Abstract
Purpose of review
Granuloma formation in giant cell arteritis (GCA) emphasizes the role of the adaptive immunity and highlights the role of antigen-specific T cells. Recent data demonstrate that at least two separate lineages of CD4 T-cells participate in vascular inflammation, providing an important clue that multiple disease instigators may initiate pathogenic immunity.
Recent finding
IFN-γ-producing Th1 cells and IL-17-producing Th17 cells have been implicated in GCA. Patients with biopsy-positive GCA underwent two consecutive temporal artery biopsies, one prior to therapy and one while on corticosteroids. In untreated patients, Th1 and Th17 cells co-existed in the vascular lesions. Following therapy, Th17 cells were essentially lost, whereas Th1 cells persisted almost unaffected. In the peripheral blood of untreated patients Th17 frequencies were increased eightfold, but normalized with therapy. Blood Th1 cells were doubled in frequency, independent of therapy. Corticosteroids functioned by selectively suppressing IL-1β, IL-6 and IL-23-releasing antigen-presenting cells (APC), disrupting induction of Th17 cells.
Summary
At least two distinct CD4 T-cell subsets promote vascular inflammation in GCA. In early disease, APCs promote differentiation of Th17 as well as Th1 cells. Chronic disease is characterized by persistent Th1-inducing signals, independent of IL-17-mediated inflammation. More than one disease instigator may trigger APCs to induce multiple T cell lineages. Cocktails of therapies will be needed for appropriate disease control.
Keywords: IL-17, IFN-γ, T cell, Antigen-presenting cell
Introduction
GCA is a granulomatous disease [1]. Highly activated macrophages and T cells come together in the wall layers of medium and large arteries and form sophisticated lymphoid microstructures named granulomatous infiltrates. Often, but not always, multinucleated giant cells contribute to the microstructures. While the early signals leading to granuloma formation remain insufficiently understood, the structuring of the infiltrates has provided invaluable clues towards disease-relevant immune responses. Granulomas are a typical response pattern in tuberculosis, syphilis and leprosy suggesting that certain classes of pathogens, such that are intracellular, rather indolent and persist for long periods of time, have a tendency to elicit granuloma-forming immunity [2]. The hypothesis that the ultimate instigator in GCA is an infectious pathogen is not new, but attempts to identify such a pathogen have so far not been fruitful [3]. However, studies of the cell types, activation patterns and inflammatory mediators in GCA arteries have confirmed that the dominant immune response depends on CD4 T helper cells which orchestrate the stimulation of macrophages, finally leading to the blood vessel's response-to-injury reaction; a reaction that either results in luminal stenosis or in wall destruction and aneurysm formation. Understanding how granulomatous reactions are being initiated and promoted will inevitably require understanding of CD4 T cells in GCA.
CD4 T cells in GCA – how they inform pathogenic concepts
The power of CD4 T cells, both in protective and in pathogenic immune responses, lies in their ability to recognize antigen with extreme specificity. Antigen recognition initiates activation of the T cell, induces its differentiation into effector and memory T cells and facilitates a 10- to 100-fold expansion of the antigen-specific population. Only few antigen-specific T cells are needed to memorize a previous antigen encounter and the immune system's memory for that prior encounter can persist indefinitely. These features of adaptive immune responses provide life-long protection against microbes but, with equal potency, build barriers against the elimination of T cell-mediated autoimmune responses. In terms of understanding pathogenesis, the identification of antigen-specific CD4 T cells holds the unparalleled promise that a disease-specific probe could be developed, capable of finding the antigen/pathogen that underlies the tissue-destructive inflammation (Table 1).
Table 1.
Potential impact of understanding functional T cell lineages in GCA
| A. | Diagnostics |
| Determine T cell frequencies in diagnostic work-up | |
| Monitor T cell frequencies as a marker of disease activity | |
| Evaluate type and frequency of tissue-infiltrating T cells | |
| Example: IL-17-producing patients with MS are non-responders to IFN-β Tx | |
| B. | Therapeutics |
| Block T cell cytokine to disrupt inflammatory end pathway | |
| Target antigen-presenting cells to inhibit T cell differentiation | |
| Interrupt T cell recruitment to vessel wall or in situ activation | |
| Example: Therapeutic anti-IL-17 antibodies in Rheumatoid Arthritis | |
| C. | Pathogenic concepts |
| Use disease-relevant T cells as a tool to identify the disease instigator(s) | |
| Separate GCA from other disease processes | |
| Example: Chromogranin-specific T cells cause islet cell destruction and diabetes |
Analyses of T cells occupying the vascular lesions in GCA have provided intriguing evidence for a restricted repertoire of disease-relevant T cells [4]. Attempts to isolate the one T cell that defines the pinnacle of the pathology, however, have suggested more heterogeneity than one would expect in an immune reaction against a single antigen [5–8]. These findings are now corroborated by recent reports [9]** that multiple T cell lineages contribute to the disease process (Table 1). A unique study design, harvesting two consecutive biopsies from the same patient prior to therapy and once on therapy, has allowed comparing the composition and functional capacities of T cells driving early and chronic GCA. These studies have demonstrated that two T cell lineages, Th1 and Th17 cells, populate the blood vessel wall prior to corticosteroid therapy. Intriguingly, Th17 cells are sensitive to immunosuppressive therapy whereas Th1 cells persist in the treated patients (Fig. 1). The presence of the two T cell lineages coincided closely with the stimulation of two immune axes, an IL-12-IFN-γ axis and an IL-1-IL-23-Th17 axis (Fig. 2). Independent APC signals recruited either the IFN-γ or the IL-17-dependent arm of adaptive immunity raising the interesting possibility that more than one disease instigator is involved in GCA and that the patient's immune system deals with a variety of disease-inducing agents.
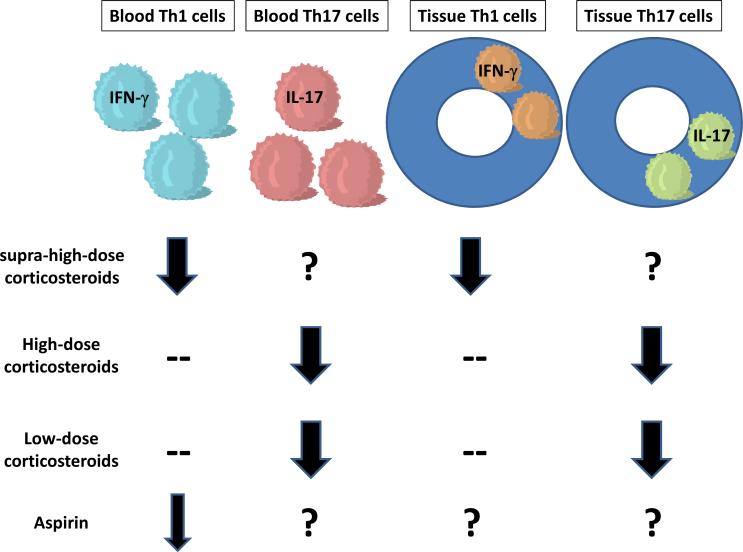
Figure 1. Therapeutic responsiveness distinguishes separate immune-inflammatory pathways in GCA.
Supra-high doses of corticosteroids (>1 mg/kg) are necessary to suppress the IFN-γ pathway. Vice versa, even low doses of corticosteroids are sufficient to inhibit IL-17 mediated immunity. Aspirin has previously been described to lower IFN-γ-production. Differences in therapeutic responsiveness strongly suggest a contribution of two independent inflammatory networks to the disease process and emphasize the requirement for therapeutic cocktails to efficiently treat the vasculitis.
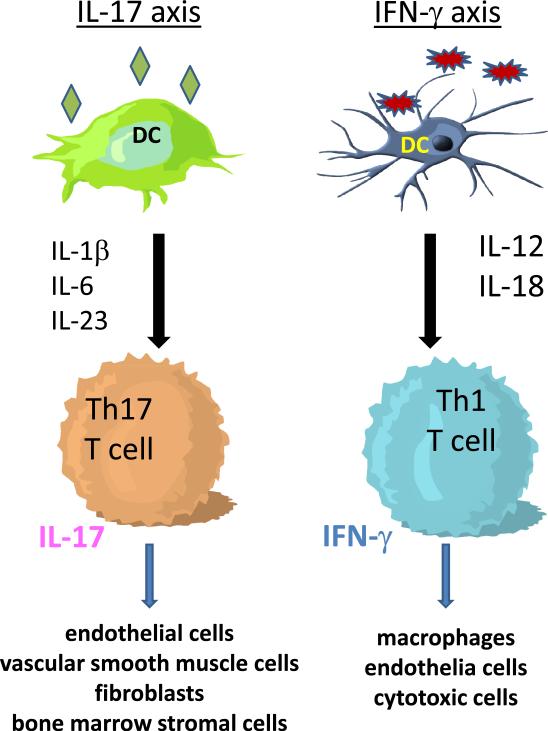
Figure 2. Two separable immune axes participate in GCA.
Studies in untreated and treated GCA patient have indicated that two, separable cytokine networks contribute to the vasculitic immune pathology. Dendritic cells producing IL-1β, IL-6 and IL-23 coax T cells to differentiate into IL-17 producers that modulate the function of endothelia cells, vascular smooth muscle cells, fibroblasts and bone marrow stromal cells. In an independent immune pathway, dendritic cells release IL-12 to induce the differentiation of IFN-γ-producing T cells. The downstream, target cells are macrophages, endothelial cells and cytotoxic cells. The IL-17 axis is steroid responsive, whereas the IFN-γ axis is steroid resistant. The separability of both axes is strongly suggestive for distinct immune instigators.
IFN-γ-producing Th1 cells in GCA
The major function through which differentiated CD4 T cells regulate inflammation is the release of cytokines. In terms of their cytokine production profile not all CD4 T cells are equal [10]. IFN-γ is the signature cytokine produced by the Th1 cell lineage. Originally called macrophage activating factor IFN-γ has a critical role against viral and intracellular bacterial infections. Aberrant production has been described in a multitude of autoimmune and chronic inflammatory diseases. Profiling of tissue cytokines in GCA-affected temporal arteries has demonstrated robust expression of IFN-γ and an association with a defined disease phenotype [11,12]. Specifically, high IFN-γ is typical for patients with ischemic complications implicating IFN-γ in the process leading to luminal occlusion. IL-2, a T-cell growth factor much more important for naïve T cells, has been less abundant in temporal arteries but its presence has been associated with clinical manifestations such as fever of unknown origin and polymyalgia rheumatica [13]. These studies established early on that GCA lesions display a pattern highly suggestive for antigen-specific immune responses and that distinct functional T cell subsets have a place in this vasculitis [4].
Efforts to identify CD4 T cells recognizing the disease relevant antigen have rested mostly on the analysis of the molecular make-up of T cell receptor genes. Antigen imposes a high selection pressure and drives the expansion of selected T cells, yet frequencies of antigen-specific T cells only reach 1 in 1,000 to 1 in 10,000 cells. Studies focusing on T cell receptor V genes in the arterial wall and the peripheral blood of GCA patients have arrived at the conclusion that the T cell repertoire is biased [14,15]. Sequence analysis of CD4 T cells isolated from the inflamed temporal arteries has strongly supported local T cell activation and expansion of only a few selected T cell specificities. Notably, CD4 T cells recovered from the right and the left temporal artery of the same patient utilized identical T cell receptors. These data provided the strongest possible evidence for the role of antigen recognition in the granulomatous lesions [5].
Localization of IFN-γ producing T cells in the inflamed arteries has shed light on the relationship between lymphoid structure and T cell activation and has placed the site of T cell/antigen-presenting-cell contact into the adventitia of blood vessels [16]. Meanwhile, it is well understood that the adventitia harbors specialized dendritic cells that shape the nature of the immune responses in the vessel wall [17,18]**.
In a recent study, IFN-γ producing lymphocytes emerged as the dominant cell populations in the intramural infiltrates [9]**. They account for the majority of CD4 T cells in the arteries harvested from patients with untreated disease. Remarkably, IFN-γ production in the vasculitic lesions continues even after months of corticosteroid therapy (Figure 1), essentially being resistant to the immunosuppression. IFN-γ-committed T cells accounted for 20% of circulating CD4 T cells, an almost 100% increase when compared to age-matched healthy donors. Corticosteroid therapy could not affect the expansion of the IFN-γ producing compartment, indicating continuous signaling of immune instigators eliciting this type of immune response. Underlying mechanisms involve the triggering of APCs that release IL-12. The steroid resistance of that mechanism is remarkable. Both in the blood and in the temporal arteries IL-12 production continued unabated during the chronic phase of GCA. In age-matched control donors only 11% of circulating CD4 T cells were dedicated to IFN-γ production. The expansion of CD4 T cells ready to secret IFN-γ to 20% in untreated and treated patients signifies strong, and steroid-resistant, stimuli that recruit capable T cells for the secretion of this potent anti-viral, anti-bacterial and pro-inflammatory cytokine.
It is currently unknown which aspects of the granulomatous inflammation depend upon IFN-γ. The ability of IFN-γ to activate monocytes and macrophages certainly has a role in supporting the lesional histiocytes. However, the profound differences in the clinical presentation of treated and untreated patients predict that IFN-γ is less relevant in the systemic manifestations of the disease, such as fever, weight loss, and polymyalgia rheumatic and instead is the major mediator of vasculotoxic responses. In line with this prediction are former reports that temporal arteries collected from patients with polymyalgia rheumatic consistently lack transcripts for IFN-γ [11]. Also, previous trials in human-GCA chimeras have demonstrated that very high doses of corticosteroids are needed for even a partial suppression of IFN-γ [7].
IFN-γ protein is measurably elevated in untreated and treated patients and may represent a useful biomarker to assess patients for persistent disease activity (Table 1).
IL-17-producing Th17 cells in GCA
Named after their signature cytokine IL-17, Th17 cells have recently been discovered as a unique T cell lineage [19]. Th17 cells play an important role in antimicrobial immunity where they regulate the recruitment of neutrophils and facilitate protection against extracellular bacterial and fungal infection [20]. Induction of IL-6 and the chemokine CXCL8 are recognized as IL-17-dependent functions [21]. Extending beyond their role in host defenses, Th17 cells have been implicated in the pathogenesis of several inflammatory and autoimmune diseases. In mice, Th17 cells promote arthritis, autoimmune encephalitis and colitis. In humans, Th17 cells are suspected to contribute to rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease [22].
In patients with untreated GCA Th17 cell frequencies are almost 10-fold elevated in the blood and accumulate in the vascular infiltrates [9]**. IL-17-secreting T cells are infrequent in healthy individuals accounting for less than 0.3% of circulating CD4 T cells. In newly diagnosed patients an average of 2.2% of circulating CD4 T cells are IL-17 producers. In some patients frequencies reached over 5%. In contrast to the Th1 lineage, however, the Th17 lineage displayed a totally different sensitivity to corticosteroid therapy (Fig. 1). Prednisone therapy led to a fast and almost complete reduction of circulating and tissue-infiltrating Th17 cells. In treated patients only 0.4% of blood CD4 T cells had the capability to release IL-17. Treatment for 2 months was sufficient to normalize Th17 frequencies and chronic therapy with less than 10 mg of Prednisone seemed capable of keeping Th17 cells in the normal range.
Like for IFN-γ, correlating the clinical picture of treated GCA and the successful suppression of IL-17-dependent immunity may allow predictions which manifestations are more closely related to IL-17 overproduction. In general, systemic manifestations of GCA promptly respond to corticosteroids, coincident with the normalization of Th17 cells. Also, polymyalgia rheumatica and Th17 seem to share superb responsiveness to corticosteroids. Recent studies have suggested that the Th17 lineage may not be as rigid as Th1 cells and that Th17 cells have substantial plasticity such that they can transition from effectors that produce predominantly IL-17A and IL-17E to effectors that release IFN-γ [23]. Thus, one could speculate that Th17 cells are precursor cells that will progress to Th1 cells in a chronic disease process. The persistence of Th1 cells at levels indistinguishable between untreated and treated GCA patients makes this scenario unlikely as the successful depression of precursor cells should eventually lead to a reduction in the end product and depleted Th1 cells from the vascular lesions. Also, a more detailed analysis of the cytokine profile pre and post treatment revealed that the entire IL-1β-IL-6-IL-23-IL-17 axis was paralyzed upon corticosteroid treatment and that the effect was not restricted to the differentiated T cell. In untreated patients, circulating monocytes/macrophages produced IL-17-polarizing cytokines IL-1β, IL-6 and IL-23, an abnormality that was essentially abrogated once patients were taking corticosteroids. Monitoring of these cytokines may give valuable information about the state of immunosuppression and co-existent disease activity (Table 1). In a recent study of patients with multiple sclerosis, high serum IL-17 levels correlated with nonresponsiveness to IFN-β treatment [24]**, emphasizing the usefulness of understanding T cell function for diagnostic and therapeutic purposes. In patients with GCA, it is currently unknown whether IL-1β, IL-6 and IL-23 production is regained in chronic flaring disease. In order to link acute phases of disease and aberrant APC function supplying IL-1β, IL-6 and IL-23 chronic patients with disease exacerbations have to be studied.
IL-17/IFN-γ double producers in vascular inflammation
Upon antigenic stimulation, naïve CD4 T cells make choices to differentiate into Th1, Th2, Th17, or even Treg cells. Ultimately, the nature of the instigator and its interaction with immune sentinels recognizing the danger signals determine the commitment of the evolving immune response. In an attempt to better define immunopathways in another inflammatory vasculopathy, atherosclerosis, Eid and colleagues [25]** have conducted an elegant study to evaluate IFN-γ and IL-17-dependent immunity in patients with coronary artery disease. Surprisingly, both IL-17 and IFN-γ were found in excessive amounts in the circulating blood. However, whereas there was good evidence for an activation of the IL-12/IFN-γ/CXCL10 axis, IL-17-polarizing cytokines (IL-1β, IL-6 and IL-23) were not elevated. Nevertheless, atherosclerotic coronary arteries contained T cells that produced IFN-γ, IL-17 or both cytokines together. Both IFN-γ and IL-17 interacted in stimulating vascular smooth muscle cells to produce IL-6, CXCL8 and CXCL10. In essence, the co-production of both T-cell cytokines created an inflammatory milieu that amplified the tissue-damaging potential of plaque inflammation and vessel wall remodeling. Coordinate actions of IFN-γ and IL-17 may also be the case in early GCA, albeit the two cytokines appears to derive from distinct and separable populations. Untreated GCA patients possess a small population of IFN-γ/IL-17 double producers, which disappears upon steroid therapy. In terms of therapeutic responsiveness, these double producers mimic the Th17 cells, suggesting that they are not important in promoting the chronic smoldering arteritis that persists in treated patients.
Multiple T-cell lineages – multiple APCs – multiple danger signals
Obviously, Th1 cells in GCA are entirely independent from Th17 immunity. Whether Th17 cells need Th1 cells is not yet clear, but unlikely, given the profound susceptibility of Th17 cells and the resistance of Th1 cells in treated patients. Lineage-specific responses of effector T cells reflect the nature of the antigen, but more importantly the cytokine milieu generated by instructing APCs (Fig. 2). In humans, Th17 effector cells develop out of CD161+ precursors upon cytokine cues from innate immune cells activated by danger signals [26]. Given the role of IL-17 in antimicrobial immunity, microbe-activated dendritic cells are the primary decision maker and modify the developmental program of naïve T cells through the release of IL-1β and IL-23 [27]. Monocyte/macrophage populations from GCA patients respond vividly to LPS, a pathogen-associated molecular pattern, and instruct the differentiation of Th17 cells. Th1 cell precursors require IL-12 (and likely additional cytokines, e.g. IL-18) to differentiate into IFN-γ producers. The ability to separate these two T cell lineages in GCA patients coincides with a complete separation of IL-12 and IL-1β/IL-6/IL-23-producing monocyte/macrophages. In essence, two distinct immune axes exist in GCA (Fig. 2).
The question then arises whether separable APCs have disease relevance. Heterogeneity of tissue macrophages within the granulomatous lesions of GCA has been reported [28–31]. A series of studies has provided compelling evidence that dendritic cells (DC) serve as the ultimate innate cell population initiating arterial wall inflammation. CD83+ DC populate the vasculitic infiltrates and co-localize with activated T cells [17]. Antibody-mediated depletion of CD83+ DC strongly inhibits disease activity [32]. In bioengineered human arteries CD83+ DC, but not CD14+ macrophages are indispensible to trigger vasculitis [33]. A careful study of normal human arteries has established that DC are an indigenous population of the vessel wall [18]. In medium-size arteries such vascular DC are posted on the adventitial side of the lamina elastic externa. They function as sentinels for pathogen-derived patterns. Upon depletion of phagocytic cells or removal of the adventitia, human arteries lose the ability to sense Toll-like receptor ligands. Gene expression profiling has revealed that human arteries express abundant levels of TLR transcripts. Intriguingly, different vascular territories display vessel-specific signatures of TLRs (Table 2). In essence, each vascular region specializes in the sensing of a different combination of TLR ligands. With abundant expression of innate pattern recognition receptors these data connect blood vessels with the innate immune system. Recognizing that TLR-expression dendritic cells are an integral part of arteries not only emphasizes the role that blood vessels have in immune surveillance. The unique signature of TRL expression in each vascular bed assigns specific functions to specific vessels. This mechanism could certainly be critical in defining the tissue tropism for diseases that emerge at the interface of the immune and vascular system. The targeting of vasculitides to selected vascular territories may simply reflect a differentially involvement of vessels in immune sensing.
Table 2.
Vessel-specific signature of TLR expression in human arteries
| Blood vessel | Dominant Toll-like receptors |
|---|---|
| Aorta | TLR 1, 2, 4, 5, 6 |
| Carotid artery | TLR 1, 2, 4, 6, 8 |
| Subclavian artery | TLR 2, 4, 8 |
| Temporal artery | TLR 2, 4, 8 |
| Mesenteric artery | TLR 2, 4, 5 |
| Iliac artery | TLR 1, 2, 6 |
Human arteries from 6 vascular beds were collected and processed for gene expression profiling for Toll-like receptor 1–9 transcripts. Each arterial territory expressed a distinct combination of TLRs. For each artery the dominant TLRs are listed.
The establishment of a humanized mouse model in which human arteries are engrafted into immune-deficient mice has created a bioincubator system in which in vivo interactions between human T cells and blood vessels can be studied. Screening of human artery-SCID chimeras with a panel of TLR ligands has demonstrated that TRL4 and TLR5 ligands are rapidly recognized by arteries [34]. TLR ligand-conditioned vascular DC are powerful partners for human T cells and provide signals that recruit and retain such T cells in the vessel wall. However, stimulation of vascular DC by either TLR4 or TLR5 ligands coaxes T cells to enter distinct pathways of inflammation. DC sensing TLR5 instruct T cells to arrange in perivascular clusters, around vasa vasorum. This pattern of vasculitis best resembles vasa vasoritis. Conversely, T cells differentiating under the guidance of TLR4-activated DC leave the adventitia, infiltrate into all wall layers and cause transmural inflammation. These studies have directed attention towards the architecture of vessel wall inflammation and the critical role of the initial instigator in shaping patterning and progression of disease. Chemokine receptor profiling of T cells mediating TLR4-initiated panarteritis suggests that such T cells are predominantly IL-17 producers, connecting initial trigger, T cell immunity and disease architecture.
Conclusion
Granuloma formation in GCA has long emphasized the central role of antigen-specific T cells. Recent studies have shed light on the diversity of T effector cells in the disease process. A direct comparison between disease lesions in untreated and treated patients has identified two separable T cell lineages, Th1 and Th17 cells. Th1 cells are corticosteroid resistant. Th17 cells are explicitly corticosteroid sensitive (Fig. 1). The two T cell lineages correlated with distinct patterns of vascular and systemic disease, suggesting that different disease components are sustain by distinct immune responses.
Mechanistic studies have drawn attention to APCs which hold responsibility for providing differentiation cues to T cells entering immune responses. In GCA, IL-12 producing APCs and IL-1β/IL-6/IL-23-producing APC function independently. The separation of two inflammatory axes strongly supports the concept that initial triggers driving these pathways are distinct. Thus, vascular disease in GCA may not only be the sequel of infection, it may require multiple pathogens to generate the clinical picture of large vessel vasculitis (Fig. 2, Table 1).
Studies of adaptive immunity in GCA have revealed a number of lessons that inform our understanding of this vasculitis:
At least two distinct T cell lineages actively participate in GCA.
Distinct T cell lineages have distinct responsiveness to corticosteroids.
APC function is not uniform. Diversity exists for the production of polarizing cytokines.
GCA persists in the blood vessels for an extended period of time; in some patients far beyond a year.
Abnormal Th1 responses continue, unaffected by corticosteroids, identifying GCA as a long-term, chronic immune-mediated disease. So far, only two therapeutic interventions appear to affect IFN-γ-dependent immunity in GCA; aspirin and supra-high doses of corticosteroids [35,36].
One therapeutic intervention can unlikely control all inflammatory lineages in GCA. Cocktails of immunosuppressive therapies need to be applied.
Th17 responses are explicitly sensitive to even low doses of steroids and represent a minor therapeutic challenge.
The major therapeutic challenge in controlling GCA disease activity lies in persistent Th1 responses.
ACKNOWLEDGMENTS
This work was funded in part by NIH grants RO1 AR42527, RO1 AI44142, RO1 EY11916, RO1 AI 57266, PO1 HL 058000 and the Vasculitis Foundation. The authors would like to thank Linda Arneson for editorial support.
This work was supported by the National Institutes of Health grants R01 EY11916, R01 AI44142, R01 AR42527, U19 AI57266, P01 HL58000 and the Vasculitis Foundation.
Footnotes
The authors have declared no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349:160–169. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 2.Saunders BM, Cooper AM. Restraining mycobacteria: role of granulomas in mycobacterial infections. Immunol Cell Biol. 2000;78:334–341. doi: 10.1046/j.1440-1711.2000.00933.x. [DOI] [PubMed] [Google Scholar]
- 3.Duhaut P, Bosshard S, Ducroix JP. Is giant cell arteritis an infectious disease? Biological and epidemiological evidence. Presse Med. 2004;33:1403–1408. doi: 10.1016/s0755-4982(04)98939-7. [DOI] [PubMed] [Google Scholar]
- 4.Weyand CM, Goronzy JJ. Giant cell arteritis as an antigen-driven disease. Rheum Dis Clin North Am. 1995;21:1027–1039. [PubMed] [Google Scholar]
- 5.Weyand CM, Schonberger J, Oppitz U, et al. Distinct vascular lesions in giant cell arteritis share identical T cell clonotypes. J Exp Med. 1994;179:951–960. doi: 10.1084/jem.179.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brack A, Geisler A, Martinez-Taboada VM, et al. Giant cell vasculitis is a T cell-dependent disease. Mol Med. 1997;3:530–543. [PMC free article] [PubMed] [Google Scholar]
- 7.Brack A, Rittner HL, Younge BR, et al. Glucocorticoid-mediated repression of cytokine gene transcription in human arteritis-SCID chimeras. J Clin Invest. 1997;99:2842–2850. doi: 10.1172/JCI119477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez-Taboada V, Hunder NN, Hunder GG, et al. Recognition of tissue residing antigen by T cells in vasculitic lesions of giant cell arteritis. J Mol Med. 1996;74:695–703. doi: 10.1007/s001090050074. [DOI] [PubMed] [Google Scholar]
- 9.Deng J, Younge BR, Olshen RA, et al. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation. 2010;121:906–915. doi: 10.1161/CIRCULATIONAHA.109.872903. [DOI] [PMC free article] [PubMed] [Google Scholar]; **In this study temporal artery biopsies were harvested before and after steroid therapy. Analysis of T cell immunity demonstrated that in untreated GCA both Th1 and Th17 cells contribute to vascular inflammation. In treated GCA, Th17 cells are minimal but Th1 cells persist unaffected. The two separable T cell arms correlated with two immune axes that produce distinct patterns of T cell-polarizing cytokines; one of them steroid resistant, the other steroid responsive. The multiplicity of disease-relevant T cells and the multiplicity of antigen-presenting cells suggest a variety of disease instigators in GCA.
- 10.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weyand CM, Hicok KC, Hunder GG, Goronzy JJ. Tissue cytokine patterns in patients with polymyalgia rheumatica and giant cell arteritis. Ann Intern Med. 1994;121:484–491. doi: 10.7326/0003-4819-121-7-199410010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Weyand CM, Tetzlaff N, Bjornsson J, et al. Disease patterns and tissue cytokine profiles in giant cell arteritis. Arthritis Rheum. 1997;40:19–26. doi: 10.1002/art.1780400105. [DOI] [PubMed] [Google Scholar]
- 13.Brack A, Martinez-Taboada V, Stanson A, et al. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum. 1999;42:311–317. doi: 10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Grunewald J, Andersson R, Rydberg L, et al. CD4+ and CD8+ T cell expansions using selected TCR V and J gene segments at the onset of giant cell arteritis. Arthritis Rheum. 1994;37:1221–1227. doi: 10.1002/art.1780370817. [DOI] [PubMed] [Google Scholar]
- 15.Schaufelberger C, Andersson R, Nordborg E, et al. An uneven expression of T cell receptor V genes in the arterial wall and peripheral blood in giant cell arteritis. Inflammation. 2008;31:372–383. doi: 10.1007/s10753-008-9088-9. [DOI] [PubMed] [Google Scholar]
- 16.Wagner AD, Bjornsson J, Bartley GB, et al. Interferon-gamma-producing T cells in giant cell vasculitis represent a minority of tissue-infiltrating cells and are located distant from the site of pathology. Am J Pathol. 1996;148:1925–1933. [PMC free article] [PubMed] [Google Scholar]
- 17.Krupa WM, Dewan M, Jeon MS, et al. Trapping of misdirected dendritic cells in the granulomatous lesions of giant cell arteritis. Am J Pathol. 2002;161:1815–1823. doi: 10.1016/S0002-9440(10)64458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, et al. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118:1276–1284. doi: 10.1161/CIRCULATIONAHA.108.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Peck A, Mellins ED. Precarious balance: Th17 cells in host defense. Infect Immun. 2010;78:32–38. doi: 10.1128/IAI.00929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol Immunol. 2007;51:1139–1147. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Lanzavecchia A. Human Th17 cells in infection and autoimmunity. Microbes Infect. 2009;11:620–624. doi: 10.1016/j.micinf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21:274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Axtell RC, de Jong BA, Boniface K, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]; **These authors show that in mice and humans Th1 as well as Th17 cells can induce central nervous system autoimmunity. Th1-dependent disease can be well controlled with IFN-β treatment whereas Th-17-dependent disease is non-responsive to IFN-β. Patients with multiple sclerosis who had higher IL-17F concentrations in serum were non-responders to IFN-β therapy, had worse disease, required more steroids and experienced more relapses.
- 25.Eid RE, Rao DA, Zhou J, et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Elegant analysis of Th1 and Th17 immune responses in coronary artery disease. The authors found that coronary artery-infiltrating T cells often co-produce INF-γ and IL-17 and that both cytokines act synergistically in inducing the inflammatory phenotype of vascular smooth muscle cells.
- 26.Kleinschek MA, Boniface K, Sadekova S, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Annunziato F, Romagnani S. Do studies in humans better depict Th17 cells? Blood. 2009;114:2213–2219. doi: 10.1182/blood-2009-03-209189. [DOI] [PubMed] [Google Scholar]
- 28.Weyand CM, Wagner AD, Bjornsson J, Goronzy JJ. Correlation of the topographical arrangement and the functional pattern of tissue-infiltrating macrophages in giant cell arteritis. J Clin Invest. 1996;98:1642–1649. doi: 10.1172/JCI118959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rittner HL, Kaiser M, Brack A, et al. Tissue-destructive macrophages in giant cell arteritis. Circ Res. 1999;84:1050–1058. doi: 10.1161/01.res.84.9.1050. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser M, Weyand CM, Bjornsson J, Goronzy JJ. Platelet-derived growth factor, intimal hyperplasia, and ischemic complications in giant cell arteritis. Arthritis Rheum. 1998;41:623–633. doi: 10.1002/1529-0131(199804)41:4<623::AID-ART9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Kaiser M, Younge B, Bjornsson J, et al. Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol. 1999;155:765–774. doi: 10.1016/S0002-9440(10)65175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma-Krupa W, Jeon MS, Spoerl S, et al. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199:173–183. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han JW, Shimada K, Ma-Krupa W, et al. Vessel wall-embedded dendritic cells induce T-cell autoreactivity and initiate vascular inflammation. Circ Res. 2008;102:546–553. doi: 10.1161/CIRCRESAHA.107.161653. [DOI] [PubMed] [Google Scholar]
- 34.Deng J, Ma-Krupa W, Gewirtz AT, et al. Toll-like receptors 4 and 5 induce distinct types of vasculitis. Circ Res. 2009;104:488–495. doi: 10.1161/CIRCRESAHA.108.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weyand CM, Kaiser M, Yang H, et al. Therapeutic effects of acetylsalicylic acid in giant cell arteritis. Arthritis Rheum. 2002;46:457–466. doi: 10.1002/art.10071. [DOI] [PubMed] [Google Scholar]
- 36.Mazlumzadeh M, Hunder GG, Easley KA, et al. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum. 2006;54:3310–3318. doi: 10.1002/art.22163. [DOI] [PubMed] [Google Scholar]