Abstract
Myalgias are the most common side effect of statin use and the commonest cause for discontinuing therapy. Vitamin D has known physiologic functions in muscle and vitamin D deficiency is known to cause myalgias, with its correction leading to disappearance of muscle symptoms. The 521T>C SLCO1B1*5 gene polymorphism decreasing function in the gene coding for a liver anion transporter that is responsible for statin uptake has been found to explain the majority of statin-associated muscle symptoms. Patients with statin-associated myalgias have been reported to improve with vitamin D supplementation. We therefore investigated (i) whether repletion of vitamin D in deficient patients with myalgias could lead to tolerance for subsequent statin therapy and (ii) whether vitamin D status modifies the effect of the SLCO1B1*5 genotype on myalgia risk. Using a retrospective cohort of 64 patients in whom 25-hydroxyvitamin D [25(OH)D] had been measured for any reason while on statin therapy, including 46 patients who consented to be genotyped, we found strong evidence showing that repletion of vitamin D in vitamin D deficient patients improved myalgias. Of 21 vitamin D deficient patients with intolerable statin-associated myalgias, 14 of 15 rechallenged with statins were subsequently symptomfree, with one patient experiencing mild and tolerable symptoms, far exceeding expected rates of acquired tolerability with no therapy (p = 0.01). In addition, while the SLCO1B1*5 genotype was associated with a three-fold increased risk of myalgias (p = 0.07), this risk was not found to differ by vitamin D status (p = 0.60).
Key words: vitamin D, statin, myalgias, SLCO1B1 genotype, vitamin D deficiency, SNPs
Introduction
Muscle abnormalities are the most common side-effect in patients treated with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor (statin) drugs and the commonest reason for discontinuing therapy.1–6 Vitamin D deficiency is common in the U.S. and worldwide7–9 and may cause muscle dysfunction.10–12 Dietary vitamin D3 or vitamin D3 formed in the skin after sunlight exposure, is rapidly converted to 25-hydroxyvitamin D3 [25(OH)D3] in the liver.11,13 25(OH)D3 is the precursor for the active hormone 1,25-dihydroxyvitamin D3 (calcitriol) that acts via the vitamin D receptor (VDR) similarly to other steroid hormones.13 25(OH)D (when written without a subscript, indicates either vitamin D2 or D3) is the circulating form of the hormone and is routinely assayed to determine vitamin D status.11,13 VDR are present in muscle14 and calcitriol regulates multiple pathways in skeletal muscle.15 It has long been known that in patients with severe vitamin D deficiency causing osteomalacia or rickets, major symptoms of muscle weakness and pain occur.16–18 Correcting vitamin D deficiency frequently leads to disappearance of muscle symptoms.17–19 Recently, several studies have assessed whether lower circulating levels of serum 25(OH)D are associated with increased risk of statin-associated myalgias.20–22 While two studies did find such an association,20,21 another larger study did not.22 Patients with vitamin D deficiency and myalgias were reported to be better able to tolerate statins on rechallenge after correction of deficiency with vitamin D supplements.20,21 Other investigators have observed a relationship between vitamin D deficiency and statinassociated myalgias, with vitamin D replacement also leading to statin tolerance.23
A recent genomewide analysis has shown that over 60% of patients with myalgias associated with simvastatin therapy have a single-nucleotide polymorphism (SNP) in the SLCO1B1 gene that codes for an organic anion transporter protein that regulates statin uptake in liver.24 Among other polymorphisms in the gene, the specific polymorphism (521T>C) (rs4149056 designated SLCO1B1*5) codes for a variant amino acid substitution (Val174Ala) in one of the transmembrane domains of the transporter protein, reducing its activity.25 Recently the SLCO1B1*5 polymorphism was found to be associated with an increased risk of side effects from simvastatin,24 and perhaps to a lesser extent from other statins.25–28
Because of the possible relationship of vitamin D to statinassociated myalgias, we retrospectively evaluated patients on statin therapy for whom a 25(OH)D level had been measured for any reason. In addition to determining whether the patients with statin-associated myalgias were more likely to be deficient in vitamin D, we evaluated both the SLCO1B1*5 genotype status and the impact of vitamin D treatment on subsequent ability to tolerate statin therapy in subsets of these patients. Our goals were two-fold: (1) to determine whether repletion of vitamin D deficient statin-taking patients with myalgias rendered patients statin-tolerant; and (2) to discover if there was a synergistic effect between SLCO1B1*5 polymorphism and vitamin D on myalgia risk.
Results
In the clinical practice of endocrinology of one of the authors (RL), 21 vitamin D deficient patients were seen who also complained of intolerable myalgias while receiving statins. All symptoms had resolved when statins were discontinued suggesting causality. The 21 patients were treated with vitamin D to repair their deficiency, with the dose varying from 1,000 IU daily of vitamin D3 to 50,000 IU once weekly of vitamin D2 depending on the degree of vitamin D deficiency. Fifteen of the 21 patients were willing and able to be re-challenged with a statin post vitamin D treatment. Six patients either refused or were not eligible for subsequent statin therapy: one patient could not be evaluated for symptom relief due to a longstanding diagnosis of fibromyalgia; one patient had an abnormal liver test and was no longer a candidate for statin therapy; one patient whose lipids normalized through weight loss no longer needed therapy; and three patients refused to be rechallenged. A posttreatment 25(OH)D level was obtained in 10 of the 15 patients treated with vitamin D (Fig. 1), with the 25(OH)D values increasing to >30 ng/mL in all patients. After 2–3 months of vitamin D repletion, the 15 patients were rechallenged with statin therapy, and 14 of these (93%) remained symptom-free while one was left with mild and tolerable symptoms and was able to continue on statin therapy. Seven of the 15 patients (47%) had previously developed myalgias on at least two different statins. Six of the patients tolerated the same statin after vitamin D repletion while nine of the 15 patients (60%) had been switched to a different statin after being given vitamin D. The decision regarding choice of rechallenge statin took into consideration clinical issues and insurance coverage and included all six available statins (see Table 1). The rechallenge statins used were fluvastatin (5 patients), pravastatin (5), rosuvastatin (2), lovastatin (1), simvastatin (1) and atorvastatin (1). The patients were all followed for at least 1 year, although changes in myalgia status were generally apparent within days to weeks.
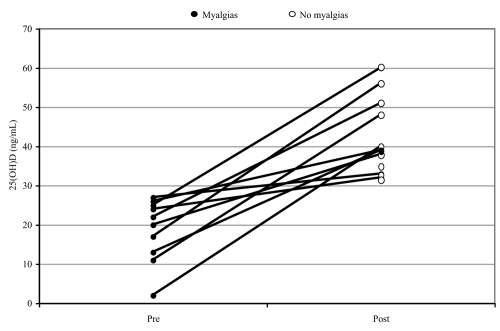
Figure 1.
Response to vitamin D treatment in patients rendered tolerant to statin therapy. Fifteen patients were able to reinstitute statin therapy after vitamin D repletion. Of these 14 were asymptomatic and one had mild tolerable symptoms on continuing statin therapy. The figure shows the vitamin D levels before and after vitamin D therapy in the 10 patients that were rechallenged with statins and in whom we have 25(OH)D levels pre- and post-intervention with vitamin D therapy. The average concentration of 25(OH)D is 17.8 ± 2.7 (n = 10) ng/ml in the pretreatment group, whereas the levels rose to 43.6 ± 3.3 (n = 10) ng/ml in the post-treatment group. p < 0.0005 when compared between groups.
Table 1.
Baseline characteristics of 64 subjects who had 25(OH)D levels measured while on statin therapy
| No myalgia | Myalgia | ||
| Subject variable | N = 25 (37.5%) | N = 39 (62.5%) | p-value* |
| Age Mean (SD) | 59.3 (13.8) | 59.5 (10.0) | 0.95 |
| Gender (Male, Female) | 16/9 | 21/18 | 0.58 |
| BMI Mean (SD) | 27.5 (5.5) | 27.9 (5.7) | 0.77 |
| 25(OH)D (ng/mL) Mean (SD) | 24.3 (10.5) | 28.2 (11.6) | 0.17 |
| Statin (mg) | 0.20 | ||
| Atorvastatin (10, 20, 40) | 7/1/1 | 13/4/1 | |
| Pravastatin (10, 20, 40) | 0/0/2 | 5/2/0 | |
| Simvastatin (10, 20, 40) | 4/4/1 | 3/2/1 | |
| Lovastatin (10, 20, 80) | 0/1/1 | 3/2/0 | |
| Rosuvastatin (5, 10, 20) | 0/1/1 | 1/2/2 | |
| Fluvastatin (20, 80) | 0 | 4/1 | |
| Taking niacin, fenofibrate, diltiazem or verapamil | 0 | 9 |
The doses for the various statins are listed in parentheses in mg and the number of subjects for each dose is indicated separated by /.
p values are based on a two-sample t-test, Chi-square test or Fisher's exact test as appropriate.
A previous study reported that re-challenging with the same or a different statin, without other intervention, relieves myalgia symptoms in 43% of patients.4 To assess whether vitamin D treatment among vitamin D deficient patients had an impact on symptom relief, we tested the null hypothesis that the proportion of patients who experienced relief is larger than 45%. Using an intent-to-treat approach, with 15 of the 21 treated patients experiencing sufficient relief in symptoms to tolerate statin therapy, we conclude that vitamin D had a strong impact in relieving myalgia- related symptoms (p = 0.01). Among only those vitamin D deficient patients who were willing and able to be re-challenged with statin therapy, there is overwhelming evidence to suggest that vitamin D was efficacious. As all 15 patients improved with vitamin D treatment, whether they received D2 (4 patients) or D3 (11 patients), the response was equally effective with either form of vitamin D.
The SLCO1B1*5 genotype was available for 8 of the 15 patients who benefited from vitamin D therapy, with 6 having TT, one having TC and one having CC genotype. Thus, vitamin D deficient patients with all 3 possible genotypes benefited from vitamin D treatment. The one patient still left with mild but tolerable symptoms had the TT genotype.
A serum level of 25(OH)D had been obtained either because of risk factors for vitamin D deficiency, osteoporosis or myalgias (defined in this study as pain or weakness) on an additional 43 patients. Baseline characteristics of all 64 patients (including the 21 treated with vitamin D) were then studied in retrospect, and are described in Table 1. Of the 64 patients on statin therapy, muscle symptoms occurred in 39 patients (61%). Vitamin D deficiency (25(OH)D <30 ng/mL) was detected in 37 patients (58%). No differences in the proportions of patients experiencing myalgia were observed between vitamin D status groups (59% of vitamin D deficient and 63% of vitamin D sufficient patients, where the Chi-squared p value was 0.98) (Fig. 2). There was no difference in age, gender, BMI or mean 25(OH)D level in the groups with and without myalgias. Although myalgias were more common with certain statins, this was not statistically significant, and our clinical impression was that this relationship mirrored the frequency with which the various statins were prescribed in these patients.
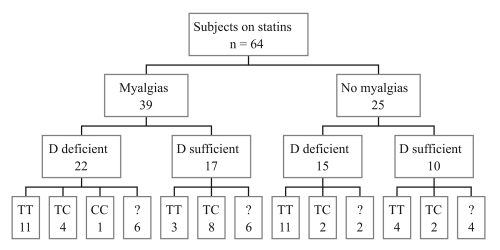
Figure 2.
SLCO1B1 genotypes, vitamin D and myalgias status of 64 patients on statin therapy. Unknown genotypes are denoted by “?”.
Analysis of the relationship between vitamin D status and severity of myalgias revealed no significant differences in levels of 25(OH)D by severity of myalgia (ANOVA p = 0.62). Levels of 25(OH)D were comparable between those with and without myalgia symptoms, where the p = 0.17, and the mean levels were 28 ng/mL and 24 ng/mL, respectively. Furthermore, there were no differences in the proportion of those with vitamin D deficiency across levels of myalgia (Chi-squared p = 0.77, Table 2).
Table 2.
Number and percentage with vitamin D deficiency by severity of Myalgia
| Myalgia severity | No symptoms | Mild | Moderate | Severe |
| Vitamin D deficient levels (%) | 15 (60) | 5 (50) | 10 (52.6) | 7 (70) |
| Vitamin D sufficient levels (%) | 10 (40) | 5 (50) | 9 (47.4) | 3 (30) |
Forty-six of the 64 patients in the study consented to SLCO1B1 genotyping. There were no differences in age, BMI or myalgia status between those who were and were not willing to be genotyped. Females, however, were less likely to provide samples for genotyping (p = 0.028), and there were marginal differences in vitamin D levels between the two groups; the mean 25(OH)D level for those who were genotyped was 25 ng/mL versus 30 ng/mL for those not genotyped (p = 0.07), suggesting that those with lower 25(OH)D levels may have been more motivated to provide DNA. There were no differences, however, in the proportion of patients who were vitamin D deficient.
Of the 46 patients genotyped at SLCO1B1*5, 29 were TT homozygotes, 16 were TC heterozygotes and 1 was homozygous for CC. Muscle symptoms occurred in 14 (48%) of TT homozygotes, 12 (75%) of TC heterozygotes and the 1 (100%) CC homozygote (Fig. 2). Although only approaching statistical significance, we found a 3-fold increased risk of myalgias for individuals with at least one C allele (p = 0.07). These data are shown in Figure 3. Although relative risks were not found to be statistically different, the reduction in myalgia risk with the TT genotype was 81% in vitamin D sufficient patients versus 60% in vitamin D deficient patients. While our study did not find an interaction effect between vitamin D deficiency and genotype (p = 0.60), the presence of a C allele increased risk of myalgias whether vitamin D deficient or not, and TT protected regardless of the vitamin D status, as shown in Figure 4. The end result is that SLCO1B1*5 genotype explained, in part, the basis for some vitamin D deficient patients not experiencing myalgias and vitamin D sufficient patients having myalgias.
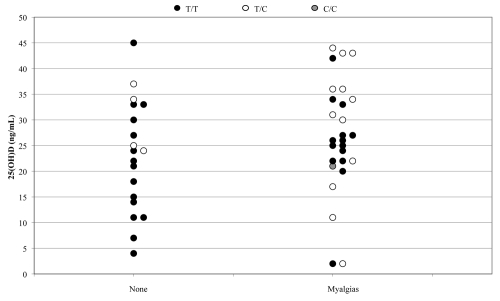
Figure 3.
25(OH)D levels, SLCO1B1 genotype and myopathy status in 46 patients on statin therapy. The data are grouped by myalgia status and displayed according to genotype and 25(OH)D concentration. Mean levels of 25(OH)D for those homozygous for the T allele and those with at least one C allele are 23.0 (10.4; n = 29) and 28.7 (12.0; n = 17) ng/ml, respectively. p = 0.11 when comparing average 25(OH)D levels between genotype groups; p = 0.17 when comparing average 25(OH)D levels between patients with and without myalgia symptoms.
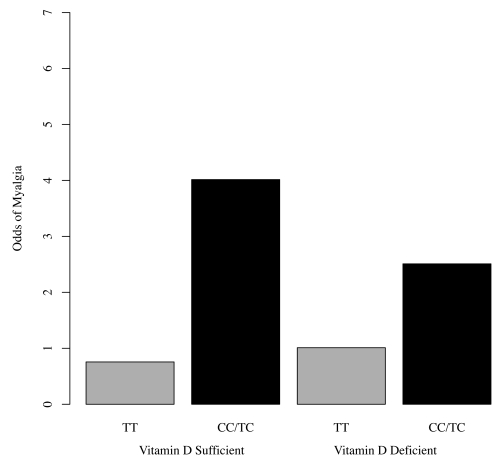
Figure 4.
Odds of Myalgia by SLCO1B1 Genotype and Vitamin D Status. The presence of a C allele increased risk of myalgias whether vitamin D sufficient or deficient.
When vitamin D deficiency was defined as 25(OH)D <20 ng/mL, analysis of these relationships yielded similar results.
As a hypothesis to explain the ability of vitamin D to protect against statin-induced muscle symptoms, we considered the possibility that calcitriol might regulate the expression of the SLCO1B1 gene in liver. In in vitro studies using cultured Huh6 human liver cells and in vivo experiments in mice, we failed to detect any change in SLCO1B1 expression in liver induced by calcitriol (Table 3). The calcitriol induction of 24-hydroxylase (CYP24) was used as a positive control.
Table 3.
Effect of calcitriol on SLCO1B1 gene expression
| In vivo in mice | In vitro in cells | |||
| mSLCO1B1 | m24OHase | hSLCO1B1 | h24OHase | |
| Control | 1 | 1 | 1 | 1 |
| Calcitriol | 0.9 | 5.1 | 1.1 | 744 |
The ability of calcitriol to induce hepatic SLCO1B1 mRNA expression in vitro in human Huh6 liver cells and in vivo in mice was assessed by realtime PCR as described in Methods. In each study, expression of SLCO1B1 in vehicle treated controls is set at 1 and relative stimulation induced by calcitriol is shown as fold-change. Cells were treated for 24 hours with 100 nM calcitriol and mice were treated for 4 weeks with 25 ng calcitriol every other day. Induction of 24-hydroxylase (CYP24) was used as a positive control. For both experiments n = 3 treated and 3 controls.
Discussion
Our study had findings in five different spheres. First, vitamin D repletion of deficient patients with myalgias allowed the reinstitution of statin therapy without symptoms. Second, we did not find that vitamin D deficiency by itself led to myalgias on statins. Third, our data suggested that SLCO1B1*5 genotype that included at least one “C” allele increased risk of myalgias and that the increased risk did not differ by vitamin D status. Fourth, we found that calcitriol did not regulate SLCO1B1 gene expression. Finally, we observed that vitamin D deficiency appears to be common, even in a sunny California location.
Regarding the first finding, we showed that vitamin D repletion of deficient patients with myalgias was associated with the ability to reinstitute statin therapy without symptoms. Of 21 patients with vitamin D deficiency and myalgias who were replaced with vitamin D, 15 patients were rechallenged with statin therapy and of those, all were able to tolerate reinstituted treatment (71%). It is notable that every one of the 15 rechallenged patients (100%) improved, with only one patient left with mild but tolerable myalgias. Even though six patients were not rechallenged, if we consider the total number of patients (21) and apply an intent-to-treat analysis, 14 of the 21 (67%) (p = 0.01) were symptom-free on statin treatment after vitamin D repletion. Nine of these 14 patients (64%) had been switched to a different statin, so that it cannot be known with certainty whether vitamin D replacement allowed for tolerance to statin re-treatment in every patient. Many of these patients, however, had been unable to tolerate multiple statins, and then were able to do so following vitamin D replacement, leaving us with the clinical impression of benefit as only 43% are expected to improve following subsequent therapy with no intervention.4 While the incidence of myalgias differed according to the statin used, our impression is that the incidence mirrored the frequency with which each statin had been prescribed, and all 6 available statins were tolerated by the 14 patients who did not have myalgias recur after vitamin D deficiency had been corrected.
Because of this clinical observation, we retrospectively evaluated the role of vitamin D sufficiency and SLCO1B1*5 genotype in 64 patients on statin therapy of whom 39 (61%) experienced myalgias and 25 (39%) did not. The high proportions of patients with myalgias and with vitamin D deficiency is consistent with the selection bias of our study, which required that the patients had been assessed with a blood level of 25(OH)D, for which many patients are screened because of muscle pain or weakness. This bias could theoretically have skewed our findings in favor of a relationship between vitamin D deficiency and statin-associated myalgias, but we observed a similar incidence of myalgias of 59% in patients with vitamin D deficiency and 63% with vitamin D sufficiency.
The second finding was that vitamin D deficiency itself did not lead to myalgias in patients receiving statins. We similarly saw no relationship between the severity of vitamin D deficiency and the development of myalgias. As a group, our symptomatic patients did not have lower 25(OH)D levels than non-symptomatic patients. In fact, more vitamin D deficiency was seen in asymptomatic patients but these were almost all TT genotype individuals. Our findings differ from that of two previous studies in which patients with statin-associated myalgias were more likely to be vitamin D deficient,20,21 although no relationship was found between vitamin D deficiency and myalgias in the largest study that examined this relationship.22 Differences in patient selection and study populations might explain these different observations. Indeed, on the basis of the statin tolerance following vitamin D repletion that occurred in our patients, we believe that vitamin D deficiency is likely to be a risk factor for statin associated myalgias in specific subgroups of patients. Additional studies need to be performed to identify additional factors that modify risk.26
Vitamin D deficiency, defined as levels of 25(OH)D <30 ng/mL, were found in 37 of 64 (58%) of our patients. Thus, the test of vitamin D status was more often abnormal than normal and therefore clinically important on its own merits since asymptomatic patients were equally or more likely to be vitamin D deficient. These data are somewhat biased, since 25(OH)D was measured in these patients for some indication; nevertheless this finding highlights the frequency of vitamin D insufficiency, seen in even relatively sunny geographical locations.7–9,11
The third finding involves the SLCO1B1*5 genotype. In a subset of 46 patients that consented to be genotyped, our data suggested that patients having the SLCO1B1*5 (521T>C) genotype that included at least one “C” allele exhibited an increased risk of myalgias, whereas the TT genotype was protective. The genetic status appeared to protect some vitamin D deficient patients with a TT genotype and increased risk of vitamin D sufficient patients with the TC or CC genotype. Based on a clear indication that not all statin-taking vitamin D deficient patients had myalgias and not all symptomatic patients were vitamin D deficient, we hypothesized an interaction between the SLCO1B1*5 genotype and vitamin D status to explain which patients developed myalgias. Our working hypothesis was that patients homozygous for the “C” or high risk variant could develop myalgias even with adequate vitamin D status, whereas heterozygous patients would be more likely to have muscle symptoms if also deficient in vitamin D and patients with both common “T” protective alleles would largely be asymptomatic even if vitamin D deficient.
In The Search Collaboration Group study,24 the prevalence of the “C” allele was 13%. In our study, the prevalence of the “C” allele was 19%, which is similar given the relatively small size of our study population. We found that 75% of the heterozygotes and 1 of 1 (100%) CC homozygote had myalgias, while this was the case in only 48% of the TT homozygotes, consistent with the observation that the “C” variant is associated with increased risk of muscle symptoms. Among vitamin D sufficient patients, 80% of those with at least one “C” allele developed myalgias versus only 43% of those with the TT genotype and among deficient patients, 71% of those with a “C” variant had myalgias versus 50% of those with the TT genotype. As the increase in myalgia risk of having a “C” allele was not found to differ significantly by vitamin D status (p = 0.60), the point estimates suggest the possibility that the TT genotype can protect patients from developing myalgias and that the “C” allele can sensitize even vitamin D sufficient patients to develop myalgias. Other factors also clearly play a role, including possibly other polymorphisms in the SLCO1B1 gene and polymorphisms that affect enzymatic metabolism of statins.29,30 This relationship will require confirmation in a larger study.
Fourth, to explain the ability of vitamin D to protect against statin-induced muscle symptoms, we considered the possibility that calcitriol might regulate the expression of the SLCO1B1 gene. We tested this possibility both in mouse liver in vivo and cultured human liver cells. In the in vivo experiments, mice treated with high doses of calcitriol for 4 weeks showed no change in SLCO1B1 gene expression whereas CYP24, a known calcitriol target gene, was dramatically induced. In in vitro studies using cultured Huh6 human liver cells treated with high dose calcitriol for 24 hours, similarly no stimulation of SLCO1B1 expression was seen (Table 3). Thus both in cultured human liver cells and intact mice calcitriol did not regulate hepatic SLCO1B1 gene expression.
The SLCO1B1*5 genotype causes a loss of statin transporter function into the liver, thereby increasing the risk of muscle symptoms by exposing muscle to increased circulating levels of statins.25 This phenomenon is well known to occur in patients treated with several drugs that interfere with the metabolism of the statins.25,26 In the 39 of our patients who had myalgias, 9 had been on one of four of these sensitizing drugs (Table 1) and 5 of the 9 patients were then able to tolerate the same drug in combination with statin therapy after vitamin D deficiency had been corrected. Genotype data were available in only 2 of these 9 patients, both of whom were symptomatic TT homozygotes who were able to tolerate fenofibrate and statin therapy together after vitamin D deficiency was corrected. Four of the 9 patients on sensitizing drugs were in the group that was not rechallenged with statin therapy.
Vitamin D has known physiologic functions in muscle and vitamin D deficiency causes myalgias and muscle weakness.10,16–18 Vitamin D signals muscle by way of a nuclear vitamin D receptor10,14 that mediates genomic effects and a cell membrane receptor that promotes non-genomic effects.15 These pathways influence the differentiation and proliferation of muscle cells and muscular contractility. Whether vitamin D deficiency and statins damage muscle by similar or different mechanisms is unknown. Clinically, some patients at increased risk of statin-associated myalgias are also more likely to be deficient in vitamin D, including the elderly and those with renal failure or obstructive liver disease. In our study, the 15 patients who were able to tolerate statin therapy after correction of their vitamin D deficiency had the same mean age as the entire study cohort and none had significant kidney or liver disease.
The fifth area for discussion is the frequency of vitamin D deficiency even in sunny latitudes. Although our study is small, a number of larger studies have shown that vitamin D deficiency is common, even in a sunny regions.7–11 Screening for vitamin D deficiency to predict who will develop myalgias is not likely to be useful since as many patients without vitamin D deficiency developed myalgias as did those with deficiency. However, our study suggests that at the latitude of northern California (37.441N), vitamin D deficiency is common, so that screening for vitamin D status is advisable on that basis alone. The decision to screen should be made individually based on geography and other risk factors for vitamin D deficiency, as well as the concomitant use of a drug that impairs statin metabolism. Screening for SLCO1B1 polymorphisms prior to initiating statin therapy would be expensive in today's marketplace and probably not clinically helpful since the need for statins is greater than the potential increased risk of myalgias in patients with C alleles. However, for patients who develop myalgias on statin therapy, we strongly recommend assaying 25(OH)D levels and treating any patient with vitamin D deficiency before rechallenging with statins. For those patients who require a second muscle sensitizing drug it may be wise to check vitamin D status and correct any deficiency found prior to initiating the second drug.
Statin therapy itself is associated with higher blood levels of 25(OH)D and 1,25(OH)D,31,32 although not in all studies,33 and this may contribute in a small way to the cardioprotective effects of statin therapy.34 In a small drug interaction study, vitamin D supplementation lowered LDL cholesterol despite lowering blood levels of the statin.33 Vitamin D deficiency has recently been associated with increased risk of cardiovascular disease,34–36 although there is inconclusive trial data to know whether vitamin D supplementation reduces cardiovascular risk.37–41
Our study grew out of clinical experience of patients with statinassociated myalgias that were able to tolerate statin rechallenge after correction of vitamin D deficiency. Among its strengths are the assessment of SLCO1B1*5 polymorphisms together with vitamin D status in patients with and without myalgias evaluated by the same clinician. Weaknesses of our study include its retrospective nature, the non-random selection of participants, the lack of a comparison arm with randomized treatment allocation and the relatively small number of patients. The data appear to indicate that patients with statin-associated myalgias are as likely to be vitamin D sufficient as deficient, although we suspect that vitamin D deficiency is a risk factor for myalgias as has been shown in some20,21 but not all other studies.22
In conclusion, we have observed that patients with statin-associated myalgias and vitamin D deficiency are able to tolerate statin therapy when vitamin D deficiency is corrected. We also found that vitamin D deficiency does not determine severity of symptoms. The SLCO1B1*5 (521T>C) “C” allele does appear to increase myalgia risk, but not differentially between those sufficient and insufficient in vitamin D. A larger study is needed to confirm these relationships. In particular, as vitamin D is a fairly simple and safe therapy for the reduction of myalgia symptoms and could allow patients to continue comfortably with their statin therapy, it is crucial that our finding regarding vitamin D repletion be replicated in a randomized placebo-controlled clinical trial.
Materials and Methods
Study design.
Two related groups of patients were used to address our questions; the first group was a subset of the second. The first group was a sample of 21 patients seen in the endocrinology practice (located within a large multispecialty medical group) of the first author and was the basis of our findings for the relation between vitamin D repletion and myalgias. More specifically, this first group consisted of a subset of the first author's patients: those who were statin-treated, developed myalgias post statin-treatment, proved to be vitamin D deficient and were subsequently treated with vitamin D. Motivated by the empirical observation that repletion seemed to be associated with tolerance to statin therapy, we expanded this sample in order to study the role of the SLCO1B1*5 genotype.
The second sample was derived after obtaining approval from both the Palo Alto Medical Foundation and the Stanford University Human Institutional Review Boards. By searching the electronic medical record database, 64 patients who were taking statins and had a 25(OH)D level measured for any reason, were identified, including the first group of 21. The patients were contacted and 46 agreed to genetic testing of the SLCO1B1 gene. At the time of blood sampling, the patients were given a brief questionnaire that was designed to confirm clinical information from their medical records. Myalgias were defined as muscular pain or weakness as reported by the patients, who graded their symptoms as mild, moderate or severe. 25(OH)D levels were measured at the Quest Diagnostics Incorporated lab using a liquid chromatography-tandem mass spectrometry assay, which has a lower limit of detection of 4 ng/mL. Parathyroid hormone levels were unavailable in most of the patients. Patients found to be vitamin D insufficient (20–29 ng/mL) or deficient (<20 ng/mL)42 were considered collectively to be “vitamin D deficient” for our analysis, although a repeat analysis using <20 ng/mL as the cut point yielded similar results. Vitamin D deficient patients were treated either with one or two 1,000 IU capsules daily of cholecalciferol (vitamin D3) purchased over the counter or with a prescription for 50,000 IU of vitamin D2 taken weekly, depending on the degree of vitamin D deficiency. The patients were not paid for their participation.
SLCO1B1 genotyping.
The genotyping of the SLCO1B1 gene at the rs4149056 SNP (521T > C or SLCO1B1*5) was performed using PCR and DNA sequencing. DNA was extracted from the whole blood using the QIAamp DNA Blood Mini Kit from Qiagen (Valencia, CA) according to the manufacturer's instructions. PCR was performed using the DNA Engine (MJ Research, Foster City, CA). The PCR reaction was set up using the primers (5′-TGA GGA ACT ATG AGT CCA TTA GAC C-3′, sense, in intron 5; 5′-TGT AAG AAA GCC CCA ATG G-3′, antisense, in exon 6). The PCR products were purified with the QIAquick PCR Purification Kit (Qiagen). The purified PCR products were sequenced using the primer (5′-GAGT/TTACAAGTAGT/TAAAT/TTGT-3′, sense, in intron 5), which was confirmed by sequencing with the antisense PCR primer. The sequencing was carried out in the Protein and Nucleic Acid Facility at Stanford University School of Medicine.
Calcitriol induction of SLCO1B1 expression, isolation of RNA and quantitative real-time reverse transcription-PCR.
Cultured human Huh6 liver cells were treated with vehicle (0.01% ethanol in buffer) or 100 nM calcitriol (a generous gift from Milan Uskokovich, BioXell Co., Nutley, NJ) for 24 hours to compare baseline with hormone treated responses. For in vivo studies, mice were treated with calcitriol (25 ng/mouse) or vehicle every other day for 4 weeks and livers harvested 14 h after the last injection. Total RNA was prepared from the cells or from the mouse liver using RNeasy Plus Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Reverse transcription and real-time PCR analyses were performed as described previously.43 The SLCO1B1*5 primers were designed (sense: 5′-TAA GGC TAA CAT CTT ATT GGG AGT C-3′; antisense: 5′-CAC AGC AGT AAA ACA TGA GAA TTT G-3′) and synthesized based on its cDNA sequence. The vitamin D-24-hydroxylase gene (CYP24) was used as a positive control for calcitriol regulation. Detailed methods for these experiments are as previously described.44
Statistics.
Two primary hypotheses were examined. The first was whether the proportion among those treated with vitamin D who were successfully rechallenged on statin therapy exceeded the proportion expected. The second was whether the SLCO1B1*5 genotype modified the relation between vitamin D status and mylagia risk. These hypotheses were tested at the 0.05 level of significance. A one-sided binomial test was used to test the first hypothesis and utilized an intent-to-treat approach. Logistic regression techniques were used to examine the second hypothesis. More specifically, we fit the following logistic regression model:
where p = the probability of having myalgia symptoms, Callele is an indicator for whether a patient has at least one C allele corresponding to the SLCO1B1*5 genotype and Ddeficient is an indicator for being vitamin D deficient. A test that β3 is significantly different from 0 corresponds to testing whether the increase in myalgia risk for those with at least one C allele versus no C alleles differs by vitamin D deficiency status. Additional statistical tools were used for descriptive purposes. Logistic regression was used to evaluate the impact of genotype on myalgia risk and the impact of genotype on vitamin D deficiency. T tests, Chi-squared tests and ANOVA techniques were also used to describe relationships especially for the purpose of comparing features between those missing and not missing genotype data. Results from analyses that were not of primary interest were considered descriptive and hypothesis generating and were mainly intended to describe our study sample and its generalizability. All analyses were performed using the statistical software R (cran.r-project.org/).
Acknowledgements
We thank Christyn Tannenbaum for help with statistical analyses, Diana Hill and Paula Whited for providing clinical support and both Bonnie Linde and Ronald Guzman for technical assistance.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- VDR
vitamin D receptor
- SNP
single-nucleotide polymorphism
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/13509
Financial Support
Supported in part by NIH grant DK042482 and research funds from Palo Alto Medical Foundation.
References
- 1.Joy TR, Hegele RA. Narrative review: Statin-related myopathy. Ann Intern Med. 2009;150:858–868. doi: 10.7326/0003-4819-150-12-200906160-00009. [DOI] [PubMed] [Google Scholar]
- 2.Mohaupt MG, Karas RH, Babiychuk EB, Sanchez-Freire V, Monastyrskaya K, Iyer L, et al. Association between statin-associated myopathy and skeletal muscle damage. CMAJ. 2009;181:11–18. doi: 10.1503/cmaj.081785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 4.Hansen KE, Hildebrand JP, Ferguson EE, Stein JH. Outcomes in 45 patients with statin-associated myopathy. Arch Intern Med. 2005;165:2671–6. doi: 10.1001/archinte.165.22.2671. [DOI] [PubMed] [Google Scholar]
- 5.Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther. 2007;29:1761–1770. doi: 10.1016/j.clinthera.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson TA. Toward “pain-free” statin prescribing: clinical algorithm for diagnosis and management of myalgias. Mayo Clin Proc. 2008;83:687–700. doi: 10.4065/83.6.687. [DOI] [PubMed] [Google Scholar]
- 7.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112:659–662. doi: 10.1016/s0002-9343(02)01091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvo MS, Whiting SJ. Prevalence of vitamin D insufficiency in Canada and the United States: importance to health status and efficacy of current food fortification and dietary supplement use. Nutr Rev. 2003;61:107–113. doi: 10.1301/nr.2003.marr.107-113. [DOI] [PubMed] [Google Scholar]
- 9.Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: a global perspective of current status. J Nutr. 2005;135:310–316. doi: 10.1093/jn/135.2.310. [DOI] [PubMed] [Google Scholar]
- 10.Boland R. Role of vitamin D in skeletal muscle function. Endocr Rev. 1986;7:434–448. doi: 10.1210/edrv-7-4-434. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D Deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 12.Ceglia L. Vitamin D and skeletal muscle tissue and function. Mol Aspects Med. 2008;29:407–414. doi: 10.1016/j.mam.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Feldman D, Malloy PJ, Krishnan AV, Balint E. Vitamin D: biology, action and clinical implications. In: Marcus R, Feldman D, Nelson DA, Rosen CJ, editors. Osteoporosis. third Ed. San Diego: Academic Press; 2007. pp. 317–382. [Google Scholar]
- 14.Costa EM, Blau HM, Feldman D. 1,25-Dihydroxyvitamin D3 receptors and hormonal responses in cloned human skeletal muscle cells. Endocrinology. 1986;119:2214–2220. doi: 10.1210/endo-119-5-2214. [DOI] [PubMed] [Google Scholar]
- 15.Boland R, Buitrago C, De Boland AR. Modulation of tyrosine phosphorylation signalling pathways by 1alpha,25(OH)2-vitamin D3. Trends Endocrinol Metab. 2005;16:280–287. doi: 10.1016/j.tem.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Francis RM, Selby PL. Osteomalacia. Balliere's Clin Endocrinol Metab. 1997;11:145–163. doi: 10.1016/s0950-351x(97)80569-1. [DOI] [PubMed] [Google Scholar]
- 17.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78:1463–1470. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 18.Pettifor JM. Vitamin D deficiency and nutritional rickets in children. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D. 2nd Ed. Boston: Elsevier Academic Press; 2005. pp. 1065–1084. [Google Scholar]
- 19.Badsha H, Daher M, Ooi Kong K. Myalgias or nonspecific muscle pain in Arab or Indo-Pakistani patients may indicate vitamin D deficiency. Clin Rheumatol. 2009;28:971–973. doi: 10.1007/s10067-009-1146-7. [DOI] [PubMed] [Google Scholar]
- 20.Duell PB, Connor WE. Vitamin D deficiency is associated with myalgias in hyperlipidemic subjects taking statins. Circulation. 2008;118:470. [Google Scholar]
- 21.Ahmed W, Khan N, Glueck CJ, Pandey S, Wang P, Goldenberg N, et al. Low serum 25 (OH) vitamin D levels (<32 ng/mL) are associated with reversible myositis-myalgia in statin-treated patients. Translational Research. 2009;153:11–16. doi: 10.1016/j.trsl.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Bittner V, Wenger NK, Waters DD, DeMicco DA, Messig M, LaRosa JC. Vitamin D levels are not related to myalgias in statin-treated patients with stable coronary disease. J Am Coll Cardiol. 2010;55:177–1659. [Google Scholar]
- 23.Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency; an important, common and easily treatable cardiovascular risk factor. J Am Coll Cardiol. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 24.The Search Collaborative Group, author. Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 25.Seithel A, Glaeser H, Fromm MF, Konig J. The functional consequences of genetic variations in transporter genes encoding human organic anion-transporting polypeptide family members. Expert Opin Drug Metab Toxicol. 2008;4:51–64. doi: 10.1517/17425255.4.1.51. [DOI] [PubMed] [Google Scholar]
- 26.Voora D, Shah SH, Spasojevic I, Ali S, Reed CR, Salisbury BA, Ginsburg GS. The SLCO1B1*5 genetic variant is associated with statin-induced side effects. J Am Coll Cardiol. 2009;54:1609–1616. doi: 10.1016/j.jacc.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi JS, McLeod HL. The pharmacogenetics of statin therapy; when the body aches the mind will follow. J Am Coll Cardiol. 2009;54:1617–1618. doi: 10.1016/j.jacc.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 28.Zair ZM, Eloranta JJ, Stieger B, Kullak-Ublick GA. Pharmacogenetics of OATP (SLC21/SLCO), OAT and OCT (SLC22) and PEPT (SLC15) transporters in the intestine, liver and kidney. Pharmacogenomics. 2008;9:597–624. doi: 10.2217/14622416.9.5.597. [DOI] [PubMed] [Google Scholar]
- 29.Knoblauch H, Schoewel V, Rosada A, Spuler S. Another side to statin-related side effects. Ann Intern Med. 2010;152:478–479. doi: 10.7326/0003-4819-152-7-201004060-00025. [DOI] [PubMed] [Google Scholar]
- 30.Ghatak A, Faheem O, Thompson PD. The genetics of statin-induced myopathy. Atherosclerosis. 2010;210:337–343. doi: 10.1016/j.atherosclerosis.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 31.Yavuz B, Ertugrul DT, Cil H, Ata N, Akin KO, Yalcin AA, et al. Increased levels of 25 hydroxyvitamin D and 1,25-dihydroxyvitamin D after rosuvastatin treatment: a novel pleiotropic effect of statins? Cardiovasc Drugs Ther. 2009;23:295–299. doi: 10.1007/s10557-009-6181-8. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Castrillon JL, Vega G, Abad L, Sanz A, Chaves J, Hernandez G, Duenas A. Effects of Atorvastatin on vitamin D levels in patients with ischaemic heart disease. Am J Card. 2007;99:903–935. doi: 10.1016/j.amjcard.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 33.Rejnmark L, Vestergaard P, Heickendorff L, Mosekilde L. Simvastatin does not affect vitamin D status, but low vitamin D levels are associated with dyslipidemia; results from a randomized, controlled trial. Int J Endocrinol. 2010;957174:1–6. doi: 10.1155/2010/957174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimes DS. Statins and Vitamin D. Cardiovasc Drugs Ther. 2009;23:261–262. doi: 10.1007/s10557-009-6182-7. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz JB. Effects of vitamin D supplementation in atorvastatin-treated patients: a new drug interaction with an unexpected consequence. Clin Pharmacol Ther. 2009;85:198–203. doi: 10.1038/clpt.2008.165. [DOI] [PubMed] [Google Scholar]
- 36.Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, et al. 1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005;94:483–492. doi: 10.1079/bjn20051544. [DOI] [PubMed] [Google Scholar]
- 38.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pittas AG, Chung M, Trikalinos T, Mitri TJ, Brendel M, Patel K, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–314. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152:307–314. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 41.Ordovas J, Stranges JS. Vitamin D supplementation in the age of lost innocence. Ann Intern Med. 2010;152:327–329. doi: 10.7326/0003-4819-152-5-201003020-00013. [DOI] [PubMed] [Google Scholar]
- 42.Bischoff-Ferrari HA, Giovannucci E, WilleT/T WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. Erratum, Am J Clin Nutr 2006; 84:1253. [DOI] [PubMed] [Google Scholar]
- 43.Peng L, Malloy PJ, Wang J, Feldman D. Growth inhibitory concentrations of androgens upregulate insulin-like growth factor binding protein-3 expression via an androgen response element in LNCaP human prostate cancer cells. Endocrinology. 2006;147:4599–4607. doi: 10.1210/en.2006-0560. [DOI] [PubMed] [Google Scholar]
- 44.Malloy PJ, Wang J, Peng L, Nayak S, Sisk JM, Thompson CC, Feldman D. A unique insertion/duplication in the VDR gene that truncates the VDR causing hereditary 1,25-dihydroxyvitamin D-resistant rickets without alopecia. Arch Biochem Biophys. 2006;460:285–292. doi: 10.1016/j.abb.2006.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]