Abstract
In the past 10 years, there has been a virtual explosion in the literature concerning the construct of mild cognitive impairment. The interest in this topic demonstrates the increasing emphasis on the identification of the earliest features of cognitive disorders such as Alzheimer’s disease and other dementias. Mild cognitive impairment represents the earliest clinical features of these conditions and, hence, has become a focus of clinical, epidemiological, neuroimaging, biomarker, neuropathological, disease mechanism and clinical trials research. This review summarizes the progress that has been made while also recognizing the challenges that remain.
Search terms: Mild cognitive impairment, Alzheimer’s disease, Imaging, Cognitive decline
During the past decade, a major transition in the clinical characterization of cognitive disorders has taken place.1 Many of the prodromal stages of conditions such as frontotemporal dementia and dementia with Lewy bodies have been recognized and we can now make the clinical diagnosis at an earlier stage in the disease process.2 At the same time, there has been a growing interest in the pre-dementia phase of these conditions because of suggestions that we may be able to identify the earliest clinical features of these illnesses before functional impairment is evident. Toward this end, the construct of mild cognitive impairment (MCI) has evolved to capture this pre-dementia phase of cognitive dysfunction.3, 4
Most investigators believe that if we wait for functional impairment, and perhaps even mild cognitive symptoms, to emerge, it may be too late to treat the underlying disease process.5 Ideally, we would like to be able to prevent or postpone the disease process by intervening early. If a disease-modifying therapy or effective lifestyle intervention were available, one would want to intervene as soon as possible, but these treatments are not on the immediate horizon. As such, the construct of MCI serves a useful purpose as a clinical stage in which meaningful interventions can take place. Mild Cognitive Impairment may be an intermediate step on the way to primary prevention but important for formulating research hypotheses.
HISTORY
Mild cognitive impairment as a term was introduced into the literature in 1988 by Reisberg and colleagues,6 but at that time, it was intended to refer to stage 3 of the Global Deterioration Scale (GDS). In a similar vein, the Clinical Dementia Rating (CDR) scale has gained popularity as an instrument for characterizing either mild impairment or very early dementia, and both instruments have been catalysts at stimulating research on early impairment.7 As the field has advanced, however, we have realized that these severity scales do not adequately characterize the subtle differences between MCI and early dementia. Participants with MCI, as currently diagnosed, can be classified as GDS stage 2 or 3 and as having a CDR of 0 or 0.5.3 Therefore, a finer grain of diagnostic acumen was necessary to distinguish these prodromal conditions from the dementia stage beyond the granularity of the GDS and CDR instruments.
In a 1999 article published in the Archives of Neurology, a group of investigators from the Mayo Clinic described their experience with participants with MCI in a community cohort and put forth diagnostic criteria outlined in Table 1.3 These criteria have been the subject of a great deal of study, validation and criticism.8 That said, there has been an explosion of interest in the literature as characterized in Figure 1.
Table 1.
Original 1999 Mild Cognitive Impairment Criteriaa
| Criterion |
|---|
|
Based on information from Petersen et al.3
Figure 1.
Number of publications with “mild cognitive impairment” in the title or abstract from 1990 through 2008.
CRITERIA
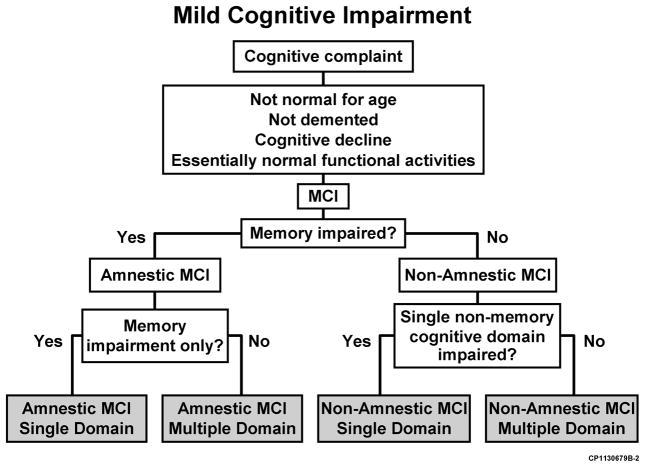
The 1999 Archives article focused on MCI as a prodromal condition for Alzheimer’s disease (AD) and, as such, emphasized the importance of memory impairment for incipient AD. Subsequently, other investigators appropriately noted that not all forms of MCI may evolve into AD, and a broader conceptualization was necessary. In 2003, Winblad et al9 convened a conference of international experts on MCI to revise criteria.10 From that conference, new, more expansive criteria for MCI were proposed, and these criteria now form the foundation for the National Institute on Aging-sponsored Alzheimer’s Disease Centers Program Uniform Data Set and the public-private neuroimaging/biomarker consortium, the Alzheimer’s Disease Neuroimaging Initiative (ADNI).11 These criteria depict the clinical phenotypes of amnestic MCI (aMCI) and non-amnestic MCI (naMCI) with the subtypes of single and multiple domain classifications (Figure 2). These clinical phenotypes are then combined with the presumed cause (Figure 3) as the next step in the diagnostic process. This is analogous to a physician diagnosing a particular syndrome and then searching for an etiologic explanation for the syndrome. This combination leads to the diagnostic impression of a possible outcome of the clinical entity of MCI. With this theoretical framework, many studies have been conducted to investigate the utility and prognostic outcome of the diagnoses.5
Figure 2.
Current flowchart for the diagnosis of mild cognitive impairment (MCI) and its subtypes.10 Reprinted with permission from Blackwell Publishing.
Figure 3.
Presumed outcome of the subtypes of mild cognitive impairment (MCI) when combined with the presumed pathogenesis. Adapted from: Petersen.4 Reprinted with permission from Oxford University Press, Inc. AD indicates Alzheimer disease; depr, depression; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; NA, not applicable; VaD, vascular dementia.
EPIDEMIOLOGY
Numerous investigations worldwide have used these criteria as an infrastructure for estimating the frequency of MCI and its subtypes.12 13–15 Some studies have retrospectively applied MCI criteria to previously acquired data sets and have provided important insights.13, 16 However, the most informative studies have been conducted prospectively to incorporate MCI criteria at the outset.12, 17. These studies have captured the subtleties of the diagnosis in a prospective fashion and, consequently, are better able to address the clinical characterization and mild features of the construct.
The Mayo Clinic Study of Aging was designed as a population-based study in Olmsted County, Minnesota, involving a random sample of nearly 3,000 participants aged 70 through 89 years who were non-demented and cognitively normal or who had MCI at entry.18 The prevalence of MCI from this study is estimated at approximately 15% of the nondemented population with a 2:1 ratio of aMCI to naMCI. The most common putative cause is degenerative, and this predominates to a greater extent for aMCI than for naMCI.
Other international studies have also addressed this issue and are summarized in Table 2.12, 17, 19–27 Although these studies incorporate a variety of tools to fulfill the diagnostic criteria for MCI, yielding some variability, there is a coalescence of prevalence rates from around the world.12, 17 In general, the rates appear to converge in the 14 to 18% range for individuals aged 70 years and older.
Table 2.
Prevalence Studies
| Source | Study Location | No. of Participants | Participant Age Range | Prevalence of MCI, (%) |
|---|---|---|---|---|
| Unverzagt et al,19 2001 | Indianapolis, IN | 2212 | ≥65 | 23.4 |
| Hanninen et al,20 2002 | Finland | 806 | 60–76 | 5.3 |
| Lopez et al,17 2003 | CHS | 1690 | ≥75 | 22 |
| Ganguli et al,13 2004 | MoVIES | 1248 | ≥65 | 3.2 |
| Busse et al,12 2006 | Leipzig, Germany | 980 | 75–79 | 19.3 |
| Das et al,22 2007 | India | 745 | ≥50 | 14.9 |
| Di Carlo et al,23 2007 | Italy | 2830 | 65–84 | 16.1 |
| Fischer et al,24 2007 | Austria | 697 | 75 | 24.3 |
| Manly et al,25 2008 | Manhattan, NY | 2364 | ≥65 | 21.8 |
| Palmer et al, 21 2008 | Kungsholmen, Stockholm, Sweden | 379 | 75–95 | 11.1 |
| Plassman et al,26 2008 | ADAMS | 856 | ≥71 | 22.2 |
| Roberts et al,272008 | Rochester, MN | 1969 | 70–89 | 14.8 |
Abbreviations: ADAMS, Aging, Demographics and Memory Study; MCI, mild cognitive impairment; MoVIES, Monongahela Valley Independent Elders Survey; CHS, Cardiovascular Health Study.
OUTCOMES
The next issue that arises regarding MCI pertains to the participants’ outcomes following a diagnosis. A major factor in determining outcome depends on the source of participants being studied. In general, it appears that participants from referral sources, such as memory disorders clinics or AD centers, likely have a progression rate to dementia, particularly AD, of 10% to 15% per year.28 This is likely also true for some of the clinical trials on MCI, such as those designed incorporating the protocols used by the Alzheimer’s Disease Cooperative Study and the ADNI.29 However, if one addresses a population from an epidemiologic perspective whereby participants are prospectively approached about participation, the progression rates are likely lower (in the 6% to 10% per year range) (Table 3).12, 24, 28, 30–32 This reflects several factors: One concerns the prior probability of having an underlying disorder such as MCI when a participant seeks treatment at a referral clinic. In the referral clinic setting, this probability is reasonably high, and hence the higher annual rate of progression to dementia in this setting (10%–15%). However, in epidemiologic studies, there is a broader spectrum of MCI severity, more heterogeneity as to the underlying condition and, likely, lower annual rates of progression (6–10%). It is noteworthy that, in both settings, the referral clinic and the epidemiologic context, the rates are greatly elevated over the base incidence rates of dementia and AD of 1% to 2% per year.3
Table 3.
Rates of Progression
| Source | Study Location | No. of Participants | Participant Age Range | Reported Rate of Progression | Annual^ Progression Rate, % |
|---|---|---|---|---|---|
| Solfrizzi et al,30 2004 | Italy | 1524 | ≥65 | 3.8/100 person-years | 3.8 |
| Busse et al,12 2006 | Leipzig, Germany | 863 | ≥75 | 44% per 4.3 y | 10.2 |
| Tschanz et al,31 2006 | Cache County, Utah | 3266 | ≥65 | 46% per 3 y | 15.3 |
| Fischer et al,24 2007 | Austria | 476 | 75–76 | 33.9% per 30 mo | 13.6 |
| Ravaglia et al,32 2008 | Italy | 937 | ≥65 | 14% | 14 |
| Farias et al,28 2009 | California | 111 | >60 | 3%* per 1 y | 3* |
| Petersen et al, 2009 (unpublished data+) | Rochester, MN | 1969 | 70–89 | 7.5% per 1 y | 7.5 |
Reported or crude rate estimated from data
Progression rate for clinic cohort reported as 13% per 1 yr
Petersen RC, Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Rocca WA. Preliminary estimates of MCI progression to dementia from 2004–2009. Unpublished data. 2009.
PREDICTORS
Among those individuals who exhibit an elevated rate of progression from MCI to AD, can we predict who will progress more rapidly? A great deal of research on this issue has been generated in the past decade. Table 4 lists the most common factors related to rate of progression.
Table 4.
Factors Influencing Rates of Progression
| Predictor of Progression |
|---|
|
Abbreviations: ApoE, apolipoprotein E; CSF, cerebrospinal fluid; FDG PET, fludeoxyglucose F 18-positron emission tomography; MRI, magnetic resonance imaging.
SEVERITY OF COGNITIVE IMPAIRMENT
As would be expected, those who are more impaired in the clinical spectrum, by virtue of degree of memory impairment or other cognitive deficits, are more likely to progress more rapidly.8 This factor has been demonstrated in several clinical studies and likely reflects the underlying extent of pathological involvement.
GENETIC CONSIDERATIONS
In 1995, the initial report of the effect of apolipoprotein E ε4 carriers progressing more rapidly from MCI to dementia was published in JAMA, and, since that time, there have been numerous replications.33, 34 This finding is particularly relevant for AD because apolipoprotein E ε4 carrier status is a major genetic risk predictor of late onset AD.35
MAGNETIC RESONANCE IMAGING
Quantitative magnetic resonance imaging (MRI) has perhaps been the most intensively studied imaging and biomarker entity in the context of MCI.36 Jack and colleagues at the Mayo Clinic37 have published the initial and many of the subsequent studies in this area. Following this lead, the National Institute on Aging sponsored ADNI was designed to assess the utility of neuroimaging and chemical biomarkers in predicting prodromal AD and characterizing features that likely predict progression.11
In general, structural MRI has been shown to predict progression from MCI to AD such that volumetric measurements of the hippocampal formation, entorhinal cortex, whole brain and ventricular volumes are commonly used in clinical studies.38 This precision has been translated into proposed design of clinical trials to reduce the sample size of the treatment groups for proposed therapies.39 As such, the sample size for therapeutic interventions can be greatly reduced using volumetric indices gained from MRI as a stratifying variable.
In addition to structural MRI, other measures such as magnetic resonance spectroscopy, diffusion tensor imaging, and arterial spin labeling have been useful in differentiating among those participants with MCI and AD and those who are cognitively normal, and may also be useful in predicting progression to dementia and AD.40 The breadth of useful measures involving MRI is impressive and clearly represents an advance in the field during the past decade.
FLUDEOXYGLUCOSE F 18-POSITRON EMISSION TOMOGRAPHY
A functional imaging modality that has been studied to a lesser extent than MRI includes the use of 18FDG PET (fludeoxyglucose F 18-positron emission tomography) scans. The available data suggest that many participants with the clinical syndrome of MCI exhibit the “AD-pattern” of hypometabolism in the temporoparietal regions and this predicts progression to clinical AD.41 This imaging modality likely detects an aspect of neurodegeneration that perhaps reflect the loss of synaptic integrity and provides dynamic information on progression.
CEREBROSPINAL FLUID
One of the more active areas of biomarker prediction of progression has arisen in the area of cerebrospinal fluid (CSF) biomarkers42 such as Amyloid-β 1 to 42 peptide (Aβ 1–42), total tau, and tau phosphorylated at the threonine 181. An influential article in 2006 by Hansson and colleagues43 highlighted the finding that among those participants with MCI who possess the profile of AD, low Aβ1–42 and elevated total tau or phosphorylated tau or the ratio of Aβ1–42 to tau is predictive of progression from MCI to AD. A recent report of a group of 12 research centers from Europe involving a large group of participants with MCI essentially replicated the findings from Hansson et al.44 A similar study from the ADNI recently reported the utility of CSF biomarkers in predicting progression, so there is a wealth of data now converging that CSF may provide useful information in predicting progression from MCI to AD 45 Because, in these studies, most of the participants have MCI of moderate severity, the real utility of these indices will reside in their ability to be accurate predictors in participants with less severe manifestations of disease.
MOLECULAR IMAGING
A recent advancement in imaging to shed light on this issue involves the use of molecular imaging of amyloid.46 The initial agent to address this issue was the carbon 11 known as Pittsburgh Compound B; this tracer has been the most widely studied in the world. But, since its introduction, several 18F compounds have been proposed, and some of these agents are likely to become commercial products; and, as such, new data are emerging. Early data from the University of Pittsburgh indicate that amyloid imaging may be important in selecting a subset of participants who are more likely to progress rapidly, and perhaps differentiating among those participants with aMCI and naMCI who might be appropriate for anti-amyloid therapies.47
COMBINATION OF MARKERS
In the final analysis, it is likely that the best prediction model will involve a combination of neuroimaging and chemical biomarker measures. Several recent studies have suggested that, depending on the stage in the clinical spectrum, certain neuroimaging and biomarker measures or their combinations may be quite informative.48 As is shown in Figure 4, it is possible that perhaps a deposition of amyloid is the initial event, characterized by a low CSF Aβ 1–42 level or a positive amyloid imaging scan, followed by measures of degeneration, such as seen on FDG PET or by CSF levels of total tau or phosphorylated tau, or, as the preponderance of evidence suggests, as characterized by structural, changes on MRIs.49
Figure 4.
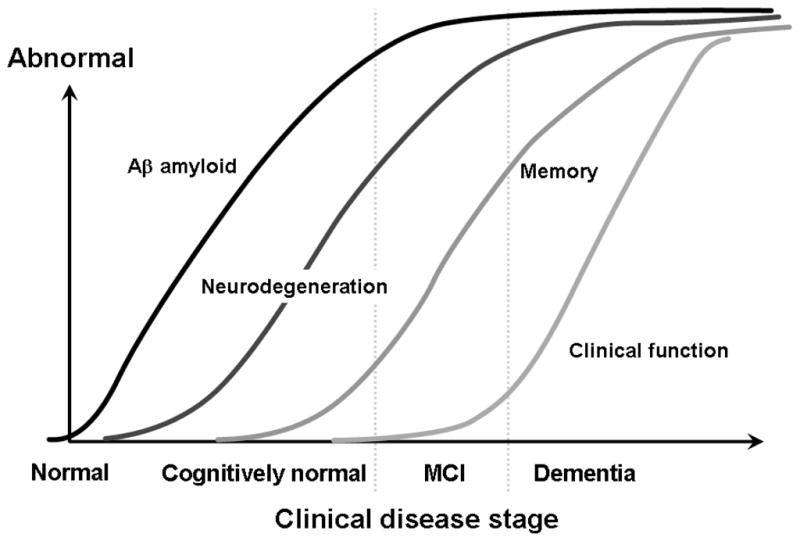
Hypothetical temporal ordering of neuropathological processes in the course of Alzheimer disease and corresponding imaging in biomarker measures. Aβ indicates amyloid β; CSF, cerebrospinal fluid; FDG PET, fludeoxyglucose F 18 positron emission tomography; MCI, mild cognitive impairment; MRI, magnetic resonance imaging.
Then, as Figure 4 indicates, clinical changes become manifest typically as a change in memory function for early AD followed by other cognitive changes and eventually functional impairment. As such, certain neuroimaging and biomarker measures may be differentially sensitive and informative at different stages in the underlying progression of the diseases. No single measure will be uniformly predictive throughout the entire disease process. Toward that end, Jack and colleagues50 have proposed that amyloid deposition as depicted on amyloid imaging may set the stage for subsequent cognitive decline.
NEUROPATHOLOGICAL ANALYSIS
Relatively few neuropathology studies have been completed on participants during the MCI stage of the illness. Some investigators contend that this stage of MCI is, in fact, AD but also indicate that these participants may be more clinically advanced than others in the literature.51, 52 The Religious Orders Study has followed up a group of nuns and priests for many years and has an excellent autopsy rate. In general, they have found that approximately 60% of the participants with MCI have neuropathological evidence of AD, but indicate that vascular disease also accounts for a significant degree of the neuropathological features.53 Other studies have highlighted the importance of neurofibrillary tangle density in accounting for the symptoms of MCI.54
Two studies from the Mayo Clinic published in the Archives of Neurology shed additional light on these participants.55, 56 One study evaluated participants who died while their clinical classification was MCI and found that most had a low probability of having the neuropathological features of AD at that point in time.55 However, it appeared as if the participants were in transition to greater degrees of pathological involvement. A second study observed participants who had been previously diagnosed with MCI and progressed to dementia and characterized these participants as having the ultimate pathological characteristics.56 This study indicated that, while most of the participants with aMCI developed AD, a considerable proportion, 20% to 30%, developed another type of dementing disorder, indicating that, while the clinical criteria for aMCI likely predict AD, they are not absolutely specific.
CLINICAL TRIALS
In the past 10 years, there have been numerous clinical trials on aMCI, testing most of the current therapies available for AD.29, 57, 58 All of the acetylcholinesterase inhibitors have been evaluated, and with one partial exception, results of all these analyses were negative (Table 5).29, 57–60 These trials, cumulatively, have involved between 4,000 and 5,000 participants. One trial with rivastigmine, two with galantamine, and one with rofecoxib failed to achieve the anticipated rates of progression from MCI to AD and, consequently, had to be extended, resulting in a lack of power.57–59 The rivastigmine trial was conducted in multiple countries with multiple languages and likely recruited a heterogeneous group of participants with very mild disease.57 Hence, the rate of progression was lower.
Table 5.
Clinical Trialsa
| Source | Study Sponsor | Duration | End Point | Medication |
|---|---|---|---|---|
| Petersen et al,29 2005 | Alzheimer’s Disease Cooperative Study | 3 yr | AD | Vitamin E donepezil |
| Thal et al,58 2005 | Merck | 3–4 yr | AD | Rofecoxib |
| Feldman et al,57 2007 | Novartis | 4 yr | AD | Rivastigmine |
| Winblad et al,59 2008 | Johnson & Johnson | 2 yr | CDR 1 | Galantamine |
| Doody et al,60 2009 | Pfizer | 48 wk | AD | Donepezil |
Abbreviations: AD, Alzheimer disease; CDR 1, Clinical Dementia Rating of 1.
All trials used the clinical diagnosis of AD as an end point.
Two trials involving galantamine used mild entry criteria and required a more advanced degree of “conversion” of CDR 1 rather than the clinical diagnosis of AD as an end point.59 Hence, this criterion may have inadvertently required participants to remain in the MCI stage for a longer period, resulting in a lower rate of progression than anticipated. However, this trial almost achieved its anticipated rate and had a suggestion of a therapeutic response.59
The rofecoxib trial initially required a more stringent degree of memory impairment and had to loosen the inclusion criteria to recruit enough participants. This may have contributed to its low rate of progression, necessitating an extension of the trial.58 Consequently, for a variety of reasons, all of these trials did not meet their anticipated therapeutic goals.
The Alzheimer’s Disease Cooperative Study conducted a therapeutic trial on participants with aMCI to test high-dose vitamin E and donepezil and achieved its anticipated progression rate of 16% per year.29 It is interesting to note that virtually the same recruitment techniques were used in the ADNI, and this study has achieved a virtually identical progression rate of 16% per year.11 The Alzheimer Disease Coopertive Study suggested a therapeutic effect of donepezil for the first 12 months in all participants with MCI and up to 24 months for the Apolipoprotein E ε4 carriers. However, because the study was designed to assess effects over 36 months, the ultimate outcome was negative.29 A subsequent 48-week trial of donepezil alone failed to replicate the Alzheimer Disease Cooperative Study results, and, hence, no treatments have been approved for MCI.60
IMPLICATIONS
From a clinical trials perspective, if one were designing a disease-modifying therapeutic trial involving participants at the MCI stage, one could consider aMCI criteria of a degenerative pathogenesis and require positive imaging and biomarker data to enrich the clinical population to enhance the likelihood of progression to clinical AD. This could very well be consistent with the presumed therapeutic target of the agent. That is, if one were testing an amyloid-specific agent, one could use aMCI clinical criteria and stratify participants according to their apolipoprotein E ε4 carrier status, amyloid imaging, or markers of CSF involving amyloid to create a subset of participants who are more likely to progress rapidly and harbor the underlying amyloid pathological substrate.
CHALLENGES
While the construct of MCI has engendered a great deal of attention (Figure 1), it has also raised a great deal of controversy. Much of the concern about the construct pertains to its heterogeneity, lack of specific ability to predict outcome, and vagueness of the criteria, e.g., the degree of cognitive impairment in non-memory cognitive domains and the degree of functional impairment. Table 6 depicts many of the sources of variability in studies on MCI. A few deserve mention.
Table 6.
Sources of Variability in MCI Studies
| Source of Variability |
|---|
|
Abbreviation: MCI, mild cognitive impairment.
The source of participants is a prominent aspect of variability in many of these studies. As mentioned earlier, participants from a referral clinic, memory disorders clinic, or an AD center likely have a higher prior probability of having AD at the outset. On the contrary, participants who are recruited proactively through an epidemiological procedure are likely more heterogeneous and have multiple medical comorbidities and are “less pure” from an AD substrate perspective. These participants would cause more “noise” in the system if used in clinical trials but likely represent the reality of MCI in the population.
Several early studies retrospectively applied MCI criteria to previously collected data sets.16 This approach necessitates the use of an algorithmic model to retrofit previously acquired neuropsychological data.
An issue inherent in the discussion of neuropsychological test scores pertains to the use of normative data on neuropsychological instruments and cutoff scores. It is important to emphasize that MCI is not just a neuropsychological entity. Although findings from neuropsychological testing constitute a cornerstone of the objective assessment, the ultimate diagnosis involves more than just a set of cognitive test scores.
Two other sources of variability merit discussion. In longitudinal studies, the blinding of the investigators to the previous clinical diagnoses is important. That is, if the investigators know that the participants were previously classified as having MCI, they would be less likely to label them as being cognitively normal on a subsequent visit. This is not necessarily inappropriate since it may reflect the natural variability of the clinical course of these participants and may actually consider this in the diagnostic process. However, in a research setting, it can confound the interpretation of the data. Hence, many studies render the clinical investigators blind to previous clinical classifications. These types of studies lead to higher degrees of “reversion” to normal.
Finally, we need to address the issues of MCI as a clinical or pathological entity and the constructs of sensitivity and specificity. That is, when we make the diagnosis of MCI, does this refer to the clinical state of the patient or the underlying pathophysiological features, leading to the symptoms, or both? It might be most productive to keep these entities separate.
CLINICAL IMPLICATIONS
These studies, coupled with the neuropathological findings, suggest that the clinical criteria for aMCI may designate a mildly impaired set of participants, many of whom, but not all, have the underlying neuropathological features of AD. This has implications for the labeling of the clinical condition of MCI and the design of future trials. It may be inappropriate to label participants at the aMCI stages as having AD, or even incipient or prodromal AD because many will not eventually evolve to AD, and hence, we cannot afford to mislabel all participants with MCI as having AD features because this is the only label that they will perceive. In other words, participants and families will only “hear” the AD part of the label, yet we will be incorrectly labeling some of them because not all will progress to AD. Therefore, it might be preferable to use an etiologically neutral term such as “MCI,” couple that with a suspected pathogenesis through history and ancillary testing, and explain to the participants the possibility that this term may imply development of AD in the future or it might imply stability or, even less commonly, improvement in their clinical symptoms. This approach may be more consistent with the longitudinal data.
In 2001, the American Academy of Neurology published an evidence-based medicine practice parameter on MCI and recommended that physicians should identify and monitor patients with MCI, because these persons had an increased risk of developing dementia.61 At that time this recommendation was based on relatively few longitudinal studies. Now, the literature has expanded greatly, and numerous prospectively designed longitudinal studies are available from which to draw conclusions. As such, the American Academy of Neurology is repeating the practice parameter exercise at present reassessing the clinical utility of MCI. In addition, to assess the clinical acceptance of the construct, a recent survey by the American Academy of Neurology indicated that 80% of neurologists use the term “MCI” and find it useful at describing this type of patient, implying that the construct of MCI is becoming clinically useful and gaining more widespread acceptance.62
SUMMARY
In conclusion, the construct of MCI has influenced the field of aging and dementia in several significant spheres. It has served to focus the attention of investigators on the earlier, prodromal states of many cognitive disorders. Research programs ranging from epidemiological studies to explorations of the mechanisms of disease have been influenced by the construct of MCI, and, hopefully, these investigations will lead to more effective therapies. This work has stimulated discussions concerning new clinical criteria for conditions such as AD and will likely have an effect on the development of international classification systems for cognitive disorders.63
Ultimately, we hope that this work will lead to the development of imaging and biomarkers for the asymptomatic stages of neurodegenerative diseases. That is, by augmenting our knowledge of the role of imaging and measures in the MCI stage, we will be able to validate their utility in predicting the progression to more advanced stages of the disorders and, hence, suggest their further utility by being applied to the asymptomatic stages of the conditions. As such, MCI will have served an important role in advancing our understanding of disease mechanisms with the ultimate goal of finding preventive therapies.
Acknowledgments
The work of the authors is supported by grants P50 AG16574, U01 AG06786, R01 AG11378, and U01 AG24904 from the National Institute on Aging; K01 MH68351 from the National Institute of Mental Health; and by the Robert H. and Clarice Smith and Abigail van Buren Alzheimer’s Disease Research Program.
Contributor Information
Ronald C. Petersen, Email: peter8@mayo.edu.
David S. Knopman, Email: knopman@mayo.edu.
Bradley F. Boeve, Email: bboeve@mayo.edu.
Yonas E. Geda, Email: geda.yonas@mayo.edu.
Robert J. Ivnik, Email: ivnik.robert@mayo.edu.
Glenn E. Smith, Email: smith.glenn@mayo.edu.
Rosebud O. Roberts, Email: roberts.rosebud@mayo.edu.
Clifford R. Jack, Jr., Email: jack.clifford@mayo.edu.
References
- 1.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Archives of Neurology. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 2.Molano J, Boeve B, Ferman T, et al. Mild Cognitive Impairment Associated with Limbic and Neocortical Lewy Body Disease: A Clinicopathological Study. Brain. 2009 doi: 10.1093/brain/awp280. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC. Conceptual Overview. In: Petersen RC, editor. Mild Cognitive Impairment: Aging to Alzheimer’s Disease. New York: Oxford University Press, Inc; 2003. pp. 1–14. [Google Scholar]
- 5.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 6.Reisberg B, Ferris S, de Leon MJ. Stage-Specific Behavioral, Cognitive, and In Vivo Changes in Community Residing Subjects with Age-Associated Memory Impairment and Primary Degenerative Dementia of the Alzheimer Type. Drug Dev Res. 1988;15(2–3):101–114. [Google Scholar]
- 7.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 8.Visser PJ, Scheltens P, Verhey FR, et al. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. Journal of Neurology. 1999;246(6):477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 9.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment - beyond controversies, towards a consensus. Journal of Internal Medicine. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 11.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical Characterization. Neurology. 2009 doi: 10.1212/WNL.0b013e3181cb3e25. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busse A, Guhne U, Angermeyer MC, Riedel-Heller SG. MCI subtypes - course and 6-years outcome: results of the Leipzig longitudinal study of aged (LEILA75+) Neurology. 2006 submitted. [Google Scholar]
- 13.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 14.Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 15.DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurology. 2003;2:15–21. doi: 10.1016/s1474-4422(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 16.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 17.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the cardiovascular health study cognition study. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 18.Roberts RO, Geda YE, Knopman D, et al. The Mayo Clinic Study of Aging: Design and Sampling, Participation, Baseline Measures and Sample Characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unverzagt FW, Gao S, Baiyewu O, et al. Prevalence of cognitive impairment: data from the Indianapolis Study of Health and Aging. Neurology. 2001;57:1655–1662. doi: 10.1212/wnl.57.9.1655. [DOI] [PubMed] [Google Scholar]
- 20.Hanninen T, Hallikainen M, Tuomainen S, Vanhanen M, Soininen H. Prevalence of mild cognitive impairment: a population-based study in elderly subjects. Acta Neurologica Scandinavica. 2002;106:148–154. doi: 10.1034/j.1600-0404.2002.01225.x. [DOI] [PubMed] [Google Scholar]
- 21.Palmer K, Backman L, Winblad B, Fratiglioni L. Mild cognitive impairment in the general population: occurrence and progression to Alzheimer disease. Am J Geriatr Psychiatry. 2008 Jul;16(7):603–611. doi: 10.1097/JGP.0b013e3181753a64. [DOI] [PubMed] [Google Scholar]
- 22.Das SK, Bose P, Biswas A, et al. An epidemiologic study of mild cognitive impairment in Kolkata, India. Neurology. 2007 Jun 5;68(23):2019–2026. doi: 10.1212/01.wnl.0000264424.76759.e6. [DOI] [PubMed] [Google Scholar]
- 23.Di Carlo A, Lamassa M, Baldereschi M, et al. CIND and MCI in the Italian elderly: frequency, vascular risk factors, progression to dementia. Neurology. 2007 May 29;68(22):1909–1916. doi: 10.1212/01.wnl.0000263132.99055.0d. [DOI] [PubMed] [Google Scholar]
- 24.Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007 Jan 23;68(4):288–291. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- 25.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63(4):494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008 Mar 18;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts R, Geda Y, Knopman D, et al. Men are More Likely to Have Mild Cognitive Impairment Than Women: The Mayo Clinic Study of Aging. Neurology. 2008 abstract (In press) [Google Scholar]
- 28.Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic-vs community-based cohorts. Arch Neurol. 2009 Sep;66(9):1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen RC, Thomas RG, Grundman M, et al. Donepezil and vitamin E in the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 30.Solfrizzi V, Panza F, Colacicco AM, et al. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882–1891. doi: 10.1212/01.wnl.0000144281.38555.e3. [DOI] [PubMed] [Google Scholar]
- 31.Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, et al. Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology. 2006 Jul 25;67(2):229–234. doi: 10.1212/01.wnl.0000224748.48011.84. [DOI] [PubMed] [Google Scholar]
- 32.Ravaglia G, Forti P, Montesi F, et al. Mild cognitive impairment: epidemiology and dementia risk in an elderly Italian population. J Am Geriatr Soc. 2008 Jan;56(1):51–58. doi: 10.1111/j.1532-5415.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- 33.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA. 1995;273:1274–1278. [PubMed] [Google Scholar]
- 34.Aggarwal NT, Wilson RS, Bienias JL, Berry-Kravis E, Bennett DA. The Apolipoprotein E4 allele and incident Alzheimer’s disease in persons with mild cognitive impairment. Neurocase. 2005;11:3–7. doi: 10.1080/13554790490903038. [DOI] [PubMed] [Google Scholar]
- 35.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 36.Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Annals of Neurology. 2000;47:430–439. [PubMed] [Google Scholar]
- 37.Jack CR, Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52(7):1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox NC, Scahill RI, Crum WR, Rossor MN. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology. 1999;52:1687–1689. doi: 10.1212/wnl.52.8.1687. [DOI] [PubMed] [Google Scholar]
- 39.Jack CR, Jr, Slomkowski M, Gracon S, et al. MRI as a biomarker of disease progression in a therapeutic trial of milameline for AD. Neurology. 2003;60(2):253–260. doi: 10.1212/01.wnl.0000042480.86872.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kantarci K, Weigand SD, Petersen RC, et al. Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2007 Sep;28(9):1330–1339. doi: 10.1016/j.neurobiolaging.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jagust W, Landau S, Shaw LM. Relationships between biomarkers in aging and dementia. Neurology. 2009 doi: 10.1212/WNL.0b013e3181bc010c. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw LM, Korecka M, Clark CM, Lee VM, JQT Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007;6(4):295–303. doi: 10.1038/nrd2176. [DOI] [PubMed] [Google Scholar]
- 43.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 44.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. Jama. 2009 Jul 22;302(4):385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 45.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009 Apr;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:303–305. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 47.Wolk DA, Price JC, Saxton JA, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009 May;65(5):557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008 Mar;131(Pt 3):665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ewers M, Bokde A, Walsh C, Hampel H. Multidemensional Biomarker-Based Diagnosis of Early Alzheimer’s Disease. Alzheimer Dement. 2009;5(4):136–137. [Google Scholar]
- 50.Jack C, Jr, Low V, Weigand S, et al. Serial PiB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris JC. Mild cognitive impairment is early-stage Alzheimer disease: time to revise diagnostic criteria. Arch Neurol. 2006;63:15–16. doi: 10.1001/archneur.63.1.15. [DOI] [PubMed] [Google Scholar]
- 52.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 53.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 54.Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol. 2003;60:729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- 55.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathology of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 56.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathological outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 57.Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007 Jun;6(6):501–512. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- 58.Thal LJ, Ferris SH, Kirby L, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30:1204–1215. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 59.Winblad B, Gauthier S, Scinto L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008 May 27;70(22):2024–2035. doi: 10.1212/01.wnl.0000303815.69777.26. [DOI] [PubMed] [Google Scholar]
- 60.Doody RS, Ferris SH, Salloway S, et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009 May 5;72(18):1555–1561. doi: 10.1212/01.wnl.0000344650.95823.03. [DOI] [PubMed] [Google Scholar]
- 61.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 62.Roberts S, Uhlmann W, Petersen R, Karlawish J, Green R. Cinical Practices Regarding Mild Cognitive Impairment (MCI) Among Neurology Service Providers. http://www.alz.org/icad/documents/abstracts/abstracts_phaseIII_ICAD09.pdf.
- 63.Petersen RC, O’Brien J. Mild cognitive impairment should be considered for DSM-V. J Geriatr Psychiatry Neurol. 2006;19:147–154. doi: 10.1177/0891988706291085. [DOI] [PubMed] [Google Scholar]