Abstract
According to the multi-process theory of prospective memory (ProM), time-based tasks rely more heavily on strategic processes dependent on prefrontal systems than do event-based tasks. Given the prominent frontostriatal pathophysiology of HIV infection, one would expect HIV-infected individuals to demonstrate greater deficits in time-based versus event-based ProM. However, the two prior studies examining this question have produced variable results. We evaluated this hypothesis in 143 individuals with HIV infection and 43 demographically similar seronegative adults (HIV−) who completed the research version of the Memory for Intentions Screening Test, which yields parallel subscales of time- and event-based ProM. Results showed main effects of HIV serostatus and cue type, but no interaction between serostatus and cue. Planned pair-wise comparisons showed a significant effect of HIV on time-based ProM and a trend-level effect on event-based ProM that was driven primarily by the subset of participants with HIV-associated neurocognitive disorders. Nevertheless, time-based ProM was more strongly correlated with measures of executive functions, attention/working memory, and verbal fluency in HIV-infected persons. Although HIV-associated deficits in time- and event-based ProM appear to be of comparable severity, the cognitive architecture of time-based ProM may be more strongly influenced by strategic monitoring and retrieval processes.
Keywords: AIDS dementia complex, Episodic memory, Executive functions, Neuropsychological assessment
Introduction
Prospective memory (ProM) is the neurocognitive capacity to successfully form, maintain, and execute an intention at a particular point in the future in response to a specific cue. Commonly cited examples of ProM in daily life include remembering to deliver a message to a friend, take a medication as prescribed, or attend a healthcare appointment. Conceptually, ProM is a form of episodic memory that is related to, but dissociable from retrospective memory (RetM; Einstein & McDaniel, 1990). It is primarily dependent on prefrontal systems, most notably Brodmann's area 10 (Burgess, Quayle, & Frith, 2001, 2003), and associated cognitive constructs, including attention/working memory and executive functions, such as planning and cognitive flexibility (McDaniel, Glisky, Rubin, Guynn, & Routhieaux, 1999). It also relies on the integrity of the medial temporal lobe, specifically the hippocampal formation, which is critical to the RetM encoding and consolidation aspects of ProM (McDaniel & Einstein, 2007; Simons, Schölvinck, Gilbert, Frith, & Burgess, 2006). Although scientific inquiry into ProM gained momentum and prominence in the aging literature (Henry, MacLeod, Phillips, & Crawford, 2004), there has been growing interest in the mechanisms and functional impact of ProM in clinical populations (Kliegel, Jager, Altgasen, & Shum, 2008), especially given its demonstrated relevance to independent living skills (Twamley et al., 2008).
Persons living with HIV infection, for example, subjectively experience more ProM failures in their daily lives than their seronegative counterparts (Woods et al., 2007) and perform more poorly on laboratory measures of ProM (Carey, Woods, Rippeth, Heaton, Grant, & The HNRC Group, 2006; Martin et al., 2007). Prior evidence of increased rates of omission (i.e., no response), commission (i.e., perseverations), and loss of time errors (i.e., the correct intention performed at the wrong time) in the context of normal recognition support the hypothesis that HIV-associated ProM deficiencies are driven by the strategic aspects of encoding and retrieval, rather than by deficits in consolidation or other failures of RetM (Carey et al., 2006). Underscoring their clinical relevance, HIV-associated ProM deficits confer a 4-fold increased risk of concurrent declines in independent activities of daily living (Woods, Iudicello, et al., 2008) and are highly predictive of a number of adverse health behaviors, including risky sexual and injection drug use practices (Martin et al., 2007), unemployment (Woods, Weber, Weisz, Twamley, & Grant, 2011), and non-compliance with antiretroviral regimens (Contardo, Black, Beauvais, Dieckhaus, & Rosen, 2009; Woods, Moran, Carey, et al., 2008; Woods et al., 2009) and general healthcare instructions (Zogg et al., 2010). Importantly, ProM displays incremental ecological validity in the prediction of successful daily functioning among HIV-infected individuals beyond the influence of traditional measures of neuropsychological impairment, psychiatric comorbidity, demographics, and HIV disease severity (Woods, Iudicello, et al., 2008; Woods, Moran, Carey, et al., 2008).
A defining feature of ProM is that an individual must independently detect a relevant environmental cue and self-initiate retrieval of a linked intention during a period of delay and distraction without an explicit prompt to do so (Einstein & McDaniel, 1990). This contrasts with RetM in which retrieval is induced by an explicit prompt, such as a direct query from an examiner. Of particular interest among researchers are the processes and conditions supporting successful cue recognition in ProM and the necessary switching of attention from ongoing activity to the task at hand (i.e., intention retrieval). According to the highly influential multi-process theory of McDaniel and Einstein (2000), characteristics of the target cue are instrumental in determining whether encoding, monitoring, and/or retrieval processes are likely to be effective. One such critical feature is whether cue detection is a function of the passage of time (TB cue) or the occurrence of an event (EB cue). In a TB task, an intention is executed after the passage of a specified period of time (e.g., taking a medication 1 hr after a meal), whereas EB task completion is contingent on an external cue (e.g., taking a medication before going to bed). Successful TB ProM is hypothesized to rely on voluntary, strategic monitoring processes that consume considerable cognitive resources and decline with dysregulation of prefrontal systems (Raskin et al., 2011). Generally speaking, EB intentions are thought to come to mind involuntarily and relatively automatically when the target event is encountered, and therefore involve less strategic monitoring and cognitive control (McDaniel & Einstein, 2000). Complicating matters, however, is the fact that not all EB tasks are created equal; that is, the strategic demands of EB tasks can also be manipulated, as has been demonstrated in the case of focal versus non-focal cues (i.e., focal cues are believed to demand less in the way of strategic resources because the PM cue [e.g., an animal word] is central to the processing of the ongoing task [e.g., a lexical decision task]) (Kliegel et al., 2008). Nevertheless, for TB and EB tasks that are well matched on these (and other) important characteristics, it is generally agreed that TB tasks are relatively more dependent on self-initiated monitoring and retrieval processes supported by frontostriatal pathways than are EB tasks (Gilbert, Gollwitzer, Cohen, Oettingen, & Burgess, 2009). Conditions affecting prefrontal systems, therefore, might be expected to negatively impact performance on TB tasks relative to EB tasks (e.g., Parkinson's disease; Costa, Peppe, Caltagirone, & Carlesimo, 2008; Raskin et al., 2011). Although HIV affects several different neural systems, a convergence of neuropathological (Masliah, Ge, & Mucke, 1996), neuroimaging (Paul et al., 2007), and neuropsychological (Woods, Carey, Tröster, Grant, & The HNRC Group, 2005) data supports a preferential impact of HIV-associated neuropathology on the fronto-striato-thalamo-cortical loops (see Ellis, Calero, & Stockin, 2009, for a review). Accordingly, one might hypothesize that the prominent frontostriatal systems pathophysiology of HIV infection would confer a disproportionate risk for deficits in TB versus EB ProM.
However, findings from the two published studies investigating this question among persons living with HIV have been mixed. In the first study, Carey and colleagues (2006) compared the ProM performance of 42 HIV-positive participants with that of 29 seronegative comparison participants on the Memory for Intentions Screening Test (MIST; Raskin, Buckheit, & Sherrod, 2010) and found significant impairment in both TB and EB, with a slightly larger effect size for EB ProM (Cohen's d = 0.84 vs. 0.68). However, a subsequent study by Martin and colleagues (2007) reported that TB, but not EB, ProM was impaired in 31 HIV-infected substances when compared with 35 uninfected comparison substance users. The EB task in this study required participants to turn over a piece of paper upon hearing a particular verbal cue from the examiner, whereas successful completion of the TB task required keeping track of elapsed time and informing the examiner each time 7min had passed. Noting that EB scores were negatively skewed and approached ceiling levels, the authors hypothesized that psychometric incomparability between the EB and TB may have influenced their findings. Another limitation of this prior literature was the relatively small samples of HIV-infected individuals, which may have restricted the statistical power of both studies. A more general weakness is the absence of a conceptually based, hypothesis-driven approach to identifying whether HIV infection is associated with a disproportionate effect on TB versus EB ProM (e.g., neither of these studies examined possible serostatus by cue interactions).
With these limitations in mind, the present study aimed to examine the relative effects of HIV on TB and EB ProM in a large sample as guided by McDaniel & Einstein's (2000) multi-process theory. Overall ProM performance was expected to be impaired among HIV-positive individuals relative to uninfected participants, with disproportionately adverse effects on TB versus EB ProM. We also sought to assess whether specific ProM errors (i.e., omissions, task substitution, loss of time, or loss of content) varied by cue type (i.e., time- or event-based). Previous researchers have reported on the prevalence of various types of errors across summary indices of ProM, but not whether error profiles differ as a function of TB relative to EB performance. Finally, we aimed to examine the cognitive correlates of TB and EB ProM in HIV using a well-validated battery of standard clinical tests and a few targeted experimental measures (e.g., time estimation).
Method
Participants
Participants were 143 adults with HIV infection and 43 demographically similar healthy, seronegative adults (HA) from the San Diego community or local HIV clinics who responded to flyers or newspaper advertisements describing the study. Those with severe psychiatric illness (e.g., schizophrenia), neurological disease (e.g., seizure disorders, stroke, closed head injury with loss of consciousness for more than 15 min, and central nervous system [CNS] neoplasms or opportunistic infections), verbal IQ scores <70 (based on Wechsler Test of Adult Reading; Psychological Corporation, 2001), diagnoses of substance dependence within the 6 months prior to the baseline evaluation, or urine toxicology screens positive for illicit drugs on the day of testing were excluded from participation. Table 1 shows the demographic, psychiatric, and HIV disease characteristics of the study cohorts. Participants were generally middle-aged with post-high school educational backgrounds and fairly well-managed HIV disease (e.g., the median CD4 t-lymphocyte count was over 500). No significant differences in age, education, ethnic identity, or estimated premorbid verbal intelligence were observed between infected and uninfected groups (ps>.10), although there was a lower proportion of women in the HIV-positive group (p<.001). HIV-infected participants were marginally more likely to be co-infected with hepatitis C and to report lifetime histories of major depressive disorder and substance dependence (ps<.10).
Table 1.
Demographic, psychiatric, and medical characteristics of the study participants
| Characteristics | HIV− (N = 43) | HIV+ (N = 143) | p |
|---|---|---|---|
| Demographic | |||
| Age (years) | 43.3 (12.5) | 45.6 (8.4) | .335 |
| Education (years) | 14.6 (2.2) | 14.1 (2.3) | .191 |
| WTAR verbal IQ | 105.7 (11.2) | 105.0 (11.9) | .834 |
| Sex (% women) | 46.5 | 12.6 | <.001 |
| Ethnicity (% Caucasian) | 53.5 | 60.8 | .340 |
| Psychiatric | |||
| Major depressive disordera (%) | 35.7 | 53.2 | .047 |
| Generalized anxiety disordera (%) | 7.1 | 5.7 | .725 |
| Substance dependencea (%) | 35.7 | 51.8 | .068 |
| POMS totalb | 39 (23, 68) | 49 (31, 69) | .124 |
| Medical | |||
| Hepatitis C (%) | 2.3 | 14 | .034 |
| HIV disease | |||
| HIV duration (years) | — | 13.8 (7.5) | |
| AIDS (%) | — | 61.5 | |
| cART (%) | — | 79.7 | |
| Nadir CD4b (cells/μl) | — | 142 (40, 310) | |
| Current CD4b (cells/μl) | — | 535.5 (325.8, 783) | |
| HIV RNAb log10 (% detectable) | — | 31.4 | |
Note: WTAR = Wechsler Test of Adult Reading; POMS = Profile of Mood States; cART = combination antiretroviral therapy.
aLifetime diagnoses.
bMedian (interquartile range).
Materials and Procedure
After providing written informed consent, participants completed a comprehensive neuropsychological, psychiatric, and medical evaluation.
ProM assessment
ProM was assessed using the research version (Woods, Moran, Dawson, et al., 2008) of the MIST (Raskin et al., 2010), which provides methodologically comparable subscales of TB and EB ProM. The MIST comprised eight trials during which participants complete a series of word search puzzles as ongoing distracter tasks. Participants respond to EB cues in four of the eight trials (e.g., “When I show you a postcard, self-address it.”) and to TB cues in the other four trials (e.g., “In 15 minutes, tell me it is time to take a break.”). The TB and EB trials are balanced for delay interval (i.e., 2 and 15 min delay periods) and response modality (i.e., verbal and action responses). Each item receives a score ranging from 0 to 2 points, which are summed to create two cue-specific subscales that range from 0 to 8 points (see Woods, Moran, Dawson, et al., 2008, for a more detailed description of MIST variables). In addition, we generated separate error type totals for the TB and EB scales (range = 0–4). Consistent with prior studies (Woods, Moran, Dawson, et al., 2008), errors were coded as no-response (i.e., response omission errors), loss of time (i.e., executing an intention ≥15% of the target time), task substitution (e.g., responding verbally instead of with an action or vice versa), or loss of content (i.e., awareness that a response is required but failure to recall the content). Finally, participants were administered an eight-item, three-choice post-test recognition task, from which we generated separate error totals for recognition of TB (range = 0–4) and EB (range = 0–4) cues.
General neuropsychological assessment
Participants also completed a standard battery of clinical tests of neuropsychological functioning. The battery was consistent with National Institutes of Health guidelines for assessing the cognitive domains that are most sensitive to HIV infection (Antinori et al., 2007). Thirty-one percent (N = 44) of the HIV sample met the Frascati criteria for an HIV-associated neurocognitive disorder (HAND; Antinori et al., 2007), which required evidence of impairment (i.e., 1 + SD below the normative mean) in ≥2 neurocognitive domains as determined by blinded clinical ratings and a multidisciplinary case conference (Woods et al., 2004). Seventy-three percent were diagnosed with Asymptomatic Neurocognitive Impairment, 25% with Mild Neurocognitive Disorder, and 2% with HIV-associated Dementia. The specific domains assessed were: (a) retrospective learning and memory, which included the immediate- and long-delayed trials of the California Verbal Learning Test—Second edition (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2000), the Logical Memory subtest of the Wechsler Memory Scale-III (Psychological Corporation, 1997), and the Rey-Osterreith Complex Figure (Stern et al., 1999); (b) information processing speed (Trail Making Test, Part A (TMT; Reitan & Wolfson, 1985) and total execution time from the Tower of London—Drexel (ToL-DX; Culbertson & Zillmer, 2001); (c) executive functions (TMT Part B and the ToL-DX total move score); (d) attention/working memory (Digit Span subtest from the Wechsler Adult Intelligence Scale-Third Edition [Psychological Corporation, 1997]) and the total errors from the Self-Ordered Pointing Test (Morgan et al., 2009); and (e) verbal fluency (animals and letter C [Benton, Hamsher, & Sivan, 1983] and actions [Woods et al., 2005]). Raw scores were converted to population-based z-scores derived separately from the HIV+ (N = 143) and HIV− (N = 43) samples then averaged across the tests in each domain to create composite z-scores for each ability area. Participants also completed an experimental measure of time estimation (Mimura, Kinsbourne, & O'Connor, 2000) in which they were asked to estimate how much time had elapsed during the four brief intervals (i.e., 15, 30, 45, and 90 s in a randomized order), without the aid of a clock. The discrepancy between the actual time elapsed and the participants’ response was used as the primary outcome (Woods et al., 2009).
Psychiatric assessment
Lifetime and current diagnoses of major depressive disorder, generalized anxiety disorder, and substance use disorders were generated from structured psychiatric interviews conducted using the Composite International Diagnostic Interview (version 2.1; World Health Organization, 1998) according to Diagnostic and Statistical Manual of Mental Disorders criteria (4th ed., American Psychiatric Association, 1994). These CIDI modules were chosen because they assess the psychiatric disorders most prevalent in HIV disease. Participants also completed the Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1981), which measures current affective distress in the areas of tension/anxiety, depression/dejection, anger/hostility, vigor/activity, fatigue/inertia, and cognitive/confusion. The POMS also provides a Total Mood Disturbance score on which higher scores signal greater distress.
Data analyses
To determine whether HIV serostatus disproportionately affected time- versus event-based ProM in this sample, we conducted a repeated-measures analysis of variance (ANOVA) with HIV serostatus as the between-subjects factor and cue type (time- or event-based) as the within-subjects factor. We included sex as a covariate given the group imbalance on this variable. Wilcoxon's Rank-Sum tests (due to unequal variance and non-normal distributions) with Cohen's d effect size estimates were implemented for planned follow-up pair-wise comparisons. Spearman's rank correlation coefficients were estimated to assess the associations between error performance on TB and EB tasks and other cognitive processes, including executive function and RetM. Finally, we used multiple regression techniques to identify whether summary scores for TB and/or EB ProM uniquely predicted cognitive functioning found to be significantly correlated in bivariate analyses. A critical α level of .05 was used for all analyses.
Results
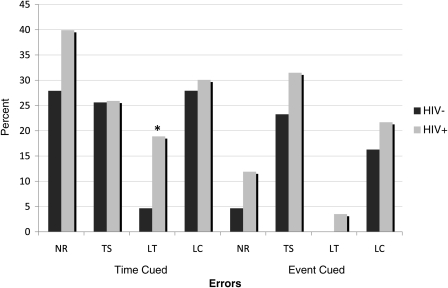
The descriptive data for the TB and EB ProM subscales in the HIV-positive and HA groups are displayed in Table 2. A repeated-measures ANOVA covarying for sex revealed main effects of HIV serostatus (F = 5.9, p = .016, η2 = 0.031) and cue type (F = 41.7, p<.001, η2 = 0.186), but no interaction between serostatus and cue (F<1, p = .861, η2 = 0.00). These findings did not change when hepatitis C co-infection or lifetime major depressive and substance use disorders were included in the model. Planned pair-wise comparisons showed a significant HIV effect on TB ProM (p = .016, Cohen's d = 0.43) and a trend-level effect on EB ProM (p = .074, Cohen's d = 0.38). Post hoc analyses suggested that the trend-level EB finding was driven primarily by the subset of participants with HAND, who performed significantly below the HA participants (p = .03, Cohen's d = 0.57). The HA and neurocognitively normal HIV-positive subjects did not differ on EB (p = .20, Cohen's d = 0.33). With regard to TB, participants with HAND similarly performed below the HA group (p = .01, Cohen's d = 0.58), but there was also a trend-level effect observed between the HA and neurocognitively normal HIV+ participants (p = .05, Cohen's d = 0.37). Analysis of error types (Table 2 and Fig. 1) revealed that TB ProM among HIV-positive participants was marked by an elevation in loss of time errors (p<.05, d = 0.37). No other TB error types differed by serostatus, nor were group differences evident on the TB recognition scale (ps>.10). There were no significant differences between HIV and HA groups on either EB error types or the recognition subscale (ps>.10). Finally, the study groups did not differ on word search task (p>.10).
Table 2.
Time- and event-based ProM performance in the study samples
| MIST variable | HIV− (N = 43) | HIV+ (N = 143) | p | Cohen's d (95% CI) |
|---|---|---|---|---|
| Time-based cues | 7 (6, 8) | 6 (5, 7) | .016 | –0.43 (–0.77, –0.09) |
| No response | 0 (0, 1) | 0 (0, 1) | .175 | 0.22 (–0.12, 0.56) |
| Task substitution | 0 (0, 1) | 0 (0, 1) | .963 | 0.02 (–0.32, 0.36) |
| Loss of time | 0 (0, 0) | 0 (0, 0) | .023 | 0.39 (0.04, 0.73) |
| Loss of content | 0 (0, 1) | 0 (0, 1) | .716 | 0.09 (–0.25, 0.43) |
| Recognition errors | 0 (0, 0) | 0 (0, 0) | .264 | 0.19 (–0.16, 0.53) |
| Event-based cues | 8 (7, 8) | 7 (6, 8) | .075 | –0.38 (–0.72, –.04) |
| No response | 0 (0, 0) | 0 (0, 0) | .177 | 0.19 (–0.15, 0.53) |
| Task substitution | 0 (0, 0) | 0 (0, 1) | .239 | 0.24 (–0.10, 0.58) |
| Loss of time | 0 (0, 0) | 0 (0, 0) | .217 | 0.19 (–0.15, 0.53) |
| Loss of content | 0 (0, 0) | 0 (0, 0) | .42 | 0.15 (–0.20, 0.49) |
| Recognition errors | 0 (0, 0) | 0 (0, 0) | .721 | –0.06 (–0.40, 0.28) |
| Word search | 17 (13, 19) | 16 (13, 19) | .42 | –0.20 (–0.54, 0.14) |
Note: Data represent medians with interquartile ranges in parentheses. ProM = prospective memory; MIST = Memory for Intentions Screening Test.
Fig. 1.
Bar chart displaying the proportion of individuals in the HIV+ and HIV− groups who committed at least one ProM error on time- and event-based scales. *p<0.05. NR = no response; TS = task substitution; LT = loss of time; LC = loss of content.
Correlational analyses of the ProM TB and EB scale summary scores and other cognitive domain scores were first conducted in the HIV-positive group. Results are shown in Table 3 and indicate that TB, but not EB, was associated with verbal fluency and attention/working memory (ps<.05). Both TB and EB were significantly correlated with RetM, retrospective learning, executive functions, and information processing speed (ps<.05). Follow-up separate linear regressions were conducted to predict TB and EB scores in the entire HIV+ sample from the domain z-scores of RetM, executive functions, and information processing speed. Results showed that the overall EB model was significant (adjusted R2 = .08, p = .003), but that RetM was the only unique contributor (p = .01). The omnibus TB model was also significant (adjusted R2 = .08, p = .003); however, both RetM (p = .004) and executive functions (p = .04) were independently associated with TB. Information processing speed did not contribute to either model (ps>.10). Neither TB nor EB ProM was associated with time estimation (ps>.10). A broadly similar pattern and magnitude of intercorrelations was observed in the HIV− sample, with the most notable exception being a stronger relationship between ProM and RetM learning and memory in this smaller seronegative group.
Table 3.
Correspondence between MIST time- and event-based scores and traditional neuropsychological tests in study participants
| Domain (z-score) | Time | Event |
|---|---|---|
| HIV+ | ||
| Retrospective learning | 0.29**** | 0.26*** |
| Retrospective memory | 0.34**** | 0.29**** |
| Executive functions | 0.31**** | 0.25*** |
| Information processing speed | 0.24*** | 0.23** |
| Verbal fluency | 0.24*** | 0.13 |
| Attention/working memory | 0.28**** | 0.14 |
| Time estimation | 0.04 | 0.05 |
| HIV− | ||
| Retrospective learning | 0.68**** | 0.39* |
| Retrospective memory | 0.63**** | 0.48*** |
| Executive functions | 0.38* | 0.26 |
| Information processing speed | 0.38* | 0.28 |
| Verbal fluency | 0.27 | 0.06 |
| Attention/working memory | 0.41** | 0.16 |
| Time estimation | 0.03 | 0.08 |
Note: Data are presented as Spearman's ρ correlation coefficients. MIST = Memory for Intentions Screening Test.
*p≤.05.
**p≤0.01.
***p<0.005.
****p<0.001.
Discussion
Conceptual models of ProM propose that all other factors being equal, TB tasks have greater resource demands on strategic, self-initiated processes related to executive functions than do EB tasks (McDaniel & Einstein, 2000). Considering the extensive prior literature on frontostriatal pathology and associated executive dysfunction in HIV infection (Woods et al., 2009), one would predict that individuals with HIV would show disproportionate impairment in TB versus EB ProM. However, the two prior studies on this topic have produced mixed findings (Carey et al., 2006; Martin et al., 2007). In an effort to address the limitations of previous work, the current study sought to clarify the possible differential effects of HIV on TB and EB by employing a theory-driven approach and using psychometrically comparable measures (with cue-specific error and recognition scores) in a large, well-characterized sample. Consistent with prior studies, we observed a mild-to-moderate main effect of HIV serostatus on ProM, which post hoc tests revealed was driven by significantly lower scores in the HIV cohort when compared with HA across ProM subscales. Similarly, there was a main effect of cue type, with lower scores evident on TB versus EB ProM across all study participants. However, contrary to our expectations, the serostatus by cue interaction term was not statistically significant. Planned comparisons showed a significant effect of HIV on TB ProM, which was associated with a medium effect size, but the effect of HIV on EB ProM was associated with a small-to-medium effect size and only approached significance. Underlying this trend-level EB ProM finding were significantly higher scores in HA when compared with participants with (but not without) HAND.
The absence of the hypothesized interaction suggests that the effects of HIV on TB and EB ProM are broadly comparable in magnitude, perhaps reflecting more generalized problems with self-initiated cue detection and retrieval. This result generally comports with those reported by Carey and colleagues (2006), who employed similar methodology and an identical measure of ProM (i.e., the MIST) and who likewise reported that HIV-associated deficits in ProM were expressed on both TB and EB tasks (i.e., overlapping effect size confidence intervals). On the other hand, Martin and colleagues (2007) found deficits in TB, but not EB, ProM among HIV-positive substance users. Our results may support those authors’ contention that the TB and EB measures used in that study were not comparable in their psychometrics or their administration procedures, which could have introduced psychometric artifact (Henry et al., 2004). Although TB tasks appear to place a greater emphasis on self-initiated monitoring and retrieval than do EB tasks (Einstein & McDaniel, 1990), the difficulty level of some EB tasks may place demands on strategic aspects of monitoring (Foster, McDaniel, Repovš, & Hershey, 2009) and retrieval (Kliegel et al., 2008) that rival those more characteristics of TB tasks. To this end, the MIST EB scale uses non-focal cues, which prior research shows are more strategically demanding than focal cues (Kliegel et al., 2008). As such, future experimental studies may wish to manipulate various aspects of the EB paradigm in HIV-infected persons, such as cue focality (Foster et al., 2009), semantic relatedness (Woods, Dawson, Weber, Grant, & The HNRC Group, 2010), and salience (Smith, Hunt, McVay, & McConnell, 2007).
Cohort differences across prior studies may have also influenced the discordant findings. One possibility is that EB deficits become more pronounced as HIV disease progresses; for example, 79% percent of participants in the Carey and colleagues (2006) study had been diagnosed with AIDS compared with 20% in Martin and colleagues (2007), where EB effects were not observed. Sixty-two percent of participants in the current study had had an AIDS diagnosis, a figure more closely resembling disease severity of Carey and colleagues (2006) than of Martin and colleagues (2007). Indeed, the trend-level EB ProM findings in this study were driven by the HIV+ participants with HAND, suggesting that the impact of CNS disease severity on ProM performance warrants further research. Another important cohort difference between the current and prior studies is the prevalence of substance use disorders, which was considerably higher in the Martin and colleagues study (100% vs. 48%). This is important because substance abuse can affect EB and TB ProM (Iudicello et al., 2011) and may be an important factor in the cue-specific contribution of ProM to everyday functioning outcomes, such as medication adherence (Contardo et al., 2009).
Although HIV-associated deficits in EB and TB appear to be of similar severity, analysis of their cognitive correlates provided some evidence that they may have at least partly separable mechanisms. Specifically, these correlational data were commensurate with the hypothesis that the cognitive architecture of TB ProM is more heavily dependent upon cognitive control mechanisms, including strategic (i.e., time) monitoring and cue detection processes (Einstein, McDaniel, Richardson, Guynn, & Cunfer, 1995; McDaniel & Einstein, 2000). For instance, TB, but not EB, ProM was significantly associated with verbal fluency and attention/working memory in the HIV cohort (with broadly similar effect sizes observed in the HIV− subjects). From these data, one might surmise that TB ProM is more strongly reliant on self-initiated retrieval and active monitoring processes than is EB ProM. Moreover, although both TB and EB were significantly (and generally comparably) correlated with executive functions, RetM, and information processing speed in univariate analyses, linear regression results indicated that EB was exclusively associated with RetM, whereas TB was predicted by both RetM and executive functions (e.g., switching and planning). Thus, although there is some overlap in the cognitive mechanisms of these two abilities, successful TB ProM in HIV seems to require greater levels of cognitive control, as would be predicted by McDaniel & Einstein's (2000) multi-process model. This pattern of results is consistent with a recent study in Parkinson's disease (Raskin et al., 2011), which showed that TB ProM was more strongly related executive dysfunction (e.g., disinhibition) than was EB ProM. Furthermore, analysis of error types showed that HIV-positive participants committed a greater number of loss of time errors in response to TB cues than did uninfected responders. Yet, TB ProM was not correlated with a measure of time estimation, indicating that our result was not exclusively a function of time perception deficits. The relative contributions of these component processes remain to be determined by future work, perhaps to include direct measures of time production and time checking, which prior research shows is an important predictor of TB ProM (Costa et al., 2008).
Several limitations should be considered when interpreting our results. Although the basic construction of the MIST's EB and TB scales is comparable (e.g., balanced on modality, semantic relatedness, delay), there was an important psychometric difference between the scales that may have influenced the findings. Consistent with prior studies (Raskin et al., 2011; Woods et al., 2010), performance on the EB scale of the MIST was relatively higher than the TB scale, which may have dampened our ability to detect a serostatus effect on EB ProM. The design of the study may also be limited in the sense that the EB and TB items overlapped on the MIST, which may have introduced some methodological contamination of the results (i.e., participants were oftentimes simultaneously maintaining both TB and EB intentions during the test). Although this measurement approach may more closely approximate real-world conditions, future studies may gain some methodological advantage by conducting separate, but parallel tests of TB and EB in a randomized fashion. Similarly, the overlapping trials issue precludes us from directly examining the potential influence of time delay (i.e., 2 vs. 15 min) or cue modality (i.e., action vs. verbal response) on the TB and EB findings. A post hoc analysis of the HIV effect sizes for these four subscales showed broadly commensurate results with those observed on TB and EB (Cohen's d range = 0.28–0.48), suggesting that such constructs might be a potentially interesting area for future research. Regarding the characteristics of the study sample, with over 60% Caucasian and less than 13% female participants, the demographic composition of the HIV-positive cohort in this study was not necessarily representative of recent trends in HIV infection in the USA (Centers for Disease Control, 2008). Finally, the HIV cohort was relatively healthy (median current CD4 count >500) and had only a 30% rate of HAND, indicating that further study of more severely compromised populations is warranted.
In summary, the present study revealed that the severity of EB and TB ProM deficits in HIV infection are broadly comparable, suggesting a more generalized deficit in the retrieval of future intentions across cue modalities. Nevertheless, a differential pattern of correlations with standard cognitive tests suggested that consistent with the predictions of the multi-process theory (McDaniel & Einstein, 2000), the cognitive architecture of the EB and TB ProM deficits might diverge, with the latter being more strongly associated with aspects of cognitive dyscontrol (e.g., self-initiated retrieval and planning). This is echoed in recent studies showing that TB ProM may be a particularly valuable predictor of everyday functioning behaviors (Martin et al., 2007; Woods et al., 2009). It will therefore be important to determine whether the EB versus TB discrepancy is applicable to more naturalistic ProM tasks (Zogg et al., 2010). Moreover, results from this study may also have some intriguing clinical implications. If, as our evidence suggests, TB ProM is supported chiefly by strategic monitoring and retrieval processes, interventions likely to target these particular aspects of ProM might be especially effective, particularly since TB ProM may be a stronger predictor of some everyday functioning outcomes (Woods et al., 2009). Training in self-regulatory strategies, for instance, could compensate for dysfunctions in strategic monitoring by helping individuals redirect attention or minimize distraction during the delay period. One such approach is goal management training, the utility of which has been supported in laboratory and naturalistic research settings among patients with traumatic brain injury (Fish et al., 2007; Levine et al., 2000). Other remedial strategies might offset deficits in cue detection and retrieval; for example, improving the salience of the cue (e.g., a pillbox) may increase the probability that it will be detected (McDaniel & Einstein, 2007). To the extent that EB ProM depends less on strategic monitoring and more on involuntary, automatic processing, effective remedial strategies might include those that strengthen the cue–intention bond during encoding (Woods et al., 2010). Widely researched examples of this approach include errorless learning (Clare & Jones, 2008), spaced retrieval (Camp, Foss, Stevens, & O'Hanlon, 1996), and implementation intentions (Gollwitzer & Brandstatter, 1997). The relative value of interventions designed to improve EB and TB ProM and their impact on activities of daily living and health-related quality of life in HIV infection clearly warrants investigation.
Funding
This research was supported by National Institute of Mental Health grants R01-MH073419 to SPW and P30-MH62512 to IG. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Conflict of Interest
None declared.
Acknowledgements
The authors thank Dr Catherine L. Carey, Lisa Moran, Matthew Dawson, Ofilio Vigil, and Sarah Gibson for their help with study management and Dr Sarah Raskin for providing us with the MIST.
Appendix
The HIV Neurobehavioral Research Programs (HNRP) Group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: IG, MD; Co-Directors: J. Hampton Atkinson, MD, Ronald J. Ellis, MD, PhD, and J. Allen McCutchan, MD; Center Manager: Thomas D. Marcotte, PhD; Naval Hospital San Diego: Braden R. Hale, MD, MPH (PI); Neuromedical Component: Ronald J. Ellis, MD, PhD (PI), J. Allen McCutchan, MD, Scott Letendre, MD, Edmund Capparelli, PharmD, and Rachel Schrier, PhD; Neurobehavioral Component: Robert K. Heaton, PhD (PI), Mariana Cherner, PhD, David J. Moore, PhD, and SPW, PsyD.; Neuroimaging Component: Terry Jernigan, PhD (PI), Christine Fennema-Notestine, PhD, Sarah L. Archibald, MA, John Hesselink, MD, Jacopo Annese, PhD, and Michael J. Taylor, PhD; Neurobiology Component: Eliezer Masliah, MD (PI), Ian Everall, FRCPsych., FRCPath., PhD, and T. Dianne Langford, PhD; Neurovirology Component: Douglas Richman, MD, (PI), and David M. Smith, MD; International Component: J. Allen McCutchan, MD, (PI); Developmental Component: Ian Everall, FRCPsych., FRCPath., PhD (PI), and Stuart Lipton, MD, PhD; Clinical Trials Component: J. Allen McCutchan, MD, J. Hampton Atkinson, MD, Ronald J. Ellis, MD, PhD, and Scott Letendre, MD; Participant Accrual and Retention Unit: J. Hampton Atkinson, MD (PI), and Rodney von Jaeger, MPH.; Data Management Unit: Anthony C. Gamst, PhD (PI), Clint Cushman, BA (Data Systems Manager), and Daniel R. Masys, MD (Senior Consultant); Statistics Unit: Ian Abramson, PhD (PI), Christopher Ake, PhD, and Florin Vaida PhD.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Antinori A., Arendt G., Becker J. T., Brew B. J., Byrd D. A., Cherner M., et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton A. L., Hamsher K., Sivan A. B. Multilingual aphasia examination. Iowa City, IA: AJA Associates; 1983. [Google Scholar]
- Burgess P. W., Quayle A., Frith C. D. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. doi:10.1016/S0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Burgess P. W., Scott S. K., Frith C. D. The role of the rostral frontal cortex (area 10) in prospective memory: A lateral versus medial dissociation. Neuropsychologia. 2003;41:906–918. doi: 10.1016/s0028-3932(02)00327-5. doi:10.1016/S0028-3932(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Camp C. J., Foss J. W., Stevens A. B., O'Hanlon A. M. Improving prospective memory task performance in persons with Alzheimer's disease. In: Brandimonte M., Einstein G. O., McDaniel M. A., editors. Prospective Memory: Theory and Applications. Mahwah, NJ: Lawrence Erlbaum; 1996. pp. 351–367. [Google Scholar]
- Carey C. L., Woods S. P., Rippeth J. D., Heaton R. K., Grant I. The HNRC Group. Prospective memory in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology. 2006;28:536–548. doi: 10.1080/13803390590949494. doi:10.1080/13803390590949494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control. ( HIV/AIDS Surveillance Report, 2008. Atlanta: U.S. Department of Health and Human Services, CDC; 2008. [Google Scholar]
- Clare L., Jones R. S. P. Errorless learning in the rehabilitation of memory impairment: A critical review. Neuropsychology Review. 2008;18:1–23. doi: 10.1007/s11065-008-9051-4. doi:10.1007/s11065-008-9051-4. [DOI] [PubMed] [Google Scholar]
- Contardo C., Black A. C., Beauvais J., Dieckhaus K., Rosen M. I. Relationship of prospective memory to neuropsychological function and antiretroviral adherence. Archives of Clinical Neuropsychology. 2009;24:547–554. doi: 10.1093/arclin/acp046. doi:10.1093/arclin/acp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A., Peppe A., Caltagirone C., Carlesimo G. A. Prospective memory impairment in individuals with Parkinson's disease. Neuropsychology. 2008;22:283–292. doi: 10.1037/0894-4105.22.3.283. doi:10.1037/0894-4105.22.3.283. [DOI] [PubMed] [Google Scholar]
- Culbertson W. C., Zillmer E. A. The Tower of London DX (TOL-DX) manual. North Tonawanda, NY: Multi-Health Systems; 2001. [Google Scholar]
- Delis D. C., Kramer J. H., Kaplan E., Ober B. A. The California Verbal Learning Test. 2nd ed. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Einstein G. O., McDaniel M. A. Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:717–726. doi: 10.1037//0278-7393.16.4.717. doi:10.1037/0278-7393.16.4.717. [DOI] [PubMed] [Google Scholar]
- Einstein G. O., McDaniel M. A., Richardson S. L., Guynn M. J., Cunfer A. R. Aging and prospective memory: examining the influences of self-initiated retrieval processes. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:996–1007. doi: 10.1037//0278-7393.21.4.996. doi:10.1037/0278-7393.21.4.996. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., Calero P., Stockin M. D. HIV infection and the central nervous system: a primer. Neuropsychology Review. 2009;19:144–151. doi: 10.1007/s11065-009-9094-1. doi:10.1007/s11065-009-9094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish J., Evans J. J., Nimmo M., Martin E., Kersel D., Bateman A., et al. Rehabilitation of executive dysfunction following brain injury: ‘Content-free’ cueing improves everyday prospective memory performance. Neuropsychologia. 2007;45:1318–1330. doi: 10.1016/j.neuropsychologia.2006.09.015. doi:10.1016/j.neuropsychologia.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Foster E. R., McDaniel M. A., Repovš G., Hershey T. Prospective memory in Parkinson disease across laboratory and self-reported everyday performance. Neuropsychology. 2009;23:347–358. doi: 10.1037/a0014692. doi:10.1037/a0014692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S. J., Gollwitzer P. M., Cohen A., Oettingen G., Burgess P. W. Separable brain systems supporting cued versus self-initiated realization of delayed intentions. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:905–915. doi: 10.1037/a0015535. doi:10.1037/a0015535. [DOI] [PubMed] [Google Scholar]
- Gollwitzer P. M., Brandstatter V. Implementation intentions and effective goal pursuit. Journal of Personality and Social Psychology. 1997;73:186–199. doi: 10.1037//0022-3514.81.5.946. doi:10.1037/0022-3514.73.1.186. [DOI] [PubMed] [Google Scholar]
- Henry J. D., MacLeod M. S., Phillips L. H., Crawford J. R. A meta-analytic review of prospective memory and aging. Psychology and Aging. 2004;19:27–39. doi: 10.1037/0882-7974.19.1.27. doi:10.1037/0882-7974.19.1.27. [DOI] [PubMed] [Google Scholar]
- Iudicello J. E., Weber E., Dawson M., Grant I., Weinborn M., Woods S. P. The HNRC Group. Misremembering future intentions in methamphetamine dependent individuals. The Clinical Neuropsychologist. 2011;25:269–286. doi: 10.1080/13854046.2010.546812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegel M., Jager T., Altgasen M., Shum D. Clinical neuropsychology of prospective memory. In: Kliegel M., McDaniel M. A., Einstein G. O., editors. Prospective Memory: Cognitive, Neuroscience, Developmental, and Applied Perspectives. New York: Taylor & Francis Group/Lawrence Erlbaum Associates; 2008. pp. 283–308. [Google Scholar]
- Levine B., Roberston I. H., Clare L., Carter G., Hong J., Wilson B. A., et al. Rehabilitation of executive functioning: An experimental-clinical validation of goal management training. Journal of the International Neuropsychological Society. 2000;6:299–312. doi: 10.1017/s1355617700633052. doi:10.1017/S1355617700633052. [DOI] [PubMed] [Google Scholar]
- Martin E. M., Nixon H., Pitrack D. L., Weddington W., Rains N. A., Nunnally G., et al. Characteristics of prospective memory deficits in HIV-seropositive substance-dependent individuals: Preliminary observations. Journal of Clinical and Experimental Neuropsychology. 2007;29:496–504. doi: 10.1080/13803390600800970. doi:10.1080/13803390600800970. [DOI] [PubMed] [Google Scholar]
- Masliah E., Ge N., Mucke L. Pathogenesis of HIV-1 associated neurodegeneration. Critical Review of Neurobiology. 1996;10:57–67. doi: 10.1615/critrevneurobiol.v10.i1.30. [DOI] [PubMed] [Google Scholar]
- McDaniel M. A., Einstein G. O. Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Applied Cognitive Psychology. 2000;14:127–144. doi:10.1002/acp.775. [Google Scholar]
- McDaniel M. A., Einstein G. O. Prospective memory: An overview and synthesis of an emerging field. Los Angeles: Sage Publications; 2007. [Google Scholar]
- McDaniel M. A., Glisky E. L., Rubin S. R., Guynn M. J., Routhieaux B. C. Prospective memory: A neuropsychological study. Neuropsychology. 1999;13:103–110. doi: 10.1037//0894-4105.13.1.103. doi:10.1037/0894-4105.13.1.103. [DOI] [PubMed] [Google Scholar]
- McNair D., Lorr M., Droppleman L. Profile of Mood States (POMS) manual. San Diego, CA: Educational and Industrial Testing Services; 1981. [Google Scholar]
- Mimura M., Kinsbourne M., O'Connor M. Time estimation by patients with frontal lesions and by Korsakoff amnesics. Journal of the International Neuropsychological Society. 2000;6:517–528. doi: 10.1017/s1355617700655017. doi:10.1017/S1355617700655017. [DOI] [PubMed] [Google Scholar]
- Morgan E. E., Woods S. P., Weber E., Dawson M. S., Carey C. L., Moran L. M., et al. HIV-associated episodic memory impairment: Evidence of a possible differential deficit in source memory for complex visual stimuli. Journal of Neuropsychiatry and Clinical Neurosciences. 2009;21:189–198. doi: 10.1176/appi.neuropsych.21.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R. H., Yiannoutsos C. T., Miller E. N., Chang L., Marra C. M., Schifitto G., et al. Proton MRS and neuropsychological correlates in AIDS dementia complex: Evidence of subcortical specificity. Journal of Neuropsychiatry and Clinical Neuroscience. 2007;19(3):283–292. doi: 10.1176/jnp.2007.19.3.283. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. WAIS-III and WMS-III technical manual. San Antonio, TX: Author; 1997. [Google Scholar]
- Psychological Corporation. Wechsler test of adult reading. Manual. San Antonio, TX: Author; 2001. [Google Scholar]
- Raskin S., Buckheit C., Sherrod C. Memory for Intentions Test (MIsT) Lutz, FL: Psychological Assessment Resources; 2010. [Google Scholar]
- Raskin S., Woods S. P., Poquette A., McTaggart A. B., Sethna J., Williams R. C., et al. A differential deficit in time-based prospective memory in Parkinson's disease. Neuropsychology. 2011;25:201–209. doi: 10.1037/a0020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. M., Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Simons J. S., Schölvinck M. L., Gilbert S. J., Frith C. D., Burgess P. W. Differential components of prospective memory? Evidence from fMRI. Neuropsychologia. 2006;44:1388–1397. doi: 10.1016/j.neuropsychologia.2006.01.005. doi:10.1016/j.neuropsychologia.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Hunt R. R., McVay J. C., McConnell M. D. The cost of event-based prospective memory: Salient target events. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33:734–746. doi: 10.1037/0278-7393.33.4.734. doi:10.1037/0278-7393.33.4.734. [DOI] [PubMed] [Google Scholar]
- Stern R. A., Javorsky D. J., Singer E. A., Singer Harris N. G., Somerville J. A., Duke L. M. The Boston Qualitative Scoring System for the Rey-Osterreith Complex Figure (BQSS): Manual. Lutz, FL: Psychological Assessment Resources; 1999. [Google Scholar]
- Twamley E. W., Woods S. P., Zurhellen C. H., Vertinski M., Narvaez J. M., Mausbach B. T., et al. Neuropsychological substrates and everyday functioning implications of prospective memory impairment in schizophrenia. Schizophrenia Research. 2008;106:42–49. doi: 10.1016/j.schres.2007.10.030. doi:10.1016/j.schres.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Carey C. L., Moran L. M., Dawson M. S., Letendre S. L., Grant I., et al. Frequency and predictors of self-reported prospective memory complaints in individuals infected with HIV. Archives of Clinical Neuropsychology. 2007;22:187–195. doi: 10.1016/j.acn.2006.12.006. doi:10.1016/j.acn.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Carey C. L., Tröster A. I., Grant I. The HNRC Group. Action (verb) generation in HIV-1 infection. Neuropsychologia. 2005;43:1144–1151. doi: 10.1016/j.neuropsychologia.2004.11.018. doi:10.1016/j.neuropsychologia.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Woods S. P., Dawson M. S., Weber E., Gibson S., Grant I., Atkinson J. H., et al. Timing is everything: Antiretroviral non-adherence is associated with impairment in time-based prospective memory. Journal of the International Neuropsychological Society. 2009;15:42–52. doi: 10.1017/S1355617708090012. doi:10.1017/S1355617708090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Dawson M. S., Weber E., Grant I. The HNRC Group. The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of Clinical and Experimental Neuropsychology. 2010;32:398–407. doi: 10.1080/13803390903130737. doi:10.1080/13803390903130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Iudicello J. E., Moran L. M., Carey C. L., Dawson M. S., Grant I., et al. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology. 2008;22:110–117. doi: 10.1037/0894-4105.22.1.110. doi:10.1037/0894-4105.22.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Moran L. M., Carey C. L., Dawson M. S., Iudicello J. E., Gibson S., et al. Prospective memory in HIV infection: Is “remembering to remember” a unique predictor of self-reported medication management? Archives of Clinical Neuropsychology. 2008;23:257–270. doi: 10.1016/j.acn.2007.12.006. doi:10.1016/j.acn.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Moran L. M., Dawson M. S., Carey C. L., Grant I. The HNRC Group. Psychometric characteristics of the Memory for Intentions Screening Test. The Clinical Neuropsychologist. 2008;23:257–270. doi: 10.1080/13854040701595999. doi:10.1016/j.acn.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Rippeth J. D., Frol A. B., Levy J. K., Ryan E., Soukup V. M., et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Journal of Clinical and Experimental Neuropsychology. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- Woods S. P., Weber E., Weisz B., Twamley E. W., Grant I. Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabilitation Psychology. 2011;56:77–84. doi: 10.1037/a0022753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Composite international diagnostic interview. Geneva, Switzerland: World Health Organization; 1998. (CIDI, version 2.1) [Google Scholar]
- Zogg J. B., Woods S. P., Weber E., Iudicello J., Dawson M. S., Grant I. The HNRC Group. HIV-associated prospective memory impairment in the laboratory predicts failures on a semi-naturalistic measure of health care compliance. The Clinical Neuropsychologist. 2010;24:945–962. doi: 10.1080/13854046.2010.501343. doi:10.1080/13854046.2010.501343. [DOI] [PMC free article] [PubMed] [Google Scholar]