Abstract
Chikungunya virus (CHIKV) is a mosquito-borne alphavirus that causes explosive outbreaks of febrile illness associated with rash, and painful arthralgia. The CHIK vaccine strain 181/clone25 (181/25) developed by the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) was shown to be well-tolerated and highly immunogenic in phase I and II clinical trials although it induced transient arthralgia in some healthy adult volunteers. In an attempt to better understand the host factors that are involved in the attenuating phenotype of CHIK 181/25 vaccine virus we conducted studies in interferon (IFN)-compromised mice and also evaluated its immunogenic potential and protective capacity. Infection of AG129 mice (defective in IFN-α/β and IFN-γ receptor signaling) with CHIK 181/25 resulted in rapid mortality within 3-4 days. In contrast, all infected A129 mice (defective in IFN-α/β receptor signaling) survived with temporary morbidity characterized by ruffled appearance and body weight loss. A129 heterozygote mice that retain partial IFN-α/β receptor signaling activity remained healthy. Infection of A129 mice with CHIK 181/25 induced significant levels of IFN-γ and IL-12 while the inflammatory cytokines, TNFα and IL-6 remained low. A single administration of the CHIK 181/25 vaccine provided both short-term and long-term protection (38 days and 247 days post-prime, respectively) against challenge with wt CHIKV-La Reunion (CHIKV-LR). This protection was at least partially mediated by antibodies since passively transferred immune serum protected both A129 and AG129 mice from wt CHIKV-LR and 181/25 virus challenge. Overall, these data highlight the importance of IFNs in controlling CHIK 181/25 vaccine and demonstrate the ability of this vaccine to elicit neutralizing antibody responses that confer short-and long-term protection against wt CHIKV-LR challenge.
Keywords: Chikungunya virus (CHIKV), 181/25 vaccine, A129 mice, interferon
1. Introduction
Chikungunya virus (CHIKV) is a mosquito borne alphavirus that has for decades been an important etiologic agent of human disease in Africa and Asia. The virus recently reemerged into the Indian Ocean islands, India and Southeast Asia to cause over two million cases of severe and often chronic arthralgia [1, 2]. There are many factors that contributed to the explosive nature of these recent outbreaks including a lack of CHIKV-specific immunity in human populations of the Indian Ocean and India and a mutation in the E1 envelope glycoprotein that adapted the epidemic strain for more efficient infection of the mosquito vector, Aedes albopictus [3, 4]. Classically, CHIKV infection is clinically indistinguishable from dengue and is characterized by fever, polyarthralgia, and myalgia, frequently associated with rash. However, the hallmark of CHIKV disease is a debilitating and prolonged arthralgia which can persist for months or years. Epidemiologic studies in La Reunion and India estimated case fatality rates of ~1:1,000 cases, principally among newborn, infants and elderly patients [5].
CHIKV is a small enveloped RNA virus in the family of Togaviridae. The RNA genome encodes four non-structural proteins (nsP1-4) that are required for virus replication and three main structural proteins C, E2 and E1. The E1 glycoprotein mediates pH-dependent fusion with endosomal membranes whereas the E2 glycoprotein interacts with cell surface receptors. In tropical regions of Africa CHIKV is maintained in an enzootic sylvatic cycle in primates while in Asia CHIKV is only known to persist in a human-mosquito-human cycle [2]. Although past epidemics of CHIK have been confined to Africa and Asia, increased air travel by humans and the new adaptation of CHIKV to transmission by Aedes albopictus have increased the risk of transmission and disease epidemics in Europe and the Western Hemisphere [6, 7, 8]. Because humans appear to be the only amplification hosts during urban transmission, the best means of controlling the spread of the infection is by vaccination. Currently there is no licensed vaccine available. Several attempts to develop CHIK vaccines have been described, including alphavirus chimeras [9], live attenuated virus [10], formalin-killed vaccines [11, 12], consensus-based DNA vaccines [13] and more recently, a virus-like particle vaccine [14].
The live-attenuated CHIKV vaccine candidate (termed 181/25) developed at the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) was derived by 18 plaque-to-plaque passages of the 15561 Southeast Asian human isolate in MRC-5 cells [10]. The CHIK 181/25 strain exhibited several attenuating characteristics including: 1) small plaque phenotype, 2) temperature-sensitivity, 3) decreased virulence for infant mice and, 4) reduced levels of viremia in monkeys. The strain was genetically stable and immunogenic in mice and Rhesus macaques [10]. The vaccine was well tolerated and immunogenic in Phase I/II human trials although several vaccinees developed transient arthralgia [15]. Given the promising results and attributes that the CHIK 181/25 vaccine exhibited in this study we further probed its attenuation in interferon (IFN)-compromised mice. Our goal was to understand host factors that control its replication and evaluate its ability to induce immune responses that protect from wild type CHIKV challenge.
2. Materials and Methods
2.1. Viruses
The CHIK 181/25 vaccine was provided by Scott Weaver (University of Texas Medical Branch, Galveston, Texas). The CHIKV strain La Reunion (LR) used for challenge experiments was isolated from a human during the 2006 La Reunion outbreak. After 5 passages in Vero cells, one passage in infant mice, and one passage in C6/36 mosquito cells viral RNA was isolated from the CHIKV-LR and was used to generate an infectious cDNA clone. CHIKV-LR virus used in these studies was generated from the infectious cDNA by electroporation of in vitro transcribed RNA into baby hamster kidney cells (BHK) as described previously [16].
2.2. Virus titer determination
A TaqMan real-time PCR assay was performed to estimate virus concentration [plaque forming equivalents (PFU) per ml] from serum samples. First, viral RNA was isolated from serum using the QiaAmp Viral RNA protocol (Qiagen, Valencia, CA). Total RNA was extracted from 50μl of sample and eluted from the kit columns into a final volume of 60μl of elution buffer. The RNA was stored at −80° C until use. The CHIKV oligonucleotide sets CHIKV 243+, CHIKV 330-, and CHIKV 273 probe were designed with the Primer Select software program and were based on the available full-length sequences. The real-time probes were labeled with 5′ end FAM reporter dye and 3′ end BHQ1 quencher dye. The QuantiTect probe RT-PCR kit (Qiagen, Valencia, CA) was used for the real-time (TaqMan) assay. Each reaction consisted of kit components, 10μl of RNA, 0.4μM of primer, and 0.15μM of probe in a total volume of 50μl. The reactions were subjected to 45 cycles of amplification in an iQ5 Real-Time PCR detection system (BioRad, Hercules, CA) according to the recommended conditions. A standard curve was used to quantify the viral nucleic acid in each serum sample. The standard curve was completed by serially diluting a CHIK virus stock for PFU conversion, titrating each dilution, and then extracting the RNA according to the RNeasy protocol. A curve correlation coefficient of ≥0.950 and a 90–100% PCR efficiency was used to validate each detection assay.
2.3. Plaque reduction neutralization test (PRNT)
Plaque reduction neutralization tests (PRNT) were used to detect neutralizing antibodies in serum samples. Briefly, the samples were heat-inactivated for 30min at 56°C and then serially diluted twofold in Dulbecco’s Minimum Essential Medium supplemented with 10% FBS, 100U/ml of penicillin, and 100mg/ml of streptomycin (Gibco, Carlsbad, CA). A suspension of 100 PFU /125μl was then mixed with the diluted serum samples and the suspension incubated for 1h at 37°C. The serum-virus suspension was then transferred onto 6-well cell culture plates (Corning, Corning, NY) containing a confluent monolayer of Vero cells. The virus/cell mixture was incubated for 1h at 37°C with the plate rocked every 20 min. Each well was then overlaid with a 0.4% Genepure LE agarose/DMEM medium layer (ISC BioExpress, Kaysville, UT) and plates were incubated for 48h at 37°C. The agarose layer was removed and the wells were covered with a fixative/staining solution (40% methanol and 0.25% crystal violet) for five minutes. Then plates were rinsed with water to remove the fixative/staining solution. Plaques were counted and titers were calculated and expressed as the reciprocal of serum dilution yielding a >80% reduction (PRNT80) in the number of plaques.
2.4. Telemetry
For measurement of body temperature, animals were implanted subcutaneously with BMDS IPTT-300 transponders (chip), purchased from BioMedic Data Systems, Inc. (BMDS, Seaford, Delaware) two to three days prior to start of the experiment and monitored for signs of infection or migration of the transponder. Chips were scanned using a DAS-6007 transponder reader (BMDS).
2.5. Animal studies
Mice with null mutations in the IFN-α/β receptor (A129) or both IFN-α/β and -γ receptors (AG129) were bred and housed at the University of Wisconsin animal facility (Charmany Farms) under specific pathogen-free conditions. The A129 heterozygous IFN-α/β−/+ mice were generated by cross-breeding male 129Sv/Ev mice with female IFN-α/β−/− homozygote A129 mice. All procedures were carried out in accordance with institutional and NIH guidelines for animal care and use.
A129 (homozygote or heterozygote) and AG129 6-8 weeks old mice (n=5) were immunized intradermally (ID) with various doses of CHIK 181/25 vaccine in their left hind footpad (in 50μl volume). Following infection mice were observed twice daily for morbidity (physical appearance, weight loss, and temperature), and survival rates were recorded. Blood samples were collected during the first four days post-infection to measure cytokine responses and viremia and on days 21 and 35 (only for A129 homozygote and heterozygote mice) to measure seroconversion. On day 38 or 247 post-primary vaccination A129 homozygote mice were challenged ID with the wild-type (wt) CHIKV-La Reunion (CHIKV-LR) virus. Mice were monitored for morbidity and mortality for two weeks and carcasses were collected for histopathological examination.
The role of antibodies in protection was tested in groups of five AG129 and A129 homozygote mice (7-9 weeks old) by passively transferring pool anti-CHIK 181/25 immune serum in 150μl or 200 μl volumes, respectively. Control mice received normal mouse serum. One day later, AG129 mice were inoculated ID with 104 PFU of CHIK 181/25 vaccine virus whereas A129 mice with 100 PFU of wt CHIKV–LR. Following challenge all mice were observed twice daily for morbidity (physical appearance, weight loss and temperature), and survival rates were recorded.
2.6. Cytokine responses
The APIX™ Murine cytokine kit, 12-plex sandwich microarray (Gentel Inc, Madison WI) was used to screen pooled sera for early cytokine responses (IL-1a, IL-1b, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-17, IFN-γ, GM-CSF and TNF-α) from each bleed following the manufacter’s protocol. Data were expressed as pg/ml and represent an average of quadruplicates for each cytokine.
2.7. Histopathology
Tissues were fixed in 10% neutral buffered formalin (Fisher Scientific) and bone was decalcified overnight using Fixative/Decalcifier (VWR International, West Cester, PA). The tissue was then embedded in paraffin and 5μm sections were cut. For hematoxylin and eosin staining sections were deparaffinized in Xylene for 15 minutes and then rehydrated by washing in ethanol and ethanol/water mixtures as follows: 100% ethanol for 9 minutes, 95% ethanol/5% deionized water for 3 minutes, 80% ethanol/20% deionized water for 5 minutes. Sections were stained with hematoxylin (Richard-Allan Scientific, Kalamazoo, MI) for 3 minutes and then rinsed with deionized water followed by another rinse in tap water for 5 minutes and then placed in Clarifier I (Richard-Allan Scientific, Kalamazoo, MI) for 5 minutes. Following a rinse in tap water for 2 minutes and deionized water for an extra 2 minutes the sections were then stained in eosin (Richard-Allan Scientific, Kalamazoo, MI) for 30 seconds. Then they were placed in 95% ethanol/5% deionized water for 15 minutes followed by 100% ethanol for 15 minutes and then placed in Xylene (Richard-Allan Scientific, Kalamazoo, MI) for 15 minutes. Cover slips were applied to the slides using Permount (Fisher Scientific, Pittsburgh, PA) and dried overnight. Deparaffinizing and hematoxylin-eosin staining was performed on the Varistain Gemini ES (Shandon, Thermo Fisher Scientific, Kalamazoo, MI).
2.8. Statistics
Student T-tests were performed using Excel (Microsoft, Redmond, WA).
3. Results
3.1. CHIK 181/25 vaccine administration to mice lacking type-I and/or type-II IFN receptor signaling pathways
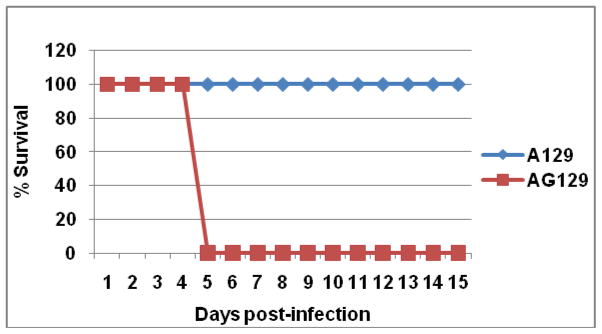
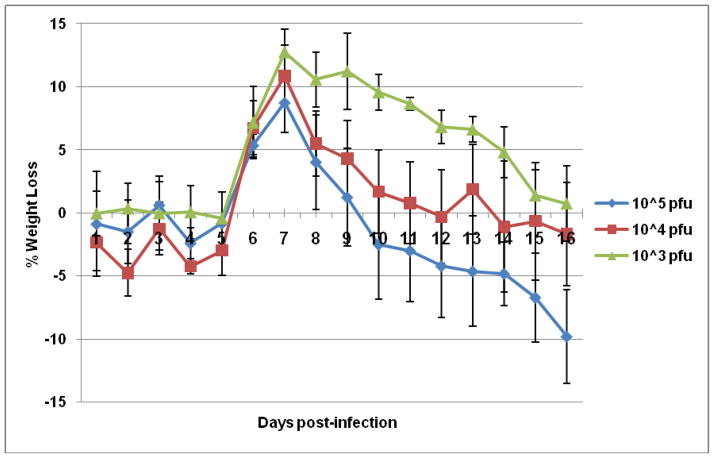
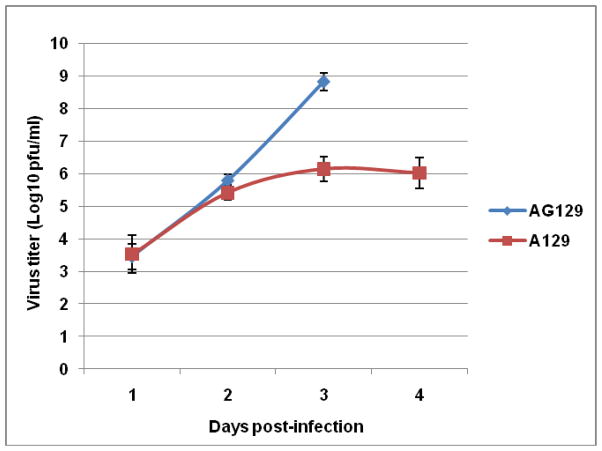
The attenuation of CHIK 181/25 vaccine was tested in adult A129 and AG129 mice that are deficient in IFN-α/β or IFN-α/β-γ receptor signaling pathways, respectively after ID administration of various doses. Inoculation of AG129 mice with 104 PFU of vaccine virus resulted in the rapid onset of clinical signs of disease, including ruffled fur and hunched posture after which animals rapidly deteriorated and died (Fig. 1). Mice injected with 105 or 103 PFU succumbed to infection three and four days post-injection, respectively (data not shown). In contrast, all infected A129 mice survived after developing temporary signs of morbidity characterized by ruffled appearance and weight loss (Fig. 1 & 2). Interestingly, there was an inverse relationship between observed morbidity and vaccine dose (Fig. 2). Injection of immunocompetent BALB/c or G129 mice (deficient in IFN-γ receptor signaling) with 105 PFU CHIK 181/25 vaccine resulted in no morbidity or mortality (data not shown). Viremia was detectable in both A129 and AG129 mice (Fig. 3). However, virus titers were significantly higher in AG129 mice by day 2 (p<0.03) and 3 (p<0.01) post-infection.
Figure 1.
Survival of adult A129 and AG129 mice (n=5) injected ID in their footpad with 104 PFU of CHIK 181/25 vaccine. Animals were monitored for two weeks.
Figure 2.
Morbidity in adult A129 mice (n=5) injected ID in their footpad with 105, 104 or 103 PFU of CHIK 181/25 vaccine. Animals were monitored for weight loss for two weeks. Each data point represents the arithmetic mean ± standard deviation.
Figure 3.
Kinetics of viremia in adult A129 and AG129 mice (n=5) injected ID with 104 PFU of CHIK 181/25 vaccine. Virus titers were determined by real-time RT-PCR relative to a standard curve of stock virus for PFU conversion (PFUeq). Each data point represents the arithmetic mean ± standard deviation.
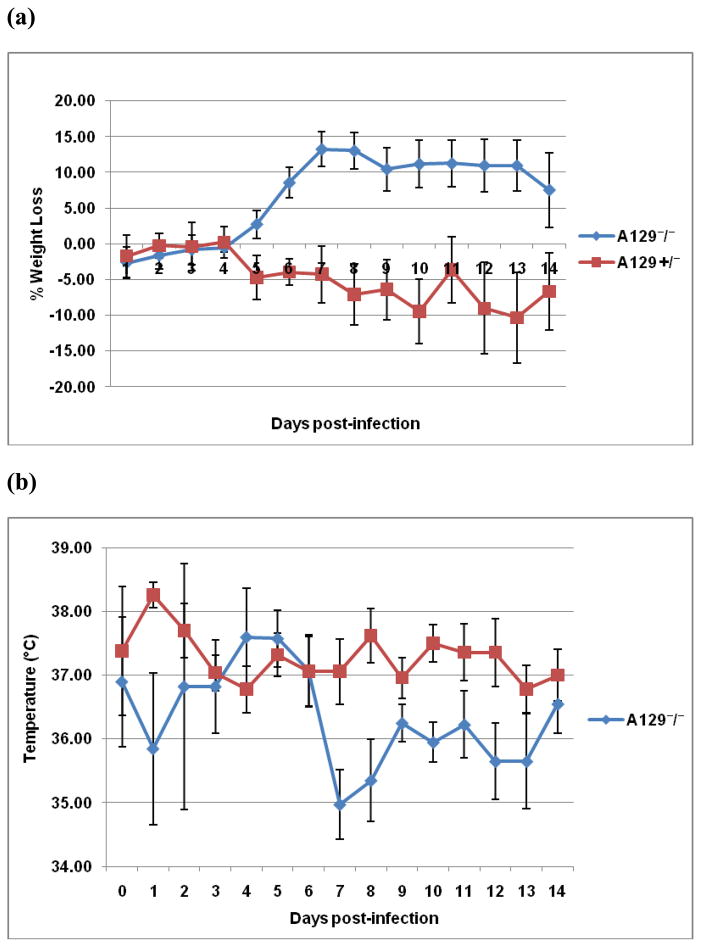
Mice heterozygous for the interferon deletion (IFN-α/β−/+) are partially deficient in interferon responses and demonstrate clinical signs when infected with the wild type CHIKV-LR [17]. They develop human-like disease that is milder than that observed in A129 homozygote mice (IFN-α/β−/−) [17]. Although ID injection of A129 (IFN-α/β−/−) or A129 (IFN-α/β−/+) mice with 104 PFU CHIK 181/25 virus did not induce any mortality, the homozygotes showed significant weight loss (p<0.01) beginning on day 5 post-infection until the end of the monitoring period, day 14. In contrast, the heterozygote mice maintained stable weights after exposure to CHIK 181/25 (Fig. 4a). In addition, the temperature of IFN-α/β−/− mice dropped by day 7 post-infection as compared to the IFN-α/β−/+ mice (Fig. 4b). IFN-α/β−/+ mice had no detectable viremia 1-4 days post-infection, whereas viremia was detectable in IFN-α/β−− mice with titers ranging from 1.27 ± 0.27, 1.81 ± 0.31, 2.90 ± 0.32 and 2.59 ± 0.20 log10 PFUeq/ml on days 1, 2, 3 and 4 post-infection, respectively. All but one A129 heterozygote mice seroconverted following a single injection with CHIK 181/25 vaccine. Measured neutralizing antibody titers were 80, 40, 10, <10, 10 and 20, 20, 10, 10, <10 three and five weeks post-primary inoculation, respectively for each mouse.
Figure 4.
Morbidity [weight loss (a) and temperature change (b)] in A129 IFN-α/β−/+ heterozygote and IFN-α/β−/− homozygote adult A129 mice (n=5) injected ID with 104 PFU of CHIK 181/25 vaccine. All mice were monitored daily for a period of two weeks. Each data point represents the arithmetic mean ± standard deviation.
3.2. Proinflammatory cytokine responses following infection of A129 and AG129 mice with CHIK 181/25 vaccine
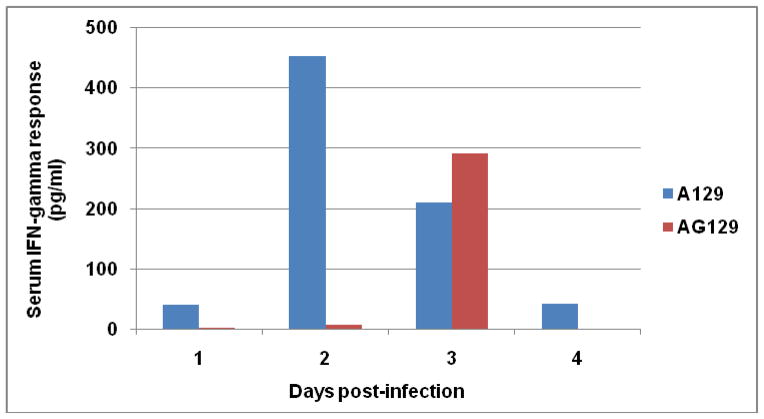
Since IFN-γ appears to have an antiviral effect in A129 mice we measured its kinetics in the serum following injection with CHIK 181/25 vaccine. As expected and shown in Figure 5, the IFN-γ response had an earlier onset and was more elevated in A129 mice (peak response on day 2) as compared to the response in AG129 mice (detectable only on day 3). Serum levels of IL-6 and TNF-α were low in both mouse strains on day 3 post-infection. The IL-6 concentrations were 16.2 and 25.2 pg/ml and TNF-α were 13.7 and 15.9 pg/ml in A129 and AG129 mice, respectively. In contrast, IL-1α and IL-1β responses were elevated by day 3. Peak concentrations for IL-1α were 354.8 and 8 pg/ml, and for IL-1β were 150.9 and 756.9 pg/ml for A129 and AG129 mice, respectively. IL-12 was detectable only in A129 mice with levels ranging from 156.5, 129.8, 305.6 and 22 pg/ml on days 1, 2, 3, and 4 post-infection, respectively.
Figure 5.
Kinetics of IFN-γ response in the serum of A129 and AG129 mice (n=5) following injection with 105 PFU CHIK 181/25. The IFN-γ levels were determined using the Gentel microarray system in pooled sera collected from each sampling day post-infection.
3.3. Control of CHIK 181/25 vaccine virus in AG129 mice by antibodies
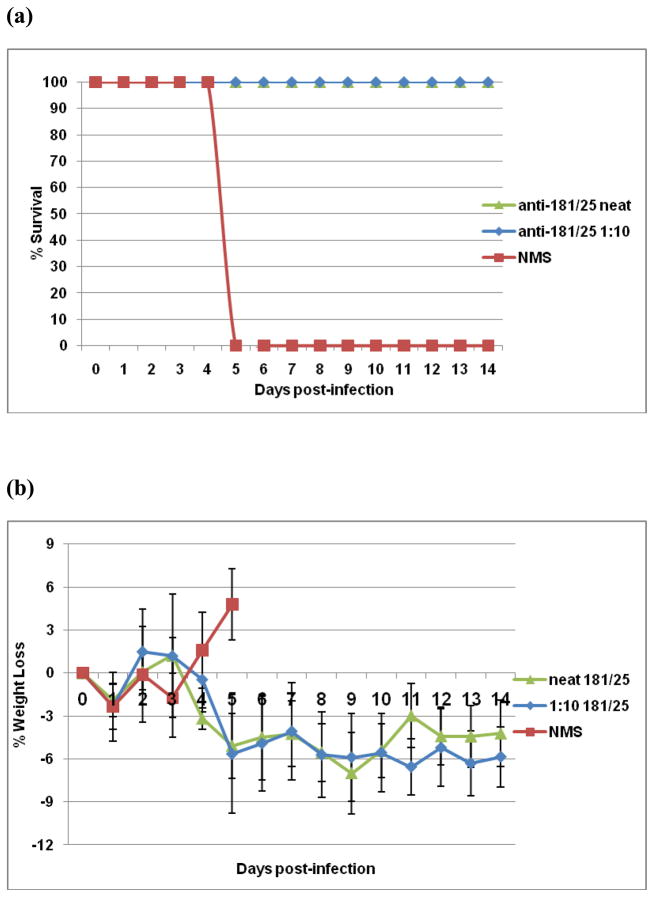
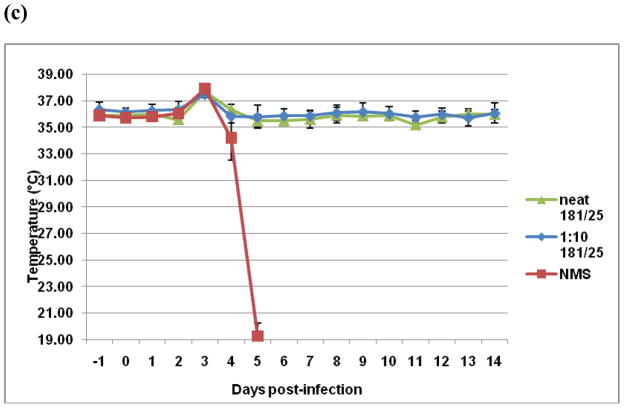
Antibodies are believed to be the primary effectors of protection against alphavirus disease [18], including CHIK [19]. Therefore, we sought to determine if anti-CHIK 181/25 vaccine antibodies can protect against fatal CHIK 181/25 virus infection independently of a functional IFN response. As shown in Figure 6a, passively transferred neat (pre-transfer neutralizing titer of 50) or 1:10 anti-CHIK 181/25 serum conferred full protection from challenge with 104 PFU CHIK 181/25 virus. Protected mice had no weight loss (Fig. 6b) nor any significant temperature change (Fig. 6c). In contrast, control animals injected with neat normal mouse serum succumbed to infection by day 5 post-challenge (Fig. 6a).
Figure 6.
Passively transferred CHIK 181/25 immune serum protects AG129 mice from infection with 104 PFU CHIK 181/25 virus. Pooled serum samples raised in A129 mice after ID injection with 104 PFU CHIK 181/25 vaccine were passively transferred IP (undiluted or diluted 1:10 in 150μl volume) in groups of 5 naïve adult AG129 mice. One day later all mice were injected ID with 104 PFU of CHIK 181/25 virus. Control mice received IP normal mouse serum (NMS). Following infection mice were monitored daily for survival (a) and morbidity (b and c).
3.4. Protective efficacy of CHIK 181/25 vaccine in A129 mice
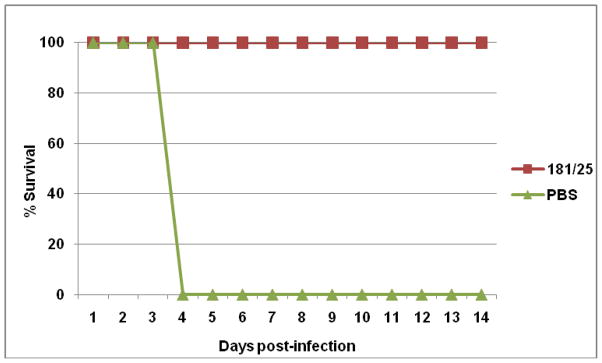
To test the efficacy of CHIK 181/25 vaccine, immunized A129 homozygote mice (IFN-α/β−/−) were challenged with 100 PFU of wt CHIKV-LR. Prior to challenge all mice were bled and seroconversion was measured by PRNT. A single injection of CHIK 181/25 vaccine elicited a strong neutralizing antibody response in all A129 mice with titers ≥160 measured five weeks post-primary inoculation. Following challenge with 100 PFU wt CHIKV-LR, temperatures and body weights of vaccinated mice remained relatively stable (data not shown) and all animals survived (Fig. 7). In contrast, PBS immunized control mice succumbed to infection by day 4 post-challenge.
Figure 7.
Protection of CHIK 181/25 immunized A129 homozygote mice following challenge with wt CHIKV-LR. Mice were injected ID with 104 PFU CHIK 181/25 vaccine (n=4) or PBS (n=5) and challenged ID with 100 PFU CHIKV-LR 38 days post-primary inoculation.
To assess tissue damage after challenge, a histopathologic analysis was conducted in both PBS immunized and CHIK 181/25 vaccinated groups of mice after challenge with CHIKV-LR. The organs from the PBS immunized group were harvested on day 4 post-challenge and the organs from CHIK 181/25 vaccinated mice were harvested at day 14 post-challenge (end of the monitoring period). There was no significant histopathologic change in the brain, lung, heart, small bowel, large bowel, pancreas, skeletal muscle, kidney or bone after CHIKV-LR challenge in the PBS immunized and CHIK 181/25 vaccinated groups (data not shown). However, there was a marked difference in the spleens of these two groups of mice (Fig. 8). The spleens of the CHIK 181/25 vaccinated groups demonstrate no significant histological change with a normal follicular pattern and architecture after CHIKV-LR challenge (Fig. 8c). In contrast, CHIKV-LR infection caused severe necrosis with a paucity of lymphocytes in the spleens of the PBS immunized control group (Fig. 8a & b). In these mice succumbing to viral challenge, there were no intact splenic lymphoid follicles and splenic architecture was disrupted. The viable splenic cells consisted of collections of monocytes with abundant eosinophilic cytoplasm along with occasional plasma cells. The liver of the PBS control group demonstrated severe congestion (data not shown) which could be due to the virus replication in the liver as demonstrated by Courdec et al [17] or due to splenic necrosis and a congestion of blood in the portal circulation.
Figure 8.
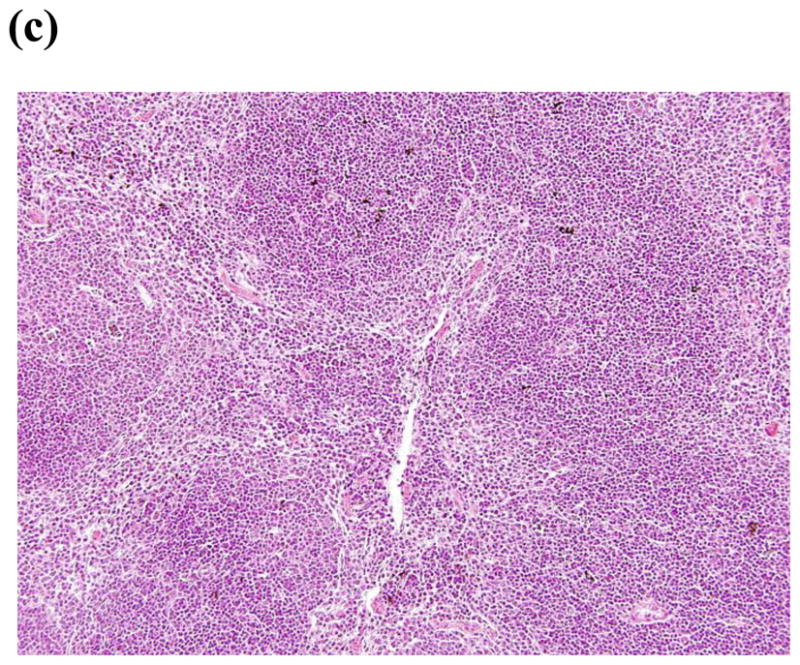
Spleens of control mice (sham vaccinated 20 X) (a) demonstrate necrosis and disruption of follicular architecture. The remaining cells are composed mainly of monocytes (arrows) (b) (40X). The spleens of 181/25 (20 X) (c) demonstrate a normal follicular architecture with appropriate amounts of white pulp and red pulp.
We studied the ability of a single dose of CHIK 181/25 vaccine to provide protection from wt CHIKV-LR challenge in A129 homozygous mice. Mice immunized with 103 or 104 PFU of CHIK 181/25 vaccine were fully protected 247 days after vaccination as compared to age-matched control mice immunized with PBS that succumbed to infection 4-6 days after challenge with CHIKV-LR. None of the protected mice manifested any sign of morbidity. In addition, serum neutralizing antibody responses were detectable in both immunized groups just prior to challenge (day 247) with mean titers of 88 ± 43.8 to 112 ± 65.7 for animals vaccinated with 103 and 104 PFU of CHIK 181/25 vaccine, respectively.
To examine the role of anti-CHIK 181/25 virus antibodies in protection against wt CHIKV-LR challenge, A129 homozygote mice were passively immunized with a pool of serum collected three weeks post-primary immunization with 104 PFU of CHIK 181/25 vaccine (neutralizing titers of individual sera prior to pooling were 160, 160, 80 and >640). Passively transferred immune serum conferred full protection against infection by 100 PFU wt CHIKV-LR, whereas all animals that received normal mouse serum succumbed to infection (data not shown).
4. Discussion
In these studies, we probed the attenuation, immunogenicity and efficacy profile of the chikungunya vaccine candidate, CHIK 181/25 in adult mice deficient in IFN-α/β and/or IFN-γ receptor signaling. In the absence of a functional IFN-α/β response, A129 mice developed only transient morbidity after administration of the CHIK 181/25 vaccine and then fully recovered. In contrast, wt CHIKV-LR administration to A129 mice lead to rapid mortality (Fig. 7). Therefore, our findings highlight the attenuated nature of the CHIK 181/25 vaccine. Surprisingly, the duration of morbidity was inversly proportional to the vaccine dose. It is possible that the higher vaccine doses induce a more potent innate immune response resulting in a quicker recovery.
The importance of IFN-α/β in controlling infection by the CHIK 181/25 virus is supported by two lines of evidence. First, G129 mice that have a functional IFN-α/β receptor showed no mortality nor morbidity after CHIK 181/25 vaccination. Secondly, A129 heterozygote mice also show no morbidity after CHIK 181/25 virus infection demonstrating that a single copy of the IFN-α/β receptor is capable of further inhibiting CHIK 181/25 virus replication and pathogenesis. These data are consistent with the findings by Couderc et al [17] highlighting the dependence of wt CHIKV infection in A129 mice on the strength of IFN-α/β receptor signaling pathway. IFN-α/β provides the first line of defense by inducing an anti-viral state that controls viral replication and modulates the adaptive immune response [20]. In the case of CHIKV, IFN-α/β controls early virus replication via its action on nonhematopoietic cells [21]. After secretion from the infected cells IFN-α/β binds to its INFAR receptor in an autocrine or paracrine manner to amplify the signal or prime uninfected cells to establish an antiviral state. However, in primate cell lines once viral replication has been established further CHIKV replication becomes resistant to inhibition by IFNs. This is due to the suppressive effect exerted by the CHIKV nonstructural protein 2 which inhibits IFN-induced JAK-STAT signaling and therefore prevents IFN–induced stimulating genes (ISGs) expression [22]. More recently, it was shown that CHIKV envades innate immune response to infection by widespread shut off of cellular protein synthesis and a targeted block of late synthesis of antiviral mRNA transcripts [23].
In contrast, AG129 mice that lack both functional IFN-α/β and/IFN-γ receptors rapidly succumbed to CHIK 181/25 virus infection. Thus, in the absence of IFN-α/β, IFN-γ is able to partially control replication of an attenuated CHIKV. These data suggest that although the IFN-γ response is subordinate to IFN-α/β in terms of antiviral activity, it contributes to the control of viral replication and spread. The pattern of cytokine expressed after infection in these mice is consistent with this hypothesis. In A129 mice, the IFN-γ response was stronger and had an earlier onset relative to the AG129 mice following CHIK 181/25 vaccination. In addition, IL-12 was detectable in A129 mice but not in AG129 mice. IFN-α/β normally regulates the induction of IL-12 and IFN-γ during a viral infection [24]. However, our findings suggest that the induction of both of these cytokines occurs in the absence of IFN-α/β.
In these studies, levels of proinflammatory cytokines TNF-α and IL-6, which are typically induced in response to an inflammatory stimulus, were low. This is in contrast to the proinflammatory cytokine responses observed following infection of A129 mice by other alphaviruses [25, 26, 27]. Levels of IL-1β were elevated in AG129 mice as compared to the A129 mice. Interestingly, high levels of IL-1β have similarly been observed in humans and associated with disease severity [28]. This cytokine is known to be involved in the immunopathogenesis of many arthritic pathologies [29] but also could participate in viral control [6, 30]. The A129 model with defective IFN-α/β signaling, may result in abnormalities in the cytokine patterns observed after infection. However, since CHIKV has evolved to blunt the human IFN-α/β signaling cascade [22, 23] or mobilize the apoptotic machinery to invade host cell defenses [31] these findings may still be reflective of viral pathogenesis in humans.
We evaluated the efficacy of passively transferred anti-CHIK 181/25 antibodies in AG129 mice that have both IFN-type responses compromised and are susceptible to CHIK 181/25 virus. Passive transfer of anti-CHIK 181/25 antibodies abrogated lethality associated with the CHIK 181/25 virus. These data are consistent with earlier findings demonstrating antibody-mediated protection of IFN-deficient mice against Sindbis virus infection [32]. The protective effect of passively transferred serum could be attributed to its capacity to directly neutralize CHIKV and/or its capacity to induce other protective immune responses such as antibody-dependent and complement–mediated cellular cytotoxicity. Our findings are in agreement with data demonstrating the protective effect of passively transferred low titer anti-Ross River Virus (RRV) antibodies against RRV challenge [33] and suggest that low levels of antibody responses might be sufficient in mediating effective protection.
The CHIK 181/25 vaccine elicited a uniformly robust neutralizing antibody response after a single injection and protected A129 immune mice after challenge with wt CHIKV-LR. This protection was shown to be at least partially mediated by antibodies confirming the important role that humoral immunity plays in controlling CHIKV infection [19]. However, studies with other alphaviruses have demonstrated that cellular immunity can protect mice against fatal disease in the absence of an antibody response [34, 35]. The contribution of cellular immune responses to protection from wt CHIK-LR virus challenge remains to be addressed in future studies. In addition, after CHIKV-LR challenge, there was no overt organ damage in the CHIK 181/25 vaccinated mice. In contrast, the spleen of PBS injected animals showed large areas of necrosis and lacked any lymphoid follicles with only a few scattered lymphocytes. The organ damage and lack of lymphocytes could be a direct consequence of wt CHIKV infection in this model [19] or could be an indirect consequence of cytokine dysregulation and immunopathogenesis.
The CHIK 181/25 vaccine conferred long-term protection against wt CHIKV-LR virus challenge in A129 mice (ca, 8 months after a single intradermal injection). Neutralizing antibody responses were detectable prior to challenge following vaccination with only 103 PFU/mouse. The long-term duration of neutralizing anti-CHIK 181/25 antibody responses is consistent with the kinetics of antibody responses observed in humans that received this vaccine. Follow-up of healthy vaccinated volunteers demonstrated persistence of antibodies with in vitro neutralizing activity after 12 months [15]. Similarly, 18 months after natural infection anti-CHIKV antibodies are still detectable in 40% of patients [36].
These studies highlight the role of IFN-α/β in controlling CHIK 181/25 virus replication and demonstrate the potential of a live, attenuated candidate CHIKV vaccine to elicit neutralizing antibody responses that confer short-and long-term protection against wt CHIKV-LR challenge.
Acknowledgments
We are grateful to Dr Joseph Santangelo for reviewing the manuscript and Joanna Cramer for technical assistance. These studies were partially supported by National Institute of Allergy and Infectious Diseases (NIAID) grant 5R43AI080041-021. JW was supported by an American Recovery and Reinvestment Act award for a summer internship by the NIAID grant 3R43AI080041-01S1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 2.Powers AM. Chikungunya. Clin Lab Med. 2010;30:209–219. doi: 10.1016/j.cll.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Tsetsarkin KA, McGee CE, Volk SM, Vanlandingham DL, Weaver SC, Higgs S. Epistatic roles of E2 glycoprotein mutations in adaptation of chikungunya virus to Ades Albopictus and Ae. Aegyptimosquitoes. PLoS One. 2009;4:e6835. doi: 10.1371/journal.pone.0006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3:e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das T, Jaffar-Bandjee MC, Hoarau JJ, Trotot PK, Denizot M, Lee-Pat-Yuen G, Sahoo R, Guiraud P, Ramful D, Robin S, Alessandri JL, Gauzere BA, Gasque P. Chikungunya fever: CNS infection and pathologies of re-emerging arbovirus. Prog Neurobiol. 2010;91:121–129. doi: 10.1016/j.pneurobio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol. 2010;7:491–500. doi: 10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]
- 7.Reiskind MH, Pesko K, Westbrook CJ, Mores CN. Susceptibility of Frorida mosquitoes to infection with Chikungunya virus. Am J Trop Med Hyg. 2008;78:422–425. [PMC free article] [PubMed] [Google Scholar]
- 8.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, Cordioli P, Fortuna C, Boros S, Magurano F, Silvi G, Angelini P, Dottori M, Ciufolini MG, Majori GC, Cassone A CHIKV study group. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang E, Volkova E, Adams AP, Forrester N, Xiao SY, Frolov I, Weaver SC. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine. 2008;26:5030–5039. doi: 10.1016/j.vaccine.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE, Jr, Lupton HW. Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine. 1986;3:157–162. doi: 10.1016/0264-410x(86)90003-4. [DOI] [PubMed] [Google Scholar]
- 11.Harrison VR, Eckels KH, Bartelloni PJ, Hampton C. Production and evaluation of a formalin-killed Chikungunya vaccine. J Immunol. 1971;107:643–647. [PubMed] [Google Scholar]
- 12.Tiwari M, Parida M, Santhosh SR, Khan M, Dash PK, Rao PV. Assessment of immunogenic potential of Vero adapted formalin inactivated vaccine derived from novel ECSA genotype of Chikungunya virus. Vaccine. 2009;27:2513–2522. doi: 10.1016/j.vaccine.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 13.Muthumani K, Lankaraman KM, Laddy DJ, Sundaram SG, Chung CW, Sako E, Wu L, Khan A, Sardesai N, Kim JJ, Vijayachari P, Weiner DB. Immunogenicity of novel consensus-based DNA vaccines against Chikungunya virus. Vaccine. 2008;26:5128–5134. doi: 10.1016/j.vaccine.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akahata W, Yang Z-Y, Andersen H, Sun S, Holdaway HA, Kong W-P, Lewis MG, Higgs S, Rossmann MG, Rao S, Nabel GJ. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16:334–338. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, Mangiafico JA. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg. 2000;62:681–685. doi: 10.4269/ajtmh.2000.62.681. [DOI] [PubMed] [Google Scholar]
- 16.Wang E, Petrakova O, Adams AP, Aguilar PV, Kang W, Paessler S, Volk SM, Frolov I, Weaver SC. Chimeric Sindbis/eastern equine encephalitis vaccine candidates are highly attenuated and immunogenic in mice. Vaccine. 2007;25:7573–7581. doi: 10.1016/j.vaccine.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couderc T, Chrétien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, Desprès P, Arenzana-Seisdedos F, Michault A, Albert ML, Lecuit M. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008;4:e29. doi: 10.1371/journal.ppat.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin D, Levine B, Tyor W, Ubol S, Despres P. The role of antibody in recovery from alphavirus encephalitis. Immunol Rev. 1997;159:155–161. doi: 10.1111/j.1600-065x.1997.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 19.Couderc T, Khandoudi N, Grandadam M, Visse C, Gangneux N, Bagot S, Prost JF, Lecuit M. Prophylaxis and therapy for Chikungunya virus infection. J Infect Dis. 2009;200:516–523. doi: 10.1086/600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Sastre A, Biron CA. Type I interferons and the virus-host relationship: A lesson in détente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- 21.Schilte C, Couderc T, Chretien F, Sourisseau M, Gangneux N, Guivel-Benhassine F, Kraxner A, Tschopp J, Higgs S, Michault A, Arenzana-Seisdedos F, Colonna M, Peduto L, Schwartz O, Lecuit M, Albert ML. Type I IFN controls chikungunya virus via its action on nonhematopoietic cells. J Exp Med. 2010;207:429–442. doi: 10.1084/jem.20090851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fros JJ, Liu WJ, Prow NA, Geertsema C, Ligtenberg M, Vanlandingham DL, Schnettler E, Vlak JM, Suhrbier A, Khromykh AA, Pijlam GP. Chikungunya virus nonstructural protein 2 inhibits type I/II interferon-stimulated JAK-STAT signaling. J Virol. 2010;84:10877–10887. doi: 10.1128/JVI.00949-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White LK, Sali T, Alvarado D, Gatti E, Pierre P, Streblow D, DeFilipps VR. Chikungunya virus induces IPS-1-dependent innate immune activation and protein kinase R-independent translational shutoff. J Virol. 2011;85:606–620. doi: 10.1128/JVI.00767-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cousens LP, Orange JS, Su HC, Biron CA. Interferon alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc Natl Acad Sci USA. 1997;94:634–639. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryman KD, Klimstra WB, Nguyen KB, Biron CA, Johnston RE. Alpha/Beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J Virol. 2000;74:3366–3378. doi: 10.1128/jvi.74.7.3366-3378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryman KD, Meier KC, Gardner CL, Adegboyega PA, Klimstra WB. Non-pathogenic Sindbis virus causes hemorrhagic fever in the absence of alpha/beta and gamma interferons. Virology. 2007;368:273–285. doi: 10.1016/j.virol.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 27.Ryman KD, Klimstra WB. Host responses to alphavirus infection. Immunol Rev. 2008;225:27–45. doi: 10.1111/j.1600-065X.2008.00670.x. [DOI] [PubMed] [Google Scholar]
- 28.Ng LF, Chow A, Sun YJ, Kwek DJ, Lim PL, Dimatatac F, Ng LC, Ooi EE, Choo KH, Her Z, Kourilsky P, Leo YS. IL-1b, IL-6, and RANTES as biomarkers of chikungunya severity. PLoS One. 2009;4:e4261. doi: 10.1371/journal.pone.0004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinarello CA. Biologic basis for interleukin -1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 30.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin -1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krejbich-Trotot P, Denizot M, Hoarau J-J, Jaffar-Bandjee M-C, Das T, Gasque P. Chikungunya virus mobilizes the apoptotic machinery to invade host cell defenses. FASEB J. 2010;25:1–12. doi: 10.1096/fj.10-164178. [DOI] [PubMed] [Google Scholar]
- 32.Byrnes AP, Durbin JE, Griffin DE. Control of Sindbis virus infection by antibody in interferon-deficient mice. J Virol. 2000;74:3905–3908. doi: 10.1128/jvi.74.8.3905-3908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu S, Aaskov JG. Development of a candidate vaccine against Ross River virus infection. Vaccine. 1994:1118–1124. doi: 10.1016/0264-410x(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 34.Linn ML, Mateo L, Gardner J, Suhrbier A. Alphavirus-specific cytotoxic T lymphocytes recognize a cross-reactive epitope from the capsid protein and can eliminate virus from persistently infected macrophages. J Virol. 1998;72:5146–51513. doi: 10.1128/jvi.72.6.5146-5153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paessler S, Yun NE, Judy BM, Dziuba N, Zacks MA, Grund AH, Frolov I, Campbell GA, Weaver SC, Estes DM. Alpha-beta T cells provide protection against lethal encephalitis in the murine model of VEEV infection. Virology. 2007;367:307–323. doi: 10.1016/j.virol.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiboutot MM, Kannan S, Kawalekar OU, Shedlock DJ, Khan AS, Sarangan G, Srikanth P, Weiner DB, Muthumani K. Chikungunya: A potentially emerging epidemic? PLoS Negl Trop Dis. 2010;4:e623. doi: 10.1371/journal.pntd.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]