Abstract
Polypeptide profile of somatic antigen of Paramphistomum epiclitum (PSAg) and Gastrothylax crumenifer (GSAg) was studied by SDS-PAGE. PSAg and GSAg showed 14 and 19 polypeptides in the range of 14.9–95.5 and 13.7–129.6 kDa with six common polypeptides of mol wt 16.8, 21.8, 23.7, 35.5, 43.4 and 70.8 kDa. P. epiclitum experimentally infected sheep sera were used for identification of specific immuno-dominant peptide in the range of 37–40 kDa against P. epiclitum by western blotting. Hyperimmune sera (HIS) was raised in rabbit against the identified polypeptide, IgG was separated from HIS and an immunoaffinity column was constructed with a binding percentage of 83.74 of IgG with CNBr activated Sepharose 4B. Purification of somatic antigen (PSAg) was done with immunoaffinity chromatography and 37–40 kDa protein antigen was isolated in pure form with recovery percentage of 2.97%. This purified fraction of somatic antigen can be used as a candidate antigen for development of serological assay for early diagnosis of paramphistomosis among livestock.
Keywords: Affinity chromatography, Gastrothylax crumenifer, Immunodiagnosis, Paramphistomum epiclitum, SDS-PAGE, Western blotting
Introduction
In India, livestock products are the most important source of animal protein and account for over 25% of the agricultural gross domestic product (Ghosh et al. 2008). In subtropical and tropical areas, the infection leads to economic losses related to mortality and low productivity (Spence et al. 1996; Kilani et al. 2003). Paramphistomosis, caused by a group of amphistome species, in cattle has a wide range of geographic distribution and is prevalent in several states of India including Punjab (Chhabra et al. 1972; Hassan et al. 2005) and Kashmir valley (Tariq et al. 2008).
For strategic application of available antiparasitic drugs, rapid and specific diagnosis of the disease is of paramount importance. However, the confirmation of most of the helminth diseases is done through detection of eggs in the faeces. In paramphistomosis, it is highly probable not to find eggs or only very few, due to massive infestation with young flukes and relatively long prepatent period therefore, early immunological diagnosis is important (Sanabria and Romero 2008). Immunological and serum antibody detection techniques are not conclusive (Horak 1967, 1971; Alabay 1981; Singh et al. 1983) as the potential of immunodiagnostic assays for early detection of helminth infections is marred by non specificity and cross-reactions, because of shared common antigenic epitopes in several trematodes (Ghosh et al. 2005). The present study was undertaken to identify and isolate immunodominant specific diagnostic polypeptides of Paramphistomum epiclitum that would be helpful in specific diagnosis of paramphistomosis at an early stage in livestock to control the mortality and the economic losses.
Materials and methods
Experimental animals
Healthy Soviet Chinchilla rabbits (two) of 4–5 months of age and 1.5–2 kg b. wt. procured from rabbit farm of Department of Livestock Production and Management, COVS, GADVASU, were used to raise specific hyperimmune sera. Healthy lambs (five) procured from Mattewara sheep farm were maintained on green fodder, wheat bhusa and water ad lib and were used for establishing experimental infection of P. epiclitum.
Raising of metacercariae
The intermediate host of P. epiclitum (Indoplanorbis exustus), were collected from pond of Hambran village of District Ludhiana, Punjab, screened and the cercariae shed were identified as per the method of Prasad et al. (1994). The metacercariae encysted on polythene sheets were counted and stored at 4°C in glass tubes containing distilled water and were used for establishing experimental infection in lambs.
Collection of sera from lambs with experimental infection
Each lamb was orally infected with 5,000 viable metacercariae of P. epiclitum. Blood samples from all the animals were collected from 7 days post-infection (PI) to 56 days PI at weekly interval. The sera was separated and stored in 1.5 ml aliquots at -20°C after adding thiomersal (10 mg/ml) @ 5 μl/ml in eppendorf tubes till further use.
Preparation of somatic antigens
Adult P. epiclitum and Gastrothylax crumenifer collected from rumen and reticulum of goats and buffaloes from the slaughter house, Bareilly, were utilized for preparation of somatic antigen of P. epiclitum (PSAg) and G. crumenifer (GSAg) as per the method of Arora et al. (2007). Both the antigens after protein estimation were stored at −20°C after addition of 10 μg/ml Phenyl methyl sulfonyl fluoride (PMSF) (Lowry et al. 1951).
Identification of immunodominant antigen by SDS-PAGE and western blotting
Both somatic antigens PSAg and GSAg were analyzed by discontinuous SDS-PAGE (Laemmli 1970) with slight modifications in 10% resolving and 5% stacking gel. The antigens from SDS-PAGE were transferred onto 0.45 μm nitrocellulose paper (NCP) as per the method of Towbin et al. (1979). Western blotting was done using sera from experimentally infected sheep as primary antibody according to Arora et al. (2007).
Preparation of specific antigen for hyperimmunization
Several runs of SDS-PAGE (10%) with somatic PSAg were done and the bands of specific immunodominant polypeptides of PSAg identified by western blotting were cut from the gel after staining and were stored in PBS (Phosphate buffer saline, pH 7.2) containing PMSF. They were homogenized in a sterilized pestle and mortar and were centrifuged @ 10,000 rpm for 20 min. The supernatant was collected and used for the raising of specific hyper immune sera in rabbit. The protein concentration was determined (Lowry et al. 1951).
Raising of hyper immune sera
Stable water in oil emulsion of equal volume of antigen and adjuvant was used as inoculum. A total of 300 μg of antigen was used for the primary immunization followed by three booster doses at 14, 28 and 42 days post immunization for raising of hyperimmune sera. Incomplete Freund’s Adjuvant (IFA) was used for all doses of immunization as Freund’s Complete Adjuvant (FCA) when used causes skin ulceration, necrosis and may even lead to temporary or permanent muscle lesion.
Confirmation and collection of hyperimmune sera
Rabbit blood was collected after 10 days of third booster intracardially after confirmation of raising of hyper immune sera by Double Immunodiffusion (DID) and Western blotting with PSAg from both the rabbits. Sera was separated and stored at −20°C after adding thiomersal (10 mg/ml) @ 5 μl/ml of sera.
Construction of immunoaffinity column
Immunoglobulins (Ig) were precipitated from the rabbit sera with ammonium sulphate as per the method of Fey et al. (1976). The precipitated Igs were extensively dialysed against coupling buffer i.e. 0.1 M NaHCO3, 0.5 M NaCl, pH 8.5 for 36 h and coupled to swelled CNBr activated Sepharose-4B.
Affinity purification of antigen
A total of 28 mg equilibrated (20 mM Tris saline, pH 8.0) PSAg was loaded on the pre-equilibrated affinity column and then washed with excess equilibrating buffer. The bound proteins were eluted using 0.2 M Glycine HCl (pH 2.2) and the pH of the eluted fractions was brought to neutral by adding 2 M Tris. The absorbance of fractions was measured at 260 and 280 nm on a spectrophotometer (Perkin Elmer Lambda 25) and the protein concentration was estimated (Aiken and Learmoth 1996). The column was regenerated using 0.1 M Tris–HCl, 0.5 M NaCl, pH 8.5, 0.1 M Sodium acetate and 0.5 M NaCl, pH 4.5 after each use. The eluted bound protein was dialysed extensively against 20 mM tris-saline buffer, pH 7.4, concentrated with PEG 20,000 and was designated as affinity purified P. epiclitum somatic antigen (Aff-PSAg). The protein content of the antigen was determined spectrophotometrically (Aiken and Learmoth 1996).
SDS-PAGE confirmation of purified antigen
Characterization of Aff-PSAg was done by SDS-PAGE followed by staining with Coomassie Brilliant blue.
Results and discussion
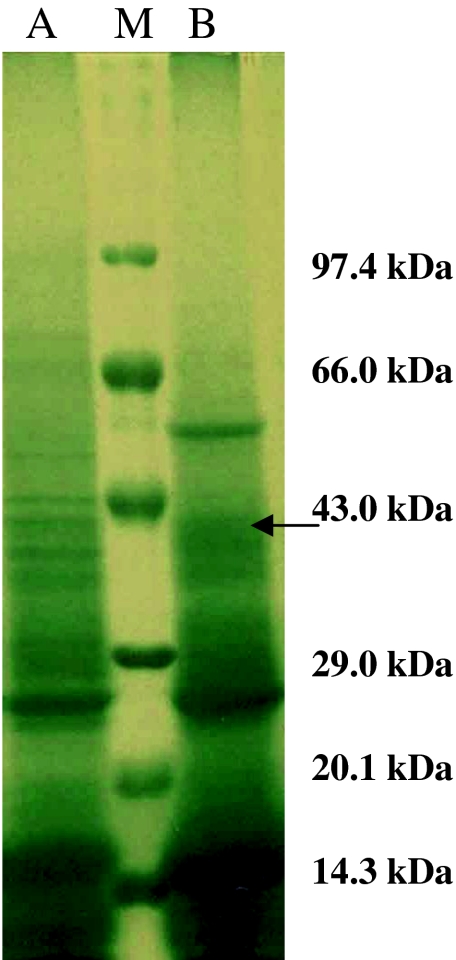
Electrophoretic analysis of referral antigens i.e. PSAg resolved into 14 polypeptides with mol wt of 95.5, 81.8, 70.8, 54.6, 51.6, 43.4, 39.8, 37.6, 35.5, 33.1, 23.7, 21.8, 16.8 and 14.1 kDa. Similarly GSAg resolved into 19 polypeptides of 129.6, 125.9, 102.9, 100.0, 77.2, 70.8, 56.2, 53.1, 43.4, 40.7, 36.7, 35.5, 32.4, 26.6, 23.7, 22.4, 21.8, 16.8 and 13.7 kDa. Six polypeptides of the mol wt 16.8, 21.8, 23.7, 35.5, 43.4 and 70.8 kDa were found common among PSAg and GSAg (Fig. 1). Somatic antigen of G. crumenifer (GSAg) was used in the study as this amphistome parasite was found to be concurrently present along with P. epiclitum in the rumen and reticulum of the infected host.
Fig. 1.
Polypeptide profile of Somatic antigen of P. epiclitum and G. crumenifer. Lane M Molecular weight marker in range of 14.3–97.4 kDa. Lane A Adult somatic antigen of G. crumenifer (GSAg). Lane B Adult somatic antigen of P. epiclitum (PSAg)
The western blot analysis of PSAg revealed 5 immunodominant polypeptides of the mol wt 39.8, 37.6, 35.5, 33.1 and 21.8 kDa, whereas GSAg using same experimental sheep sera raised against P. epiclitum revealed 2 immunodominant polypeptides of mol wt 35.5 and 21.8 kDa (Arora et al. 2007). Two polypeptides of mol wt 37.6 and 39.8 kDa in the range of 37–40 kDa were utilized to raise P. epiclitum specific hyperimmune sera in rabbits, since no such polypeptides were evident in the polypeptide profile of G. crumenifer. A strong precipitin line was obtained against the rabbit sera collected on 10th day of last immunization (52 days PI) using the specific antigen. Further confirmation was done by western blotting when polypeptides in the NCM were probed with hyperimmune sera raised against PSAg.
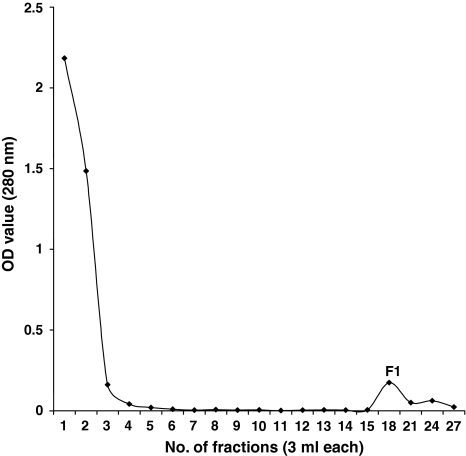
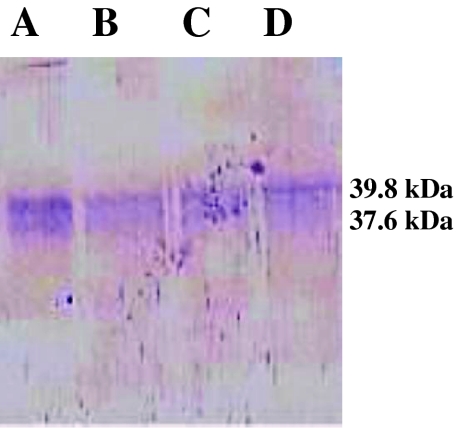
The percentage binding of IgG with CNBr activated Sepharose-4B was 83.74%. Out of a total of 28.0 mg of PSAg loaded in 2 batches on the immunoaffinity column, 0.83 mg Aff-PSAg was eluted as F1 with a recovery percentage of 2.97 (Fig. 2). Electrophoretic separation of Aff-PSAg resolved into 2 polypeptides of mol wt 39.8 and 37.6 kDa (Fig. 3). Hence the isolation of these polypeptides was achieved in pure form. In similar study, low molecular weight polypeptides of Mr <14, 15–19, 23–24, 27–28 and 30–33 kDa pertaining to fractions F1–F8 were predominantly antigenic in G. crumenifer (Saifullah et al. 2000).
Fig. 2.
Affinity chromatographic elution pattern of Somatic antigen of P. epiclitum (PSAg) F1: affinity purified P. epiclitum somatic antigen (Aff-PSAg)
Fig. 3.
Gel of affinity purified P. epiclitum somatic antigen (Aff PSAg) (Lane A–D)
The probability of diagnosis of animals infected with paramphistomes is more by detecting the circulating antibodies in the sera samples of infected animals as compared to the functional/structural antigen of the parasite by ELISA (Maji et al. 1999). However, the success of the diagnostic test depending upon the antibody detection depends heavily on identification of suitable antigen specific for paramphistomosis, because the efficacy of immunodiagnostic test is hampered by the possession of common antigenic epitopes by various helminths, thus leading to lack of specificity. To overcome these disadvantages encountered with the use of somatic antigens, identification and purification of specific antigen was carried out in the study. Similar observations has been reported in past with related concomitant parasitic infections i.e. fasciolosis, dicrocoeliosis, gastrointestinal strongylosis (Otranto and Traversa 2002).
Several immunogenic diagnostic polypeptides have been identified for diagnosis of Fasciola spp. and other helminths till date (Ruppel et al. 1985; Indrawati et al. 1991; Attallah et al. 2002; Sarimehmetoglu 2002; Ghosh et al. 2005). Efficacy of various stage specific antigens of P. epiclitum was compared by ELISA (Jyoti 2001) and revealed that immature intestinal stage antigen was more immunogenic as compared to metacerial, immature ruminal and adult somatic antigens. However, till date no specific diagnostic polypeptide against P. epiclitum has been identified and purified. Affinity chromatography has been shown to be a very effective tool for isolation of candidate diagnostic and vaccine molecules (Ghosh et al. 1999; Sharma et al. 2001; Singh and Ghosh 2003; Yokananth et al. 2005). The affinity purified antigens for Fasciola spp. have shown high sensitivity and specificity (Fagbemi and Guobadia 1995; Intapan et al. 2003; Ghosh et al. 2005; Yokananth et al. 2005). Hence, on similar lines affinity chromatography was employed in the present study for the purification of specific immunodominant polypeptides against P. epiclitum.
As a proven fact that purified proteins are better antigens for serological diagnosis as compared to crude somatic antigens, the purified polypeptides of the present study can be a candidate antigen for diagnostic tests like ELISA. The problem of cross-reactivity which hampers the development of any specific diagnostic test for helminthic infection can be overcome by the use of purified antigen and can be achieved by the antigen isolated in the present study for early diagnosis of paramphistomosis. However, ELISA at large scale need to be carried out with sera collected from field, slaughter house and experimentally infected animals to work out the sensitivity and specificity of the test. Thus this purified antigen can lay the possible foundation for development of a serological kit for early diagnosis of paramphistomosis.
Acknowledgements
The authors are grateful to Guru Angad Dev Veterinary and Animal Sciences University (GADVASU), Ludhiana for providing necessary help for conduction of the work. The experimental animals used in the study were permitted by Institutional Animal Ethics committee of GADVASU.
Footnotes
Part of MVSc thesis of first author submitted to GADVASU, Ludhiana.
References
- Aiken A, Learmoth M. Protein determination by UV absorption. In: Walker JM, editor. The protein protocols hand book. Totowa, NJ: Humana Press; 1996. [Google Scholar]
- Alabay M. Comparative diagnostic studies on Paramphistomum cervi infection in sheep with the immunoperoxydase (ELISA) and indirect immunofluorescence (IFAT) techniques. Veteriner Fakultesi Dergesi Ankara Universitesi. 1981;28:72–88. [Google Scholar]
- Arora R, Singh NK, Hassan SS, Juyal PD. Identification of immunodominant antigens of Paramphistomum epiclitum. J Vet Parasitol. 2007;21:117–120. [Google Scholar]
- Attallah AM, Karawia EA, Ismail HI, Tabll AA, Nawar AA, Ragab WA, Abdel AMM, El-Dosoky. Identification and characterization of a 26–28 kDa circulating antigen of Fasciola gigantica. Ann Trop Med Parasitol. 2002;96:271–282. doi: 10.1179/000349802125000754. [DOI] [PubMed] [Google Scholar]
- Chhabra RC, Kwatra MS, Hothi DS. Immature paramphistomosis in sahiwal and cross bred calves in Punjab. Indian J Anim Sci. 1972;43:272. [Google Scholar]
- Fagbemi BO, Guobadia EE. Immunodiagnosis of fasciolosis in ruminants using a 28 kDa cysteine protease of Fasciola gigantica adult worms. Vet Parasitol. 1995;57:309–318. doi: 10.1016/0304-4017(94)00684-5. [DOI] [PubMed] [Google Scholar]
- Fey H, Phster H, Messerli J, Sturzenegger N, Grolimund F. Methods of isolation, purification and quantitation of bovine immunoglobulins. A technical review. Zentralblatt fur Veterinar Medizin. 1976;23:269. doi: 10.1111/j.1439-0450.1976.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Khan MH, Ahmed N. Cross-bred cattle protected against Hyalomma anatolicum anatolicum by larval antigens purified by immunoaffinity chromatography. Trop Anim Health Prod. 1999;31:263–273. doi: 10.1023/A:1005218821889. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Rawat P, Velusamy R, Joseph D, Gupta SC, Singh BP. 27 kDa Fasciola gigantica glycoprotein for the diagnosis of prepatent fasciolosis in cattle. Vet Res Commun. 2005;29:123–135. doi: 10.1023/B:VERC.0000047497.57392.8c. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Ray DD, Vanlahmuaka, Das G, Singh NK, Sharma JK, Azhahianambie P. Progress in development of vaccine against Hyalomma anatolicum anatolicum—Indian scenario. Vaccine. 2008;26S:G40–G47. doi: 10.1016/j.vaccine.2008.09.067. [DOI] [PubMed] [Google Scholar]
- Hassan SS, Kaur K, Joshi K, Juyal PD. Epidemiology of paramphistomosis in domestic ruminants in different districts of Punjab and other adjoining areas. J Vet Parasitol. 2005;19:43–46. [Google Scholar]
- Horak IG. Host-parasite relationships of Paramphistomum microbothrium Fischoeder, 1901, in experimentally infested ruminants, with particular reference to sheep. Onderstepoort J Vet Res. 1967;34:451–454. [PubMed] [Google Scholar]
- Horak IG. Paramphistomosis of ruminants. Adv Parasitol. 1971;9:33–72. doi: 10.1016/S0065-308X(08)60159-1. [DOI] [PubMed] [Google Scholar]
- Indrawati I, Chaicumpa W, Setasuban P, Ruangkunaporn P. Studies on immunodiagnosis of human paragonimiasis and specific antigen of Paragonimus heterotremus. Int J Parasitol. 1991;121:395–401. doi: 10.1016/0020-7519(91)90096-P. [DOI] [PubMed] [Google Scholar]
- Intapan PM, Maleewong W, Nateeworanart S, Wongkham C, Pipitgool V, Sangmaneedet S. Immunodiagnosis of human fasciolosis using an antigen of Fasciola gigantica adult worm with molecular mass of 27 kDa by a dotELISA. Southeast Asian J Trop Public Health. 2003;34:713–717. [PubMed] [Google Scholar]
- Jyoti (2001) Studies on immunodiagnosis of Paramphistomum epiclitum in kids using stage specific antigens, M.V.Sc. thesis, IVRI, Deemed University, Izatnagar
- Kilani K, Guillot J, Chermette R (2003) Amphistomoses digestives. In: Principales maladies infectieuses et parasitaires du be’tail. Ed. Tec & Doc, Paris, pp 1400–1410
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maji BP, Dwivedi P, Rao JR, Yadav SC. Cross antigenicity between ruminal and hepatic paramphistomes and liver flukes of buffalo origin. J Appl Anim Res. 1999;16:53–57. [Google Scholar]
- Otranto D, Traversa D. A review of dicrocoeliosis of ruminants, recent advances in the diagnosis and treatment. Vet Parasitol. 2002;107:317–335. doi: 10.1016/S0304-4017(02)00121-8. [DOI] [PubMed] [Google Scholar]
- Prasad A, Malviya HC, Varma TK, Dwivedi P. Development of Paramphistomum epiclitum Fischoeder 1904 in the snail host. Indian J Anim Sci. 1994;64:568–573. [Google Scholar]
- Ruppel A, Diesfeld HJ, Rother U. Immunoblot analysis of Schistosoma mansoni antigens with sera of schistosomiasis patient: diagnostic potential of an adult schistosome polypeptide. Clin Exp Immunol. 1985;62:499–506. [PMC free article] [PubMed] [Google Scholar]
- Saifullah MK, Ahmad G, Nizami WA, Abidi SMA. Partial purification and characterization of Gastrothylax crumenifer somatic antigens. Vet Parasitol. 2000;89:23–29. doi: 10.1016/S0304-4017(00)00192-8. [DOI] [PubMed] [Google Scholar]
- Sanabria REF, Romero JR. Review and update of paramphistomosis. Helminthologia. 2008;45(2):64–68. doi: 10.2478/s11687-008-0012-5. [DOI] [Google Scholar]
- Sarimehmetoglu OH. Application of western blotting for the immunodiagnosis of Fasciola hepatica in cattle using excretory/secretory antigens. Turkish J Vet Anim Sci. 2002;26:1061–1065. [Google Scholar]
- Sharma JK, Ghosh S, Khan MH, Das G. Immunoprotective efficacy of 39 kDa purified nymphal antigen of Hyalomma anatolicum anatolicum. Trop Anim Health Prod. 2001;33:103–113. doi: 10.1023/A:1005281429652. [DOI] [PubMed] [Google Scholar]
- Singh NK, Ghosh S. Experimental immunization of crossbred cattle with glycoprotein isolated from the larvae of Hyalomma anatolicum anatolicum and Boophilus microplus. Exp Appl Acarol. 2003;3:297–314. doi: 10.1023/B:APPA.0000010382.73735.23. [DOI] [PubMed] [Google Scholar]
- Singh RP, Prasad KD, Ansari MZ, Sahai BN. Observations on intradermal skin test for diagnosis of Paramphistomum cervi in goats. Indian J Anim Health. 1983;22:67–68. [Google Scholar]
- Spence SA, Fraser GC, Chang S. Response in milk production to the control of gastro intestinal nematode and paramphistomeparasites in dairy cattle. Aust Vet J. 1996;74:456–459. doi: 10.1111/j.1751-0813.1996.tb07569.x. [DOI] [PubMed] [Google Scholar]
- Tariq KA, Chishti MZ, Ahmad F, Shawl AS. The epidemiology of paramphistomosis of sheep (Ovis aries L.) in the north west temperate Himalayan region of India. Vet Res Commun. 2008;32:383–391. doi: 10.1007/s11259-008-9046-x. [DOI] [PubMed] [Google Scholar]
- Towbin HT, Staehelin T, Gordon J. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokananth S, Ghosh S, Gupta SC, Suresh MG, Saravanan D. Characterization of specific and cross-reacting antigens of Fasciola gigantica by immunoblotting. Parasitol Res. 2005;97:41–48. doi: 10.1007/s00436-005-1371-1. [DOI] [PubMed] [Google Scholar]