% are women (see Brinton 2008 for review; Brookmeyer et al, 1998; Gao et al, 1998). At menopause, women experience a dramatic drop in circulating levels of endogenous estrogens, including 17β-estradiol (Boron and Boulpaep, 2009). While a common perception is that more women suffer from AD due to their increased longevity (Alzheimer’s Association, 2010), basic research indicates that the increased incidence of AD in women may be associated with this decline in ovarian hormone levels after menopause. Loss of ovarian hormones has been shown to lead to decreases in brain glucose bioavailability (Mosconi et al, 2007), mitochondrial bioenergetics and function (for reviews, see Brinton, 2008 and Brinton, 2009; Irwin et al, 2008; Yao et al, 2009), and synaptic transmission and cognitive function (for review see Brinton, 2009; Foy et al, 2008a; Foy et al, 2008b; Zadran et al, 2009). In addition, loss of ovarian hormones increases beta amyloid generation in rodents and mouse models of AD (for review see Pike et al, 2009; Yao et al, 2009; Yao et al, 2010; Zheng et al, 2002).
Through membrane-associated receptors, estrogen activates neuronal PI3K signaling pathways which converge on the mitochondria to promote mitochondrial bioenergetics (for reviews see Brinton, 2008 and Brinton, 2009; Mannella and Brinton, 2006). Two emerging bodies of recent research indicate that there is 1) an increased risk of neurodegenerative diseases after premenopausal loss of ovarian hormones, and 2) a critical window for efficacy of estrogen therapy in menopausal women. Epidemiological data regarding cognitive health in women who underwent bilateral oophorectomy before the onset of natural menopause indicate a long-term increased risk of many neurological disorders, including dementia (Rocca et al, 2009). Further, the critical window hypothesis states that estrogen supplementation initiated early in menopause may have beneficial effects on the bioenergetic function of the brain; however, estrogen therapy that is initiated several years post-menopause may be ineffective or even detrimental (for review, see Henderson and Brinton, 2010). A recent re-analysis of published data conducted by Rocca and colleagues supported this hypothesis, as they found that estrogen therapy tended to have a neuroprotective effect when initiated in women soon after the onset of menopause, but was associated with an increased risk of dementia when initiated many years after menopause (for review, see Rocca et al, 2010). Together, these data support the notion that hormonal status can strongly influence cognitive status, and properly-timed hormone therapy is crucial to maintaining healthy cognitive function.
We have previously shown in rodent models that treatment with 17β-estradiol immediately following ovariectomy increases the expression of enzymes involved in glycolysis (multiple subunits of PDH) and mitochondrial function (tricarboxylic acid [TCA] cycle: decreased MDH2 and increased aconitase; oxidative phosphorylation: increased expression of complex-I and V subunits) (Irwin et al 2008; Nilsen et al, 2007). This mechanistic pathway is critical for the neuroprotective, neurotrophic and bioenergetic functions regulated by estrogen. Additionally, 17β-estradiol treatment following ovariectomy has been shown to improve cognitive function in aged non-human primates (NHPs) (Rapp et al, 2003). This improvement in cognitive function may be a result of estrogen’s ability to reverse age-dependent decreases in prefrontal cortex dendritic spine density (Hao et al, 2006; Hao et al, 2007). Further, 17β-estradiol is known to enhance neurogenesis in the dentate gyrus of the hippocampus in adult animals (for reviews see Brinton, 2009; and Galea et al, 2008). However, it is worth noting that data from rodent models indicates that the ability of estrogen to induce neurogenesis is dependent on time since ovariectomy; if the rodent was not exposed to estrogen until 4 weeks after ovariectomy, there was no effect of estrogen supplementation in neurogenesis (Tanapat et al, 2005). Thus, data from animal models suggests that post-menopausal estrogen replacement could have beneficial cognitive effects in women, and it also agrees with the critical window hypothesis.
17β-estradiol has the potential to protect women from the risk of both cardiovascular and neurodegenerative diseases after menopause (for reviews, see Brinton 2008; Wagner et al, 2002). However, its use is contraindicated in some women because of the increased risk of endometrial cancer in the absence of a blocking agent such as a progestin (North American Menopause Society, 2010). Development of selective ER modulators (SERMs) has now become a therapeutic approach to promote the beneficial effects of ER signaling while decreasing the adverse effects of 17β-estradiol. One such potential SERM is equol, an ERβ-preferring agonist (Zhao et al, 2009). Equol is a product of intestinal bacterial processing of the soy isoflavone daidzein; however, only about a third of humans produce equol after consuming soy (Lampe et al, 1998). In women who are equol producers, soy foods have been reported to have cardiovascular benefits including lowering plasma concentrations of total cholesterol, low-density lipoprotein cholesterol, triglycerides, and lipoprotein(a) (Setchell et al, 2002). Data indicates that soy may also prevent postmenopausal bone loss in women who are equol producers (Lydeking-Olsen et al, 2002), and high urinary excretion of equol has been linked to reduced breast cancer risk in women (Ingram et al, 1997). Similarly, NHP studies have shown equol to have minimal proliferative effects on the breast (Wood et al, 2006). Recent studies in the Brinton lab have shown that, in rats, equol administered in conjunction with select other phytoestrogens is able to increase mitochondrial respiration (Zhao et al, 2009). This makes equol an attractive candidate for a post-menopausal hormone therapeutic, particularly for women who are not natural equol producers.
A considerable volume of research exists on how hormone therapy affects aging in rodent models (for review see Brinton, 2005), but less work has been done in NHP models. While rodent models are a foundation of biomedical research, they are dissimilar enough from humans in some aspects that our ability to generalize between species is limited. A critical issue for translational research relevant to aging women’s health is that reproductive senescence in NHPs is more similar to humans than it is in rodents (Dumitriu et al, 2010; Shively and Clarkson, 2009). In the current study, we took advantage of an opportunity to assess the effect of chronic daily exposure to 17β-estradiol or equol after long-term ovariectomy on glucose uptake and mitochondrial metabolism in the brains of adult female cynomolgus macaques (Macaca fascicularis). Using hippocampal tissues from these animals, we investigated expression of proteins involved in glucose uptake, glycolysis, the TCA cycle, oxidative phosphorylation, antioxidant function, and mitochondrial fission/fusion.
2. Results
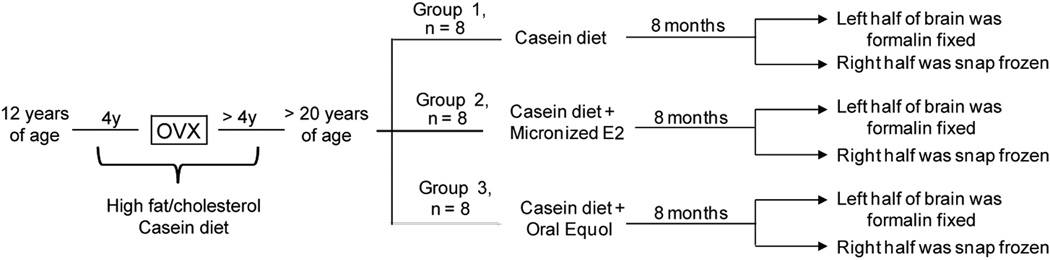
Female cynomolgus macaques (Macaca fascicularis) were obtained from Indonesia at an average age of 12 years (equivalent to 36 human years). After a 30-day quarantine, monkeys were placed into stable social groups of 3–4 animals, and consumed a semi-purified, atherogenic diet formulated to match a typical North American diet for 8 years (Table 1). At the start of this current study, monkeys were an average age of 20 (equivalent to 60 human years), and had been surgically postmenopausal for approximately 4 years. For this study, monkeys were randomized to one of three hormone treatment paradigms: placebo (control) (n = 8), oral micronized 17β-estradiol (E2) (1 mg/1800 calories, n = 8), or oral equol (52 mg S equol/1800 calories, n = 8) (Figure 1). Monkeys received the specified hormone treatment in addition to their normal diet for 8 months prior to euthanization. This allowed us to study the effect of hormone treatment after long-term ovariectomy on hippocampal mitochondrial markers of glucose uptake, bioenergetics, antioxidant capacity, and fission/fusion. Hippocampal punches encompassing the entire hippocampus (anterior to posterior, including all sub-regions) were used in these analyses.
Table 1.
Composition of the diets
| Ingredient | g/100 grams |
|---|---|
| Control Diet | |
| Casein | 9.0 |
| Lactalbumin | 9.0 |
| Wheat Flour, self-rising | 30 |
| Dextrin | 12 |
| Sucrose | 12 |
| Alphacel (non-nutrient bulk) | 7.9 |
| Lard | 5.0 |
| Butter, lightly salted | 1.5 |
| Safflower Oil | 5.5 |
| Crystalline Cholesterol | 0.07 |
| Complete Vitamin Mix | 2.5 |
| Modified Ausman-Hayes Mineral Mix #2 | 5.0 |
| Calcium Carbonate | 0.34 |
| Calcium Phosphate, monobasic | 0.15 |
| Equol Diet (same base diet + equol) | 0.0237 |
| Estradiol (same base diet + 17β-estradiol) | 0.220 |
| Composition | |
| Protein (% of calories) | 19.8 |
| Carbohydrate (% of calories) | 50.9 |
| Lipid (% of calories) | 29.4 |
| Saturated (% of calories) | 29.6 |
| Monounsaturated (% of calories) | 29.0 |
| Polyunsaturated (% of calories) | 41.4 |
| Cholesterol (mg/calorie) | 0.20 |
| Calcium (mg/1800 calories) | 1179 |
| Phosphorus (mg/1800 calories) | 1224 |
Figure 1. Experimental paradigm and diets.
A detailed description of the experimental protocol, showing the two hormone treatment conditions and the control condition.
Expression levels of the proteins investigated in this study were quantified using Western blotting, and protein expression levels were normalized to β-actin or β-tubulin. Bar graphs are presented as an average of the protein levels measured from all samples within each group; however, it is worth noting that there was a fair amount of within-group variability, which affected measurements of statistically significant differences between groups. In certain conditions, variability was due to the fact that some monkeys would show an increase in enzyme expression, and others would not. In the results presented below, p-values for significant differences (p < 0.05) and trends (p < 0.35) are presented.
2.1 Effect of post-ovariectomy hormone treatment on expression levels of glycolytic enzymes
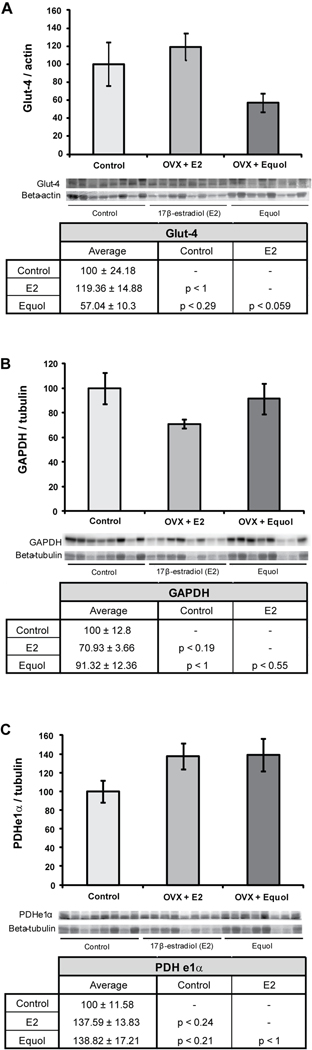
To determine the impact of chronic 17β-estradiol or equol exposure in long-term ovariectomized NHPs on proteins involved in glucose uptake and glycolysis, expression of three proteins (glucose transporter-4, the glycolytic enzyme GAPDH, and the pyruvate conversion enzyme PDH subunit e1α) was assessed. Neither of the hormone treatments had a significant effect on glucose transporter-4 (Glut-4) protein expression in the hippocampus (F2, 23 = 3.34, p < 0.054) (Figure 2A). There was, however, a trend towards decreased Glut-4 expression in hippocampal samples from NHPs which were treated with equol when compared to those treated with 17β-estradiol (p < 0.059) and compared to control animals which received only a placebo (p < 0.29). Hormone treatment also had no significant effect on GAPDH expression in the hippocampus (F2, 23 = 2.02, p < 0.16) (Figure 2B), although there was a trend towards decreased GAPDH expression in animals treated with 17β-estradiol compared to controls (p < 0.19). Similarly, there was no significant effect of hormone treatment on PDHe1α expression in the hippocampus (F2, 23 = 2.35, p < 0.12) (Figure 2C). There were non-significant trends towards increased PDHe1α expression both in the 17β-estradiol-treated animals compared to the control animals (p < 0.24) and in the equol-treated animals compared to the control animals (p < 0.21).
Figure 2. 17β-estradiol and equol modulation of glucose uptake and glycolysis.
Western blot analysis of Glut-4 (A), GAPDH (B), and PDHe1α (C) expression was performed on NHP hippocampal samples from both of the hormone treatment paradigms as well as controls. Expression levels for each sample were normalized to beta-actin levels (Glut-4) and beta-tubulin levels (GAPDH and PDHe1α). Expression levels were then normalized to the control animals (controls were set to 100%). Statistically significant differences were calculated using a two-tailed, one-way analysis of variance (ANOVA) followed by a Bonferroni post-hoc correction. Hormone treatment did not significant affect expression levels of these three proteins in the NHP hippocampus. Bars represent % control ± S.E.M., n = 8 for each condition.
2.2 Effect of post-ovariectomy hormone treatment on expression levels of TCA cycle enzymes
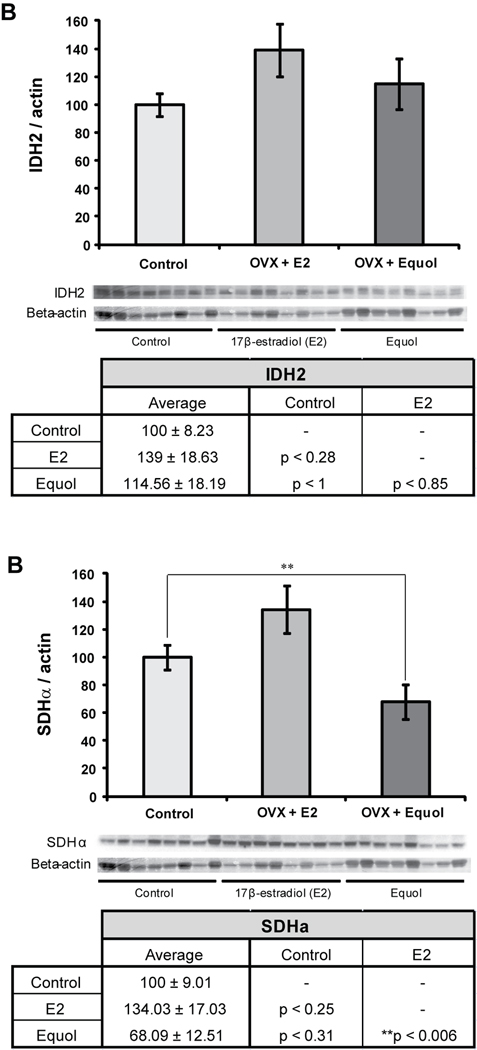
Expression levels of two enzymes involved in the TCA cycle, isocitrate dehydrogenase (IDH2) and succinate dehydrogenase (SDHα), were also measured in the NHP hippocampal samples. Neither of the hormone treatments had a significant effect on IDH2 protein expression in the hippocampus (F2, 23 = 1.57, p < 0.23) (Figure 3A). However, there was a trend towards increased IDH2 expression in hippocampal samples from NHPs treated with 17β-estradiol compared to controls (p < 0.28). Hormone treatment did have a significant effect on expression levels of SDHα (F2, 23 = 6.18, p < 0.008) (Figure 3B). SDHα expression was significantly decreased in animals treated with equol compared to animals treated with 17β-estradiol (**p < 0.006). There was a trend towards increased SDHα expression in NHPs treated with 17β-estradiol compared to control animals (p < 0.25), as well as a trend towards decreased SDHα expression in NHPs treated with equol compared to control animals (p < 0.31).
Figure 3. 17β-estradiol and equol modulation of TCA cycle.
Western blot analysis of IDH2 (A) and SDHα (B) expression was performed on NHP hippocampal samples from both of the hormone treatment paradigms as well as controls. Expression levels for each sample were normalized to beta-actin levels. Expression levels were then normalized to the control animals (controls were set to 100%). Statistically significant differences were calculated using a two-tailed, one-way analysis of variance (ANOVA) followed by a Bonferroni post-hoc correction. Hormone treatment significantly affected expression of SDHα in the NHP hippocampus. Bars represent % control ± S.E.M., n = 8 for each condition, **p<0.01.
2.3 Effect of post-ovariectomy hormone treatment on expression levels of the enzyme complexes of oxidative phosphorylation
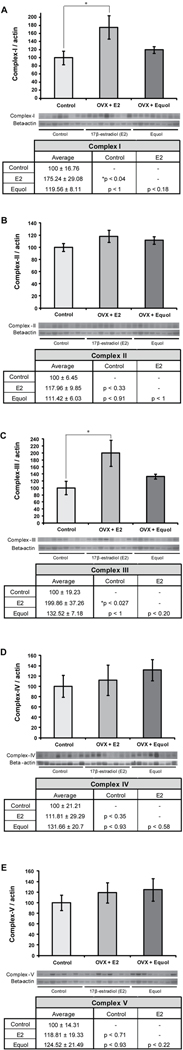
To determine the impact of chronic 17β-estradiol or equol exposure in long-term ovariectomized NHPs on oxidative phosphorylation, expression levels of complexes I through V were assessed. Complex I expression was significantly affected by hormone treatment (F2, 23 = 3.83, p < 0.038) (Figure 4A). Hippocampal samples from NHPs treated with 17β-estradiol showed a significant increase in expression levels of complex I compared to controls (* p < 0.04). Additionally, there was a trend towards increased complex I expression in the 17β-estradiol-treated animals when compared to the animals treated with equol (p < 0.18). Complex II expression was not affected by hormone treatment (F2, 23 = 1.42, p < 0.26) (Figure 4B), although there was a trend towards increased expression in the animals treated with 17β-estradiol when compared to control animals (p < 0.33). Hippocampal expression levels of complex III were significantly affected by hormone treatment (F2, 23 = 4.30, p < 0.027) (Figure 4C), and the NHPs treated with 17β-estradiol showed a significant increase in complex III expression compared to the control animals (* p < 0.027). There was also a trend towards increased complex III expression in the animals treated with 17β-estradiol compared to the animals treated with equol, although it was not significant (p < 0.20).
Figure 4. 17β-estradiol and equol modulation of oxidative phosphorylation.
Western blot analysis of complex-I (A), complex-II (B), complex-III (C), complex-IV-II (D) and complex-Vα (E) expression was performed on hippocampal samples from long-term ovariectomized NHP in both the hormone treatment conditions as well as controls. Expression levels for each sample were normalized to beta-actin levels. Statistically significant differences were determined by a two-tailed one-way analysis of variance (ANOVA) assuming unequal variances, followed by a Bonferroni post-hoc correction. 17β-estradiol therapy induced an increase in expression levels of complex-I (A) and complex-III (C) when compared to the control condition. No other significant changes from control were seen. Bars represent % control ± S.E.M., n = 8 for each condition, *p<0.05.
Complex IV expression was not significantly affected by hormone treatment (F2, 23 = 0.44, p < 0.65) (Figure 4D), but there was a slight trend towards increased expression in NHPs treated with 17β-estradiol compared to controls (p < 0.35). It is also worth noting that 17β-estradiol treatment did cause a large increase in complex IV expression in 4 out of the 8 animals. Hormone treatment had no effect on hippocampal expression levels of complex Vα (F2, 23 = 0.47, p < 0.63) (Figure 4E), although there was a trend towards increased complex Vα expression in NHPs treated with equol compared to those treated with 17β-estradiol (p < 0.22).
2.4 Effect of post-ovariectomy hormone treatment on expression levels of antioxidant enzymes
To determine the effect of hormone treatment on expression levels of antioxidant enzymes in NHPs after long-term ovariectomy, we measured hippocampal expression of manganese superoxide dismutase (MnSOD) and peroxiredoxin-V (PRDX-V). There was no significant effect of hormone treatment on MnSOD expression levels in the NHP hippocampus (F2, 23 = 0.95, p < 0.40) (Figure 5A). There was also no significant effect of hormone treatment on PRDX-V expression levels in the hippocampus (F2, 23 = 3.08, p < 0.07) (Figure 5B). However, animals treated with equol showed a trend towards decreased PRDX-V expression both when compared to control animals (p < 0.31) and when compared to animals treated with 17β-estradiol (p < 0.07).
Figure 5. 17β-estradiol and equol modulation of antioxidant defense.
Western blot analysis of MnSOD (A) and PRDX-V (B) expression was performed on hippocampal samples from long-term ovariectomized NHP. Expression levels for each sample were normalized to beta-actin levels. Statistically significant differences were determined by a two-tailed one-way analysis of variance (ANOVA) assuming unequal variances, followed by a Bonferroni post-hoc correction. Neither hormone treatment paradigm induced significant changes from control. Bars represent % control ± S.E.M., n = 8 for each condition.
2.5 Effect of post-ovariectomy hormone treatment on expression of mitochondrial fission and fusion proteins
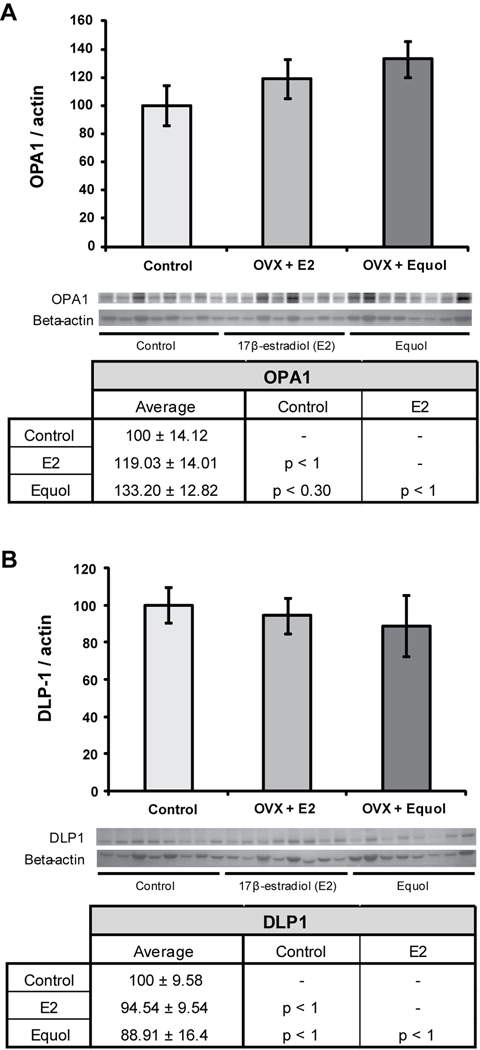
Effects of hormone treatment paradigms on mitochondrial dynamics were measured by looking at the expression of a protein which promotes fusion (optic atrophy 1; OPA1) and a protein which promotes fission (dynamin-like protein; DLP1). Hormone treatment had no significant effect on OPA1 expression (F2, 23 = 1.49, p < 0.25) (Figure 6A), although there was a slight trend towards increased expression in NHPs treated with equol compared to control animals (p < 0.30). Similarly, there was no effect of hormone treatment on DLP1 expression (F2, 23 = 0.20, p < 0.81) (Figure 6B).
Figure 6. 17β-estradiol and equol modulation of mitochondrial fission and fusion.
Western blot analysis of OPA1 (A) and DLP-1 (B) expression was performed on hippocampal samples from long-term ovariectomized NHP. Expression levels for each sample were normalized to beta-actin levels. Statistically significant differences were determined by a two-tailed one-way analysis of variance (ANOVA) assuming unequal variances, followed by a Bonferroni post-hoc correction. Neither hormone treatment paradigm induced significant changes from control. Bars represent % control ± S.E.M., n = 8 for each condition
3. Discussion
Much of the previous research regarding the effects of 17β-estradiol is based on an experimental paradigm of short-term ovariectomy and acute estrogen replacement (for review, see Brinton 2005). Animal models in these experiments experience only a short delay between reproductive senescence and initiation of hormone supplementation, and undergo an acute exposure to the hormone before the hormone’s effects are investigated (Brinton, 2005). Data from these experiments indicate that 17β-estradiol supplementation after reproductive senescence has numerous beneficial effects on mitochondrial function and neuronal viability in the brain (Brinton, 2005). This model of hormone exposure, however, has limited applicability to the range of clinical situations. Our current study, which included both a long delay between hormone loss and replacement as well as chronic exposure to the replacement hormone, was constructed so as to more closely model the effects of hormone supplementation in postmenopausal women initiated after a significant period of estrogen deficiency. The aim of this study was to determine the outcomes of estrogen treatment following long-term ovarian hormone depletion (the human equivalent of >12 years post-menopause) on proteins important in enzymatic, antioxidant, and fission/fusion aspects of hippocampal mitochondria. Results from this study indicate that after approximately 4 years of hormone loss in NHP, (a) chronic 17β-estradiol treatment influenced only a small subset of markers of mitochondrial bioenergetics, and (b) chronic treatment with equol, a low-affinity estrogenic molecule, has no effect on reversing the decline in mitochondrial bioenergetics.
Basic and clinical evidence indicate a bioenergetic decline in brain with age and loss of ovarian hormones (Kadish et al, 2009; Yao et al, 2009). Yao and colleagues (Yao et al, 2009) found that a decline in the bioenergetic capacity of the brain coincided with reproductive senescence in the mouse. This decline in bioenergetic capacity was evidenced by a decline in aerobic glycolysis and mitochondrial respiration. In particular, there was a significant decline in the activity of PDH and complex-IV, indicating that the neurons are no longer metabolizing glucose and generating ATP efficiently after estrogen depletion. In addition, a commensurate increase in the expression of enzymes involved in ketogenic metabolism, including HADHA and SCOT occurred. These findings in the rodent brain suggest that natural reproductive senescence is associated with a metabolic shift in the brain. The decline in bioenergetics induced by natural reproductive senescence was comparable to that induced by 3 month ovariectomy (Yao et al, 2010). These findings in the rodent brain are consistent with results obtained in the current study in long-term ovariectomized NHP brain.
In both the WHI and WHIMS studies the women who responded best to hormone replacement therapies, in terms of reduction in risk of developing cognitive impairment, were those women who had used hormone therapy prior to menopause or initiated hormone therapy early in menopause (Zandi et al, 2002). This provides insight into a therapeutic window of opportunity and suggests that the beneficial effects of estradiol treatment diminish once this window of efficacy has closed. However, there has been much debate as to whether decreased efficacy is based on the "healthy cell bias" (for review, see Brinton 2008) or whether the tissues become non-responsive to estradiol therapy via decline and/or change in ER expression. A change in ER expression and responsiveness to estrogen signaling is known to occur during aging: ERα is more highly expressed in the brains of young female rats than old rats (Adams et al. 2002) and old female rats remain responsive to estrogen signaling though ERβ (Waters et al., 2011; Zhao et al, 2002). This shift in the ratio between ERα- and ERβ-mediated signaling in mitochondria could play a role in age-related changes in responses of the brain to estrogens.
The increase in responsiveness to ERβ-mediated signaling with age would have predicted greater efficacy of an ERβ-preferring ligand such as equol. However, the data indicate that equol was largely ineffective and in certain instances induced a trend towards decline. These findings in the NHP brain are consistent with earlier in vitro and in vivo findings indicating that individual phytoestrogens, including equol, are ineffective alternatives to 17β-estradiol (Zhao et al, 2002). In contrast, in vivo data indicate that a combination of ERβ-preferring phytoestrogens is able to induce responses comparable to 17β-estradiol (Zhao et al, 2009). The caveat to these studies is that the experimental animal paradigm was one of short-term ovariectomy and acute hormone exposure in otherwise healthy young rodents. (Zhao et al, 2009)
Although results from the current study in long-term ovariectomized NHP brain are consistent with our previous results in rodents, there is some evidence that estrogen has different effects in rodent and monkey brains (Kordower et al, 2010). In studies on dendritic spine induction, aged female rhesus monkeys were shown to have a prolonged window of estrogen responsiveness after ovarian hormone loss when compared to aged female rats (Adams et al, 2001; Hao et al, 2003). Thus, there is a possibility that the NHP model in the current study does not have the same mitochondrial bioenergetic response to 17β-estradiol as the rodent model used in our previous studies, and that could explain the lack of a response to 17β-estradiol or equol therapy that we saw in this study. However, there is research indicating that treatment with 17β-estradiol shortly after ovariectomy upregulates mRNA and protein expression of glucose transporters Glut3 and Glut4 in the frontal cortex of NHPs (Cheng et al, 2001). Further studies on the effects of 17β-estradiol or equol treatment immediately following ovariectomy in NHPs would be necessary to resolve whether the effects of hormone treatment on mitochondrial bioenergetic function really do diminish with time post-ovariectomy.
It is worth noting that these monkeys were obtained as adults, and they had likely given birth to and raised at least one offspring before this experiment was conducted. Research indicates that both reproductive experience and motherhood can have an effect on hippocampal structure and function in rats, including decreased neurogenesis early in the post-partum period in first-time mothers but enhanced hippocampal cell survival in rats which had reared offspring several times (for review, see Galea et al, 2008). Additionally, rats which had raised offspring at least once had better spatial working memory than virgin rats (Galea et al, 2008). One study has also shown that the hippocampus is more responsive to late-life estrogen supplementation if rats have had previous reproductive and motherhood experience (Barha and Galea, 2009). Thus, there is a potential that previous reproductive experience may have altered the response of NHP hippocampal mitochondria to post-ovariectomy hormone therapy in the current study.
In summary, results of these analyses using the long-term ovariectomized cynomolgus macaque model support the hypothesis of a critical window for responsiveness to estrogen interventions, after which time these compounds have reduced efficacy. Further, the data derived from the equol intervention are consistent with numerous studies indicating modest to no efficacy for low-affinity, natural source estrogenic alternatives (for review, see Zhao and Brinton, 2007). While limited by potential trans-species specificities, these results provide suggestive evidence for mechanisms which may be important in functional responses to estrogen and support the critical window for efficacy by demonstrating that neither estrogen nor an estrogen-like compound sustains bioenergetic system function in brain after long-term ovarian hormone deprivation.
4. Experimental Procedure
4.1 Animals and Experimental Design
Female cynomolgus macaques (Macaca fascicularis) were obtained as adults (average age of 12 years; equivalent to 36 human years) from Indonesia. Following their arrival, monkeys were individually housed during a 30-day quarantine, after which they were placed into social groups of 3–4 animals. Monkeys were housed in these stable social groups and consumed a semipurified, atherogenic diet for 8 years. The diets were formulated to mimic a typical diet consumed by people in North America, containing ~0.20 mg cholesterol/calorie of diet and the protein source was mainly animal based (casein/lactalbumin) (Table 1). At the start of the study presented here, monkeys were on average > 20 years old (equivalent to > 60 human years) and had been surgically postmenopausal for approximately 4 years.
For this study, monkeys were randomized to receive one of the following three dietary hormone treatments in addition to their normal diet: placebo (n = 8), oral micronized 17β-estradiol (E2) at a human equivalent dose of 1mg/day (n = 8), or oral equol at a human equivalent dose of 105 mg/day (n = 8) (Table 2). The equol supplement contained a 96.0% pure racemic mixture of S- and R-equol enantiomers in a 1:1 ratio and was provide by Solae, a division of Dupont (St. Louis, MO, USA). The amount of equol added to the diet was based on several previous monkey studies in which isolated soy protein containing ~ 52 mg/1800 calories of daidzein was fed and found to have beneficial effects on cardiovascular risk (for review, see Clarkson 2002). A similar amount of total isoflavones has been shown to have beneficial effects on plasma lipids in women (Merz-Demlow et al, 2000). In monkeys, nearly all of the dietary daidzein is metabolized to equol during absorption from the gastrointestinal tract. To adjust for the use of a racemic mixture of R (inactive) and S (active) equol, a total of 104.8mg of racemic equol/1800 calories was added to the diet (Appt et al, 2005). A per caloric basis was used to determine the dose of equol in order to account for metabolic differences between women and monkeys. Estradiol (Estrace®, 1mg tablets) was administered at a dose equivalent to 1mg/day. The estradiol dose was chosen because 1mg E2/day or 1mg E2/1800 calories was a typical dose taken by women for treatment of vasomotor symptoms at the time this study was designed (for review, see Nelson 2004).
Table 1.
Hormone treatment diet paradigms
| Protein | Cholesterol (mg/calorie) |
Estradiol dose Human equiv. |
Equol dose Human equiv. |
|
|---|---|---|---|---|
| Control diet | Casein/lactalbumin | 0.20 | 0 | 0 |
| Estradiol diet | Casein/lactalbumin | 0.20 | 1 mg/day | 0 |
| Equol diet | Casein/lactalbumin | 0.20 | 0 | 105 mg/day |
After 8 months of hormone treatment, NHP were euthanized and brains were collected, with the right hemisphere formalin fixed and the left hemisphere cut into 2 mm slices and snap frozen. Hippocampal punches were taken from the left hemisphere slices to be analyzed by Western blot. The punches encompassed the whole anterior-to-posterior extent of the left hippocampus, including all sub-regions, and measured 1 cm thick by 2 mm deep. All procedures were conducted in compliance with state and federal laws, standards of the U.S. Department of Health and Human Services, and regulations and guidelines established by the Wake Forest University Animal Care and Use Committee. Wake Forest University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
4.2 Western Blot
Protein was extracted from hippocampal tissues using Tissue Protein Extraction Reagent (Thermoscientific, Rockford, IL) with phosphatase and protease inhibitors (Sigma, St. Louis, MO), and protein concentrations were determined with the Bio-Rad Bradford assay. 20–50 mg of protein was loaded per well on 12.5% SDS PAGE criterion gels (Bio-Rad, Hercules, CA). Proteins were transferred to PVDF membranes and probed with primary antibodies as listed in Table 3. Antibodies were then each probed with their corresponding HRP-conjugated secondary antibody (1:10000 dilution, Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. All membranes were stripped once for 15–20 minutes with stripping buffer, and were then re-probed with antibody/secondary antibody as described above for either another mitochondrial protein or β-actin/β-tubulin. β-actin (1:5000, mouse) (MS1501, Millipore, Billerica MD) or β-tubulin (1:3000, mouse) (ABCAM, Cambridge, MA, (AB6046)) was used as a loading control. Bands were visualized using an ECL kit (Thermoscientific, Pittsburgh PA) followed by a TMB colorimetric kit (Vector Laboratories, Burlingame, CA). The relative intensities of the immunoreactive bands were captured using a Molecular Imager ChemiDoc XRS+ System (Bio-Rad, Hercules, CA) and quantified with Quantity One Analysis Software, Version 4.6.4 (Bio-Rad, Hercules, CA). Expression levels for each sample were normalized to β-actin levels, with the exception of GAPDH and PDHe1α, which were normalized to β-tubulin levels due to their bands overlapping in size with β-actin. Following this, all expression levels were normalized to the average expression level of the control animals for each protein of interest.
Table 3.
List of antibodies used for Western blotting
| Antibody | Size | Catalog # | Company | Dilution | Species | Reference |
|---|---|---|---|---|---|---|
| aComplex I | 20 KDa | MS601 | Mitosciences, Eugene, OR | 1/1000 | Mouse | Liang et al, 2008 |
| aComplex II-FeS subunit | 30 KDa | MS601 | Mitosciences, Eugene, OR | 1/1000 | Mouse | |
| aComplex III subunit, Core 2 | 46 KDa | MS601 | Mitosciences, Eugene, OR | 1/1000 | Mouse | |
| aComplex IV subunit II | 22 KDa | MS601 | Mitosciences, Eugene, OR | 1/1000 | Mouse | |
| aComplex Vα | 55 KDa | MS601 | Mitosciences, Eugene, OR | 1/1000 | Mouse | |
| Dynamin-like protein-1 (DLP-1) | 79–84 KDa | 611113 | BD Biosciences, San Jose, CA | 1/1000 | Mouse | Wang et al, 2008 |
| Glucose transporter-4 (Glut-4) | 55 KDa | 07-140455 | Millipore, Billerca, MD | 1/1000 | Rabbit | Weisová et al, 2009 |
| Glyceraldehyde phosphate dehydrogenase (GAPDH) | 38 KDa | AB8245 | ABCAM, Cambridge, MA | 1/1000 | Mouse | Thomson et al, 2010 |
| Isocitrate dehydrogenase 2 (IDH2) | 44 KDa | H00003418 | Novus Biologicals, Littleton, CO | 1/1000 | Mouse | Brinton Lab, unpublished data |
| Manganese superoxide dismutase (MnSOD) | 25 KDa | 611581 | BD Biosciences, San Jose, CA | 1/1000 | Mouse | Irwin et al, 2008 |
| Optic atrophy protein-1 (OPA1) | 80, 100 KDa | 612607 | BD Biosciences, San Jose, CA | 1/1000 | Mouse | Wang et al, 2008 |
| Peroxiredoxin-V (PRDX-V) | 15 KDa | 612085 | BD Biosciences, San Jose, CA | 1/1000 | Mouse | Irwin et al, 2008 |
| Pyruvate dehydrogenase e1α subunit | 44 KDa | MSP03 | Mitosciences, Eugene, OR | 1/1000 | Mouse | Yao et al, 2009 |
| Succinate dehydrogenase α (SDHα) | 78 KDa | SC-59687 | Santa Cruz Biotechnology, Santa Cruz, CA | 1/1000 | Mouse | Costford et al, 2008 |
These antibodies were purchased from MitoSciences as MitoProfile® Total OXPHOS Human WB Antibody Cocktail (Catalog # MS601).
4.3 Statistical analysis
Data are presented as group mean normalized to the control ovariectomy group ± S.E.M. Statistically significant differences between treatment paradigms were obtained by a two-tailed one-way analysis of variance (ANOVA) assuming unequal variances, followed by a Bonferroni post-hoc correction. All statistical analysis was conducted using Origin data analysis and graphing software (OriginLab Corporation, Northampton, MA).
Acknowledgements
The authors would like to thank Dr. Jon Nilsen for his contributions to this study. This work was supported by NIA USC ADRC pilot grant to RDB (P50AG005142 H. Chui PI), HL 45666 (JRK), HL 079421 (JRK), National Center for Research Resources (NCRR, T32 RR07009-32 to SA) and Norris Foundation to RDB.
Abbreviations
- E2
17β-estradiol
- DLP-1
Dynamin like protein-1
- ER
Estrogen receptor
- Glut-4
Glucose transporter type 4
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- IDH2
Isocitrate dehydrogenase-2
- MDH2
Malate dehydrogenase 2
- MnSOD
Manganese superoxide dismutase
- NHP
Non-human primate
- OPA-1
Optic atrophy-1
- PRDX-V
Peroxiredoxin-V
- PDHe1α
Pyruvate dehydrogenase e1α subunit
- SERM
Selective Estrogen Receptor Modulator
- SDHα
Succinate dehydrogenase α
- SCOT
Succinyl-CoA oxoacid transferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22(9):3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's Association. Alzheimer's disease facts and figures. 2010 Mar; doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Appt SE, Clarkson TB, Register TC, Chen H. Dietary equol does not reproduce effects of dietary soy on cardiovascular disease risk variables; observations from postmenopausal monkeys. The North American Menopause Society; 16th Annual Meeting; September 28–October 1, 2005; San Diego. 2005. [Google Scholar]
- Barha CK, Galea LA. Motherhood alters the cellular response to estrogens in the hippocampus later in life. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Boron WF, Boulpaep EL. Medical Physiology. second ed. Philadelphia, PA: Saunders Elsevier; 2009. [Google Scholar]
- Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30(4):212–222. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Cohen M, Wang J, Bondy CA. Estrogen augments glucose transporter and IGF1 expression in primate cerebral cortex. FASEB J. 2001;15(6):907–915. doi: 10.1096/fj.00-0398com. [DOI] [PubMed] [Google Scholar]
- Ciarmiello A, Cannella M, Lastoria S, Simonelli M, Frati L, Rubinsztein DC, Squitieri F. Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington's disease. J Nucl Med. 2006;47(2):215–222. [PubMed] [Google Scholar]
- Clarkson TB. Soy, soy phytoestrogens and cardiovascular disease. J Nutr. 2002;132(3):566S–569S. doi: 10.1093/jn/132.3.566S. [DOI] [PubMed] [Google Scholar]
- Costford SR, Chaudhry SN, Crawford SA, Salkhordeh M, Harper ME. Long-term high-fat feeding induces greater fat storage in mice lacking UCP3. Am J Physiol Endocrinol Metab. 2008;295(5):E1018–E1027. doi: 10.1152/ajpendo.00779.2007. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH. Estrogen and the aging brain: an elixir for the weary cortical network. Ann N Y Acad Sci. 2010;1204:104–112. doi: 10.1111/j.1749-6632.2010.05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling JL, Richardson BC, Reed BR, Wolfe N, Jagust WJ. Cortical glucose metabolism in Parkinson's disease without dementia. Neurobiol Aging. 1994;15(3):329–335. doi: 10.1016/0197-4580(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Foy MR, Baudry M, Foy JG, Thompson RF. 17beta-estradiol modifies stressinduced and age-related changes in hippocampal synaptic plasticity. Behav Neurosci. 2008a;122(2):301–309. doi: 10.1037/0735-7044.122.2.301. [DOI] [PubMed] [Google Scholar]
- Foy MR, Baudry M, Brinton RD, Thompson RF. Estrogen and hippocampal plasticity in rodent models. J Alzheimers Dis. 2008b;15(4):589–603. doi: 10.3233/jad-2008-15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Uban KA, Epp JR, Brummelte S, Barha CK, Wilson WL, Lieblich SE, Pawluski JL. Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Can J Exp Psychol. 2008;62(4):247–260. doi: 10.1037/a0014501. [DOI] [PubMed] [Google Scholar]
- Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55:809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J. Comp. Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J. Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer's disease risk and therapy. Brain Res. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram D, Sanders K, Kolybaba M, Lopez D. Case-control study of phyto-estrogens and breast cancer. Lancet. 1997;350:990–994. doi: 10.1016/S0140-6736(97)01339-1. [DOI] [PubMed] [Google Scholar]
- Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology. 2008;149:3167–3175. doi: 10.1210/en.2007-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Landfield PWJ. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J Neurosci. 2009;29(6):1805–1816. doi: 10.1523/JNEUROSCI.4599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Chen EY, Morrison JH. Long-term gonadal hormone treatment and endogenous neurogenesis in the dentate gyrus of the adult female monkey. Exp Neurol. 2010;224(1):252–257. doi: 10.1016/j.expneurol.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostanyan A, Nazaryan K. Rat brain glycolysis regulation by estradiol-17 beta. Biochim Biophys Acta. 1992;1133:301–306. doi: 10.1016/0167-4889(92)90051-c. [DOI] [PubMed] [Google Scholar]
- Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with soy challenge:influence of habitual diet. Proc Soc Exp Biol Med. 1998;217:335–339. doi: 10.3181/00379727-217-44241. [DOI] [PubMed] [Google Scholar]
- Liang WS, Reiman EM, Valla J, Dunckley T, Beach TG, Grover A, Niedzielko TL, Schneider LE, Mastroeni D, Caselli R, Kukull W, Morris JC, Hulette CM, Schmechel D, Rogers J, Stephan DA. Alzheimer's disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc Natl Acad Sci U S A. 2008;105(11):4441–4446. doi: 10.1073/pnas.0709259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydeking-Olsen E, Jensen J, Setchell K, Damhus M, Jensen T. Isoflavone-rich soymilk prevents bone loss in the lumbar spine of postmenopausal women - a 2 year study. J Nutr. 2002;132:581. [Google Scholar]
- Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci. 2006;26:9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz-Demlow BE, Duncan AM, Wangen KE, Xu X, Carr TP, Phipps WR, Kurzer MS. Soy isoflavones improve plasma lipids in normocholesterolemic, premenopausal women. Am J Clin Nutr. 2000;71(6):1462–1469. doi: 10.1093/ajcn/71.6.1462. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Brys M, Switalski R, Mistur R, Glodzik L, Pirraglia E, Tsui W, De Santi S, de Leon MJ. Maternal family history of Alzheimer's disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U.S.A. 2007;104:19067–19072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291(13):1610–1620. doi: 10.1001/jama.291.13.1610. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Irwin RW, Gallaher TK, Brinton RD. Estradiol in vivo regulation of brain mitochondrial proteome. J Neurosci. 2007;27:14069–14077. doi: 10.1523/JNEUROSCI.4391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17(2):242–255. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009;30(2):239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23(13):5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U.S.A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, Melton LJ., III Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond Engl) 2009;5(1):39–48. doi: 10.2217/17455057.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen, and cognitive aging: the timing hypothesis. Neurodegener Dis. 2010;7(1–3):163–166. doi: 10.1159/000289229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol - a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB. The unique value of primate models in translational research. Nonhuman primate models of women's health: introduction and overview. Am J Primatol. 2009;71(9):715–721. doi: 10.1002/ajp.20720. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Yang SH, Sarkar SN, Pearce V. Estrogen actions on mitochondria--physiological and pathological implications. Mol Cell Endocrinol. 2008;29:51–59. doi: 10.1016/j.mce.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481(3):252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- Thomson SE, McLennan SV, Hennessy A, Boughton P, Bonner J, Zoellner H, Yue DK, Twigg SM. A novel primate model of delayed wound healing in diabetes: dysregulation of connective tissue growth factor. Diabetologia. 2010;53(3):572–583. doi: 10.1007/s00125-009-1610-6. [DOI] [PubMed] [Google Scholar]
- Wagner JD, Kaplan JR, Burkman RT. Reproductive hormones and cardiovascular disease mechanism of action and clinical implications. Obstet Gynecol Clin North Am. 2002;29(3):475–493. doi: 10.1016/s0889-8545(02)00011-6. [DOI] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105(49):19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WGM, Lou WYW, McEwen BS, Morrison JH, Milner TA. Estrogen and aging affect the synaptic distribution of estrogen receptor beta immunoreactivity in the CA1 region of female rat hippocampus. Brain Res (in progress) 2010 doi: 10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisová P, Concannon CG, Devocelle M, Prehn JH, Ward MW. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J Neurosci. 2009;29(9):2997–3008. doi: 10.1523/JNEUROSCI.0354-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CE, Appt SE, Clarkson TB, Franke AA, Lees CJ, Doerge DR, Cline JM. Effects of high-dose soy isoflavones and equol on reproductive tissues in female cynomolgus monkeys. Biol Reprod. 2006;75:477–486. doi: 10.1095/biolreprod.106.052142. [DOI] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106(34):14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Hamilton RT, Cadenas E, Brinton RD. Decline in mitochondrial bioenergetics and shift to ketogenic profile in brain during reproductive senescence. Biochim Biophys Acta. 2010;1800(10):1121–1126. doi: 10.1016/j.bbagen.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadran S, Qin Q, Bi X, Zadran H, Kim Y, Foy MR, Thompson R, Baudry M. 17-Beta-estradiol increases neuronal excitability through MAP kinase-induced calpain activation. Proc Natl Acad Sci U S A. 2009;106(51):21936–21941. doi: 10.1073/pnas.0912558106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen Q, Brinton RD. Neuroprotective and neurotrophic efficacy of phytoestrogens in cultured hippocampal neurons. Exp Biol Med (Maywood) 2002;227:509–519. doi: 10.1177/153537020222700716. [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. WHI and WHIMS follow-up and human studies of soy isoflavones on cognition. Expert Rev Neurother. 2007;7:1549–1564. doi: 10.1586/14737175.7.11.1549. [DOI] [PubMed] [Google Scholar]
- Zhao L, Mao Z, Brinton RD. A select combination of clinically relevant phytoestrogens enhances estrogen receptor beta-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology. 2009;150:770–783. doi: 10.1210/en.2008-0715. [DOI] [PubMed] [Google Scholar]
- Zheng H, Xu H, Uljon SN, Gross R, Hardy K, Gaynor J, Lafrancois J, Simpkins J, Refolo LM, Petanceska S, Wang R, Duff K. Modulation of A(beta) peptides by estrogen in mouse models. J Neurochem. 2002;80(1):191–196. doi: 10.1046/j.0022-3042.2001.00690.x. [DOI] [PubMed] [Google Scholar]