Abstract
The present study has been carried out to find out the prevalence of ectoparasites of carp fingerlings during different months of the year 2008–2009. Four groups of ectoparasites viz. myxozoan, ciliophoran, monogenean and crustacean were recorded from 400 fingerlings of Rohu (Labeo rohita), Catla (Catla catla), Mrigal (Cirrhinus mrigala), Bata (Labeo bata), Common carp (Cyprinus carpio) and Lata (Channa punctatus) collected from different ponds of Nadia and Hooghly district of West Bengal from June 2008 to May 2009. The highest prevalence (51.66%) of infection has been recorded in ciliophorans and the lowest was in crustacean (17.5%) between the months of December and February. The highest ectoparasitic prevalence (36.85%) was recorded during winter season (December–February) while the lowest prevalence (9.16%) recorded during rainy season (June–August).
Keywords: Prevalence, Ectoparasite, Fingerlings, West Bengal
Introduction
Aquaculture is one of the most economically important applied strategies all over the world and fishes are one of the most beneficial and nutritional resources of human beings. The entire water area of West Bengal supports the potential fish farming of the state as well as the whole country. The major carp farming mainly dominates fresh water aquaculture of West Bengal. The most important prerequisite of fish production is availability of healthy fish fingerlings of carps. It is evident from the available literature that parasitic diseases caused significant damage in nursery system of carp mostly affecting the fry and fingerlings (Gopalkrisnan 1961). The parasitic community of fish show considerable variation with the environmental conditions in which fish live (Hossain et al. 2008). Certain environmental conditions are more conducive to disease among which water temperature is one of the important criteria associated with disease out break.
It was observed that the prevalence of the disease was more in the winter season (Ahmed et al. 1991) than the other months of the year. The present study deals with ectoparasitic prevalence of carp fingerlings as well as the correlation between ectoparasitic prevalence and environmental conditions.
Materials and methods
The present study was done during the period of June 2008–May 2009. Live carp fingerlings of Rohu (Labeo rohita), Catla (Catla catla), Mrigal (Cirrhinus mrigala), Bata (Labeo bata), Common carp (Cyprinus carpio) and Lata (Channa punctatus) were randomly collected from the adjacent fish market and ponds of Nadia and Hooghly. The fishes were brought to the laboratory and kept in the small water bodies (vats) containing pond water in the vicinity of the university. A total number of 400 fingerlings were observed for ectoparasitic infection. The infected fishes were collected and examined in every month of the year. The gill, body, and tail fin smear were prepared on grease free clean slides with a drop of 0.5% NaCl solution and air-dried. The Indian ink method of Lom and Vavrá (1963) was employed to identify the myxozoan spore and for permanent preparation, the air-dried smears were stained with Giemsa. The ciliophoran parasites were subjected to silver impregnation after the method of Klein (1958). The monogenean parasites were observed following the method of Yamaguti (1963). The crustacean parasites were stained with Cotton blue stain for permanent preparation.
The months were divided into four groups viz June–August, September–November, December–February and March–May indicating four seasons i.e., rainy, autumn, winter and summer on the basis of seasonal variation. The prevalence was calculated as number of infested fish divided by number of observed fish multiplied by hundred. The three main water quality parameters viz water temperature; pH and dissolved oxygen were measured each month from the local ponds. These are the main water quality parameters related to fish health. For the analysis of dissolved oxygen water was collected from column region in DO bottles and fixed with MnSO4 and alkaline KI. Water temperature is measured by mercury thermometer and pH by Pen pH meter.
Results and discussion
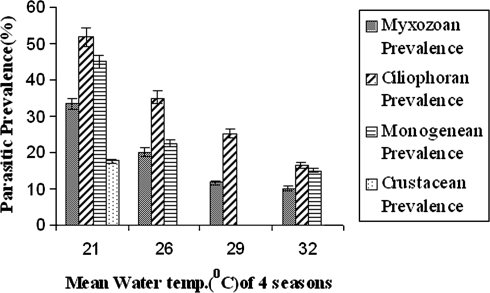
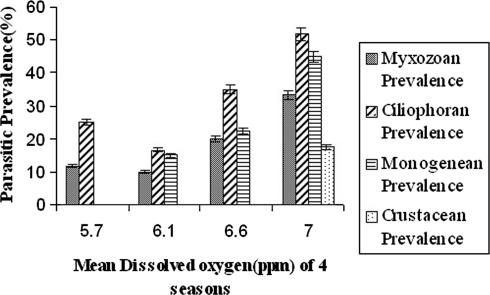
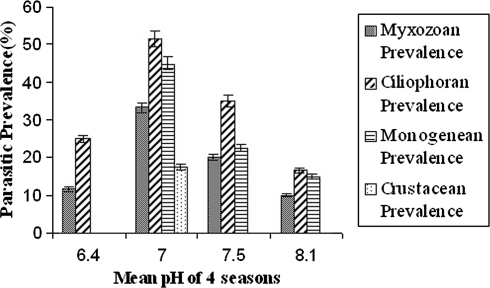
The percentages of infection by different ectoparasites in four groups with months have been incorporated in Table 1. The average prevalence of each group of ectopaarasites i.e., myxozoan, ciliophoran, monogenean and crustacean have been listed in Table 2. It is clear that prevalence of ectoparasitic infection changes with month. In winter season i.e., December–February it reaches its peak (Table 2). The correlation between mean water temperature of four different seasons i.e., winter, autumn, rainy and summer, with the parasitic prevalence of four different groups i.e., myxozoans, ciliophorans, monogeneans and crustaceans are given in Fig. 1. The correlation between mean dissolved oxygen (ppm) of four different seasons i.e., rainy, summer, autumn, and winter with the parasitic prevalence of four different groups i.e., myxozoans, ciliophorans, monogeneans and crustaceans are given in Fig. 2. The correlation between mean pH value of four different seasons i.e., rainy, winter, autumn, and summer with the parasitic prevalence of four different groups i.e., myxozoans, ciliophorans, monogeneans and crustaceans are given in Fig. 3. It is also noticed that some group of parasites are totally absent in some particular season, such as the crustacean parasites are found only in the months of December–February but not in rest of the year. The monogenean parasites are also not found during the rainy months i.e., June–August. Among the myxozoan parasites, Henneguya is reported only during the winter months i.e., December–February. The Ich infection is reported only during September–February when the water temperature is low in comparison to other months (Table 1). The monthly prevalence data reveals that the ectoparasitic infection is heavy during December–February which leads to the conclusion that biological factors of the host as well as the water quality are responsible for infestation. The parasite infects the adult fishes. Since the fingerlings are delicate, they are more susceptible to infection. The water temperature, pH and dissolved oxygen are three water parameters that are related to disease infestation as they fluctuate more rapidly. The data of these three parameters with their mean and ±SD have been shown in Table 3. Ectoparasitic prevalence decreases with the increasing temperature of water (Fig. 1) and pH (Fig. 3).
Table 1.
Prevalence of individual ectoparasites in Carp fingerlings in different months of the year
| Months | Myxozoans | Prevalence (%) | Ciliophorans | Prevalence (%) | Monogeneans | Prevalence (%) | Crustacean | Prevalence (%) |
|---|---|---|---|---|---|---|---|---|
| June–August | Myxobolus sp. | 25 | Trichodina sp. | 30 | Dactylogyrus sp. | 0 | Ergasilus sp. | 0 |
| Thelohanellus sp. | 10 | Tripertiella sp. | 45 | Gyrodactylus sp. | 0 | Argulus sp. | 0 | |
| Henneguya sp. | 0 | Ichthyophthirius multifilis | 0 | |||||
| September–November | Myxobolus sp. | 40 | Trichodina sp. | 55 | Dactylogyrus sp. | 30 | Ergasilus sp. | 0 |
| Thelohanellus sp. | 20 | Tripertiella sp. | 35 | Gyrodactylus sp. | 15 | Argulus sp. | 0 | |
| Henneguya sp. | 0 | Ichthyophthirius multifilis | 15 | |||||
| December–February | Myxobolus sp. | 60 | Trichodina sp. | 65 | Dactylogyrus sp. | 55 | Ergasilus sp. | 30 |
| Thelohanellus sp. | 35 | Tripertiella sp. | 40 | Gyrodactylus sp. | 35 | Argulus sp. | 5 | |
| Henneguya sp. | 5 | Ichthyophthirius multifilis | 50 | |||||
| March–May | Myxobolus sp. | 20 | Trichodina sp. | 20 | Dactylogyrus sp. | 20 | Ergasilus sp. | 0 |
| Thelohanellus sp. | 10 | Tripertiella sp. | 25 | Gyrodactylus sp. | 10 | Argulus sp. | 0 | |
| Henneguya sp. | 0 | Ichthyophthirius multifilis | 5 | |||||
| Average in total year | Myxozoans | 18.7 | Ciliophorans | 32 | Monogeneans | 20.6 | Crustacean | 4.3 |
Table 2.
Monthly prevalence (%) of ectoparasites in carp fingerlings
| Parasites | June–August | September–November | December–February | March–May |
|---|---|---|---|---|
| Myxozoans | 11.6 | 20 | 33.3 | 10 |
| Ciliophorans | 25 | 35 | 51.6 | 16.6 |
| Monogeneans | 0 | 22.5 | 45 | 15 |
| Crustaceans | 0 | 0 | 17.5 | 0 |
| Average of total parasitic prevalence | 9.1 | 19.3 | 36.8 | 10.4 |
Fig. 1.
Correlation between mean water temperature (°C) of four seasons and parasitic prevalence (%) of four different groups
Fig. 2.
Correlation between mean dissolved oxygen level (ppm) of four seasons and parasitic prevalence (%) of four different groups
Fig. 3.
Correlation between mean pH value of four seasons and parasitic prevalence (%) of four different groups
Table 3.
Monthly fluctuations of water quality parameters in nursery pond
| Months | Water temperature (°C) | DO (ppm) | pH |
|---|---|---|---|
| June–August | 29 | 5.7 | 6.4 |
| September–November | 26 | 6.6 | 7.5 |
| December–February | 21 | 7 | 7.0 |
| March–May | 32 | 6.1 | 8.1 |
| Mean ± SD | 27 ± 3.7 | 6.4 ± 0.5 | 7.25 ± 0.6 |
Fish fingerlings become more susceptible to infection because of their immature immune system (Anderson 1974), which corroborated with the present findings. It has been previously reported that shallow ponds and stagnant water favors multiplication of ciliate like Trichodina (Kabata 1985). Trichodina sp. is the most prevalent ectoparasite followed by Dactylogyrus sp. in Philippines (Lumanlan et al. 1992). These two parasites were most common and caused mass mortality in fish in peninsular Malaysia as well (Shariff and Vijiarungam 1986). The findings in Sri Lanka also corroborated with the work done by Subashinghe (1992). In Bangladesh a survey report on ectoparasitic infestation revealed that Trichodina is the most prevalent ectoparasite followed by Myxobolus sp. (Hossain et al. 2008).
The ectoparasitic infection is highest during December–February when the water quality detoriates due to decrease of the temperature and dissolved oxygen level. Since the fingerlings require more O2 and due to lack of O2 they become more prone to infection. High stocking density of fingerlings is another reason for ectoparasitic disease outbreak (Hossain et al. 2008). High stocking density increases the chance of ectoparasite transmission from fish to fish easily. The availability of host for the ectoparasitic infection increases with the increasing stocking density. Thus it can be concluded that the water quality has a great impact on the abundance of pathogens and their ability to survive on host. So the stocking density and water quality should be maintained properly to avoid parasitic infestation in pond.
Acknowledgment
One of the authors (SB) is thankful to the Department of Science and Technology, Govt. of India, New Delhi for financial support to run the project (Project Ref. No. ST (WB)/ST/LSR/461).
References
- Ahmed A, Ali SMK, Samad A. Probable cause of fish ulcer in Bangladesh. Nutri News. 1991;14(1):3. [Google Scholar]
- Anderson DP (1974) Fish immunology. In: Sneiszko SF, Axelrod HR (eds) Diseases of fishes. F. H. Publication Inc., New Jersey
- Gopalkrisnan V. Observation on a new epidemical eye disease affecting the Indian Carp Catla catla (Hamilton-Buchanan) Ind J Fish. 1961;8:222–232. [Google Scholar]
- Hossain MD, Hossain MK, Rahaman MH, Akter A, Khanom DA. Prevalence of ectoparasites of carp fingerlings at Santaher, Bogra. Univ J Zool Rajshahi Univ. 2008;27:17–19. [Google Scholar]
- Kabata Z. Parasites and diseases of fish cultured in the tropics. London: Taylor and Francis; 1985. [Google Scholar]
- Klein BM. The dry silver method and its proper use. J Protozool. 1958;5:99–103. [Google Scholar]
- Lom J. Trichodina reticulata Hirschmann and Partsch 1955 from Crucian carp, and T. domergueif. latispina Dogel 1940 from Diaptomus. Acta Soc Zol. 1960;3:246–257. [Google Scholar]
- Lom J, Vavrá J. Mucous envelope of spores of the subphylum Cnidospora (Deflein, 1901) Vist Esl Spol Zool. 1963;27:4–6. [Google Scholar]
- Lumanlan SC, Albaladejo JD, Bondad-Reartaso MG, Arthur JR. Fresh-water fishes imported into the Philippines. In: Shariff M, Subashinghe RP, Arthur JR, editors. Diseases in Asian aquaculture. 1. Philippines: Asian Fisheries Society; 1992. [Google Scholar]
- Shariff M, Vijiarungam A (1986) Occurrence of parasites at the fish breeding station in peninsular Malaysia and their control. In: Chan HH, Ang KJ, Law AT et al (eds) International conference on development and management of tropical living aquatic resources. Universiti Pertanian, Malaysia, 2–5 Aug 1983, pp 68–73
- Subashinghe RP. Hatchery disease of fresh water fishes in Sri Lanka. In: Sharif M, Subashinghe RP, Arthur JR, editors. Diseases in Asian aquaculture. 1. Philippines: Asian Fisheries Society; 1992. [Google Scholar]
- Yamaguti S. Systema Helminthum vol. Acanthocephala of vertebrates. New York: Interscience Publishers; 1963. [Google Scholar]