Abstract
Thymidylate synthase is a target of 5-fluoruracil, a pyrimidine analog used to treat gastrointestinal and other cancers. The 5-fluorouracil metabolite, fluoro-deoxyuridine monophosphate, forms a ternary complex with thymidylate synthase and 5,10-methylene tetrahydrofolate. The purpose of this study was to evaluate the time-honored connection between thymidylate synthase and 5-fluorouracil. From our literature search spanning reports from 1995 to 2007 published in journals having an impact factor greater than two, we stratified the tumors within each article, according to low versus high thymidylate synthase expression. These groups were subdivided into responders, stable disease or disease progression. The relationship between thymidylate synthase expression and 5-fluorouracil response was analyzed for the overall group, as well as for subsets. Overall, the literature supported an approximately two-fold inverse relationship between thymidylate synthase expression and response to 5-fluoruracil. We found no change in the trend for a relationship between thymidylate synthase and 5-fluorouracil when the literature was stratified by date of publication, impact factor of the journal in which the report was published, or substrate (mRNA versus protein) for measuring thymidylate synthase expression. Of note, there is no significant change in the trend when comparing 5-fluorouracil treatment alone or in combination with leucovorin. We found a decline of this trend when certain chemotherapeutics were used in combination with 5-fluorouracil. In sum, the connection between thymidylate synthase expression and patient response to 5-fluorouracil does not satisfy expectations for an effective drug-target relationship; and thus, studies of the thymidylate synthase tandem repeat status might only be clinically valuable in regards to patient toxicity. Thus, we question the reliability of thymidylate synthase expression as a clinical predictor of 5-fluorouracil response. Future research could perhaps be directed towards alternate targets and metabolites of 5-fluorouracil, in an effort to find a clinically relevant biomarker panel for response and to optimize fluoropyrimidine-based therapy.
Keywords: thymidylate synthase, 5-fluorouracil, clinical response, pharmacogenomics, chemotherapeutic resistance, pharamocogenetic window, resistance, sensitivity
Introduction
A short history of 5-fluorouracil
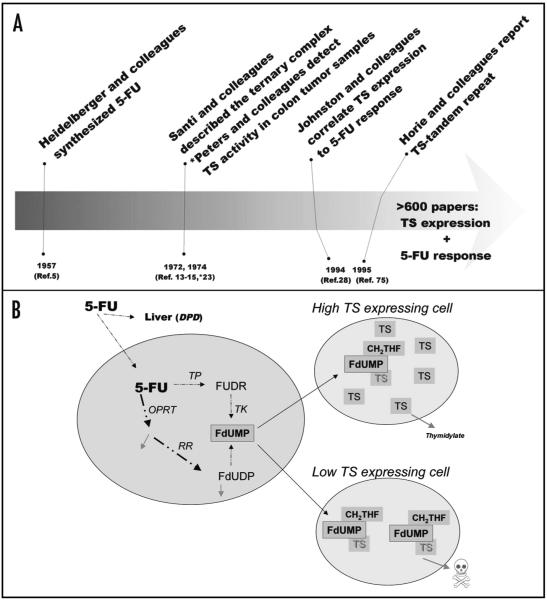
5-fluorouracil (5-FU) was originally developed as a chemotherapeutic agent over 50 years ago and remains in wide use as treatment for a variety of tumor types, both alone and in combination with other drugs.1,2 The genesis of 5-FU was based on the observation that uracil utilization differs between malignant and non-malignant cells, as studied in a rat model of hepatoma.3,4 As an early example of rational drug design, Heidelberger and colleagues synthesized 5-FU in 1957 by substituting a fluorine atom for a hydrogen atom in the pyrimidine ring to create a competitive antagonist of uracil (Fig. 1A).5 In the interval since its introduction into the clinic in the 1950’s, considerable information regarding the metabolism of 5-FU has contributed to the understanding of its mechanisms of action.2,6 5-FU enters cells via facilitated transport, where it is then converted into several metabolites (Fig. 1B). The rate-limiting step of the catabolic process is the conversion of 5-FU to the inactive metabolite dihydrofluorouracil (DHFU) by the enzyme dihydropyrimidine dehydrogenase (DPD).2 5-FU has multiple metabolites, however, the metabolite fluoro-deoxyuridine monophosphate (FdUMP), and its relationship to thymidylate synthase (TS or TYMS) expression has been the primary focus of the field of research (Fig. 1B).
Figure 1.
History and metabolites of 5-FU. (A) A timeline of the genesis of the relationship between 5-FU and TS. (B) The metabolic pathway to FdUMP. On the left of the figure we show the main metabolic pathways to the 5-FU metabolite FdUMP. TP is thymidine phosphorylase; TK is thymidine kinase; OPRT is orate phosphoribosyltransferase; RR is ribonucleotide reductase. Note that the common pathway in cancer cells metabolize 5-FU to FdUMP is through ribonucleotide reductase. On the right is a simple schematic showing the central dogma of the most publicized 5-FU resistant mechanism. Increase numbers of TS molecules (top) in a cell will be able to resist FdUMP; while low amounts of TS in a cell will be inhibited by FdUMP (bottom) causing cellular death.
After 5-FU was established in initial clinical trials, much subsequent research focused on improving its efficacy through the use of modulatory methods such as changes in infusion schedules or the co-administration of agents with biomodulatory or synergistic effects, that are intended to improve the efficacy of 5-FU-based chemotherapy regimens.6,7 Currently, 5-FU is included as a component of chemotherapy protocols for breast, head and neck, and alimentary tract cancers.2,8,9 Its impact has perhaps been strongest in colorectal cancer treatment, where it is a standard element of adjuvant therapy for node-positive patients.10 Phase III trials have demonstrated a 5–6% benefit in 5-year overall survival using adjuvant 5-FU in properly-selected patients, representing a one-third relative reduction of five-year mortality from colorectal cancer.11
The evolution of the relationship between 5-fluorouracil and thymidylate synthase
The efficacy of 5-FU is thought to be related to TS inhibition by the formation of a stable complex.12 In a seminal publication, Santi and others reported in the 1970’s that TS forms a stable complex (labeled the ternary complex) by a covalent modification by the 5-FU metabolite, FdUMP and to 5,10-methylenetetrahydrofolate (MTHF) (Fig. 1A).13-15 More recent work has demonstrated that ternary complexes can be detected in tumor specimens collected from patients. To this end, Johnston et al. introduced an immunoblot technique for the identification of the ternary complex produced after 5-FU administration.16-18 Recently, Brody et al. advanced this technique to visualize TS ternary complexes (described as classic complexes) in an in vivo mouse model and proposed that this technique be used clinically to monitor drug metabolism and targeting to tumor cells in patients.19 With this facile assay, clinicians could stratify patients upfront as being able to properly metabolize the drug or not; or, perhaps even alter the dosing in individual patients that are inefficient in the formation of the ternary complex.19 The detection of the ternary complexes in tumors may confirm the presence of the ternary complex in the tumor sample, however it may not necessarily be a clinically useful marker for response.
Historically, an inverse relationship is reported between TS expression levels and response to 5-FU (Fig. 1A).20-23 This relationship has been investigated using a variety of techniques. While the initial reports of TS measurement in tumor samples relied upon enzymatic activity or binding,23-26 it was the subsequent development of immunological methods of TS detection that allowed for the quantification of TS levels in patient tumor samples.16,27 Johnston and colleagues first used immunohistochemical analysis to demonstrate a correlation between low TS levels and improved 5-year progression-free survival (PFS), 49% versus 27%, and overall survival (OS), 60% versus 40%, for rectal cancer patients who received adjuvant chemotherapy on the pivotal NSABP R-01 trial (Fig. 1A).28 Subsequently, Johnston et al. reported a strong correlation between TS mRNA and protein expression in colorectal and gastric tumor specimens. These investigators found both indices (protein and mRNA) levels to be predictive of response to 5-FU.20
A modern perspective of thymidylate synthase expression as a 5-fluorouracil target
After decades of research on the subject, the inhibition of TS by 5-FU continues to be considered a classic drug-target interaction.29 If one is to regard this relationship as an example of targeted therapy, then we propose that this interaction be reconsidered through the perspective of the anticipated qualities of a modern pharmacologic relationship. A framework for the evaluation of the TS/5-FU relationship has been provided by advances in molecular biology that have created new expectations for efficient, well-characterized drug-target relationships with effects that translate into clinical applications in a predictable fashion. Unfortunately, despite intentions for rational drug design, there are no widely accepted guidelines or protocols for the validation of promising drug-target relationships.30 A good candidate target in cancer cells is defined as either being altered or having dysregulated expression in cancer cells compared to normal cells. Overwhelming evidence exists that cancer cells have a disrupted cell cycle and an increased need for DNA synthesis. Therefore, TS is viewed as a good candidate target because it functions as an essential enzyme for DNA synthesis and it is often upregulated or over active in cancer cells.
Hucl et al. recently published an insightful exposition of the rational use of preclinical treatment models to the development of therapeutic strategies in which they articulated the concept of a pharmacogenetic window for the assessment of potential opportunities for targeted therapies.31 A pharmacogenetic window as determined by the ratio of IC50 values in preclinical models, is defined as the magnitude of the advantage conferred by the presence of the genetic condition that is the basis for the therapy. In the case of genetic mutations, a pharmacogenetic window of 10- to 30-fold, is promising for further drug development.31 One might extend this definition to include changes in gene expression levels, with the threshold differences for favorable pharmacogenetic windows lowered to 5- to 10-fold. Smaller windows, on the order of 1.5- to 3-fold difference, may be acceptable for lead compounds that will undergo further refinement.31,32
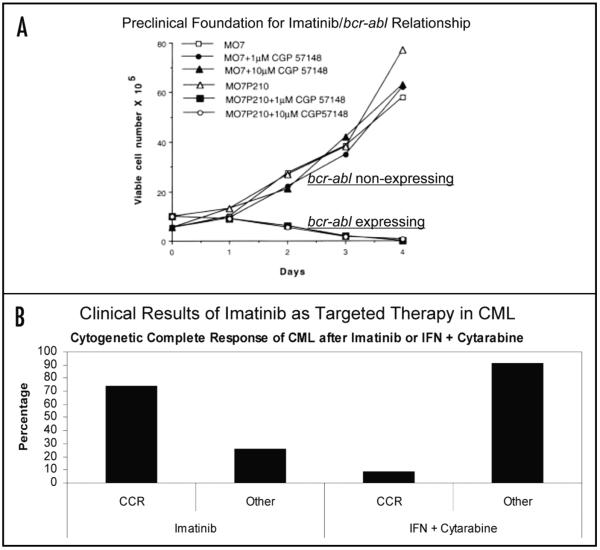
The relationship between bcr-abl, and the drug, imatinib, is a useful example in the discussion of the pharmacogenetic window. The bcr-abl fusion protein was identified in chronic myeloid leukemia (CML) cells, and it was demonstrated in preclinical studies that imatinib selectively inhibited growth of cells expressing bcr-abl (Fig. 2A).33 These discoveries translated into impressive clinical results when imatinib was administered to patients with CML (Fig. 2B).34 In this sense, overactivity of the protein, found as a consequence of the bcr-abl translocation in CML patients, represents a true drug target. An overwhelming number of patients with bcr-abl positivity respond to targeted therapy and the success of this approach has generated a paradigm shift in regards to expectations of targeted therapy in cancer (Fig. 2). Imatinib and another drug, gefitinib, are agents that exhibited windows of 10- to 20-fold magnitude when applied to appropriately selected subjects.31 Thus, the pharmacogenetic window concept and the imatinib success story help to frame the expectations of an effective rational drug-target interaction. We will extend these notions to the relationship between the target enzyme (TS) and the drug (5-FU).
Figure 2.
The pharmacogenetic window of 5-FU compared to imatinib. (A) Preclinical foundation for imatinib, demonstrating promising pharmacogenetic window. In this in vitro model of CML, imatinib exposure inhibited cell growth selectively in leukemia cells expressing the bcr-abl protein. Adapted with permission from Druker et al.33 (B) Clinical results of imatinib (left) versus previous standard therapy (right) for newly diagnosed chronic-phased CML, as reported by O’Brien et al.34 Imatinib was developed for human trials based upon preclinical data (A) that demonstrated promising activity in cell lines expressing the bcr-abl protein. CCR = complete cytogenetic response. Other = less than complete cytogenetic response.
Much research has attempted to elucidate the nature and significance of the interaction between TS expression levels and 5-FU. In general, resistance to 5-FU has been associated with increased TS expression.35-37 It is important to note that clinical scientists may define TS positivity quite differently from one another, hence methods and results may be somewhat subjective and ambiguous as compared to relying on sequencing data (e.g., bcr-abl translocations). Given the extensive literature concerning TS expression and 5-FU, it is useful to evaluate this particular drug-target relationship from a modern, molecular perspective. In this report, we review the existing literature in order to critically appraise the usefulness of continuing to study the TS/5-FU relationship.38
Results
Overall analysis
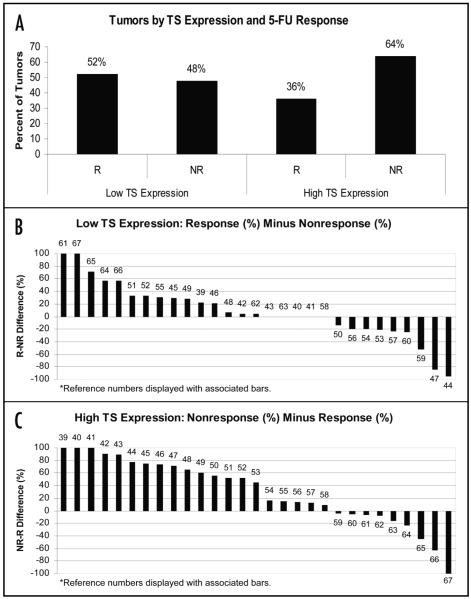
Thirty-seven articles were included in the analysis, encompassing a total of 1,839 tumor specimens. Based on cumulative data for tumors organized according to TS expression levels and response to 5-FU chemotherapy in 29 selected articles,39-67 the response to 5-FU in tumors with low TS expression was 52% and 36% in tumors with high TS expression, respectively (Fig. 3A). Forty-eight percent of the tumors with low TS expression showed no response to 5-FU therapy versus 64% of the tumors with high TS expression. The 5-FU response window between the two TS expression groups was 1.4, and the resistance window was 1.3 (Fig. 3A, Calculations A & B). The differences between the percentages of responding and non-responding tumors with low TS expression in the 29 articles reviewed ranged from positive 100% to negative 95% (Fig. 3B). The differences between the percentages of non-responding and responding tumors with high TS expression ranged from negative to positive 100% (Fig. 3C).
Figure 3.
(A) Cumulative data for tumors organized according to TS expression level and response to 5-FU chemotherapy. For the subset of journals in which articles with an impact factor greater than five, 35% of tumors with high TS expression demonstrate a response to 5-FU versus 53% of tumors with low TS expression. For tumors treated with 5-FU in combination with leucovorin, 83% of tumors with low TS expression had a response to treatment whereas 64% of tumors with low TS expression had no response to this combination treatment. R Response; NR No response. (B) Percent difference of response rates predicted by low TS expression. For tumors with low TS expression from 29 selected papers, the differences between percentages of responders minus non-responders. The differences between these percentages are displayed in descending order. The results above the x-axis support the ability of low TS levels to predict response to 5-FU, while the results below the x-axis contradict this conclusion. Each bar is marked with the reference number of the article it represents. (C) Percent difference of nonresponse rates predicted by high TS expression. For tumors with high TS expression from 29 selected papers, the differences between percentages of non-responders minus responders. The differences between these percentages are displayed in descending order. The results above the x-axis support the ability of high TS levels to predict resistance to 5-FU, while the results below the x-axis contradict this conclusion. Each bar is marked with the reference number of the article it represents.
Study-related variables
The weighted-average of the impact factor of the journals for all 37 articles reviewed was 6.55. For the subgroup of studies from journals with an impact factor ≥5, the response window was 1.5 and the resistance window was 1.4. The response windows were 1.9 and 1.5 during the periods 1995–2002 and 2003–2007, respectively.20,39-74 The TS/5-FU resistance windows were 1.6 and 1.3 during 1995–2002 and 2003–2007, respectively, suggesting that neither measure improved between the time periods. In the group of articles published between 2003–2007, 66% of tumors with high TS expression did not respond to 5-FU. In the group with low TS expression published during the same time period, 51% of tumors responded to 5-FU. For the subset of tumors that were treated with leucovorin and 5-FU, the response window was 2.1 and the resistance window was 1.7 (legend, Fig. 3).
Tumor-related variables
The TS/5-FU response windows were 0.9 and 1.2, and the resistance windows were 0.9 and 1.2, for primary tumors and metastatic tumors, respectively. In the tumors with low TS expression, response rates were 49% and 59% for primary tumors and metastases, respectively. Fifty percent of the metastatic tumors with high TS expression responded to 5-FU. Similarly, in the primary tumors with high TS expression, 54% of these tumors showed a response to 5-FU therapy.
Each primary site was also evaluated separately. The TS/5-FU window response windows were 1.0 and 2.4, and the resistance windows were 0.9 and 1.6, for pancreatic and gastric tumors, respectively. For colorectal tumors, the tumor type widely treated with 5-FU, the TS/5-FU response window was 2.3 and the resistance window was 1.6. Although the response window was greater than two, the response rate for tumors with low TS expression was only 52%.
Discussion
We found in our diverse paper cohort that a modest and consistent inverse trend exists between TS expression levels and chemotherapeutic response to 5-FU, and we estimate that the difference is approximately between 1.5- to 2.5-fold. A more thorough evaluation would require collection of primary source data from each individual publication concerning TS expression levels and objective response measurements, whereas our study relied upon the authors’ definitions. Our analysis does have the inherent limitations of a systematic literature review, but an inclusive approach allowed us to assemble a pool of clinical studies that show the same trend regardless of the subset analyzed and that provide material for revisiting the TS/5-FU relationship. The percent differences displayed in Figure 3B and C represent a wide range of results regarding the ability of TS expression to predict tumor response to 5-FU. Although a strong predictive capacity has been reported in some studies (on the left side of Fig. 3B and C), contradictory results of similar magnitude have also been described (on the right side of Fig. 3B and C). Despite the isolated success stories of TS expression for the prediction of response or resistance to 5-FU chemotherapy, the overall relationship in the literature is suboptimal, as suggested by the response and resistance windows calculated.
We suggest that although the documented response and resistance windows reported for the TS/5-FU relationship are consistent, they are not clinically informative. The magnitude of difference does not meet the criteria of a promising pharmacogenetic window when one applies the definitions previously discussed (Fig. 2B).31 Although the current review of TS/5-FU is an academic exercise, real-life confirmation is available in the results of the prospective trial by Smorenburg et al. in which TS expression failed to serve as a useful predictor of response to 5-FU-based chemotherapy.64 The results from Smorenburg et al. are in agreement with our analysis. We must reflect upon the TS/5-FU target-drug relationship and whether TS alone should remain an integral predictive marker of 5-FU response. We will discuss the literature regarding TS and 5-FU and consider the logical questions: (1) Should we move forward beyond reiterating the often published trend of TS expression and 5-FU response to consider other markers or targets of 5-FU? (2) Should TS be considered with additional predictive markers such as 5-FU metabolizing enzymes (e.g., DPD)? (3) Is TS an important therapeutic target?
Methods of TS evaluation
Vast resources have been applied towards the development of new methods for dividing tumors into groups according to their resistance and response to 5-FU chemotherapy. In this respect, the literature has shifted in its approach to studying the TS/5-FU relationship. Peters and colleagues published a seminal study that detected TS enzymatic activity in tumor samples shortly after 5-FU treatment.23 Unfortunately, perhaps due to the complexity of the assay, clinical scientists rapidly transitioned into measuring TS expression levels and not enzymatic activity in regards to 5-FU clinical response. Recent new methods of separating tumors on the basis of TS characteristics have been reported, including sequencing of the TS gene polymorphism. In general, the 5′ region of the TS gene has been found to be polymorphic, with either two (2R) or three (3R) repeats.75 The allele described in the literature as having the highest allelic expression contains a 3G repeat.76 Sarbia et al. attempted to relate TS polymorphisms to outcomes of patients with advanced squamous cell esophageal cancer after surgery and 5-FU-based chemotherapy. They extracted DNA from tumor samples obtained prior to 5-FU and then genotyped for three genes involved in folate metabolism. In their study, determination of TS tandem repeat provided no value in predicting the outcome of patients with locally advanced squamous cell esophageal cancer after treatment with 5-FU based chemotherapy.58 These data are supported by the study from Nief et al. in which 60 human cancer cell lines from the NCI were evaluated for three relevant TS gene polymorphisms, and no relationship was found between 5-FU cytotoxicity and TS expression or polymorphisms.77 However, there are studies that contradict these findings and suggest that TS polymporphism status may be a useful predictor of 5-FU response. For instance, Lecomte et al. investigated the relationship between two TS gene polymorphisms and the efficacy and treatment-related toxicity of 5-FU-based chemotherapy in colorectal cancer patients. Individuals in this study who were homozygous for the double repeat (2R) in the TS promoter region had more severe side effects to 5-FU. The authors concluded that TS genotyping may be helpful in predicting toxicity to 5-FU-based chemotherapy, and therefore TS genotyping might make it possible to individualize treatment for patients with colorectal cancer.57 Although Lecomte and colleagues found an association with toxicity, the TS polymorphism literature does not clearly demonstrate a relationship between TS and tumor response to 5-FU-based chemotherapy.
Technical limitations of experimental reports
One potential technical limitation is that the TS tandem repeat status from tumor cells from a patient will in some instances be different than the constitutional DNA from a patient,78 since the TS gene resides on chromosome 18 where loss of heterozygosity and copy number changes commonly occur in gastrointestinal cancers.69,79 The studies of the TS untranslated repeat are perhaps deeply flawed because they include investigation into the efficiency of ribosome translation, which generally depends on the efficiency of polypeptide initiation at the Kozak consensus sequence. Unfortunately, for their studies, Yawata et al. and Kawakami and Watanabe seem to have used the Kozak sequence of the firefly luciferase gene, and not the different Kozak sequence of the human TS gene.76,80 The relevant laboratory experiment may not yet have been performed. Currently, we are reasonably informed as to whether the human variants of the TS repeat interfere with a human-fly inter-species interaction, one of no obvious clinical relevance.
The differences in TS expression between primary tumors and metastases create a challenge for the use of TS as a predictive marker and suggest an additional significance of TS beyond its role as a drug target for 5-FU. Recent reports propose that TS expression in metastatic lesions is a more critical determinant of 5-FU response than TS expression levels in the primary tumor. Johnston et al., the same group who first reported primary tumor TS expression and 5-FU response, demonstrated that measurement of TS protein levels in primary tumor tissue does not predict survival after 5-FU chemotherapy for patients with metastatic or recurrent colorectal cancer. There was a trend towards higher response rates for tumors with high TS expression.44 This finding is difficult to reconcile with prior reports that low TS expression predicts superior disease-free survival after adjuvant 5-FU for colorectal cancer.20,28 How should one resolve these contradictory findings regarding the proposed relationship of TS expression in primary tumors and patient response to 5-FU?20,28,67,81,82
Perhaps the best clinical example of 5-FU targeting TS is from Wang et al.83 TS amplification has been identified as a mechanism of 5-FU resistance in colorectal cancer patients with liver metastasis. These investigators used digital karyotyping to identify genomic alterations present in liver metastases that were resistant to 5-FU-based chemotherapy. Fluoresence in situ hybridization analysis revealed that one quarter of metastases treated with 5-FU had TS amplification, while no metastases that had not been treated with 5-FU showed TS amplification.83 Although a sound and informative study, the question remains as to what happened in the other 75% of 5-FU-resistant metastatic lesions where no TS amplification was detected?
Predictive models that include additional variables
Since the analysis of TS expression alone for the prediction of 5-FU response has produced unsatisfying results, other variables have been explored in combination with TS in approaches that are based upon levels of key metabolic enzymes (Fig. 1B). It is well established that in some cases over 80% of 5-FU is degraded in the liver before reaching cancer cells. Therefore these preliminary studies may have future clinical value. Metzger et al. reported a 2.6-fold higher mRNA level of thymidine phosphorylase (TP) in colorectal tumors that responded to 5-FU versus non-responders. Interestingly, TS and TP were independent predictive variables in the tumors, and the combination of TS and TP improved the ability to predict 5-FU response.39 Thymidine kinase (TK) is the enzyme that converts FdUrd, a 5-FU metabolite, into FdUMP.4 Grem and others found no association between TK activity and 5-FU sensitivity in in vitro experiments using the National Cancer Institute cell line screen.84 DPD is the rate-limiting enzyme for 5-FU metabolism. It is known that patients who are deficient in DPD are susceptible to profound toxicity after 5-FU administration.85 Intratumoral mRNA levels of DPD have been associated with response to 5-FU in colorectal tumors, and the combination of TS and DPD expression may predict response of cancer cells to 5-FU better than either marker alone.46,86,87 In a prospective Phase II study by Smorenburg and colleagues the combination of DPD and TS was tried as a means to assign patients to tailored chemotherapy. Low DPD/TS expression failed as method to select patients for 5-FU chemotherapy and did not result in the high response rates anticipated based on previous retrospective studies.64 In a separate study, Salonga et al. analyzed the genes DPD, TS, and TP, and found that low expression of all three enzymes predicted a longer survival for colorectal cancer patients than did low expression of any single gene.70
Other investigators have correlated different enzymes involved in the stabilization and formation of the ternary complex. Orotate phosphoribosyl-transferase (OPRT), an enzyme that phosophorylates 5-FU to produce 5-fluoruridine monophosphate in cancer cells, was investigated by Fujii and colleagues as a potential predictor of 5-FU response. This study related enzyme activity of TS, DPD, and OPRT in colorectal tumor specimens to 5-FU response, as well as to proliferation using the Ki-67 proliferative index. Both TS and OPRT levels, as well as Ki-67, were all inversely related to 5-FU response. The authors suggest that OPRT may be closely related to 5-FU response, while TS may be linked more closely to proliferation.68 In a study by Jakobsen et al. metastatic colorectal tumors that contain the MTHR polymorphism, MTHFR 677 TT, were found to be responsive to 5-FU.59 MTHF is involved in stabilizing the ternary complex of TS, FdUMP and MTHF, and the sensitization to 5-FU conferred by the MTHFR 677 TT polymorphism may be explained by increased formation and stability of the complex.88 The authors suggested an approach that includes assessment of both this polymorphism and the TS 3R/3R polymorphism for 5-FU response.59 The conclusions of the above studies exemplify the unsatisfactory predictive capacity of our current understanding of 5-FU and its intracellular targets in the clinic, as well as the need for alternative markers to predict response to 5-FU based chemotherapy.
Other caveats of 5-FU resistance and TS expression
Investigators have explored other 5-FU targets and mechanisms of drug resistance. Using DNA microarrays of colon cancer cells, Schmidt and colleagues studied the gene expression profiles associated with 5-FU-resistance phenotypes. They observed gene expression changes that are not classically associated with 5-FU response, such as cytoskeleton, cell adhesion and cell-matrix interactions. These findings support the theory that 5-FU resistance can not be explained solely by the expression of TS and other enzymes involved in 5-FU metabolism.89 Another DNA microarray study, reported by Wang et al. found reduced expression of G1-S and S phase transition-related genes. These results support increased cell cycle checkpoint stringency and the resultant decreased proportion of cells in S phase as a means to 5-FU resistance in colon cancer cell.90
The importance of TS expression in cancer cells for predicting patient outcomes may be distinct from its role as a target of 5-FU chemotherapy. TS expression in tumors is directly related to proliferation,68 and increased TS levels predict higher rates of both distant metastasis and local recurrence, in addition to lower survival rates.82 Rahman et al. demonstrated behaviors of TS that are characteristic of an oncogene. Ectopic overexpression of TS transformed cells in vitro into a neoplastic phenotype. Also, TS-transformed cells underwent apoptosis after serum starvation.91 TS levels vary throughout the cell cycle, with a 20-fold amplification during S phase as opposed to G0.92 Cell lines that are resistant to 5-FU have been shown to have reduced expression of genes involved in G1-S and S phase transition, with corresponding slower growth rates and lower proportion of S-phase cells.90 Therefore, TS expression levels may correspond to cell cycle related changes, and 5-FU resistance may be related to alterations in cell cycle checkpoints. Moreover, TS levels are regulated by a number of mechanisms which may make it difficult to utilize as a predictive marker in certain settings (for a concise review of TS regulation and 5-FU, see ref. 93). Investigations of the gene expression changes induced by 5-FU exposure, as well as the various potential other roles of TS in cancer cells, support suspicions that the predictive and prognostic capabilities of this enzyme may be independent of the effects of 5-FU.
Methods
Literature search
We performed an on-line search of the PubMed database in order to identify articles published between 1994 and 2007 that reported a correlation of TS expression levels with response to 5-FU-based chemotherapy. Our query of the PubMed database identified 48,432 articles containing one of the keywords “5-fluorouracil,” “fluorouracil,” “5-FU,” or “FU;” 15,536 articles with “thymidylate synthase,” “TS,” or “TYMS;” 1,638 articles with both one of the 5-FU and one of the TS search terms; and, 628 articles that contained 5-FU and TS search terms in addition to the keyword “response.” The abstracts of these articles were reviewed to identify 89 articles that associated TS expression with tumor response to 5-FU chemotherapy.
We limited our review to those articles that were published in journals with an impact factor greater than two. Thirty-seven articles satisfied these criteria; a subset of 29 of these reports described objective methods for evaluating response. These 37 articles, and the subset of 29, comprised our review. In order to assess changes in the literature over time, we subdivided the articles into two groups based on the date of publication (1995–2002 vs. 2003–2007). Information was collected regarding site of primary tumor (i.e., colorectal, stomach, pancreas, head/neck or lung), and the use of 5-FU alone versus in combination with other anti-cancer drugs.
Sub-classification criteria
The majority of the articles included in this review studied TS levels in tumors from metastatic and unresectable disease (see references for clinical and specimen collection details, Fig. 3). The tumors described within the articles were divided into two groups based on high or low TS expression. We accepted the individual authors’ determination of TS expression levels without modification when incorporating the data into our own analysis. Although this approach is less rigorous than would be required in a formal meta-analysis, the divisions established by the authors of each paper were maintained with the intent to maximize the difference between the two groups. We evaluated the effect of TS expression on 5-FU response by further dividing tumors into responders and non-responders based upon the report of objective response (either complete or partial) versus the absence of objective response (stable or progressive disease), respectively. In most instances, radiographic response was determined in the publications based upon bidimensional tumor measurements. Two authors (Havens R and Showalter SL) independently reviewed the results of each study and agreed upon categorizations of TS expression and tumor response.
Resistance and response window calculations
In order to arrive at a quantifiable estimate of pharmacogenetic window, we performed two calculations for each subset analyzed, yielding the “resistance window” and the “response window.” For the overall analysis, we used the selected subset of 29 articles that provided objective response rates of tumors after 5-FU based chemotherapy as well as tumor TS expression levels. Subset analyses were performed using the larger group of 37 articles. We determined the ratio of the percentage of non-responders in the high TS expression group to the percentage of non-responders in the low TS expression group, and referred to this number as the “resistance window” (see calculation A). The “response window” is the ratio of the percentage of responders in the low TS expression group versus the percentage of responders in the high TS expression group (see calculation B).
| Calculation A |
| Calculation B |
These ratios are reported for each subset evaluated, and are referred to in the text as a “resistance window” and a “response window.” We considered these values as surrogates for the pharmacogenetic window in our analysis regarding the TS/5-FU relationship.
An additional set of calculations was performed for evaluation of the TS/5-FU relationship in the selected group of 29 articles. For each individual study, two calculations were performed to assess the association of low TS expression with 5-FU response (calculation C) and of high TS expression with 5-FU resistance (calculation D):
| Calculation C |
| Calculation D |
Conclusion
There is a measurable inverse relationship between TS expression and 5-FU response, and this forms the basis for dividing tumors into groups with roughly a 2-fold difference in response to 5-FU. Over the past several decades, important strides towards understanding the significance of TS and 5-FU have been made by a number of investigators. Although we suggest that the nature of the drug-target relationship must be reconsidered using the expected standards of the pharmacogenetic window, it is also important to retain many of the advances produced on this subject through the work of talented researchers.
There is ample evidence that TS expression levels alone should not be used in clinical settings to predict patient response to fluoropyrimidine-based therapy.64 However, the literature regarding TS polymorphisms and expression may be applied in the future to schemas that can used to identify small groups of patients for whom chemotherapy could be tailored. This is distinct from dividing all patients into two separate groups based on TS levels. Perhaps a more sound use of TS evaluation in clinical trials should be the detection of post-therapy TS covalent modification in order to provide information regarding targeting and metabolism of a fluoropyrimidine-based agent to tumor cells. One can envision monitoring chemotherapy using such a clinically informative protocol.18,19 The identification of novel biomarkers for response prediction may contribute to the development of more powerful predictive strategies that are based upon multiple factors that may include TS expression.73 Steps have already been made in this direction, and some such studies have been discussed. Gene expression analyses have identified additional candidate genes that may be relevant targets for chemotherapy.
In the era of “rational” cancer therapy, we propose that it is time to decouple 5-FU and TS, and to populate our understandings of both drug and enzyme with a new cast of related targets. If one were to reconsider 5-FU as a “lead compound,” the two-fold difference might be acceptable.31 Decades of research pertaining to the modulation of 5-FU chemotherapy have not broadened the window. This suggests that any further gains are likely to be incremental at best. Taken together the documentation that a modest trend exists between TS expression and 5-FU response, and the overwhelming biochemical reports that TS is inhibited by FdUMP, provides evidence that TS alone is a ‘poor target’ of 5-FU. Beyond the scope of this study is a discussion about the development of derivative drugs of 5-FU and other TS inhibitors such as tomudex and pemetrexed.94 We provocatively ask, perhaps TS does not deserve its own class of inhibitors? We argue that an inverse relationship, weak as it may be, does exist between TS expression and 5-FU response. This relationship has been shown by many investigators over countless trials and patients. We urge investigators to reconsider the relevance of the TS/5-FU relationship and to instead focus on optimizing fluoropyrimidine-based cancer therapy by working to uncover better candidate targets and correlates.
References
- 1.Lokich J. Infusional 5-FU: Historical evolution, rationale and clinical experience. Oncology (Williston Park) 1998;12:19–22. [PubMed] [Google Scholar]
- 2.Longley DB, Harkin DP, Johnston PG. 5-Flurouracil: Mechanisms of action and clinical strategies. Nature Rev Cancer. 2003;3:330–8. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 3.Rutman RJ, Cantarow A, Paschkis KE. Studies on 2-acetylaminofluorene carcinogenesis: III. The utilization of uracil-2-C 14 by pre-neoplastic rat liver. Cancer Res. 1954;14:119–26. [PubMed] [Google Scholar]
- 4.Grem JL. 5-fluorouracil: Forty-plus and still ticking. A review of its preclinical and clinical development. Invest New Drugs. 2000;18:299–313. doi: 10.1023/a:1006416410198. [DOI] [PubMed] [Google Scholar]
- 5.Heidelberger C, Chaudhuri NK, Dannenberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Scheiner J. Fluorinated pyrimidines: A new class of tumour inhibitory compounds. Nature. 1957;179:663–6. doi: 10.1038/179663a0. [DOI] [PubMed] [Google Scholar]
- 6.Thomas DM, Zalcberg JR. 5-fluorouracil: A pharmacological paradigm in the use of cytotoxics. Clin Exp Pharmacol Physiol. 1998;25:887–95. doi: 10.1111/j.1440-1681.1998.tb02339.x. [DOI] [PubMed] [Google Scholar]
- 7.Sobrero AF, Aschele C, Bertino JR. Fluorouracil in colorectal cancer: A tale of two drugs. Implications for biochemical modulation. J Clin Oncol. 1997;15:368–81. doi: 10.1200/JCO.1997.15.1.368. [DOI] [PubMed] [Google Scholar]
- 8.Rustum YM, Harstrick A, Cao S, Vanhoefer U, Yin MB, Wilke H, Seeber S. Thymidylate synthase inhibitors in cancer therapy: Direct and indirect inhibitors. J Clin Oncol. 1997;15:389–400. doi: 10.1200/JCO.1997.15.1.389. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Welde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 10.IMPACT Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995;345:939–44. [PubMed] [Google Scholar]
- 11.Tebbutt NC, Cattell E, Midgley R, Cunningham D, Kerr D. Systemic treatment of colorectal cancer. Eur J Cancer. 2002;38:1000–15. doi: 10.1016/s0959-8049(02)00062-x. [DOI] [PubMed] [Google Scholar]
- 12.Carreras CW, Santi DV. The catalytic mechanism and structure of thymidylate synthase. Annu Rev Biochem. 1995;6:721–62. doi: 10.1146/annurev.bi.64.070195.003445. [DOI] [PubMed] [Google Scholar]
- 13.Sommer H, Santi DV. Purification and amino acid analysis of an active site peptide from thymidylate synthetase containing covalently bound 5-fluoro-2′-deoxyuridylate and methylenetetrahydrofolate. Biochem Biophys Res Commun. 1974;57:689–95. doi: 10.1016/0006-291x(74)90601-9. [DOI] [PubMed] [Google Scholar]
- 14.Santi DV, McHenry CS, Sommer H. Mechanism of interaction of thymidylate synthestase with 5-fluorodeoxyuridylate. Biochemistry. 1974;13:471–81. doi: 10.1021/bi00700a012. [DOI] [PubMed] [Google Scholar]
- 15.Santi DV, McHenry CS. 5-Fluoro-2′-deoxyuridylate: Covalent complex with thymidylate synthetase. Proc Natl Acad Sci USA. 1972;69:1855–7. doi: 10.1073/pnas.69.7.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston PG, Liang CM, Henry S, Chabner BA, Allegra CJ. Production and characterization of monoclonal antibodies that localize human thymidylate synthase in the cytoplasm of human cells and tissue. Cancer Res. 1991;51:6668–76. [PubMed] [Google Scholar]
- 17.Chu E, Koeller DM, Johnston PG, Zinn S, Allegra CJ. Regulation of thymidylate synthase in human colon cancer cells treated with 5-fluorouracil and interferon-gamma. Mol Pharmacol. 1993;43:527–33. [PubMed] [Google Scholar]
- 18.Drake JC, Allegra CJ, Johnston PG. Immunological quantitation of thymidylate synthase-FdUMP-5,10-methylenetetrahydrofolate ternary complex with the monocloncal antibody TS 106. Anticancer Drugs. 1993;4:431–5. doi: 10.1097/00001813-199308000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Brody J, Gallmeier E, Yoshimura K, Hucl T, Kulesza P, Canto M, Hruban R, Schulick R, Kern S. A proposed clinical test for monitoring fluoropyrimidine therapy: Detection and stability of thymidylate synthase ternary complexes. Cancer Biol Ther. 2006;5:923–7. doi: 10.4161/cbt.5.8.2976. [DOI] [PubMed] [Google Scholar]
- 20.Johnston PG, Lenz HJ, Leichman CG, Danenberg KD, Allegra CJ, Danenberg PV, Leichman L. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res. 1995;55:1407–12. [PubMed] [Google Scholar]
- 21.Davis ST, Berger SH. Variation in human thymidylate synthase is associated with resistance to 5-fluoro-2′-deoxyuridine. Mol Pharmacol. 1993;43:702–8. [PubMed] [Google Scholar]
- 22.Hughey CT, Barbour KW, Berger FG, Berger SH. Functional effects of a naturally occurring amino acid substitution in human thymidylate synthase. Mol Pharmacol. 1993;44:316–23. [PubMed] [Google Scholar]
- 23.Peters GJ, van der Wilt CL, van Groeningen CJ, Smid K, Meijer S, Pinedo HM. Thymidylate synthase inhibition after administration of fluorouracil with or without leucovorin in colon cancer patients: Implications for treatment with fluorouracil. J Clin Oncol. 1994;12:2035–42. doi: 10.1200/JCO.1994.12.10.2035. [DOI] [PubMed] [Google Scholar]
- 24.Moran RG, Spears CP, Heidelberger C. Biochemical determinants of tumor sensitivity to 5-fluorouracil: ultrasensitive methods for the determination of 5-fluoro-2′-deoxyuridylate and thymidylate synthase. Proc Natl Acad Sci USA. 1979;76:1456–60. doi: 10.1073/pnas.76.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockshin A, Danenberg PV. Biochemical factors affecting the tightness of 5-fluorodeoxyuridylate binding to human thymidylate synthase. Biochem Pharmacol. 1981;30:247–57. doi: 10.1016/0006-2952(81)90085-x. [DOI] [PubMed] [Google Scholar]
- 26.Lockshin A, Danenberg PV. Thymidylate synthase and 2-deoxyuridylate form a tight complex in the presence of pteroyltriglutamate. J Biol Chem. 1979;254:12285–8. [PubMed] [Google Scholar]
- 27.Johnston PG, Drake JC, Trepel J, Allegra CJ. Immunological quantitation of thymidylate synthase using the monoclonal antibody TS 106 in 5-fluorouracil-sensitive and -resistant human cancer cell cines. Cancer Res. 1992;52:4306–12. [PubMed] [Google Scholar]
- 28.Johnston PG, Fisher ER, Rockette HE, Fisher B, Wolmark N, Drake JC, Chabner BA, Allegra CJ. The role of thymidylate synthase expression in prognosis and outcome of adjuvant chemotherapy in patients with rectal cancer. J Clin Oncol. 1994;12:2640–7. doi: 10.1200/JCO.1994.12.12.2640. [DOI] [PubMed] [Google Scholar]
- 29.Kummar S, Noronha V, Chu E. Antimetabolites. Lippincott Williams & Wilkins; Philadelphia: 2004. [Google Scholar]
- 30.Benson JD, Chen YNP, Cornell-Kennon SA, Dorsch M, Kim S, Leszczyniecka M, Sellers WR, Lengauer C. Validating cancer drug targets. Nature. 2006;441:451–6. doi: 10.1038/nature04873. [DOI] [PubMed] [Google Scholar]
- 31.Hucl T, Gallmeier E, Kern SE. Distinguishing rational from irrational applications of pharmacogenetic synergies from the bench to clinical trials. Cell Cycle. 2007;6:1336–41. doi: 10.4161/cc.6.11.4359. [DOI] [PubMed] [Google Scholar]
- 32.Gallmeier E, Kern SE. Targeting Fanconi Anemia/BRCA2 pathway defects in cancer: The significance of preclinical pharmacogenomic models. Clin Cancer Res. 2007;13:4–10. doi: 10.1158/1078-0432.CCR-06-1637. [DOI] [PubMed] [Google Scholar]
- 33.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of the Bcr-Abl positive cells. Nat Med. 1996;2:561–5. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, Hochhaus A, Hughes T, Lechner K, Nielsen JL, Rousselot P. Imatinib compared with interferon and low-dose cytarabine for newly diagnossed chronic-phased chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 35.Giovannetti E, Mey V, Nannizzi S, Pasqualetti G, Del Tacca M, Danesi R. Pharmacogenetics of anticancer drug sensitivity in pancreatic cancer. Mol Cancer Ther. 2006;5:1387–95. doi: 10.1158/1535-7163.MCT-06-0004. [DOI] [PubMed] [Google Scholar]
- 36.Lee W, Lockhart C, Kim RB, Rothenberg ML. Cancer pharmacogenomics: Powerful tools in cancer chemotherapy and drug development. Oncologist. 2005;10:104–11. doi: 10.1634/theoncologist.10-2-104. [DOI] [PubMed] [Google Scholar]
- 37.Nagasubramanian R, Innocenti F, Ratain MJ. Pharmacogenetics in cancer treatment. Annu Rev Med. 2003;54:437–52. doi: 10.1146/annurev.med.54.101601.152352. [DOI] [PubMed] [Google Scholar]
- 38.Brody JR, Kern SE. Stagnation and herd mentality in the biomedical sciences. Cancer Biol Ther. 2004;3:903–10. doi: 10.4161/cbt.3.9.1082. [DOI] [PubMed] [Google Scholar]
- 39.Metzger R, Danenberg K, Leichman CG, Salonga D, Schwartz EL, Wadler S, Lenz HJ, Groshen S, Leichman L, Danenberg PV. High basal level gene expression of thymidine phosphorylase (platelet-derived endothelial cell growth factor) in colorectal tumors is associated with nonresponse to 5-fluorouracil. Clin Cancer Res. 1998;4:2371–6. [PubMed] [Google Scholar]
- 40.Cascinu S, Catalano V, Aschele C, Barni S, Debernardis D, Gallo L, Bandelloni R, Staccioli MP, Baldelli AM, Brenna A, Valenti A, Muretto P, Catalano G. Immunohistochemical determination of p53 protein does not predict clinical response in advanced colorectal cancer with low thymidylate synthase expression receiving a bolus 5-fluorouracil-leucovorin combination. Ann Oncol. 2000;11:1053–6. doi: 10.1023/a:1008362511552. [DOI] [PubMed] [Google Scholar]
- 41.Cascinu S, Aschele C, Barni S, Debernardis D, Baldo C, Tunesi G, Catalano V, Staccioli MP, Brenna A, Muretto P, Catalano G. Thymidylate synthase protein expression in advanced colon cancer: correlation with the site of metastasis and the clinical response to leucovorin-modulated bolus 5-fluorouracil. Clin Cancer Res. 1999;5:1996–9. [PubMed] [Google Scholar]
- 42.Leichman CG, Lenz HJ, Leichman L, Danenberg K, Baranda J, Groshen S, Boswell W, Metzger R, Tan M, Danenberg PV. Quantitation of intratumoral thymidylate synthase expression predicts for disseminated colorectal cancer response and resistance to protracted-infusion fluorouracil and weekly leucovorin. J Clin Oncol. 1997;15:3223–9. doi: 10.1200/JCO.1997.15.10.3223. [DOI] [PubMed] [Google Scholar]
- 43.Lenz HJ, Hayashi K, Salonga D, Danenberg KD, Danenberg PV, Metzger R, Banerjee D, Bertino JR, Groshen S, Leichman L, Leichman CG. p53 point mutations and thymidylate synthase messenger RNA levels in disseminated colorectal cancer: an analysis of response and survival. Clin Cancer Res. 1998;4:1243–50. [PubMed] [Google Scholar]
- 44.Johnston PG, Benson AB, III, Catalano P, Rao MS, O’Dwyer PJ, Allegra CJ. Thymidylate synthase protein expression in primary colorectal cancer: Lack of correlation with outcome and response to fluorouracil in metastatic disease sites. J Clin Oncol. 2003;21:815–9. doi: 10.1200/JCO.2003.07.039. [DOI] [PubMed] [Google Scholar]
- 45.Okonkwo A, Musunuri S, Talamonti M, Benson AI, Small WJ, Stryker SJ, Rao MS. Molecular markers and prediction of response to chemoradiation in rectal cancer. Oncol Rep. 2001;8:497–500. [PubMed] [Google Scholar]
- 46.Ichikawa W, Takahashi T, Suto K, Shirota Y, Nihei Z, Shimizu M, Sasaki Y, Hirayama R. Simple combinations of 5-FU pathway genes predict the outcome of metastatic gastric cancer patients treated by S-1. Int J Cancer. 2006;119:1927–33. doi: 10.1002/ijc.22080. [DOI] [PubMed] [Google Scholar]
- 47.Shirota Y, Stoehlmacher J, Brabender J, Xiong YP, Uetake H, Danenberg KD, Groshen S, Tsao-Wei DD, Danenberg PV, Lenz HJ. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol. 2001;19:4298–304. doi: 10.1200/JCO.2001.19.23.4298. [DOI] [PubMed] [Google Scholar]
- 48.Lenz HJ, Leichman CG, Danenberg KD, Danenberg PV, Groshen S, Cohen H, Laine L, Crookes P, Silberman H, Baranda J, Garcia Y, Li J, Leichman L. Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a predictor for primary tumor response and overall survival. J Clin Oncol. 1996;14:176–82. doi: 10.1200/JCO.1996.14.1.176. [DOI] [PubMed] [Google Scholar]
- 49.Link KH, Kornmann M, Butzer U, Leder G, Sunelaitis E, Pillasch J, Salonga D, Danenberg KD, Danenberg PV, Beger HG. Thymidylate synthase quantitation and in vitro chemosensitivity testing predicts responses and survival of patients with isolated nonresectable liver tumors receiving hepatic arterial infusion chemotherapy. Cancer. 2000;89:288–96. [PubMed] [Google Scholar]
- 50.Boku N, Chin K, Hosokawa K, Ohtsu A, Tajiri H, Yoshida S, Yamao T, Kondo H, Shirao K, Shimada Y, Saito D, Hasebe T, Mukai K, Seki S, Saito H, Johnston PG. Biological markers as a predictor for response and prognosis of unresectable gastric cancer patients treated with 5-fluorouracil and cis-platinum. Clin Cancer Res. 1998;4:1469–74. [PubMed] [Google Scholar]
- 51.Aschele C, Debernardis D, Bandelloni R, Cascinu S, Catalano V, Giordani P, Barni S, Turci D, Drudi G, Lonardi S, Gallo L, Maley F, Monfardini S. Thymidylate synthase protein expression in colorectal cancer metastases predicts for clinical outcome to leucovorinmodulated bolus or infusional 5-fluorouracil but not methotrexate-modulated bolus 5-fluorouracil. Ann Oncol. 2002;13:1882–92. doi: 10.1093/annonc/mdf327. [DOI] [PubMed] [Google Scholar]
- 52.Aschele C, Debernardis D, Casazza S, Antonelli G, Tunesi G, Baldo C, Lionetto R, Maley F, Sobrero A. Immunohistochemical quantitation of thymidylate synthase expression in colorectal cancer metastases predicts for clinical outcome to fluorouracil-based chemotherapy. J Clin Oncol. 1999;17:1760–70. doi: 10.1200/JCO.1999.17.6.1760. [DOI] [PubMed] [Google Scholar]
- 53.Paradiso A, Xu J, Mangia A, Chiriatti A, Simone G, Zito A, Montemurro S, Giuliani F, Maiello E, Colucci G. Topoisomerase-I, thymidylate synthase primary tumour expression and clinical efficacy of 5-FU/CPT-11 chemotherapy in advanced colorectal cancer patients. Int J Cancer. 2004;111:252–8. doi: 10.1002/ijc.20208. [DOI] [PubMed] [Google Scholar]
- 54.Belvedere O, Puglisi F, Loreto CD, Cataldi P, Guglielmi A, Aschele C, Sobrero A. Lack of correlation between immunohistochemical expression of E2F-1, thymidylate synthase expression and clinical response to 5-fluorouracil in advanced colorectal cancer. Ann Oncol. 2004;15:55–8. doi: 10.1093/annonc/mdh018. [DOI] [PubMed] [Google Scholar]
- 55.Marcuello E, Altes A, del Rio E, Cesar A, Menoyo A, Baiget M. Single nucleotide polymorphism in the 5′ tandem repeat sequences of thymidylate synthase gene predicts for response to fluorouracil-based chemotherapy in advanced colorectal cancer patients. Int J Cancer. 2004;112:733–7. doi: 10.1002/ijc.20487. [DOI] [PubMed] [Google Scholar]
- 56.Kwon HC, Roh MS, Oh SY, Kim SH, Kim MC, Kim JS, Kim HJ. Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann Oncol. 2007;18:504–9. doi: 10.1093/annonc/mdl430. [DOI] [PubMed] [Google Scholar]
- 57.Lecomte T, Ferraz JM, Zinzindohoue F, Loriot MA, Tregouet DA, Landi B, Berger A, Cugnenc PH, Jian R, Beaune P, Laurent-Puig P. Thymidylate synthase gene polymorphism predicts toxicity in colorectal cancer patients receiving 5-fluorouracil-based chemotherapy. Clin Cancer Res. 2004;10:5880–8. doi: 10.1158/1078-0432.CCR-04-0169. [DOI] [PubMed] [Google Scholar]
- 58.Sarbia M, Stahl M, von Weyhern C, Weirich G, Puhringer-Oppermann F. The prognostic significance of genetic polymorphisms (Methylenetetrahydrofolate Reductase C677T, Methionine Synthase A2756G, Thymidilate Synthase tandem repeat polymorphism) in multimodally treated oesophageal squamous cell carcinoma. Br J Cancer. 2006;94:203–7. doi: 10.1038/sj.bjc.6602900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jakobsen A, Nielsen JN, Gyldenkerne N, Lindeberg J. Thymidylate synthase and methylenetetrahydrofolate reductase gene polymorphism in normal tissue as predictors of fluorouracil sensitivity. J Clin Oncol. 2005;23:1365–9. doi: 10.1200/JCO.2005.06.219. [DOI] [PubMed] [Google Scholar]
- 60.Aschele C, Debernardis D, Tunesi G, Maley F, Sobrero A. Thymidylate synthase protein expression in primary colorectal cancer compared with the corresponding distant metastases and relationship with the clinical response to 5-fluorouracil. Clin Cancer Res. 2000;6:4797–802. [PubMed] [Google Scholar]
- 61.Jakob C, Liersch T, Meyer W, Baretton GB, Hausler P, Schwabe W, Becker H, Aust DE. Immunohistochemical analysis of thymidylate synthase, thymidine phosphorylase and dihydropyrimidine dehydrogenase in rectal cancer (cUICC II/III): Correlation with histopathologic tumor regression after 5-fluorouracil-based long-term neoadjuvant chemoradiotherapy. Am J Surg Pathol. 2005;29:1304–9. doi: 10.1097/01.pas.0000170346.55304.88. [DOI] [PubMed] [Google Scholar]
- 62.Meropol NJ, Gold PJ, Diasio RB, Andria M, Dhami M, Godfrey T, Kovatich AJ, Lund KA, Mitchell E, Schwarting R. Thymidine phosphorylase expression is associated with response to capecitabine plus irinotecan in patients with metastatic colorectal cancer. J Clin Oncol. 2006;24:4069–77. doi: 10.1200/JCO.2005.05.2084. [DOI] [PubMed] [Google Scholar]
- 63.Backus HHJ, van Riel JMGH, van Groeningen CJ, Vos W, Dukers DF, Bloemena E, Wouters D, Pinedo HM, Peters GJ. Rb, mcl-1 and/755 expression correlate with clinical outcome in patients with liver metastases from colorectal cancer. Ann Oncol. 2001;12:779–85. doi: 10.1023/a:1011112227044. [DOI] [PubMed] [Google Scholar]
- 64.Smorenburg CH, Peters GJ, van Groeningen CJ, Noordhuis P, Smid K, van Riel AMGH, Dercksen W, Pinedo HM, Giaccone G. Phase II study of tailored chemotherapy for advanced colorectal cancer with either 5-fluouracil and leucovorin or oxaliplatin and irinotecan based on the expression of thymidylate synthase and dihydropyrimidine dehydrogenase. Ann Oncol. 2006;17:35–42. doi: 10.1093/annonc/mdj046. [DOI] [PubMed] [Google Scholar]
- 65.Shiga H, Heath EI, Rasmussen AA, Trock B, Johnston PG, Forastiere AA, Langmacher M, Baylor A, Lee M, Cullen KJ. Prognostic value of p53, glutathione S-transferase π, and thymidylate synthase for neoadjuvant cisplatin-based chemotherapy in head and neck cancer. Clin Cancer Res. 1999;5:4097–104. [PubMed] [Google Scholar]
- 66.Hu YC, Komorowski RA, Graewin S, Hostetter G, Kallioniemi OP, Pitt HA, Ahrendt SA. Thymidylate synthase expression predicts the response to 5-fluorouracil-based adjuvant therapy in pancreatic cancer. Clin Cancer Res. 2003;9:4165–71. [PubMed] [Google Scholar]
- 67.Johnston PG, Mick R, Recant W, Behan KA, Dolan ME, Ratain MJ, Beckmann E, Weichselbaum RR, Allegra CJ, Vokes EE. Thymidylate synthase expression and response to neoadjuvant chemotherapy in patients with advanced head and neck cancer. J Natl Cancer Inst. 1997;89:308–18. doi: 10.1093/jnci/89.4.308. [DOI] [PubMed] [Google Scholar]
- 68.Fujii R, Seshimo A, Kameoka S. Relationships between the expression of thymidylate synthase, dihydropyrimidine dehydrogenase, and orotate phosphoribosyltransferase and cell proliferative activity and 5-fluorouracil sensitivity in colorectal carcinoma. Int J Clin Oncol. 2003;8:72–8. doi: 10.1007/s101470300013. [DOI] [PubMed] [Google Scholar]
- 69.Villafranca E, Okruzhnov Y, Dominguez MA, Garcia-Foncillas J, Azinovic I, Martinez E, Illarramendi JJ, Arias F, Monge RM, Salgado E, Angeletti S, Brugarolas A. Polymorphisms of the repeated sequences in the enhancer region of the thymidylate synthase gene promoter may predict downstaging after preoperative chemoradiation in rectal cancer. J Clin Oncol. 2001;19:1779–86. doi: 10.1200/JCO.2001.19.6.1779. [DOI] [PubMed] [Google Scholar]
- 70.Salonga D, Danenberg KD, Johnson M, Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman L, Diasio RB, Danenberg PV. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase and thymidine phosphorylase. Clin Cancer Res. 2000;6:1322–7. [PubMed] [Google Scholar]
- 71.Kornmann M, Link KH, Lenz HJ, Pillasch J, Metzger R, Butzer U, Leder GH, Weindel M, Safi F, Danenberg KD, Beger HG, Danenberg PV. Thymidylate synthase is a predictor for response and resistance in hepatic artery infusion chemotherapy. Cancer Letters. 1997;118:29–35. doi: 10.1016/s0304-3835(97)00220-6. [DOI] [PubMed] [Google Scholar]
- 72.Inoue T, Hibi K, Nakayama G, Komatsu Y, Fukuoka T, Kodera Y, Ito K, Akiyama S, Nakao A. Expression level of thymidylate synthase is a good predictor of chemosensitivity to 5-fluorouracil in colorectal cancer. J Gastroenterol. 2005;40:143–7. doi: 10.1007/s00535-004-1512-9. [DOI] [PubMed] [Google Scholar]
- 73.Ichikawa W, Uetake H, Shirota Y, Yamada H, Nishi N, Nihei Z, Sugihara K, Hirayama R. Combination of dihydropyrimidine dehydrogenase and thymidylate synthase gene expressions in primary tumors as predictive parameters for the efficacy of fluoropyrimidine-based chemotherapy for metastatic colorectal cancer. Clin Cancer Res. 2003;9:786–91. [PubMed] [Google Scholar]
- 74.Yanagisawa Y, Maruta F, Iinuma N, Ishizone S, Koide N, Nakayama J, Miyagawa S. Modified irinotecan/5FU/leucovorin therapy in advanced colorectal cancer and predicting therapeutic efficacy by expression of tumor-related enzymes. Scandinavian Journal of Gastroenterology. 2007;42:477–84. doi: 10.1080/00365520600994418. [DOI] [PubMed] [Google Scholar]
- 75.Horie N, Aiba H, Oguro K, Hojo H, Takeishi K. Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5-terminal regulatory region of the human gene for thymidylate synthase. Cell Struct Funct. 1995;20:191–7. doi: 10.1247/csf.20.191. [DOI] [PubMed] [Google Scholar]
- 76.Kawakami K, Watanabe G. Identification and functional analysis of single nucleotide polymorphism in the tandem repeat sequence of thymidylate synthase gene. Cancer Res. 2003;63:6004–7. [PubMed] [Google Scholar]
- 77.Nief N, Le Morvan V, Robert J. Involvement of gene polymorphisms of thymidylate synthase in gene expression, protein activity and anticancer drug cytotoxicity using the NCI-60 panel. Eur J Cancer. 2007;43:955–62. doi: 10.1016/j.ejca.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 78.Lenz HJ. Pharmacogenomics and colorectal cancer. Ann Oncol. 2004;15:173–7. doi: 10.1093/annonc/mdh923. [DOI] [PubMed] [Google Scholar]
- 79.Brody JR, Hucl T, Gallmeier E, Winter JM, Kern SE, Murphy KM. Genomic copy number changes affecting the thymidylate synthase (TYMS) gene in cancer: A model for patient classification to aid fluoropyrimidine therapy. Cancer Res. 2006;66:9369–73. doi: 10.1158/0008-5472.CAN-06-2165. [DOI] [PubMed] [Google Scholar]
- 80.Yawata A, Kim SR, Miyajima A, Kubo T, Ishida S, Saito Y, Nakajima Y, Katori N, Matsumoto Y, Fukuoka M, Ohno Y, Ozawa S, Sawad JI. Polymorphic tandem repeat sequences of the thymidylate synthase gene correlates with cellular-based sensitivity to fluoropyrimidine antitumor agents. Cancer Chemother Pharmacol. 2005;56:465–72. doi: 10.1007/s00280-005-1018-z. [DOI] [PubMed] [Google Scholar]
- 81.Alexander HR, Grem JL, Hamilton JM, Pass HI, Hong M, Fraker DL, Steinberg SM, McAtee N, Allegra BC, Johnston PG. Thymidylate synthase protein expression: Association with response to neoadjuvant chemotherapy and resection for locally advanced gastric and gastroesophageal adenocarcinoma. Cancer J Sci Am. 1995;1:49–54. [PubMed] [Google Scholar]
- 82.Edler D, Hallstrom M, Johnston PG, Magnusson I, Ragnhammar P, Blomgren H. Thymidylate synthase expression: an independent prognostic factor for local recurrence, distant metastasis, disease-free and overall survival in rectal cancer. Clin Cancer Res. 2000;6:1378–84. [PubMed] [Google Scholar]
- 83.Wang TL, Diaz LA, Jr, Romans K, Bardelli A, Saha S, Galizia G, Choti M, Donehower R, Parmigiani G, Shih IM, Iacobuzio-Donahue C, Kinzler KW, Vogelstein B, Lengauer C, Velculescu VE. Digital karyotyping identifies thymidylate synthase amplification as a mechanism of resistance to 5-fluorouracil in metastatic colorectal cancer patients. Proc Natl Acad Sci USA. 2003;101:3089–94. doi: 10.1073/pnas.0308716101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grem JL, Danenberg KD, Behan KA, Parr A, Young L, Danenberg PV, Nguyen D, Drake J, Monks A, Allegra CJ. Thymidine kinase, thymidylate synthase and dihydropyrimidine dehydrogenase profiles of cell lines of the National Cancer Institute’s anticancer drug screen. Clin Cancer Res. 2001;7:999–1009. [PubMed] [Google Scholar]
- 85.Lyss AP, Lilenbaum RC, Harris BE, Diasio RB. Severe 5-fluorouracil toxicity in a patient with decreased dihydropyrimidine dehydrogenase activity. Cancer Investig. 1993;11:239–40. doi: 10.3109/07357909309024846. [DOI] [PubMed] [Google Scholar]
- 86.Kornmann M, Schwabe W, Sander S, Kron M, Strater J, Polat S, Kettner E, Weiser HF, Baumann W, Schramm H, Hausler P, Ott K, Behnke D, Staib L, Beger HG, Link KH. Thymidylate synthase and dihydropyrimidine dehydrogenase mRNA expression levels: Predictors for survival in colorectal cancer patients receiving adjuvant 5-fluorouracil. Clin Cancer Res. 2003;9:4116–24. [PubMed] [Google Scholar]
- 87.Kakimoto M, Uetake H, Osanai T, Shirota Y, Takagi Y, Takeshita E, Toriya Y, Danenberg K, Danenberg PV, Sugihara K. Thymidylate synthase and dihydropyrimidine dehydrogenase gene expression in breast cancer predicts 5-FU sensitivity by a histocultural drug sensitivity test. Cancer Letters. 2005;223:103–11. doi: 10.1016/j.canlet.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 88.Sohn KJ, Croxford R, Yates Z, Lucock M, Kim YI. Effect of the methylenetetrahydrofolate reductase C677T polymorphism on chemosensitivity of colon and breast cancer cells to 5-fluorouracil and methotrexate. J Natl Cancer Inst. 2004;96:134–44. doi: 10.1093/jnci/djh015. [DOI] [PubMed] [Google Scholar]
- 89.Schmidt WM, Kalipciyan M, Dornstauder E, Rizovski B, Steger GG, Sedivy R, Mueller MW, Mader RM. Dissecting progressive stages of 5-fluorouracil resistance in vitro using RNA expression profiling. Int J Cancer. 2004;112:200–12. doi: 10.1002/ijc.20401. [DOI] [PubMed] [Google Scholar]
- 90.Wang W, Cassidy J, O’Brien V, Ryan KM, Collie-Duguid E. Mechanistic and predictive profiling of 5-fluorouracil resistance in human cancer cells. Cancer Res. 2004;64:8167–76. doi: 10.1158/0008-5472.CAN-04-0970. [DOI] [PubMed] [Google Scholar]
- 91.Rahman L, Voeller D, Rahman M, Lipkowitz S, Allegra C, Barrett JC, Kaye FJ, Zajac-Kaye M. Thymidylate synthase as an oncogene: A novel role for an essential DNA synthesis enzyme. Cancer Cell. 2004;5:341–51. doi: 10.1016/s1535-6108(04)00080-7. [DOI] [PubMed] [Google Scholar]
- 92.Navalgund LG, Rossana C, Muench AJ, Johnson LF. Cell cycle regulation of thymidylate synthetase gene expression in cultured mouse fibroblasts. J Biol Chem. 1980;255:7386–90. [PubMed] [Google Scholar]
- 93.Peters GJ, Backus HHJ, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J, Calvert AH, Marsh S, McLeod HL, Bloemena E, Meijer S. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochimica et Biophysica Acta. 2002;1587:194–205. doi: 10.1016/s0925-4439(02)00082-0. [DOI] [PubMed] [Google Scholar]
- 94.Costi MP, Ferrari S, Venturelli A, Calo S, Tondi D, Barlocco D. Thymidylate synthase structure, function and implication in drug discovery. Curr Med Chem. 2005;12:2241–58. doi: 10.2174/0929867054864868. [DOI] [PubMed] [Google Scholar]