Abstract
Objectives
This study sought to test the hypothesis that pressure stress of the adenylyl cyclase 6-deleted (AC6-KO) heart would result in excessive hypertrophy, early dilation and dysfunction, and increased fibrosis.
Background
Cardiac-directed AC6 expression attenuates left ventricular (LV) hypertrophy and dysfunction in cardiomyopathy.
Methods
AC6-KO and control (CON) mice underwent transverse aortic constriction (TAC) to induce pressure overload. Measures of LV hypertrophy, function, and fibrosis were obtained 3 weeks after TAC, and LV samples were assessed for alterations in expression of FHL1 and periostin.
Results
Three weeks after TAC, female AC6-KO mice had preserved left ventricular (LV) ejection fraction (CON: 22 ± 2%; AC6-KO: 52 ± 4%; p < 0.001) and reduced LV end-diastolic dimension (CON: 4.6 ± 0.1 mm; AC6-KO: 3.6 ± 0.1 mm; p < 0.001). Reduced LV/tibial length ratio (CON: 10.4 ± 1.5 mg/mm; AC6-KO: 7.5 ± 2.3 mg/mm; p < 0.001) and reduced LV expression of atrial natriuretic factor (p < 0.05), α-skeletal muscle actin (p < 0.05), and beta-myosin heavy chain (p < 0.05) were observed in AC6-KO mice. In addition, AC6 deletion was associated with less LV fibrosis (p < 0.01) and reduced collagen types I (p < 0.05) and III (p < 0.05) expression 3 weeks after TAC. LV protein expression of FHL1 (p < 0.02) and periostin (p = 0.04) were reduced after TAC in AC6-KO mice. The roles of AC6 deletion in cardiac myocytes and fibroblasts were examined in vitro using pharmacological hypertrophy and AC6 knockdown (small interfering ribonucleic acid), which recapitulated in vivo findings.
Conclusions
The deleterious effects of LV pressure overload were reduced in female mice with AC6 deletion. Reductions in FHL1 and periostin expression, direct consequences of reduced AC6 in cardiac myocytes and fibroblasts, appear to be of mechanistic importance for these unanticipated beneficial effects.
Keywords: heart failure, left ventricular function, left ventricular remodeling, transgenic animal models
Cardiac-directed expression of adenylyl cyclase (AC) attenuates left ventricular (LV) hypertrophy in cardiomyopathy (1), suggesting a link between AC6 and signaling pathways regulating LV hypertrophy. We reasoned that use of the potentially superior strategy of AC6 gene deletion (vs. gain-of-function) in a dissimilar model of hypertrophy (pressure overload) would help delineate how AC6 influences hypertrophy. For example, LV pressure overload, by activating different signaling pathways than those seen with cardiomyopathy, would provide insight regarding how AC6 influences the hypertrophic response. Furthermore, this approach might also enable us to identify potential targets for treating the dysfunctional heart.
Chronic pressure overload, such as occurs with persistent hypertension, is associated with a higher risk of the development of clinical heart failure. Although this process generally takes decades to develop in patients, severe LV pressure overload associated with transverse aortic constriction (TAC) leads to LV hypertrophy and impaired LV function in weeks in mice, thereby providing an efficient model to study the effects of AC6 deletion (2) on the pressure-stressed LV.
AC5 and AC6, the predominant adenylyl cyclase types in cardiac myocytes (2,3), have been associated with altered cell survival, fibrosis, and collagen production. For example, AC5 deletion reduces apoptosis in pressure overload (4) and decreases fibrosis in the aging heart (3). AC6 influences cardiac fibroblast function in vitro (5). TAC, which is associated with increased LV fibrosis, therefore, is a well-suited model to use to determine whether AC6 deletion alters LV fibrosis in pressure overload.
Previous reports have shown that increased levels of cardiac AC6 have beneficial effects on the normal heart, in acute myocardial infarction, in congestive heart failure due to myocardial infarction, in pacing congestive heart failure in pigs, and murine cardiomyopathy (1,6–8). In addition, AC6 deletion impairs LV function in otherwise normal mice (2). Our hypothesis, therefore, was that pressure stress of the AC6-deleted heart would result in excessive hypertrophy, early dilation and dysfunction, and increased fibrosis.
Methods
Animals
Three- to 6-month-old homozygous AC6-deleted mice (AC6-KO) and their littermate control mice (CON) were used in this study (2). These mice have a congenic C57BL/6 genetic background because this AC6-KO line has been back-crossed with C57BL/6 (Harlan Laboratories, Indianapolis, Indiana) for more than 10 generations. Genotyping was performed using genomic DNA purified from tail clip as previously described (2). Absence of AC6 protein expression was confirmed by Western blotting. The Animal Use and Care Committee of the VA San Diego Healthcare System, in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines, approved this study.
TAC
Mice were anesthetized with 5% isoflurane in oxygen (1 l/min), intubated, and ventilated (pressure-controlled). Anesthesia was maintained with 1% isoflurane in oxygen. The chest was entered at the second intercostal space at the left upper sternal border, and a segment of the aortic arch between the innominate and left carotid arteries dissected. A 7-0 silk suture was tied against a 27-gauge needle, which yields a substantial aortic constriction.
Echocardiography
Echocardiography was performed with the mice under light anesthesia before and 3 weeks after TAC using previously reported methods (2). Interobserver variability for murine echocardiography measurements in our laboratory is small, with a very tight correlation between 2 independent readers (r2 = 0.89).
LV physiological studies
A 1.4-F conductance-micromanometer catheter was used to measure LV hemodynamics using a closed chest method, as previously reported (7) (see also Online Supplement).
Necropsy and LV fibrosis assessment
Body and LV weights (including the septum) and tibial lengths were recorded. A short-axis mid-wall LV ring was formalin fixed and paraffin embedded. Dewaxed sections (6 µm) were rehydrated, stained with picrosirius red (1 h), and counterstained with hematoxylin (1 min). LV sections were then dehydrated using graded concentrations of ethanol and mounted in Permont. Collagen fractional area was quantified using NIH Image J software.
Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
See the Online Supplement.
Western blotting
See the Online Supplement.
Gelatin zymography
See the Online Supplement.
Pharmacological hypertrophy and AC6 knockdown in cultured cardiac myocytes and fibroblasts
We isolated cardiac myocytes and fibroblasts from 2-day-old Sprague-Dawley rats as previously reported (9). Left ventricles were minced and digested with collagenase II and pancreatin at 37°C. The dissociated cardiac cells were centrifuged through discontinuous Percoll gradients to separate cardiac myocytes, fibroblasts, and other cardiac cell types. Isolated cardiac fibroblasts were washed with phosphate-buffered saline and cultured overnight at 37°C in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum; isolated cardiac myocytes were washed with phosphate-buffered saline, seeded in gelatin-coated dishes, and cultured overnight at 37°C in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum and 5% horse serum. Transfection of rat AC6 small interfering ribonucleic acid (siRNA) (ON-TARGETplus SMARTpool L-100104-01-0010, Dharmacon, Lafayette, Colorado) was achieved by using X-tremeGENE siRNA Transfection Reagent (Cat. #04476093001, Roche, Indianapolis, Indiana) according to the manufacturer’s instructions. Twenty-four hours after AC6 siRNA transfection, cells were starved for 8 h and then treated with 20 µmol/l phenylephrine or 100 nmol/l angiotensin II for 16 h. Total RNA was extracted and purified, and quantitative RT-PCR was performed to compare messenger ribonucleic acid (mRNA) contents using glyceraldehyde-3-phosphate dehydrogenase as internal control. AC6 siRNA specifically knocked down AC6 mRNA expression and did not alter mRNA expression of AC5, another cardiac adenylyl cyclase isoform with high homology to AC6. To compare protein contents of FHL1 and periostin, cells were homogenized in homogenization buffer in the presence of protease inhibitors. Denatured cell homogenates in Laemmli buffer were subject to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blotting, as stated previously.
The presence of hypertrophy was evaluated by assessment of cardiac myocyte size. Photomicrographs of cardiac myocytes were obtained in randomly selected areas using a Nikon microscope. Cell area was measured with NIH Image J software, and only cardiac myocytes with an area >20 µm2 were used. Cardiac myocytes that were adjoining or apoptotic in appearance were excluded from the analyses.
Statistical analysis
Results are shown as mean ± SE. Group differences in mortality were assessed using Kaplan-Meier analysis and the log-rank test. Group means of other data were compared using Student t test (unpaired, 2-tailed) or 2-way analysis of variance with post hoc comparisons made using t testing. Multiple comparisons were corrected using the Bonferroni method. The null hypothesis was rejected when p < 0.05. Data from echocardiography and physiology studies were collected and analyzed blinded to group identity.
Results
Mortality
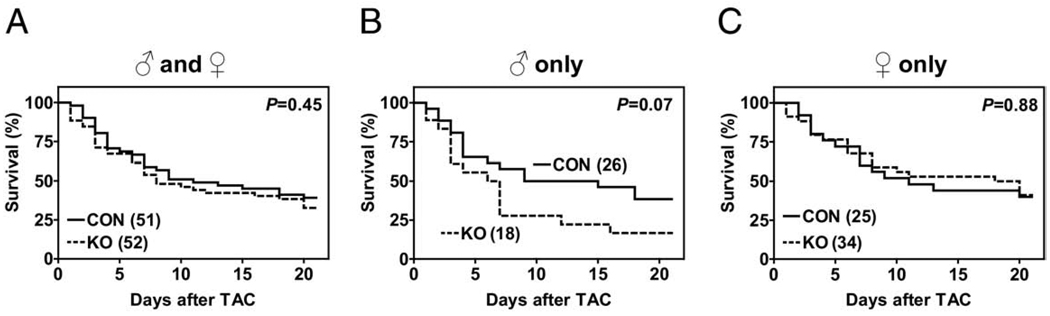
No group difference in mortality was observed 3 weeks after TAC in male and female mice combined (CON: 61%; AC6-KO: 67%; p = 0.45), or in female mice (CON: 60%; AC6-KO: 59%; p = 0.88) (Figs. 1A and 1C). However, male AC6-deleted mice tended to have higher mortality rates (CON: 62%; AC6-KO: 83%; p = 0.07) (Fig. 1B). Indeed, almost 75% of male AC6-KO mice died within 1 week of TAC, necessitating that biochemical and molecular studies over the 3-week course be conducted in female mice alone. The control group (both sexes combined) showed 61% mortality, higher than that of a previous report (10). This likely reflects a strain difference. Although C57BL/6J mice show only 20% to 50% mortality rates 3 weeks after TAC, the C57BL/6 strain, which were used in the present study, have a higher mortality rate.
Figure 1. Kaplan-Meier Analysis Showing Survival After TAC in AC6-Deleted Mice and Their Intact Littermates.
(A) No group difference in mortality 3 weeks after transverse aortic constriction (TAC) was observed in male and female mice combined. (B) Mortality was 83% in male adenylyl cyclase (AC) 6-deleted mice (p = 0.07 vs. control mice [CON]) 3 weeks after TAC. (C) No group difference was seen in mortality 3 weeks after TAC in female mice, in which the mortality rate of both groups was 60%. Because 75% of male AC6-deleted mice were dead 1 week after TAC, biochemical and molecular studies were performed only in female mice. Numbers in parentheses indicate group sizes. KO = AC6-deleted mice.
Echocardiography
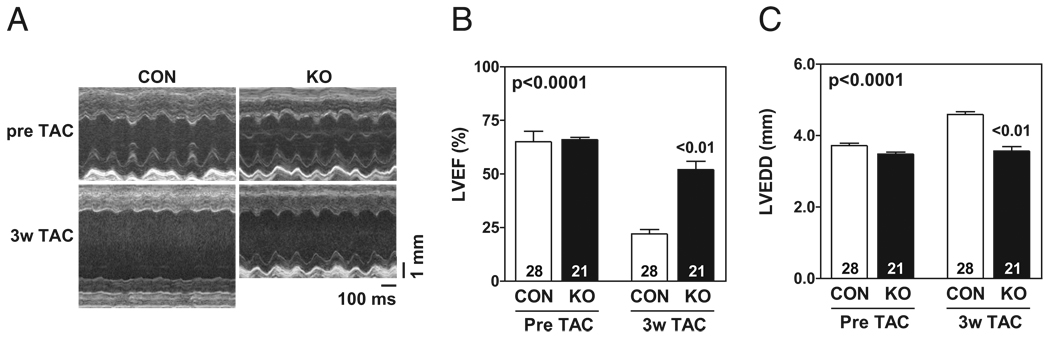
There was a decline in LV function in pressure-overloaded hearts of CON mice. There was a relative reduction of 66% in LV ejection fraction in CON mice 3 weeks after TAC; AC6-deleted mice showed a 22% relative reduction (Figs. 2A and 2B, Table 1). CON mice showed LV dilation 3 weeks after TAC, but AC6-deleted mice showed no LV dilation (Figs. 2A and 2C, Table 1). Heart rates showed no group differences during echocardiography studies (Table 1).
Figure 2. Reduced LV Dilation and Preserved LV Function 3 Weeks After TAC in AC6-Deleted Mice.
(A) Echocardiogram of a control (CON) mouse shows greater left ventricular (LV) dilation 3 weeks (3w) after transverse aortic constriction (TAC) versus an adenylyl cyclase 6-deleted (KO) mouse). (B) AC6-deleted mice showed preserved LV ejection fraction (EF) 3 weeks after TAC versus control mice. (C) Control mice, but not AC6-deleted mice, showed LV dilation 3 weeks after TAC. There were no group differences in heart rate. Two-way analysis of variance was performed for statistical analysis and p value for AC6 effect is shown in upper left corner of B and C. p Values above bars are from post-hoc testing (CON vs. KO, 3 weeks [3w] after TAC). Error bars = 1 SE; numbers in bars = group size. LVEDD = left ventricular end-diastolic diameter.
Table 1.
Echocardiography Measurements
| Before TAC |
3 Weeks After TAC |
p Value |
|||||
|---|---|---|---|---|---|---|---|
| CON (n = 28) | AC6-KO (n = 21) | CON (n = 28) | AC6-KO (n = 21) | Inter | AC6 Effect | TAC Effect | |
| End-diastolic diameter (mm) | 3.7 ± 0.1 | 3.5 ± 0.1 | 4.6 ± 0.1* | 3.6 ± 0.1 | <0.001 | <0.001 | <0.001 |
| End-systolic diameter (mm) | 2.4 ± 0.1 | 2.2 ± 0.1 | 4.1 ± 0.1* | 2.6 ± 0.2† | <0.001 | <0.001 | <0.001 |
| Posterior wall thickness (mm) | 0.7 ± 0.01 | 0.6 ± 0.01 | 0.9 ± 0.02 | 1.0 ± 0.04 | 0.78 | 0.79 | <0.001 |
| IVS wall thickness (mm) | 0.7 ± 0.01 | 0.7 ± 0.01 | 1.0 ± 0.02 | 1.0 ± 0.03 | 0.61 | 0.89 | <0.001 |
| Heart rate (beats/min) | 491 ± 13‡ | 487 ± 11§ | 521 ± 9‡ | 507 ± 12§ | 0.69 | 0.43 | <0.03 |
| Ejection time (ms) | 48 ± 1‖ | 47 ± 1¶ | 51 ± 1‖ | 50 ± 1¶ | 0.49 | 0.63 | <0.003 |
| LV fractional shortening (%) | 35 ± 1 | 36 ± 1 | 10 ± 1 | 27 ± 2 | <0.001 | <0.001 | <0.001 |
| LV ejection fraction (%) | 65 ± 2 | 66 ± 2 | 22 ± 2 | 52 ± 4 | <0.001 | <0.001 | <0.001 |
| Vcf (circ/s) | 7.4 ± 0.3‖ | 7.9 ± 0.4¶ | 2.1 ± 0.2*‖ | 5.5 ± 0.5¶# | <0.001 | <0.001 | <0.001 |
p < 0.01 vs. CON mice before TAC from post hoc testing.
p = NS vs. AC6-KO mice before TAC from post hoc testing.
n = 26.
n = 20.
n = 27.
n = 19.
p < 0.01 vs. AC6-KO mice before TAC from post hoc testing. p Values are from 2-way analysis of variance for interaction (Inter), AC6 effect, and TAC effect. Values shown are mean ± SE.
AC6-KO = AC6-deleted mice; CON = control mice; IVS = interventricular septum; LV = left ventricular; TAC = transverse aortic constriction; Vcf = velocity of circumferential fiber shortening.
LV physiological studies
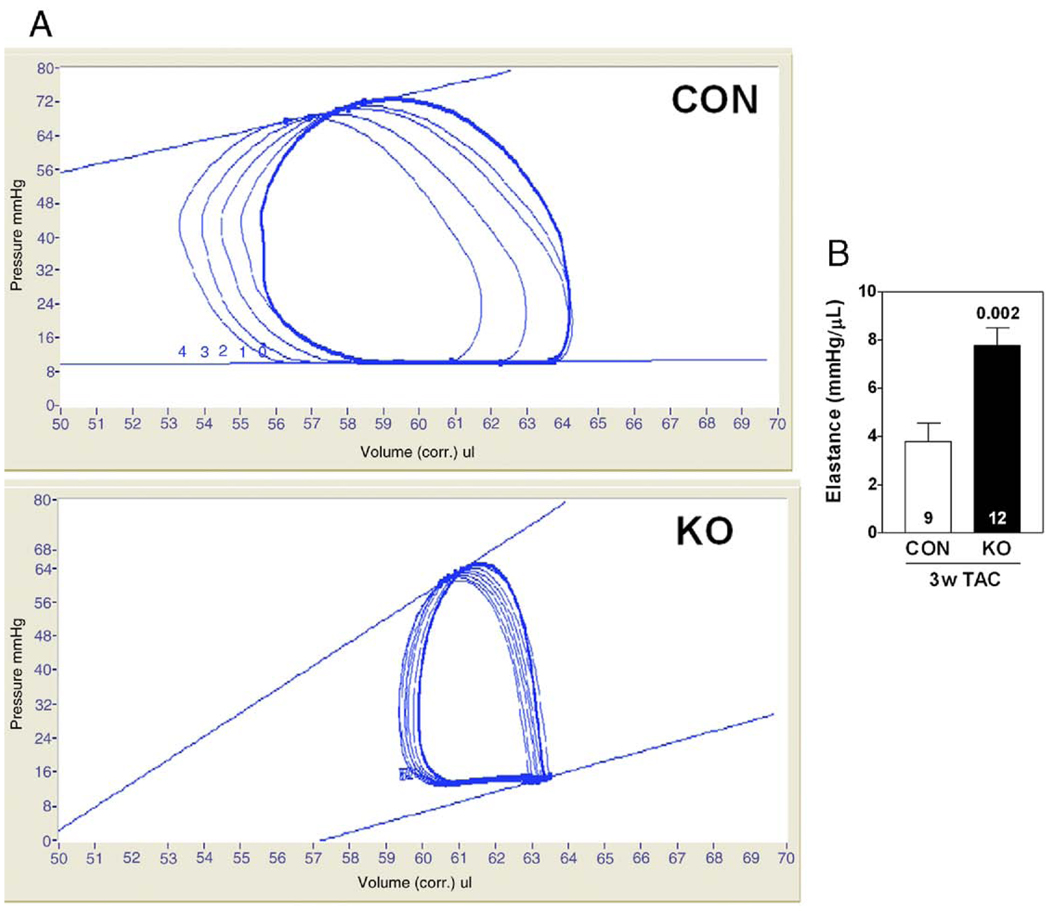
Three weeks after TAC-induced pressure overload, AC6-deleted mice showed higher stroke volume, stroke work, and LV +dP/dt (Table 2); heart rate for both groups was similar. LV −dP/dt for the AC6-deleted group tended to be lower than that for CON (Table 2). Cardiac output was higher in AC6-deleted mice 3 weeks after TAC (Table 2), and LV elastance, a measure of LV contractility, was 2-fold higher (p = 0.002) (Fig. 3, Table 2).
Table 2.
Left Ventricular Physiology 3 Weeks After Transaortic Constriction
| CON (n = 15) |
AC6-KO (n = 11) |
p Value | |
|---|---|---|---|
| Heart rate (beats/min) | 453 ± 14 | 432 ± 16 | 0.34 |
| Cardiac output (ml/min) | 2.2 ± 0.4* | 3.6 ± 0.3† | 0.01 |
| Stroke volume (µl) | 5 ± 1 | 8 ± 1 | 0.05 |
| LV pressure (mm Hg) | 87 ± 6 | 93 ± 5 | 0.45 |
| Stroke work (mm Hg·µl) | 176 ± 45* | 490 ± 62† | 0.002 |
| LV +dP/dt (mm Hg/s) | 2,793 ± 251 | 3,610 ± 406 | 0.05 |
| LV −dP/dt (mm Hg/s) | −2,842 ± 343 | −3,895 ± 286 | 0.07 |
| LV end-diastolic pressure (mm Hg) | 13 ± 2 | 7 ± 1 | 0.03 |
| Tau (ms) | 7.1 ± 0.3 | 6.6 ± 0.2 | 0.19 |
| LV elastance (mm Hg/µl) | 3.8 ± 0.7‡ | 7.7 ± 0.7* | 0.002 |
| PRSW (mm Hg) | 26 ± 6§ | 58 ± 9* | 0.02 |
Elastance is the slope of the end-systolic pressure-volume relationship. p Values are from Student t test (2-tailed). Values shown are mean ± SE.
n = 12.
n = 7.
n = 9.
n = 8.
LV = left ventricular; PRSW = preload recruitable stroke work.
Figure 3. Effects of AC6 Deletion on LV Contractility 3 Weeks After TAC.
(A) LV pressure-volume loops, generated by altering LV preload, are shown with elastance (slope of the end-systolic pressure-volume relationship). (B) The bar graph summarizes data from all animals. p Value shown is from Student t test (2 tailed). Error bars = 1 SE; numbers in bars = group size. Abbreviations as in Figure 1.
LV hypertrophy
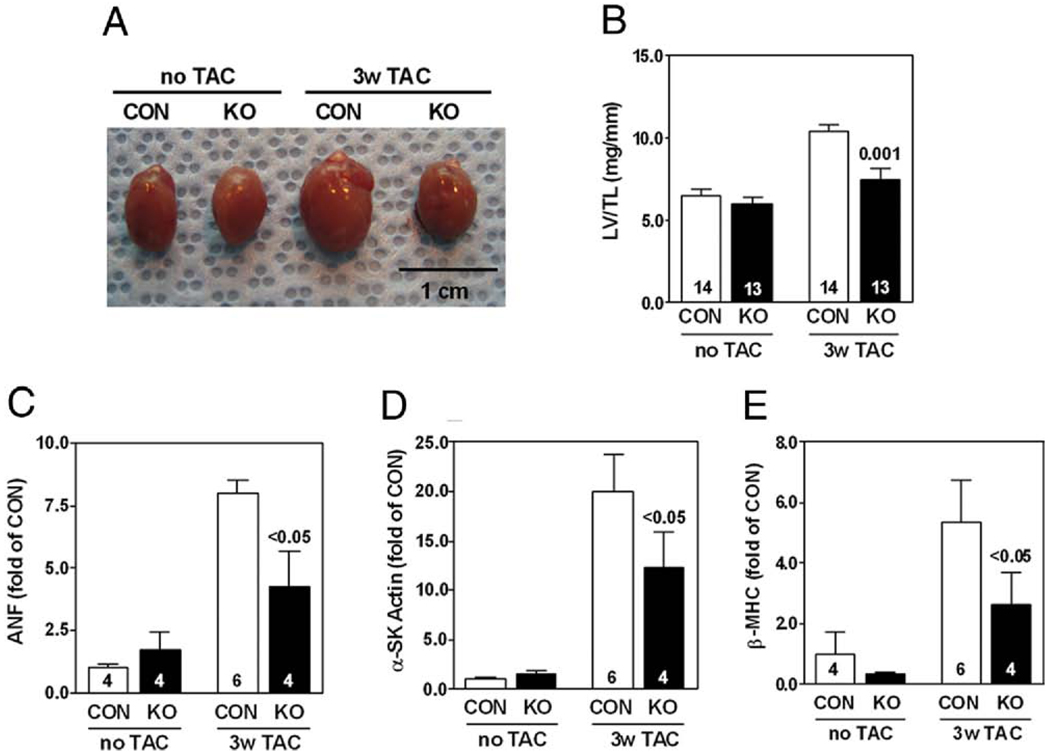
Hearts from CON mice showed greater LV hypertrophy than hearts of AC6-deleted mice (Fig. 4A). The LV weight/tibial length ratio increased from 6.5 mg/mm to 10.4 mg/mm in CON, but increased from 6.0 mg/mm to 7.5 mg/mm in AC6-deleted mice 3 weeks after TAC (p = 0.001) (Fig. 4B). LV samples from pressure-overloaded CON mice showed increased expression of atrial natriuretic factor (Fig. 4C), α-skeletal muscle actin (Fig. 4D), and β-myosin heavy chain (Fig. 4E). In contrast, LV samples from AC6-deleted mice showed less expression of these genes (Figs. 4C to 4E). These data indicate that AC6 deletion not only reduces LV hypertrophy in response to pressure overload, but also is associated with reduced expression of fetal genes.
Figure 4. Reduced LV Hypertrophy 3 Weeks After TAC in AC6-Deleted Mice.
(A) There was substantial LV hypertrophy 3 weeks after TAC, which was attenuated by AC6-deleted mice (AC6-KO). (B) AC6-deleted mice showed reduced LV weight/tibial length ratio versus control mice 3 weeks after TAC. (C, D, E) AC6 deletion reduced LV expression (quantitative reverse transcriptase-polymerase chain reaction) of atrial natriuretic factor (ANF), α-skeletal muscle actin (SK actin), and β-myosin heavy chain (MHC) 3 weeks after TAC. Two-way analysis of variance showed a significant AC6 deletion effect (p < 0.05). p Values above bars are from post-hoc testing (CON vs. KO, 3 weeks after TAC). Error bars = 1 SE; numbers in bars = group size. LV/TL = left ventricular/tibial length; other abbreviations as in Figures 1 and 2.
LV fibrosis
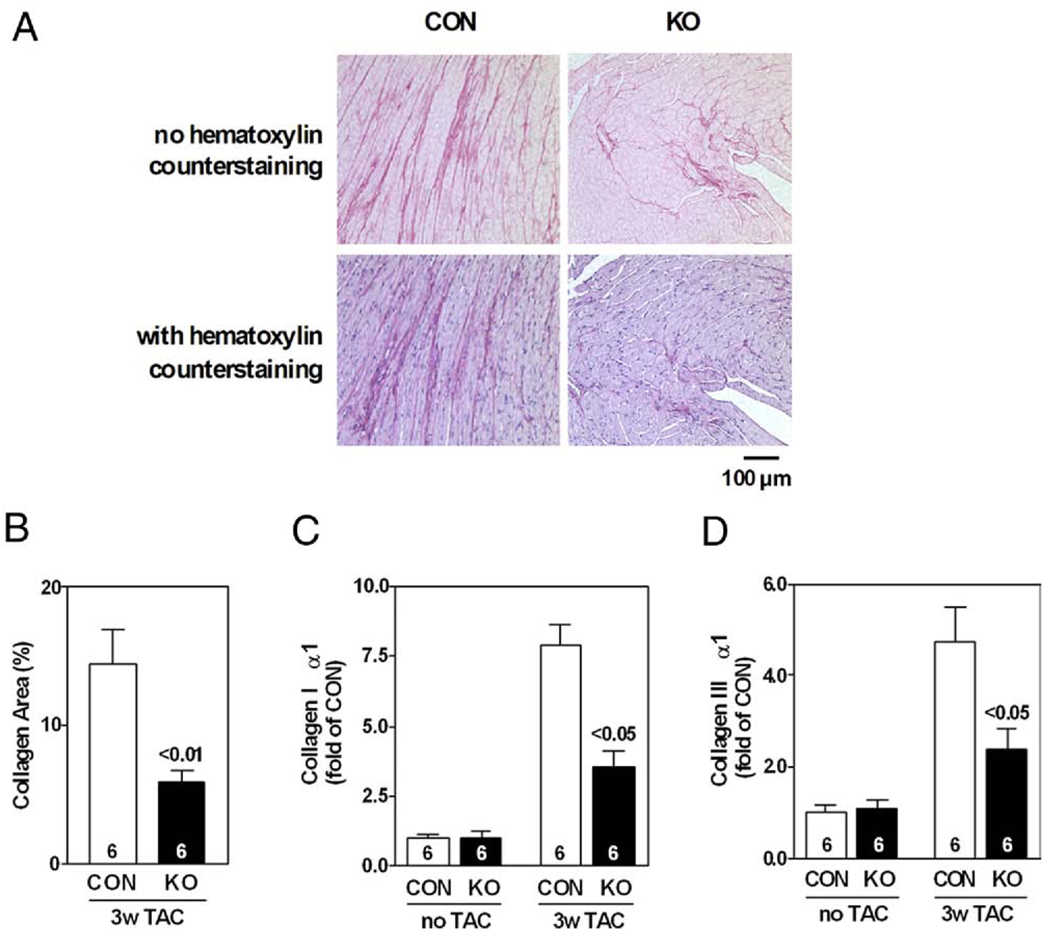
As shown in the bright-field microscopic images (Fig. 5A), there was less collagen deposition in sections of the left ventricles from AC6-deleted mice under pressure overload stress compared with those from CON mice. Quantification by NIH Image J software revealed that AC6 deletion decreased the collagen fractional area in pressure-overloaded hearts (CON: 14 ± 3%, AC6-KO: 6 ± 1%; p < 0.01, n = 6) (Fig. 5B).
Figure 5. AC6 Deletion Reduced LV Fibrosis 3 Weeks After TAC.
(A) Images of picrosirius red stained LV sections 3 weeks after TAC. (B) Analysis shows less collagen fractional area in LV samples from AC6-KO mice; p value (above bar) from Student t test (2-tailed). AC6 deletion reduced expression of collagen I α1 (C) and collagen III α1 (D). Two-way analysis of variance showed a significant AC6 effect (p < 0.05). p Values above bars are from post hoc testing (CON vs. KO, 3 weeks after TAC). Error bars = 1 SE; numbers in bars = group size. Abbreviations as in Figures 1, 2, and 4.
To determine whether reduced collagen deposition was associated with reduced collagen expression, we compared collagen mRNA content in LV samples from CON and AC6-deleted mice by quantitative RT-PCR (Figs. 5C and 5D). Pressure overload of AC6-KO mice was associated with reduced LV mRNA expression of types I (p < 0.05) and III (p < 0.05) collagen, 2 major constituents of the extracellular connective tissue matrix contributing approximately 95% of the total collagen content in the heart (11). These data indicate that AC6 deletion decreases LV collagen gene expression and LV fibrosis 3 weeks after TAC.
LV expression of genes associated with hypertrophy and fibrosis
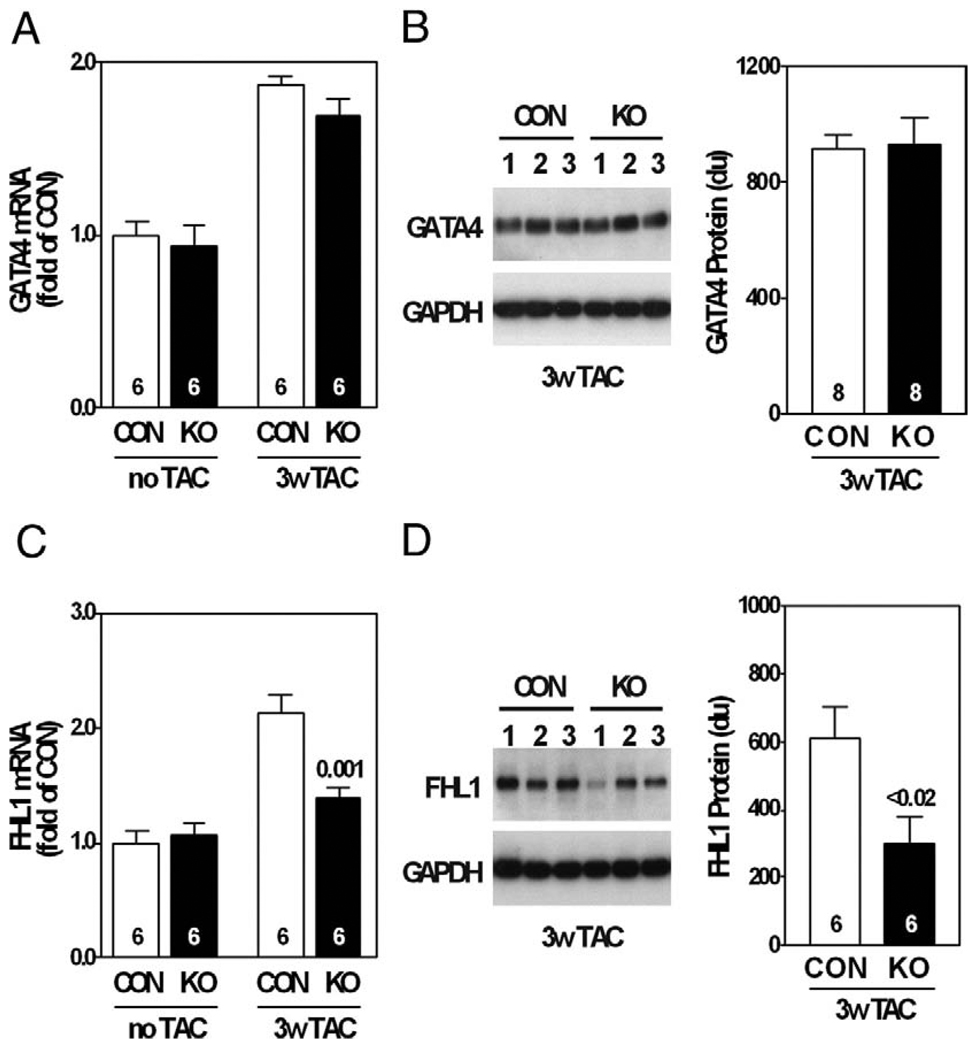
GATA4 expression was increased in pressure-overloaded hearts, as previously reported (12), but was unaffected by AC6 deletion (Figs. 6A and 6B). AC6 deletion did not alter LV protein expression of HDAC5, phospho-HDAC5, GSK-3β, or phospho-GSK3β 3 weeks after TAC (data not shown).
Figure 6. LV GATA4 and FHL1 Expression.
(A) LV GATA4 messenger ribonucleic acid (mRNA) expression increased 3 weeks after TAC from similar pre-TAC levels; no group differences were observed. (B) LV GATA4 protein expression was similar in both groups 3 weeks after TAC. LV FHL1 mRNA (C) and protein expression (D) were reduced 3 weeks after TAC in AC6-deleted mice. Gene expression was assessed by quantitative real-time polymerase chain reaction and Western blotting; p values from Student’s t test (2-tailed). Error bars = 1 SE; numbers in bars = group size. du = densitometric unit; GAPDH = glyceraldehyde-3-dehydrogenase; other abbreviations as in Figures 1 and 2.
We assessed expression of elements of the β-adrenergic receptor signaling pathway and found that AC6 deletion decreased NKH477-stimulated cyclic adenosine monophosphate generation in LV homogenates from pressure-overloaded hearts (CON: 197 ± 14 fmol/min/µg; AC6-KO: 70 ± 11 fmol/min/µg; p < 0.0001, n = 8), as was anticipated (2). Protein contents of PDE3A and PDE4D, 2 important cyclic adenosine monophosphate–hydrolyzing phosphodiesterases in cardiac myocytes, were not altered (Online Appendix). Furthermore, we found that AC6 deletion did not alter mRNA contents of adenylyl cyclase isoforms (AC2, 3, 4, 5, 7, and 9) in pressure-overloaded hearts (Online Appendix). The absence of LV AC5 protein in AC6-deleted mice was noted 3 weeks after TAC (data not shown), a finding similar to what we reported previously in AC6-deleted mice (2).
We then focused on FHL1, a protein associated with the contractile apparatus that appears to be required for pressure overload-induced LV hypertrophy (13). No group differences were seen in LV FHL1 protein expression pre-TAC. However, the increase in FHL1 expression associated with pressure overload was inhibited by AC6 deletion (Fig. 6C). Western blotting confirmed that AC6 deletion reduced LV FHL1 protein content by 51% 3 weeks after TAC (p < 0.02) (Fig. 6D).
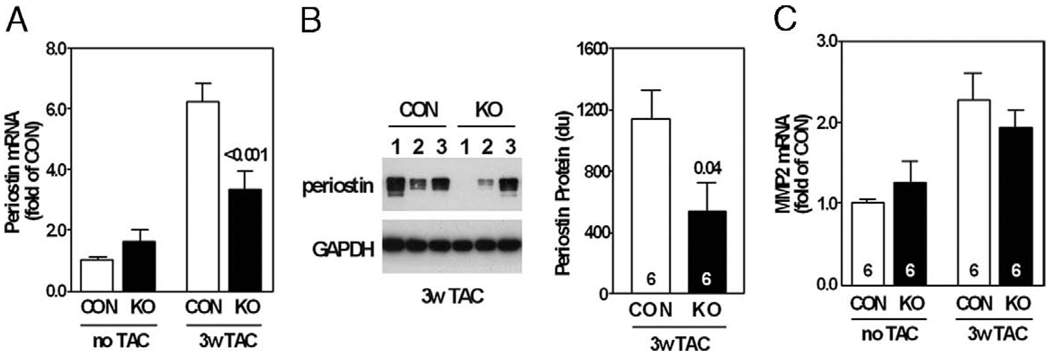
Quantitative RT-PCR showed that periostin mRNA content was increased by pressure overload, and the increase in mRNA content of periostin, a regulator of cardiac fibrosis, was attenuated by AC6 deletion (Fig. 7A). AC6 deletion decreased LV periostin protein content by 53% 3 weeks after TAC (Fig. 7B). Matrix metalloproteinase 2 mRNA expression was increased in pressure-overloaded hearts, as previously reported (14), but was unaffected by AC6 deletion (Fig. 7C); matrix metalloproteinase 2 gelatinase activity was not altered by AC6 deletion (data not shown).
Figure 7. LV Periostin and MMP-2 Expression.
(A) AC6 deletion reduced LV expression (quantitative reverse transcriptase-polymerase chain reaction) of periostin. Two-way analysis of variance showed a significant AC6 effect (p < 0.05) for periostin mRNA expression; p value above bar from post-hoc testing (CON vs. KO, 3 weeks after TAC). (B) LV periostin protein (Western blotting) was reduced 3 weeks after TAC in AC6-deleted mice; p value above bar from Student t test (2-tailed). (C) LV matrix metalloproteinase (MMP)-2 mRNA expression (quantitative reverse transcriptase-polymerase chain reaction) increased 3 weeks after TAC from similar pre-TAC levels; no group differences were observed. Error bars = 1 SE; numbers in bars = group size. Abbreviations as in Figures 1, 2, and 6.
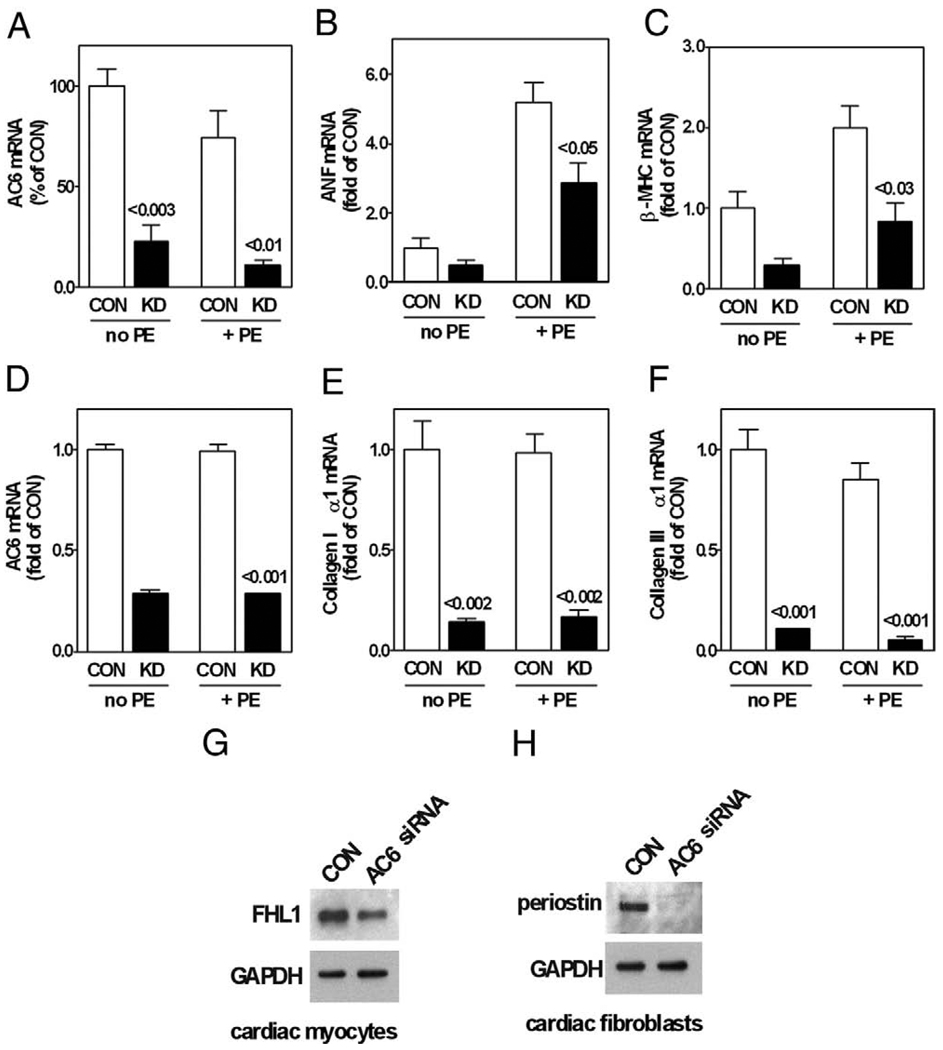
Cultured cardiac myocytes
Transfection of cardiac myocytes with AC6 siRNA specifically decreased AC6 mRNA expression by 88% (Fig. 8A). Knockdown of AC6 decreased phenylephrine-induced hypertrophy of cardiac myocytes (Table 3) and reduced mRNA expression of atrial natriuretic factor and β-myosin heavy chain (Figs. 8B and 8C). Phenylephrine- and angiotensin II–induced cardiac myocyte hypertrophy were both inhibited by reductions in AC6, indicating that AC6 levels influence Gq-mediated hypertrophy. Just as was the case in vivo, reduced AC6 content decreased protein expression of FHL1 in phenylephrine-stimulated cultured cardiac myocytes (Fig. 8G). These results suggest that reduction of AC6 decreases hypertrophy by decreasing expression of FHL1 in cardiac myocytes.
Figure 8. Cultured Cardiac Myocytes.
AC6 knockdown by specific small interfering ribonucleic acid (siRNA) followed by phenylephrine stimulation (10 µmol/l, 16 h) reduced mRNA expression (quantitative reverse transcriptase-polymerase chain reaction) of AC6 (A), ANF (B), and β-MHC (C) and also reduced FHL1 protein expression (Western blotting) in cultured cardiac myocytes (G) Cultured cardiac fibroblasts. AC6 knockdown reduced mRNA expression of AC6 (D), collagen type Iα1 (E), and collagen type IIIα1 (F) and also reduced periostin protein expression (Western blotting) in cultured cardiac fibroblasts (H). p Values above bars derived from Student t test (2-tailed) versus same condition for CON. Error bars = 1 SE. Data in A through F are from 3 independent experiments. KD = AC6 knockdown; other abbreviations as in Figures 1, 2, 4, and 6.
Table 3.
AC6 Knockdown, Phenylephrine, and Cardiac Myocyte Size
| Cardiac Myocyte Area (µm2) | |||||
|---|---|---|---|---|---|
| CON | AC6 Knockdown | p Interaction | AC6 Effect | PE Effect | |
| No phenylephrine | 305 ± 17 (n = 89) | 311 ± 21 (n = 85) | 0.02 | 0.04 | 0.0001 |
| Phenylephrine (10 µM, 16 h) | 459 ± 28 (n = 83) | 364 ± 20 (n = 80) | |||
Values represent mean ± SE. p values from 2-way analysis of variance.
CON = control mice.
Cultured cardiac fibroblasts
Transfection of cardiac fibroblasts with AC6 siRNA specifically decreased AC6 mRNA expression by 71% (Fig. 8D). AC6 knockdown reduced mRNA expression of collagen types I and III (Figs. 8E and 8F). AC6 knockdown also decreased expression of periostin (Fig. 8H). These results indicate that reduction of AC6 directly decreases gene expression related to fibrotic responses in cardiac fibroblasts.
TAC in male AC6-deleted mice
Almost 75% of male AC6-KO mice died within 1 week of TAC, and very few remained after 3 weeks—insufficient numbers to evaluate in a statistically meaningful way. The male AC6-KO mice that survived 1 week of TAC, when compared with their female cohorts, had lower ejection fractions, more LV dilation and hypertrophy, and reduced LV fetal gene expression. Expression of FHL1 and periostin mRNA was also higher in male than in female AC-KO mice 1 week after TAC. These data are presented in detail in the Online Supplement.
Discussion
The most important finding of this study is that AC6 deletion is associated with attenuation of LV hypertrophy, absence of LV dilation, and relative preservation of LV function in female mice in response to pressure overload. Reactivation of fetal gene expression and expression of collagens (types I and III) are reduced in hearts from these AC6-deleted mice after pressure overload. These striking alterations in the heart’s response to pressure stress in the absence of AC6 were associated with decreased expression of FHL1 and periostin, 2 mediators for cardiac hypertrophy and fibrosis.
Reduced LV FHL1 expression
It is intriguing that decreased expression of LV FHL1 and periostin is associated with relative preservation of cardiac function in pressure-overloaded AC6-deleted mice. FHL1 is a protein in the contractile apparatus and belongs to the family of proteins with 4 complete LIM domains and an N-terminal half LIM domain. Increased LV FHL1 expression has been reported in cardiomyopathy associated with cardiac-directed expression of β1-adrenergic receptor and Gαs (15) and in clinical hypertrophic cardiomyopathy (16). FHL1-deleted mice show reduced LV hypertrophy and dysfunction in pressure overload (17). Our data indicate that reduced LV FHL1 expression may be an important mechanism for reduced LV hypertrophy and preserved LV function associated with AC6 deletion. The precise mechanism linking AC6 deletion and reduced LV FHL1 expression in pressure overload will require additional studies, but it is noteworthy that we see reduced FHL1 expression acutely after combined AC6 knockdown and pharmacological hypertrophy in isolated cardiac myocytes, suggesting a relationship between AC6 and FHL1 in cardiac myocytes.
Reduced periostin expression
Periostin is an extracellular matrix protein expressed predominantly by fibroblasts (18–20). In connective tissues, periostin interacts with collagen and increases collagen fibrillogenesis (19). Periostin also plays an important role in cardiac hypertrophy and fibrosis. Pathological stress, such as pressure overload and myocardial infarction, increases cardiac periostin expression. Cardiac-directed expression of periostin increases both aging-associated LV hypertrophy and LV collagen deposition (18). In contrast, periostin deletion decreases cardiac hypertrophy and fibrosis and preserves LV function after pressure overload (18). Our data indicate that reduced LV periostin expression, a consequence of AC6 deletion in the pressure-overloaded heart, may be an important mechanism for reduced LV fibrosis and improved cardiac relaxation. The precise mechanism linking AC6 deletion and reduced LV periostin expression in pressure overload will require additional studies, but it is noteworthy that we see reduced periostin expression acutely after AC6 knockdown in isolated cardiac fibroblasts, suggesting a relationship between AC6 and periostin in cardiac fibroblasts.
AC6 versus AC5 and LV function in pressure overload
AC5 and AC6 are the AC types most abundantly expressed in the heart (2,3). In previous studies, we have shown that these 2 AC types have different effects on cardiac function, despite their homology in amino acid sequence (2). In the present study, we have shown that absence of AC6 reduces the LV hypertrophic response to pressure overload (Fig. 2). In contrast, AC5 deletion does not impede the hypertrophic response to pressure overload (4). Furthermore, LV Bcl2 expression was unchanged by TAC in AC6-deleted mice (data not shown), but increased in AC5-deleted mice (4). These data provide further evidence that AC5 and AC6 play divergent roles in regulating cardiac function under pathophysiological conditions.
Studies in isolated cardiac myocytes and fibroblasts
Our AC6 deletion line was whole body, not cardiac limited, so it was important to determine whether the effects observed in vivo resulted from AC6 deletion in cardiac myocytes and fibroblasts or instead reflected extracardiac effects such as altered renin-angiotensin-aldosterone signaling. Studies conducted in cultured cardiac myocytes and fibroblasts provided an answer to this question, but also enabled additional mechanistic insights. We used the combination of AC6 knockdown and pharmacological hypertrophy in cultured cardiac myocytes and established that 1) phenylephrine- (Table 3) and angiotensin II–induced hypertrophy (data not shown) were inhibited by AC6 knockdown, indicating that AC6 levels influence Gq-mediated hypertrophy; 2) AC6 knockdown reduced expression of atrial natriuretic factor and β-myosin heavy chain. Reinduction of these fetal genes is associated with a variety of LV hypertrophy models; and 3) reduced AC6 is associated with decreased cardiac myocyte FHL1 protein content. It was recently reported that FHL1 deletion reduces Gq-induced ERK2 activation, reinduction of fetal gene expression, and cardiac hypertrophy (17). Our results, obtained in vivo and in isolated cardiac myocytes, indicate that FHL1 may be an important mediator for decreased cardiac myocyte hypertrophy after AC6 reduction.
Reduction of AC6 in cultured cardiac fibroblasts decreased expression of collagen types I and III (Figs. 8D to 8F), indicating that the effects of AC6 deletion on collagen expression and LV fibrosis in vivo resulted from cardiac fibroblasts per se and did not require systemic changes in the reninangiotensin-aldosterone axis. Associated with reduced fibroblast collagen production was reduced periostin expression, which also was observed in vivo. Although it is not clear whether periostin directly regulates expression of collagen types I and III, periostin deletion is associated with decreased cardiac collagen content in pressure-overloaded hearts (18). We infer from these results that, in addition to its role in modifying collagen fiber diameter and crosslinking, reduced periostin expression may mediate AC6 knockdown-reduced expression of collagen types I and III.
AC6 expression is beneficial in heart failure; AC6 deletion is beneficial in pressure overload (in females)
A key finding in this study—that AC6 deletion is associated with improved LV function in pressure-overloaded hearts—was not anticipated. Indeed, because we have found consistently that increased cardiac AC6 content is associated with beneficial effects in the failing heart (1,6–8), our hypothesis was that its deletion, in the setting of pressure overload, would have unfavorable consequences. This unexpected result may reflect a variety of factors including: 1) Different pathophysiological models. LV dysfunction due to pressure overload involves signaling pathways different from cardiomyopathy. Thus, the expectation that reduced AC6 expression would have directionally opposite effects from increased AC6 expression independent of pathophysiological context seems to be incorrect. 2) Differences in experimental strategy vis-à-vis AC6 expression. In the present study, we explored the effects of AC6 deletion on the LV response to pressure stress. In previous studies, we tested the effects of AC6 expression on a failing heart (1,6–8). One is a strategy to determine the consequence of a gene deletion, the other a therapeutic challenge. It is not axiomatic that the results of such strategies should be directionally opposite. Finally, AC6 deletion has adverse effects in catecholamine-induced cardiomyopathy associated with sustained isoproterenol infusion, in which LV hypertrophy, dilation, dysfunction, and increased mortality are observed (unpublished data from our laboratory, November 2009), indicating that the beneficial effects of AC6 deletion may be linked specifically with the pressure-stressed heart.
In the AC6-deleted mouse, response to pressure overload is different in males versus females
The higher mortality rates in males was not determined, but correlates with early LV dysfunction and chamber dilation (see Online Supplement). Although we do not have a precise mechanism for this sex difference, the modulation of sex steroid hormones on cardiac function is a plausible contributing factor in this mortality difference (21).
Study limitations
Although our studies have identified altered signaling pathways that likely are of mechanistic importance in the favorable adaptation of the female AC6-deleted heart to pressure stress, the effect of prolonged pressure stress, in excess of 3 weeks, was not studied but may provide additional important insights. For the terminal study, we used pentobarbital in a high dose (100 mg/kg) to obtain the deep level of anesthesia demanded by our animal use committee for surgical procedures. The consequent negative inotropic effect likely influenced the LV physiological studies, although other measures of LV contractile function (fractional shortening, ejection fraction, velocity of circumferential fiber shortening) confirm a group difference. Finally, we did not examine how intracellular cyclic adenosine monophosphate compartmentalization and concentrations (22) are altered by AC6 deletion—factors that may be of additional mechanistic importance.
Conclusions
The deleterious effects of sustained LV pressure overload were reduced in female mice with AC6 deletion. Reductions in FHL1 and periostin expression, direct consequences of reduced AC6 in cardiac myocytes and fibroblasts, appear to be of mechanistic importance for these unanticipated beneficial effects.
Supplementary Material
Acknowledgments
This work was supported by Beginning Grants-In-Aid from the American Heart Association Western States Affiliate (Drs. Tang and Gao), NIH grants P01HL066941, HL081741, and HL088426), and VA Merit Review Awards.
Abbreviations and Acronyms
- AC
adenylyl cyclase
- AC6-KO
AC6-deleted mice
- CON
control mice
- LV
left ventricular
- mRNA
messenger ribonucleic acid
- RT-PCR
reverse transcriptase-polymerase chain reaction
- siRNA
small interfering ribonucleic acid
- TAC
transverse aortic constriction
APPENDIX
For supplemental material, please see the online version of this article.
REFERENCES
- 1.Roth DM, Bayat H, Drumm JD, et al. Adenylyl cyclase increases survival in cardiomyopathy. Circulation. 2002;105:1989–1994. doi: 10.1161/01.cir.0000014968.54967.d3. [DOI] [PubMed] [Google Scholar]
- 2.Tang T, Gao MH, Lai NC, et al. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation. 2008;117:61–69. doi: 10.1161/CIRCULATIONAHA.107.730069. [DOI] [PubMed] [Google Scholar]
- 3.Yan L, Vatner DE, O’Connor JP, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 4.Okumura S, Takagi G, Kawabe J, et al. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci U S A. 2003;100:9986–9990. doi: 10.1073/pnas.1733772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci U S A. 2005;102:437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi T, Tang T, Lai NC, et al. Increased cardiac adenylyl cyclase expression is associated with increased survival after myocardial infarction. Circulation. 2006;114:388–396. doi: 10.1161/CIRCULATIONAHA.106.632513. [DOI] [PubMed] [Google Scholar]
- 7.Lai NC, Tang T, Gao MH, et al. Activation of cardiac adenylyl cyclase expression increases function of the failing ischemic heart in mice. J Am Coll Cardiol. 2008;51:1490–1497. doi: 10.1016/j.jacc.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai NC, Roth DM, Gao MH, et al. Intracoronary adenovirus encoding adenylyl cyclase VI increases left ventricular function in heart failure. Circulation. 2004;110:330–336. doi: 10.1161/01.CIR.0000136033.21777.4D. [DOI] [PubMed] [Google Scholar]
- 9.Tang T, Gao MH, Miyanohara A, Hammond HK. Galphaq reduces cAMP production by decreasing Galphas protein abundance. Biochem Biophys Res Commun. 2008;377:679–684. doi: 10.1016/j.bbrc.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradshaw AD, Baicu CF, Rentz TJ, et al. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation. 2009;119:269–280. doi: 10.1161/CIRCULATIONAHA.108.773424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeGrice I, Pope A, Smaill B. The architecture of the heart: myocyte organization and the cardiac extracellular matrix. In: Villarreal FJ, editor. Interstitial Fibrosis in Heart Failure. New York, NY: Springer; 2005. pp. 5–21. [Google Scholar]
- 12.Heineke J, Auger-Messier M, Xu J, et al. Cardiomyocyte GATA4 functions as a stress-responsive regulator of angiogenesis in the murine heart. J Clin Invest. 2007;117:3198–3210. doi: 10.1172/JCI32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu PH, Ruiz-Lozano P, Zhou Q, Cai C, Chen J. Expression patterns of FHL/SLIM family members suggest important functional roles in skeletal muscle and cardiovascular system. Mech Dev. 2000;95:259–265. doi: 10.1016/s0925-4773(00)00341-5. [DOI] [PubMed] [Google Scholar]
- 14.Matsusaka H, Ide T, Matsushima S, et al. Targeted deletion of matrix metalloproteinase 2 ameliorates myocardial remodeling in mice with chronic pressure overload. Hypertension. 2006;47:711–717. doi: 10.1161/01.HYP.0000208840.30778.00. [DOI] [PubMed] [Google Scholar]
- 15.Gaussin V, Tomlinson JE, Depre C, et al. Common genomic response in different mouse models of beta-adrenergic-induced cardiomyopathy. Circulation. 2003;108:2926–2933. doi: 10.1161/01.CIR.0000101922.18151.7B. [DOI] [PubMed] [Google Scholar]
- 16.Lim DS, Roberts R, Marian AJ. Expression profiling of cardiac genes in human hypertrophic cardiomyopathy: insight into the pathogenesis of phenotypes. J Am Coll Cardiol. 2001;38:1175–1180. doi: 10.1016/s0735-1097(01)01509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheikh F, Raskin A, Chu PH, et al. An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J Clin Invest. 2008;118:3870–3880. doi: 10.1172/JCI34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oka T, Xu J, Kaiser RA, et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norris RA, Damon B, Mironov V, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borg TK, Markwald R. Periostin: more than just an adhesion molecule. Circ Res. 2007;101:230–231. doi: 10.1161/CIRCRESAHA.107.159103. [DOI] [PubMed] [Google Scholar]
- 21.Luczak ED, Leinwand LA. Sex-based cardiac physiology. Annu Rev Physiol. 2008;71:1–18. doi: 10.1146/annurev.physiol.010908.163156. [DOI] [PubMed] [Google Scholar]
- 22.Fischmeister R, Castro LR, Abi-Gerges A, et al. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99:816–828. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.