Abstract
The cestode parasite, Raillietina echinobothrida and the trematode, Gastrothylax crumenifer were exposed to the ethanolic root peel extract of Potentilla fulgens, an antiparasitic local medicinal plant of Meghalaya, India, to evaluate the anthelmintic efficacy of the plant. The parasites were incubated in 1, 5, 10, 20, 50 and 100 mg crude alcoholic extract per ml of phosphate buffered saline (PBS) at a temperature of 37 ± 1°C. Paralysis and death were observed at 2.00 ± 0.05 and 2.80 ± 0.06 h for the cestode and 1.21 ± 0.06 and 2.18 ± 0.04 h for the trematode parasites at the highest test concentration of the plant extract. The commercial anthelmintic, Praziquantel (PZQ) showed higher activity at the tested concentration (0.02 mg/ml). To further investigate the efficacy of the plant extract, vital tegumental enzymes of the parasite viz. Acid phosphatase (AcPase), Alkaline phosphatase (AlkPase) and Adenosine triphosphatase (ATPase) were studied. Quantitatively, the total enzyme activity of AcPase, AlkPase and ATPase was found to be reduced significantly by 69.20, 66.43 and 29.63% for R. echinobothrida and 47.96, 51.79 and 42.63% for G. crumenifer, respectively compared to the respective controls; histochemical study also showed reduction in the visible staining of the enzymes. The reference drug, PZQ also showed more or less similar effect like that of the plant extract. The result suggests that phytochemicals of P. fulgens have anthelmintic potential.
Keywords: Potentilla fulgens, Anthelmintic, Raillietina echinobothrida, Gastrothylax crumenifer
Introduction
Helminthiasis is one of the major problems in the productivity of grazing livestock as well as health of human beings throughout the world (WHO 2002). It causes loss of production through mortality, weight loss, reduced milk, meat, wool production etc., which impact the livelihood of marginal farmers (Sykes 1994; Perry et al. 2002). To control helminth infection, a variety of commercial anthelmintics are available; however, due to increasing development of anthelmintic resistance, the limited availability of commercial drugs to the rural people as well as the high cost of such synthetic medicines, a growing interest in the ethno-veterinary approach to examine the anthelmintic properties of plants traditionally used by local farmers in different parts of the globe is emerging (Kaplan 2004; Jegede et al. 2007; Mali and Mehta 2008). A large number of plant products are being used for treatment of gastro-intestinal parasites of livestock and also humans (Kozan et al. 2006; Lyndem et al. 2008; Roy et al. 2008; Al-Shaibani et al. 2009; Kosalge and Fursule 2009; Challam et al. 2010; Manolaraki et al. 2010).
North-east India is a major biodiversity hotspot with diverse flora and fauna. Ethnic groups of this region use plant parts like leaves, bark, roots, shoot, etc. as a source of medicine, though the knowledge about their mode of action is very limited. Potentilla fulgens L (Family: Rosaceae) is a local medicinal plant, the aqueous root peel extract of which is consumed to get rid of intestinal parasitic infections; also, the tap root of the plant is traditionally chewed along with betel nut (Areca catechu) and betel leaves (Piper betel) for various other ailments (Syiem et al. 2002). The plant extract is also reported to have anti-diabetic and antioxidant properties (Syiem et al. 2009a, b). Though the plant is known as antiparasitic, particularly in remote areas of Meghalaya, no scientific record is available about its efficacy towards curing worm infections. Therefore, the present study was taken up to investigate in vitro anthelmintic activity of the ethanolic root peel extract of the plant, choosing histochemical and biochemical parameters for the same.
Materials and methods
Preparation of the plant crude extract
The fresh roots of Potentilla fulgens were purchased from the local markets of Shillong, Meghalaya. After washing with water, the root bark was peeled off and shade-dried, grounded by motor-driven grinder into powder form. The powder was then refluxed in 90% alcohol for 12 h at 60°C, and the solution filtered through whatman filter paper no. 1. The collected solution was evaporated to dryness at 50°C to recover the plant extract as dry powder, which was stored at 4°C till further use. Praziquantel (PZQ), a broad-spectrum anthelmintic, was used as the reference drug (WHO 2010).
Test parasites
Live mature tapeworms (Raillietina echinobothrida) were collected from the intestine of naturally infected domestic fowl in 0.9% phosphate buffered saline (PBS, pH 7.2). The fluke worms, Gastrothylax crumenifer, were collected from the rumen of freshly sacrificed cattle at local abattoirs.
In vitro experiment
After washing in PBS, the test parasites were incubated at 37 ± 1°C with different concentrations of the plant extract viz., 1, 5, 10, 20, 50 and 100 mg/ml in PBS containing 1% dimethyl sulfoxide (DMSO). Control parasites were incubated in PBS having 1% DMSO. Three replicates for each set of incubation medium were used and the time taken for attaining a paralytic state as well as death was recorded as described earlier (Roy et al. 2008). Immediately after the onset of paralysis, the parasites incubated in 20 mg of plant extract were selected for histochemical and biochemical studies because of the early effect of the dose when compared with other concentrations. PZQ was used in the concentration of 0.02 mg/ml of PBS containing 1% DMSO.
Histochemical localization of enzymes
The major enzymes, associated with the tegumental interphase of the platyhelminthes parasites were investigated histochemically using duly processed frozen sections cut at a thickness of 10–12 μm using a Leica CM 1850 cryostat. The AcPase activity was detected in cold formol-calcium fixed specimen following the modified Lead nitrate method (Pearse 1968), using sodium β-phosphoglycerate as the substrate; a brownish precipitate indicates the sites of the enzyme activity. The calcium-cobalt method for AlkPase activity at room temperature (17–20°C), as described by Pearse (1968) was used; the brownish black staining of the various structures in the tissue sections indicated the AlkPase activity. For the localization of ATPase activity, the calcium method of Pearse (1968) was followed; ATP was used as the substrate and the enzyme activity was determined through observation of blackish brown deposit.
Biochemical assays
Activities of AcPase and AlkPase were measured by estimating the p-nitrophenol formation following the method of Helwig et al. (1977). Using p-nitrophenyl phosphate as a substrate for both the enzymes, activity was measured by increase or decrease in the absorbance at λ 412 nm in a double beam UV–Visible spectrophotometer (Beckman Model-26). Similarly, ATPase activity was assayed by estimating the free phosphate released, following the method of Kaplan (1957) with ATP-Na2 as a substrate with little modification, following Zaidi et al. (1981). The inorganic phosphate of the supernatant was determined by the method of Fiske and SubbaRow (1925) at 700 nm similar to our earlier work (Roy and Swargiary 2009).
One unit of enzyme activity is defined as the amount of enzyme which would catalyze the transformation of 1 μM of substrate per hour. Enzyme activities are expressed as total and specific activities. Total enzyme activity is calculated by dividing the total units with the wet weight of the sample taken. Specific activity is expressed as units/mg tissue protein.
The protein content of all the tissue was estimated following the method of Lowry et al. (1951) using bovine serum albumin as a standard protein.
Statistical calculations
Data collected from three replicates were statistically analyzed and are presented as means ± SEM. Comparisons of the paired mean values between the experimental data and respective controls were made using Student’s t-test with P < 0.05 taken as the threshold of significance.
Results
Following the exposure to different concentrations of the plant extract, the parasites contracted sharply for some time and then went into a relaxed state and continued in the same state till they attained a condition of flaccid paralysis, which was followed by death after some interval of time. Table 1 shows the motility and mortality of worms treated with various concentrations of root peel extract of P. fulgens and PZQ. The controls of R. echinobothrida and G. crumenifer survived for 28.35 ± 0.12 and 22.47 ± 0.18 h, respectively. The treated parasites showed a steady decline in their motility and survival time with exposure to ascending concentrations of the test dosage. Thus, a dose-dependent paralytic effect and subsequent loss of motility of the parasite by the extract was evident in the order-trematocide>cestocide.
Table 1.
In-vitro effect of root peel-extract of P. fulgens and PZQ on the test parasites
| Incubation medium | Dose (mg/ml) PBS | Time taken in hours | |||
|---|---|---|---|---|---|
| R. echinobothrida | G. crumenifer | ||||
| Paralysis | Death | Paralysis | Death | ||
| P. fulgens | 100 | 2.00 ± 0.05 | 2.80 ± 0.06 | 1.21 ± 0.06 | 2.18 ± 0.04 |
| 50 | 3.08 ± 0.06 | 3.70 ± 0.05 | 2.33 ± 0.08 | 3.15 ± 0.05 | |
| 20 | 4.22 ± 0.05 | 6.37 ± 0.08 | 4.20 ± 0.02 | 5.15 ± 0.07 | |
| 10 | 6.45 ± 0.03 | 8.12 ± 0.09 | 5.16 ± 0.02 | 5.50 ± 0.04 | |
| 5 | 8.42 ± 0.06 | 10.43 ± 0.07 | 7.30 ± 0.05 | 8.37 ± 0.04 | |
| 1 | 21.22 ± 0.07 | 22.44 ± 0.10 | 19.46 ± 0.04 | 21.32 ± 0.04 | |
| PZQ | 0.02 | 0.40 ± 0.05 | 3.25 ± 0.07 | 0.57 ± 0.09 | 4.20 ± 0.08 |
| Control | PBS in 1% DMSO | 28.35 ± 0.12 | 22.47 ± 0.18 | ||
Values are taken as SEM, n number of replicates (3)
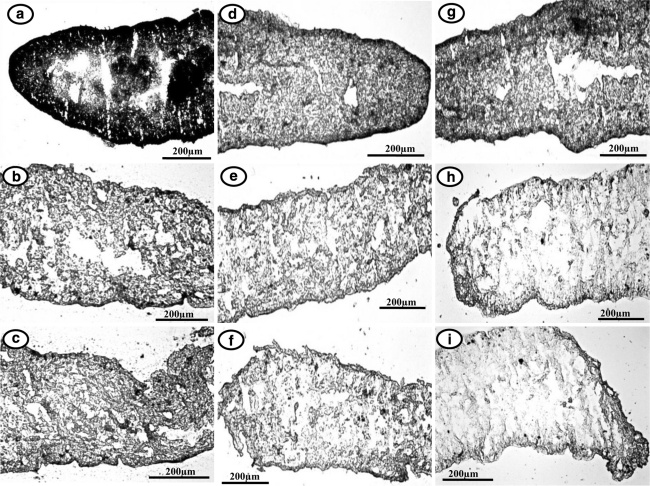
In histochemical analysis, exposure to the crude root-peel extract of P. fulgens and PZQ to R. echinobothrida showed a visible reduction in the activities of all the three enzymes (Fig. 1). Almost all the enzymes in the plant-extract- and PZQ-treated sectioned tissue were found to be diminished in the staining activity. Both cestodes and trematodes showed similar extent of stain intensity for different enzymes (in control and treated worms); therefore, photographs of only cestode material are presented herein.
Fig. 1.
AcPase, AlkPase and ATPase activities in the model test parasite Raillietina echinobothrida; photographs of fresh frozen sections (light microscopy) a–c AcPase activity a Control, bP. fulgens-treated, c PZQ treated. d–f AlkPase activity, d Control, eP. fulgens-treated, f PZQ treated, g–i ATPase activity g Control, hP. fulgens-treated, i PZQ treated
Biochemical analysis also showed that all the three enzymes in the treated parasites exhibit significant reduced activity; AcPase, AlkPase and ATPase were found to be reduced by 69.20, 66.43 and 29.63% for R. echinobothrida and by 47.96, 51.79 and 42.63% for G. crumenifer, respectively compared to their respective controls. Decreased enzyme activities were also recorded in the parasites treated with PZQ (62.31, 72.33 and 20.48% for R. echinobothrida and 52.24, 54.47 and 32.43% for G. crumenifer, respectively) (Table 2).
Table 2.
Biochemical effects of root peel-extract of P. fulgens and PZQ on test parasites in vitro
| Treatment (mg/ml) | Enzyme Activities (total/specific*) | Decrease (%) | ||||
|---|---|---|---|---|---|---|
| AcPase | AlkPase | ATPase | AcPase | AlkPase | ATPase | |
| R. echinobothrida | ||||||
| Control (PBS) |
17.83 ± 0.28 1.43 ± 0.03* |
126.01 ± 1.53 10.25 ± 0.16* |
232.42 ± 3.32 11.19 ± 0.16* |
|||
|
P. fulgens (20 mg/ml) |
5.49 0.50 ± 0.01* ± 0.05 |
42.30 ± 0.81 3.57 ± 0.13* |
163.54 ± 12.58 8.61 ± 0.68* |
69.20 | 66.43 | 29.63 |
| P < 0.0001 | P < 0.0001 | P < 0.05 | ||||
| PZQ (0.02 mg/ml) |
6.72 ± 0.23 0.59 ± 0.03* |
34.87 ± 0.78 3.15 ± 0.09* |
184.80 ± 1.87 9.58 ± 0.11* |
62.31 | 72.33 | 20.48 |
| P < 0.0001 | P < 0.0001 | P > 0.0001 | ||||
| G. crumenifer | ||||||
| Control (PBS) |
108.94 ± 0.54 5.39 ± 0.03* |
174.46 ± 1.78 8.05 ± 0.10* |
153.31 ± 7.10 7.48 ± 0.35* |
|||
|
P. fulgens (20 mg/ml) |
56.69 ± 2.58 3.27 ± 0.15* |
84.10 ± 5.27 4.13 ± 0.26* |
87.95 ± 2.80 5.02 ± 0.17* |
47.96 | 51.79 | 42.63 |
| P < 0.0001 | P < 0.0001 | P > 0.001 | ||||
| PZQ (0.02 mg/ml) |
52.03 ± 2.23 3.07 ± 0.18* |
79.43 ± 1.21 3.83 ± 0.07* |
103.58 ± 10.48 6.03 ± 0.63* |
52.24 | 54.47 | 32.43 |
| P < 0.0001 | P < 0.0001 | NS | ||||
Values are given as mean (SEM) from three replicates (n = 3)
Total activity formation of 1 μmol of product/h/g of wet tissue
* Specific activity activity/mg protein
P < 0.05, statistically significant; P < 0.0001, extremely significant; NS not significant
Discussion
Different classes of anthelmintics are established to show profound effects on the physical activities, generally culminating into loss of mobility and mortality of helminth parasites in a dose dependent manner (Urrea-Paris et al. 2000; Tippawangkosal et al. 2004; Xiao et al. 2004). Following this basic technique, several plants/plant parts such as Allium sativum, Albizzia lebbek, Artemesia santonica, Buchholzia coriaceae, Cardiospermum halicacabum, Gynandropsis gynandra, Houttuynia cordata, Neurolaena lobata, Ocimum sanctum, Perilla frutescens, Polyalthia suaveolens, Psidium guajava, Spilenthes oleracea, have been reported as potent cestocides, trematocides or nematocides (Kasuya et al. 1990; Singh and Nagaich 2000, 2002; Ajaiyeoba et al. 2001; Iqbal et al. 2001, 2004; Roy 2001; El Garhy and Mahmoud 2002; Boonmars et al. 2005; Fujimaki et al. 2005; Temjenmongla and Yadav 2005; Nyasse et al. 2006).
Results accrued from the present investigation indicate that P. fulgens exhibits cestocidal and trematocidal properties comparable with other in-vitro studies involving cestodes and trematodes (Roy and Tandon 1996, 1999; Roy et al. 2007; Tandon et al. 1997). The trematode appears to be the more susceptible helminth when treated in vitro.
The modes of action of anthelmintics are diverse, reflecting the natural differences in the physiology of the parasite and its potential hosts. It has been firmly documented that one of the hallmark effects of any anthelmintic is the destruction of the worm’s surface. This is due to the fact that the tegumental and/or cuticular structures are the primary parasite-host interfaces, vital for absorption of nutrients and perception of the surrounding micro-environment provided by the host (William et al. 2001; Mckinstry et al. 2003; Rivera et al. 2004; Xiao et al. 2004; Roy et al. 2008). Histological studies have revealed that anthelmintic agents induce considerable changes in the internal structural features of helminths, resulting in loss of normal cellular conformity in vital tissues (Roy et al. 2008; Dasgupta et al. 2010). In the present investigation, the plant extract was shown to cause reduction in the staining activity of the tegumental enzymes. In biochemical quantification also the enzyme activities in P. fulgens and PZQ treated flukes were found to be reduced significantly compared to the control ones. Other plant extract such as that of Alpinia nigra shoot-extract showed similar effect on AcPase, AlkPase and ATPase activities of Fasciolopsis buski (Roy and Swargiary 2009).
The effects of the test plant on the motility and survival of the parasite and alterations caused in their tegumental architecture clearly indicate that the phytochemicals of P. fulgens root bark may act as potential vermifuge or vermicide. In view of these observations further biochemical studies involving isolated active component(s) of this plant are warranted to confirm its anthelmintic efficacy.
Acknowledgments
Infrastructural support from DSA (UGC-SAP) programme to the Department of Zoology and UPE-Biosciences programme to the School of Life Sciences, NEHU is gratefully acknowledged.
References
- Ajaiyeoba EO, Onocha PA, Olarenwaju OT. In vitro anthelmintic properties of Buchholzia coriaceae and Gynandropsis gynandra extracts. Pharmaceut Biol. 2001;39:217–220. doi: 10.1076/phbi.39.3.217.5936. [DOI] [Google Scholar]
- Al-Shaibani IRM, Phulan MS, Shiekh M. Anthelmintic activity of Fumaria parviflora (Fumariaceae) against gastrointestinal nematodes of sheep. Int J Agr Biol. 2009;11:431–436. [Google Scholar]
- Boonmars T, Khunkitti W, Sithithaworn P, Fujimaki Y. In vitro antiparasitic activity of extracts of Cardiospermum halicacabum against third-stage larvae of Strongyloides stercoralis. Parasitol Res. 2005;97:417–419. doi: 10.1007/s00436-005-1470-z. [DOI] [PubMed] [Google Scholar]
- Challam M, Roy B, Tandon V. Effect of Lysimachia ramosa (Primulaceae) on helminth parasites: motility, mortality and scanning electron microscopic observations on surface topography. Vet Parasito. 2010;169:214–218. doi: 10.1016/j.vetpar.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Roy B, Tandon V. Ultrastructural alterations of the tegument of Raillietina echinobothrida treated with the stem bark of Acacia oxyphylla (Leguminosae) J Ethnopharmacol. 2010;127:568–571. doi: 10.1016/j.jep.2009.10.017. [DOI] [PubMed] [Google Scholar]
- El-Garhy MF, Mahmoud LH. Anthelmintic efficacy of traditional herbs on Ascaris lumbricoides. J Egypt Soc Parasitol. 2002;32:893–900. [PubMed] [Google Scholar]
- Fiske CH, SubbaRow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- Fujimaki Y, Kamachi T, Yanagi T, Caceres A, Maki J, Aoki Y. Macrofilaricidal and microfilaricidal effects of Neurolaena lobata, a Guatemalan medicinal plant, on Brugia pahangi. J Helminthol. 2005;79:23–28. doi: 10.1079/JOH2004262. [DOI] [PubMed] [Google Scholar]
- Helwig JJ, Farooqui AA, Bollack C, Mandel P. Distribution of lysosomal hydrolases in glomerular and tubular fractions of rabbit kidney cortex. Int J Biochem. 1977;8:323–327. doi: 10.1016/0020-711X(77)90140-9. [DOI] [Google Scholar]
- Iqbal Z, Nadeem QK, Khan MN, Akhtar MS, Waraich FN. In vitro anthelmintic activity of Allium sativum, Zingiber officinale, Curcurbita mexicana and Ficus religiosa. Int J Agri Biol. 2001;3:454–457. [Google Scholar]
- Iqbal Z, Lateef M, Ashraf M, Jabbar A. Anthelmintic activity of Artemisia brevifolia in sheep. J Ethnopharmacol. 2004;93:265–268. doi: 10.1016/j.jep.2004.03.046. [DOI] [PubMed] [Google Scholar]
- Jegede OC, Ikani IE, Dafwang II, Bolorunduro PI, Annatte AI. Traditional animal healthcare practices in disease prevention and control by small ruminant farmers in Oyo State, Nigeria. J Food Agr Environ. 2007;5:163–164. [Google Scholar]
- Kaplan C. Methods in enzymology, Vol III. New York: Academic Press; 1957. [Google Scholar]
- Kaplan RM. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kasuya S, Goto C, Koga K, Ohtomo H, Kagei N, Honda G. Lethal efficacy of leaf extract from Perilla frutescens (traditional Chinese medicine) or perillaldehyde on Anisakis larvae in vitro. Jpn J Parasitology. 1990;39:220–225. doi: 10.1007/BF00931082. [DOI] [PubMed] [Google Scholar]
- Kosalge SB, Fursule RA. Investigation of in vitro anthelmintic activity of Thespesia lampas (CAV) AJPCR. 2009;2:69–71. [Google Scholar]
- Kozan E, Kupeli E, Yesilada E. Evaluation of some plants used in Turkish folk medicine against parasitic infections for their in vivo anthelmintic activity. J Ethnopharmacol. 2006;108:211–216. doi: 10.1016/j.jep.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biological Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lyndem LM, Tandon V, Das B. Anthelmintic efficacy of medicinal plants from Northeast India against hookworms: an in vitro study on Ancylostoma cylanicum. Pharmacologyonline. 2008;3:697–707. [Google Scholar]
- Mali RG, Mehta AA. A review on anthelmintic plants. Nat Prod Rad. 2008;7:466–475. [Google Scholar]
- Manolaraki F, Sotiraki S, Stefanakis A, Skampardonis V, Volanis M, Hoste H. Anthelmintic activity of some Mediterranean browse plants against parasitic nematodes. Parasitology. 2010;137:685–696. doi: 10.1017/S0031182009991399. [DOI] [PubMed] [Google Scholar]
- McKinstry B, Fairweather I, Brennan GP, Forbes AB. Fasciola hepatica: tegumental surface alterations following treatment in vivo and in vitro with nitroxynil (Trodax) Parasitol Res. 2003;91:251–263. doi: 10.1007/s00436-003-0930-6. [DOI] [PubMed] [Google Scholar]
- Nyasse B, Ngantchou I, Nono JJ, Schneider B. Antifilarial activity in vitro of Polycarpol and 3-O-acetyl aleuritolic acid from Cameroonian medicinal plants against Onchocerca gutturosa. Nat Prod Res. 2006;20:391–397. doi: 10.1080/14786410600661377. [DOI] [PubMed] [Google Scholar]
- Pearse AGE. Histochemistry: theoretical and applied, Churchill Livingstone. London, New York: Edinburgh; 1968. [Google Scholar]
- Perry BD, Randolph TF, McDermott JJ, Sones KR, Thornton PK. Investing in animal health research to alleviate poverty; ILRI (International Livestock Research Institute) Kenya: Nairobi; 2002. p. 148. [Google Scholar]
- Rivera N, Ibarra F, Zepeda A, Fortoul T, Hernandez A, Castillo R, Canto G. Tegumental surface changes in adult Fasciola hepatica following treatment in vitro and in vivo with an experimental fasciolicide. Parasitol Res. 2004;93:283–286. doi: 10.1007/s00436-004-1127-3. [DOI] [PubMed] [Google Scholar]
- Roy B. Stereoscan observations on the surface alteration of Orthocoelium dinniki induced by extract of Spilanthes oleracea L. Riv Parasitol. 2001;18:9–14. [Google Scholar]
- Roy B, Swargiary A. Anthelmintic efficacy of ethanolic shoot extract of Alpinia nigra on tegumental enzymes of Fasciolopsis buski, a giant intestinal parasite. J Parasit Dis. 2009;33:48–53. doi: 10.1007/s12639-009-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B, Tandon V. Effect of root-tuber extract of Flemingia vestita, a leguminous plant, on Artyfechinostomum sufrartyfex and Fasciolopsis buski: a scanning electron microscopy study. Parasitol Res. 1996;82:248–252. doi: 10.1007/s004360050104. [DOI] [PubMed] [Google Scholar]
- Roy B, Tandon V. Flukicidal activity of Alpinia nigra (Zingiberaceae) against the trematode, Fasciolopsis buski, in humans. Biomedical Lett. 1999;60:23–29. [Google Scholar]
- Roy B, Lalchhandama K, Dutta BK. Anticestodal efficacy of Acacia oxyphylla on Raillietinaechinobothrida: a light and electron microscopic studies. Pharmacologyonline. 2007;1:279–287. [Google Scholar]
- Roy B, Dasgupta S, Tandon V. Ultrastructural observations on tegumental surface of Raillietina echinobothrida and its alterations caused by root-peel extract of Millettia pachycarpa. Microsc Res Tech. 2008;71:810–815. doi: 10.1002/jemt.20623. [DOI] [PubMed] [Google Scholar]
- Singh K, Nagaich S. Studies on the anthelmintic activity of Allium sativum (garlic) oil on common poultry worms Ascardia galli and Heterakis gallinae. J Parasitol App Anim Biol. 2000;9:47–52. [Google Scholar]
- Singh K, Nagaich S. Anthelmintic efficacy of the alcoholic extract of Ocimum sanctum against common poultry worm Ascardia galli and Heterakis gallinae. J Parasit Dis. 2002;26:42–45. [Google Scholar]
- Syiem D, Syngai G, Khup PZ, Khongwir BS, Kharbuli B, Kayang H. Hypoglycemic effects of Potentilla fulgens L in normal and alloxan-induced diabetic mice. J Ethnopharmacol. 2002;83:55–61. doi: 10.1016/S0378-8741(02)00190-3. [DOI] [PubMed] [Google Scholar]
- Syiem D, Sharma R, Saio V. In vitro study of the antioxidant potential of some traditionally used medicinal plants of North-East India and assessment of their total phenolic content. Pharmacologyonline. 2009;3:952–965. [Google Scholar]
- Syiem D, Khup PZ, Syiem AB. Effects of Potentilla fulgens Linn on carbohydrate and lipid profiles in diabetic mice. Pharmacologyonline. 2009;2:787–795. [Google Scholar]
- Sykes AR. Parasitism and production in farm animals. Anim Prod. 1994;59:155–172. doi: 10.1017/S0003356100007649. [DOI] [Google Scholar]
- Tandon V, Pal P, Roy B, Rao HSP, Reddy KS. In vitro anthelmintic activity of root-tuber extract of Flemingia vestita, an indigenous plant in Shillong, India. Parasitol Res. 1997;83:492–498. doi: 10.1007/s004360050286. [DOI] [PubMed] [Google Scholar]
- Temjenmongla, Yadav AK. Anticestodal efficacy of folklore medicinal plants of naga tribes in North-east India. Afr J Trd CAM. 2005;2:129–133. [Google Scholar]
- Tippawangkosal P, Choochote W, Na-Bangchang K, Jitpakdi A, Pitasawat B, Riyong D. The in vitro effect of albendazole, ivermectin, diethylcarbamazine, and their combinations against ineffective third stage larvae of nocturnally subperiodic Brugia malayi (Narathiwat strain): scanning electron microscopy. J Vector Ecol. 2004;29:101–108. [PubMed] [Google Scholar]
- Urrea-Paris MA, Moreno MJ, Casado N, Rodriguez-Caabeiro F. In vitro effect of praziquantel and albendazole combination therapy on the larval stage of Echinococcus granulosus. Parasitol Res. 2000;86:957–964. doi: 10.1007/PL00008526. [DOI] [PubMed] [Google Scholar]
- WHO (2002) “WHO Traditional Medicine Strategy 2002–2005”. WHO/EDM/TRM/2002.1, p 61
- WHO (2010) “WHO Model List of Essential Medicines. 16th edn (March 2010)”, pp 1–39. http://www.int/medicines/publications/essentialmedicines/en/index.html
- William S, Botros S, Ismail M, Farghally A, Day TA, Bennett JL. Praziquantel-induced tegumental damage in vitro is diminished in schistosomes derived from praziquantel-resistant infections. Parasitology. 2001;122:63–66. doi: 10.1017/S0031182000007137. [DOI] [PubMed] [Google Scholar]
- Xiao SH, Guo J, Chollet J, Wu JT, Tanner M, Utzinger J. Effect of artemether on Schistosoma mansoni: dose-efficacy relationship, and changes in worm morphology and histopathology. Chin J Parasist Dis. 2004;22:148–153. [PubMed] [Google Scholar]
- Zaidi SIM, Pandey RN, Kidwai AM, Krishnamurti CR. A rapid method for preparation of sarcolemma from frog skeletal muscle. J Biosci. 1981;3:293–302. doi: 10.1007/BF02702940. [DOI] [Google Scholar]