Abstract
Babesiosis, caused by tick-borne haematozoan organisms of the genus Babesia, is a parasitic disease of domestic and wild mammals. Canine Babesidae have historically been classified as “large Babesia” (Babesia canis) and “small Babesia” (Babesia gibsoni) based on the size of their intraerythrocytic forms. Recent publications, however, suggest that the diversity of piroplasm species infecting dogs might be greater than previously appreciated. Sequencing and phylogenetic analysis of the ssrRNA gene has revealed that canine piroplasms are found in three clades, ‘true’ Babesia sp. (B. canis and B. gibsoni), Theileria annae and Theileria-like group (B. conradae). This newly recognised piroplasm T. annae appears to be hyperendemic in northwest Spain. The vector for this emergent canine infection remains undescribed, although the Ixodeshexagonus is suspected based on their presence upon the dogs in NW Spain and the relative absence of others. These observations have introduced a major change in the approach to the epidemiology of babesiosis in dogs. More clinical samples and data will need to be collected and analyzed to understand the importance of Theileria species in dogs.
Keywords: Babesia gibsoni, Canine babesiosis, Ixodeshexagonus, Molecular detection, Sequence analysis, Thrombocytopenia, Theileria annae
The spectrum of Babesia pathogens that infect dogs has been increased in recent years and is gradually being elucidated with the aid of new molecular techniques and thorough clinical investigations. Accurate detection and species and subspecies recognition are important for the selection of the correct therapy and predicting the course of the disease. Babesia species are often referred to as piroplasm, a collective term for phenotypically similar protozoan parasites that utilize mammalian erythrocytes in their life cycle. The classification of the various Babesias that infect domestic animals rests mainly on their size, morphology and elements in their life cycles (Mehlhorn and Schein 1987; Telford and Spielman 1999) and is in a state of flux. The large piroplasms causing babesiosis in domestic dogs were generally referred to as “Babesia canis” until Uilenberg et al. (1989) drew attention to the fact that there were three separate, vector-specific taxa involved: B. canis (senso stricto), transmitted by Dermacentor reticulatus; Babesia vogeli, transmitted by Rhipicephalus sanguineus; and Babesia rossi transmitted by Haemaphysalis elliptica (previously lumped with Haemaphysalis leachi (Apanaskevich et al. 2007)). B. rossi occurs only in sub-Saharan Africa and Eastern Sudan (Oyamada et al. 2005) and is the most virulent species causing either hemolytic anemia and/or an acute and overwhelming inflammatory response (Reyers et al. 1998). On the other hand, B. canis infection is distributed in Europe and Asia, including India and is capable of causing a wide range of clinical signs such as lethargy, anorexia, fever, jaundice, anemia and thrombocytopenia (Boozer and Macintire 2003). B. vogeli infection is distributed in the Northern and Southern America (Boozer and Macintire 2003), in Europe (Duh et al. 2004), in Eastern and South Africa (Matjila et al. 2004), Australia (Jefferies et al. 2003) and Japan (Inokuma et al. 2004) and leads to a relatively mild disease, often without evidence of clinical signs (Caccio et al. 2002).
Small, solitary ring-like parasites with a diameter of 1–3 μm in Romanowsky-stained thin blood smears from dogs, for example, have routinely been identified as Babesia gibsoni (Anderson et al. 1979).The distribution of small babesiae of dogs which are indiscriminately attributed to Babesia gibsoni includes many countries in Asia as well as the USA (Anderson et al. 1979), Brazil (Braccini et al. 1992), Egypt, Nigeria and Mali (Yamane et al. 1993). Small babesiae cause diseases whose clinical manifestations in dogs are variable and mainly characterized by anemia (Groves and Dennis 1972). Other symptoms frequently found with varying strength include fever, lethargy, anorexia and spleenomegaly (Tiwari and Varshney 2002). These clinical signs are however, not exclusive to small babesiae but occur also with a large babesia of dogs i.e. Babesia canis (Taboada and Merchant 1991).
It was originally assumed that the only small piroplasm infecting dogs is B. gibsoni. The Babesia gibsoni was first described by Patton (1910) for pathogens occurring in dogs in India. This species has a global distribution and infections have been reported in Asia, Northern Africa, Middle East, United States, Australia, Brazil and Europe (Jefferies et al. 2003; Zahler et al. 2000a; Criado-Fornelio et al. 2003a; Varshney et al. 2003, 2004, 2008; Trapp et al. 2006; Hartelt et al. 2007). Recent molecular researches have shown that Californian isolate of small Babesia is genotypically and phenotypically different from the B. gibsoni group and has thus been named Babesia conradae (Kjemtrup et al. 2006).
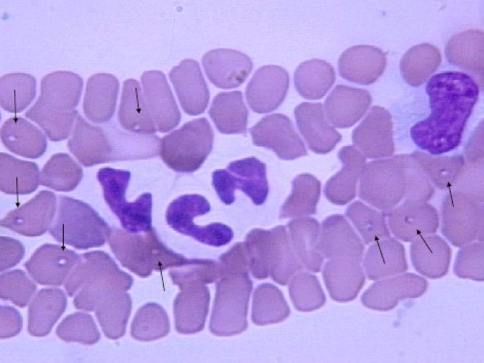
Using molecular characterization, several other small piroplasms have been detected in dogs. Zahler et al. (2000b) performed the genotypic characterization of small piroplasms found in the blood of a dog which was suffering from signs of clinical babesiosis, including apathy, fever and anemia and showing small ring-shaped bodies of 1–2 μm, never more than one per erythrocyte. Pairwise identities as well as, distance, parsimony and maximum likelyhood analysis of the 18S rDNA demonstrated that this isolate was only distantly related to other canine piroplasms characterized genetically so far, including B. gibsoni. It was more closely related to the genus Theileria and was named as Theileriaannae (Fig. 1). In another exhaustive study including 62 dogs of Spain, Garcia (2006) found that severe regenerative anemia and thrombocytopenia were almost constant characteristic of infection with T. annae. Many cases were azotemic. Abnormally high serum concentrations of urea and creatinine, together with elevated concentrations of inorganic phosphorus, hypoalbuminemia, hypercholesterolemia, high protein/creatinine and presence of hyaline and granular casts in the microscopic examination of urine sediment suggested a glomerular component to the disease while hepatic dysfunction was not a common finding. Unfortunately neither urine specific gravity nor urine osmolality was reported in these dogs, but elevated urine proteine:creatinine ratios, hypoalbuminaemia and hypercholesterolaemia in affected dogs led to the authors to suggest that glomerular injury was occurring and that renal failure was most likely a feature of Theileria annae infection. The level of parasitemia was low and not statistically related to the severity of the anemia. Small piroplasm species have been also described in Hungary and Italy based on microscopic observations (Casapulla et al. 1998; Farkas et al. 2004) but since genetic characterization was not attempted, the identity of the parasite species remained unknown.
Fig. 1.
Giemsa-stained thin-smear blood film showing erythrocytes parasitized by Theileria annae piroplasms (Courtesy: Garcia, 2006)
In South Africa, Matjila et al. (2008) examined some dogs with an immune mediated condition with severe thrombocytopenia and detected Theileria species in one sample from Pietermaritzburg and samples from Onderstepoort Veterinary Academic Hospital (OVAH) by PCR in blood samples. Smear examination of three OVAH sample did not show any piroplasms, but may have been under the detection limits for routine light microscopy as often encountered in T. annae infections (Garcia 2006). Although pathophysiology of the detected Theileria sp. in dogs is unknown, it is apparent from a few cases described here that anaemia (possibly hemolytic), splenomegaly and a possible immune mediated syndrome may be associated with this organism. Similar clinical signs were observed in T. annae infection including hematological disorders such as thrombocytopenia which is a common finding in absence of Ehrlichia infection in 75% of dogs infected with T. annae (Garcia 2006).
Other Theileria species that have been reported from dogs are Theileria annulata (Criado et al. 2006) and Theileriaequi (Criado-Fornelio et al. 2003b). T. annulata was detected from an asymptomatic dog (Criado et al. 2006), where as T. equi was detected from three asymptomatic dogs and one symptomatic dog (Criado-Fornelio et al. 2003b). Beck et al. (2009) on the basis of PCR and sequence analysis of a fragment of the ssrRNA gene found 3.42% prevalence of Babesia species in asymptomatic dogs. In the group of symptomatic dogs in Croatia which were all positive by PCR, B. canis was the predominant species (69%) followed by B. gibsoni (21%), B. vogeli (7%) and T. annae (3%).
Ixodes hexagonus has been proposed as the vector of T. annae in NW Spain, the first area where the condition has been reported to be endemic among the canine population (Camacho et al. 2003). Since this tick species is not known to occur in Croatia, additional studies are needed to identify other potential vectors of T. annae. Beck et al. (2009) detected two equine piroplasms, T. equi and B. caballi, in two symptomatic dogs. T. equi and B. caballi are transmitted by a number of tick species within the genera Boophilus, Hyalomma, Dermacentor and Rhipicephalus. All of these vectors, except Boophilus, have been recorded in Croatia and this suggests a potential role of Dermacentor and Rhipicephalus sp. as vectors. However, Rhipicephalus sanguineous seldom bites hosts other than dogs and thus their role in the transmission of these pathogens from horses to dogs in nature is probably minor (Dantas-Torres 2008).
Although Goethert and Telford (2003) questioned the use of Theileria as a genus name since evidence was not presented by Zahler et al. (2000) for a preerythrocytic or lymphocyte-infecting stage, nor was there any evidence for the absence of transovarial transmission in ticks. The provisional assignment to the genus Theileria reflects a controversial argument by some parasitologists working with piroplasms that the small Babesia should be removed from the genus Babesia (Guitián et al. 2003). It is not known whether biological characters classify them as Theileria or Babesia, although phylogenetic analysis indicated their affiliations to the genus Theileria. In the light of above reports based on molecular tools (PCR and sequence analysis), new micro organism has been added to the list of haemoprotozoans infecting dogs. There is paucity of robust evidence regarding the efficacy of drugs that can be used against this species. Drug trials against this species in dogs are scarce. There is only one report of using imidocarb dipropionate @ 4 mg/kg body weight subcutaneously but unfortunately that dog died (Camacho et al. 2002). Buparvaquone like compounds can be tried as these are effective against Theileria sp. in other animals but to our knowledge there is no such published report till date of its use for Theileria sp. in dogs. Efficacy only on the basis of improvement of clinical signs and clearance from blood films is not sufficient but absence of parasite DNA from blood as well as tissues tested by PCR can be sufficient evidence of cure.
The relationship between parasite species and clinical signs, morphological characteristics and drug trials against the infection in dogs deserves further investigation as babesiosis in dogs is being increasingly diagnosed in India on the basis of cytological examination of peripheral blood smears. This new species of piroplasm will almost certainly be described and the geographical range of established piroplasm will expand due to international movements of dogs and expansion over vector tick habitats. The challenges for the researchers are to provide practitioners with readily accessible and accurate diagnostic tools, safer and more efficacious drugs against the parasite. As babesiosis is a zoonotic infection, a description of both epidemiological and clinical features of this infection is of paramount importance for both human and veterinary parasitology.
Contributor Information
Pooja Dixit, Email: poojavet@yahoo.co.in.
Alok K. Dixit, Email: alokdixit7@yahoo.com
References
- Anderson JF, Magnarelli LA, Donner CS, Spielman A, Piesman J. Canine babesia new to North America. Science. 1979;204:1431–1432. doi: 10.1126/science.451574. [DOI] [PubMed] [Google Scholar]
- Apanaskevich DA, Horak IG, Camica JL. Redescription of Haemaphysalis (Rhipistoma) elliptica (Koch, 1844), an old taxon of the Haemaphysalis (Rhipistoma) leachi group from East and Southern Africa, and of Haemaphysalis (Rhipistoma) leachi (Audouin, 1826) (Ixodida, Ixodidae) Onderstepoort J Vet Res. 2007;74:181–208. doi: 10.4102/ojvr.v74i3.122. [DOI] [PubMed] [Google Scholar]
- Beck R, Vojta L, Mrljak V, Marinculic A, Beck A, Zivicnjak T, Caccio SM. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int J Parasitol. 2009;39:843–848. doi: 10.1016/j.ijpara.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Boozer AL, Macintire DK. Canine babesiosis. Vet Clin North Am Small Anim Pract. 2003;33:885–904. doi: 10.1016/S0195-5616(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Braccini GC, Chaplin EL, Stobbe NS, Araujo FAP, Santos NR. Resultados de exames laboratoriais realizados no setor de protozoologia da Faculdade de Veterinaria da Universidade Federal do Rio Grande Do Sul, Porto Alegre, nos anos 1986 a 1990. Arq Fac Vet UFRGS. 1992;20:134–149. [Google Scholar]
- Caccio SM, Antunovic B, Moretti A, Mangili V, Marinculic A, Baric RR, Slemenda SB, Pieniazek NJ. Molecular characterisation of Babesia caniscanis and Babesia canisvogeli from naturally infected European dogs. Vet Parasitol. 2002;106:285–292. doi: 10.1016/S0304-4017(02)00112-7. [DOI] [PubMed] [Google Scholar]
- Camacho AT, Pallas E, Gestal JJ, Guitian FJ, Olmeda AS. Natural infection by a Babesia microti-like piroplasm in a splenectomised dog. Vet Rec. 2002;150:381–382. [PubMed] [Google Scholar]
- Camacho AT, Pallas E, Gestal JJ, Guitián FJ, Olmeda AS, Telford SR, Spielma A. Ixodes hexagonus is the main candidate as vector of Theileria annae in northwest Spain. Vet Parasitol. 2003;112:57–63. doi: 10.1016/S0304-4017(02)00417-X. [DOI] [PubMed] [Google Scholar]
- Casapulla R, Baldi L, Avallone V, Sannino R, Pazzanese L, Mizzoni V. Canine piroplasmosis due to Babesia gibsoni: clinical and morphological aspects. Vet Rec. 1998;142:168–169. doi: 10.1136/vr.142.7.168. [DOI] [PubMed] [Google Scholar]
- Criado A, Martinez J, Buling A, Barba JC, Merino S, Jefferies R, Irwin PJ. New data on epizootiology and genetics of piroplasms based on sequences of small ribosomal subunit and cytochrome b genes. Vet Parasitol. 2006;142:238–247. doi: 10.1016/j.vetpar.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Criado-Fornelio A, Gónzalez-del-Río MA, Buling-Saraña A, Barba-Carretero JC. Molecular characterization of a Babesia gibsoni isolate from a Spanish dog. Vet Parasitol. 2003;117:123–129. doi: 10.1016/j.vetpar.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Criado-Fornelio A, Martinez-Marcos A, Buling-Sarana A, Barba-Carretero JC. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe. Part I. Epizootiological aspects. Vet Parasitol. 2003;113:189–201. doi: 10.1016/S0304-4017(03)00078-5. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173–185. doi: 10.1016/j.vetpar.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Duh D, Tozon N, Petrovec M, Strasek K, Avsic-Zupanc T. Canine babesiosis in Slovenia: molecular evidence of Babesia caniscanis and Babesia canisvogeli. Vet Res. 2004;35:363–368. doi: 10.1051/vetres:2004018. [DOI] [PubMed] [Google Scholar]
- Farkas R, Foldvari G, Fenyves B, Kotai I, Szilagyi A, Hegedus GT. First detection of small babesiae in two dogs in Hungary. Vet Rec. 2004;154:176–178. doi: 10.1136/vr.154.6.176. [DOI] [PubMed] [Google Scholar]
- Garcia ATC. Piroplasma infection in dogs in northern Spain. Vet Parasitol. 2006;138:97–102. doi: 10.1016/j.vetpar.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Goethert HK, Telford SRI. What is Babesia microti? Parasitology. 2003;127:301–309. doi: 10.1017/S0031182003003822. [DOI] [PubMed] [Google Scholar]
- Groves MG, Dennis GL. Babesia gibsoni: field and laboratory studies of canine infections. Exp Parasitol. 1972;31:153–159. doi: 10.1016/0014-4894(72)90057-4. [DOI] [PubMed] [Google Scholar]
- Guitián FJ, Camacho AT, Telford SR., III Case-control study of canine infection by a newly recognised Babesia microti like piroplasm. Prev Vet Med. 2003;61:137–145. doi: 10.1016/S0167-5877(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Hartelt K, Rieker T, Oehme RM, Brockmann SO, Müller W, Dorn N. First evidence of Babesia gibsoni (Asian genotype) in dogs in Western Europe. Vector Borne Zoonotic Dis. 2007;7:163–166. doi: 10.1089/vbz.2006.0580. [DOI] [PubMed] [Google Scholar]
- Inokuma H, Yoshizaki Y, Matsumoto K, Okuda M, Onishi T, Nakagome K, Kosugi R, Hirakawa M. Molecular survey of Babesia infection in dogs in Okinawa, Japan. Vet Parasitol. 2004;121:341–346. doi: 10.1016/j.vetpar.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Jefferies R, Ryan UM, Muhlnickel CJ, Irwin PJ. Two species of canine Babesia in Australia: detection and characterization by PCR. J Parasitol. 2003;89:409–412. doi: 10.1645/0022-3395(2003)089[0409:TSOCBI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kjemtrup AM, Wainwright K, Miller M, Carreno RA. Babesia conradae sp. Nov., a small canine Babesia identified in California. Vet Parasitol. 2006;138:103–111. doi: 10.1016/j.vetpar.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Matjila PT, Penzhorn BL, Bekker CP, Nijhof AM, Jongejan F. Confirmation of occurrence of Babesia canisvogeli in domestic dogs in South Africa. Vet Parasitol. 2004;122:119–125. doi: 10.1016/j.vetpar.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Matjila PT, Leisewitz AL, Oosthuizen MC, Jongejan F, Penzhorn BL. Detection of a Theileria species in dogs in South Africa. Vet Parasitol. 2008;157:34–40. doi: 10.1016/j.vetpar.2008.06.025. [DOI] [PubMed] [Google Scholar]
- Mehlhorn H, Schein E. The piroplasms: life cycle and sexual stages. Adv Parasitol. 1987;23:37–103. doi: 10.1016/S0065-308X(08)60285-7. [DOI] [PubMed] [Google Scholar]
- Oyamada M, Davoust B, Boni M, Dereure J, Bucheton B, Hammad A, Itamoto K, Okuda M, Inokuma H. Detection of Babesia canisrossi, B. canisvogeli, and Hepatozoon canis in dogs in a village of eastern Sudan by using a screening PCR and sequencing methodologies. Clin Diagn Lab Immunol. 2005;12:1343–1346. doi: 10.1128/CDLI.12.11.1343-1346.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton WS. Preliminary report on a new piroplasm (Piroplasma gibsoni sp. nov.) found in the blood of the hounds of the Madras Hunt and subsequently discovered in the blood of the jackal Canis aureus. Bull Soc Pathol Exot. 1910;3:274–280. [Google Scholar]
- Reyers F, Leisewitz AL, Lobetti RG, Milner RJ, Jacobson LS, Zyl M. Canine babesiosis in South Africa: more than one disease. Does this serve as a model for falciparum malaria? Ann Trop Med Parasitol. 1998;92:503–511. doi: 10.1080/00034989859474. [DOI] [PubMed] [Google Scholar]
- Taboada J, Merchant SR. Babesiosis of companion animals and man. Vet Clin North Am Small Anim Pract. 1991;21:103–123. doi: 10.1016/s0195-5616(91)50011-5. [DOI] [PubMed] [Google Scholar]
- Telford SR, Spielman A (1999) Babesiosis of humans. In: Kreier JP, Wakelin D (eds), Topley and Wilsons Microbiology and Microbial Infections, 9th edn, vol 5. Parasitology, Edward Arnold, London, pp 349–360
- Tiwari Pooja, Varshney JP. Canine babesiosis—an overview. Int J Anim Sci. 2002;17:25–32. [Google Scholar]
- Trapp SM, Messick JB, Vidotto O, Jojima FS, Morais HS. Babesia gibsoni genotype Asia in dogs from Brazil. Vet Parasitol. 2006;141:177–180. doi: 10.1016/j.vetpar.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Uilenberg G, Franssen FF, Perie NM, Spanjer AA. Three groups of Babesia canis distinguished and a proposal for nomenclature. Vet Q. 1989;11:33–40. doi: 10.1080/01652176.1989.9694194. [DOI] [PubMed] [Google Scholar]
- Varshney JP, Varshney VP, Hoque M. Clinico-haematological, biochemical, endocrinological and ultrasonographic findings in canine babesiosis. Indian J Anim Sci. 2003;73:1099–1101. [Google Scholar]
- Varshney JP, Kumar A, Hoque M. Ehrlichia platys and Babesia canis infection in a dog: a clinical report. J Vet Parasitol. 2004;18:35–37. [Google Scholar]
- Varshney JP, Deshmukh VV, Chaudhary PS. Multisystemic effects of canine babesiosis and management of critical cases. Intas Polivet. 2008;9:281–287. [Google Scholar]
- Yamane I, Conrad PA, Gardener IA. Babesia gibsoni infections in dogs. J Protozool Res. 1993;3:111–125. [PubMed] [Google Scholar]
- Zahler M, Rinder H, Zweygarth E, Fukata T, Maede Y, Schein E, Gothe R. Babesia gibsoni of dogs from North America and Asia belong to different species. Parasitology. 2000;120:365–369. doi: 10.1017/S0031182099005557. [DOI] [PubMed] [Google Scholar]
- Zahler M, Rinder H, Schein E, Gothe R. Detection of a new pathogenic Babesia microti-like species in dogs. Vet Parasitol. 2000;89:241–248. doi: 10.1016/S0304-4017(00)00202-8. [DOI] [PubMed] [Google Scholar]