Abstract
Progress towards a vaccine against malaria is advancing rapidly with several candidate antigens being tested for their safety and efficacy. In present investigation, two polypeptides (43 and 48 kDa) of Plasmodium berghei (NK-65) were identified by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Immunogenicity and protective efficacy of both these polypeptides formulated in saponin has been compared in Balb/c mice against challenge infection with P. berghei. Antibody responses were evaluated by indirect fluorescent antibody test and enzyme-linked immunosorbent assay. Merozoite invasion inhibition assay and challenge infections revealed that 48 kDa antigen is better immunogen as compared to 43 kDa and provide better protection against rodent malaria infection.
Keywords: Antibody, Immunity, 43 kDa, 48 kDa, Malaria, Plasmodium, Vaccine
Introduction
Malaria is one of the most prevalent parasitic diseases and a major health problem with significant economic and social consequences for many developing countries (Dwivedi et al. 2009). Despite many interventional efforts to control malaria, morbidity and mortality estimates continue to rise. Chemotherapy and vector control programs have been largely ineffective due to the emergence and spread of insecticide resistant mosquito vectors and drug resistant Plasmodia (Huaman et al. 2009). One of the most appealing strategies for eradicating this disease is the development of a vaccine.
A completely effective vaccine must contain not one but most of the molecules involved in different stages of parasite invasion of host cells (Manuel and Patarroyo 2008). Thus a vaccine targeting erythrocytic stage requires continuous efforts to identify and characterize protective antigens. Sustained antigenic stimulation and antibody production may be important in maintaining the anti disease state (Burns et al. 1997). Blood stage merozoites express a number of surface antigens that are involved in the initial interaction between the parasite and the host erythrocyte (Holder 1994).
Tan et al. (1996) identified 43 kDa P. berghei antigen with homology to the exp-1 antigen from Plasmodium falciparum on the basis of immunological cross reactivity. This antigen is located on the surface of the parasite and shares peptide sequence homology with the P. chabaudi antigen Ag 3008. Oeuvray et al. (1994) identified 48 kDa antigen by targeting the antibodies, which inhibit P. falciparum in vitro growth in an ADCI assay and it is located on surface of merozoite stage (MSP-3) of Plasmodium. More exploration of both 43 and 48 kDa antigens of P. berghei is required to check their role in generation of immune response. Hence, immunogenicity imparted by 43 and 48 kDa antigens and their protective efficacy against rodent malaria has been checked in present study.
Materials and methods
Animals
Five to six-weeks old inbred white Swiss mice, Mus musculus (Balb/c strain), weighing 25–30 g each, of either sex, were obtained from National Institute of Pharmaceutical Education and Research, Mohali (NIPER) and kept in Central Animal House, Panjab University, Chandigarh.
Parasites and infection
Asexual erythrocytic stages of P. berghei (NK-65) were maintained in Balb/c mice. Infections were initiated by intraperitoneal (i.p.) injection of 1 × 106P. berghei parasitized cells obtained from infected to naive mice. The course of infection was monitored by Giemsa-stained thin blood films as described by Santiyanont (1985).
Isolation of cell free parasites
When the parasitaemia averaged 35–40%, blood was aspirated from P. berghei infected mice by jugular vein incision. The infected blood contained a mixture of ring, trophozoite and schizont-stage parasites. Blood was centrifuged (REMI, 8C) at 800g for 10 min at room temperature. Plasma and white leukocyte layer were aspirated using Pasteur pipette. Red blood cells were collected and lyzed with 0.2% saponin in phosphate-buffered saline (PBS, pH 7.2). Intact parasites were collected by centrifugation and washed to remove soluble RBC proteins (Upma et al. 1998).
Homogenization of cell free parasites
Cell free parasite pellet suspended in sucrose (0.25 M in PBS, pH 7.2) was homogenized at 4°C by Potter Elvehjam, pre-cooled homogenizer (REMI, Bombay, India). Total parasite homogenate (TPH) thus obtained was suspended in 1–2 ml of PBS (pH 7.2, 0.01 M) and stored at −20°C for further use (Banyal et al. 1979).
Protein estimation
The concentration of protein in TPH was estimated by slightly modified method of Lowry et al. (1951) using bovine serum albumin (BSA) as standard.
SDS-PAGE
The TPH was subjected to polyacrylamide gel electrophoresis (SDS-PAGE) in tris–glycine buffer, pH 8.3 to localize the proteins of molecular weights, 43 and 48 kDa, by the method of Laemmli (1970). Gels were stained with Coomassie blue R250 followed by destaining (50% methanol + 10% acetic acid). Proteins were electro-eluted, dialyzed and suspended in PBS. Eluted proteins were quantified by Lowry’s method.
Immunization of mice
Animals were immunized by the method of Helmby et al. (1993). Balb/c mice (ten animals per group) were immunized subcutaneously (s.c.) with 20 μg of protein (43/48 kDa) along with equal amount of saponin as adjuvant on day zero. Two booster doses were given at 2 weeks interval i.e., on day 14 and 28. Control mice were injected s.c. with PBS (0.01 M, pH 7.2) and saponin (20 μg) without any protein on similar days.
Indirect immunofluorescent assays
Anti-43 kDa and anti-48 kDa sera from pre- and post-challenged mice were assayed for binding to acetone fixed whole parasites (Hui et al. 1992). Sera samples were serially diluted (1:32) in PBS (0.15 M, pH 7.2) and end-point IFA titers were determined as the highest serum dilutions that gave detectable fluorescence microscopically (Leica DMLS, Germany).
Parasite challenge and parasitaemia assessment
Six immunized mice from each group along with control mice were challenged i.p. with 1 × 106P. berghei infected erythrocytes on day 38 post-first immunization. The course of parasitaemia was monitored by studying Giemsa-stained blood smears till death of control mice or clearance of parasite from immune mice.
Antibody detection by ELISA
The presence of antigen specific antibodies was monitored in the sera of immunized mice on day 38 post-first immunization by ELISA. 96-well, polystyrene micro-ELISA plate was incubated overnight at 4°C with 50 μg/well of P. berghei antigen diluted in freshly prepared 0.1 M carbonate buffer (pH 9.6). After washing with washing buffer (PBS, pH 7.2) and blocking steps, the plate was incubated with 50 μl of sera samples (1:500 dilutions) from different immunized groups at 37°C for 1 h and washing procedure was repeated. It was further incubated with 50 μl of HRP-conjugated goat anti-mouse IgG1 and IgG2a antibodies for 1 h. After three washings, 50 μl of substrate (0.05% DAB, 0.015% H2O2 in 0.01 M PBS, pH 7.2) was added to each well and incubated for 20–30 min at 37°C. Reaction was stopped by adding 50 μl of 3 N H2SO4 to wells. After this, optical density (OD) of each well was determined by ELISA reader (Dynatech Micro-reader 11) set to 490 nm (Agrewala et al. 1998).
In vitro merozoite invasion inhibition assay
The ability of anti-43 kDa and anti-48 kDa sera to inhibit in vitro invasion of parasite was evaluated by maintaining short term P. berghei culture (Upma et al. 1998). One ml of complete medium (RPMI-1640 supplemented with 0.06% (w/v) HEPES; 5% (w/v) sodium bicarbonate; antibiotics—gentamycin (50 μg/ml), penicillin (100 μg/ml), and streptomycin (100 μg/ml); and 10% (v/v) inactivated FCS), having 2% haematocrit with 0.1–0.5% parasitaemia along with pre- and post-challenge sera of immunized groups (50 μl of each) were added to each well of 24-well culture tray (Laxbro, India). Each sample was run in duplicate. The tray was shaken gently to mix the contents. Zero hour smears were prepared and stained with Giemsa stain. The tray was then placed in a candle jar at 37°C in an incubator. After 21 h of incubation, the tray was removed from the incubator and smears from each well were prepared. Differential counting of the parasite (rings, trophozoites and schizonts) was done by studying these Giemsa-stained smears. The percent inhibition of merozoite invasion was calculated by using following formula:
 |
Statistical analysis
Student’s t-test was used for statistical analysis; results with P < 0.05 were considered to be statistically significant.
Results
Parasite isolation, homogenization and SDS-PAGE
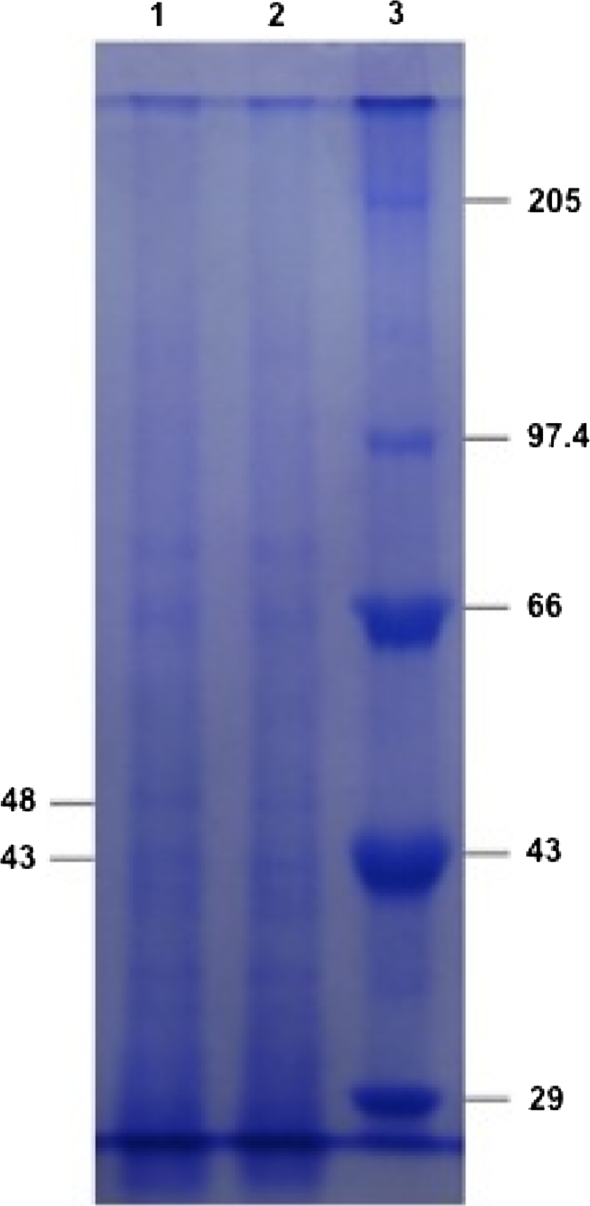
Cell free parasite (CFP) obtained after saponin lysis of infected RBCs, appeared as brightly colored nuclei in Giemsa-stained smears. 9.8 mg/ml of protein was estimated in TPH by Lowry’s method. TPH was electrophoresed to determine the molecular weights of various proteins by SDS-PAGE. Proteins of interest i.e., 43 and 48 kDa were identified by comparing their Rf values with that of protein standard (Genei) exhibiting six distinct polypeptides having molecular mass 29, 43, 66, 97.4 and 205 kDa (Fig. 1).
Fig. 1.
Coomassie-blue-stained SDS-PAGE gel of P. berghei proteins (Lane-1, 2) and protein standard (Lane-3)
Immunization of mice and antibody detection by IFA
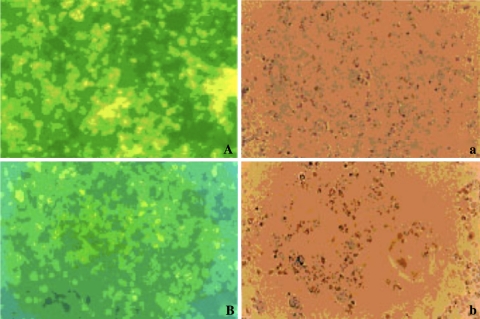
Two groups of mice were immunized using 43 and 48 kDa antigens as described earlier. Antibody titers and the specificity of antigen–antibody reaction were analyzed by IFA in serum samples of unchallenged, challenged and control mice (Fig. 2). Unchallenged sera from mice immunized with 48 kDa antigen were found to have almost double amount (1:2048) of antibody titer as compared to 43 kDa immunized mice (1:1024). After challenge, no significant rise in antibody titers was observed in sera of immunized mice as checked on day 45-post challenge. Antimalarial antibodies were not observed in sera of the control mice.
Fig. 2.
IFA reaction of immune sera raised against 43 kDa A, a and 48 kDa B, b against P. berghei antigen (A, B: under UV; a, b: under phase contrast) (×1890)
Post-challenge course of infection
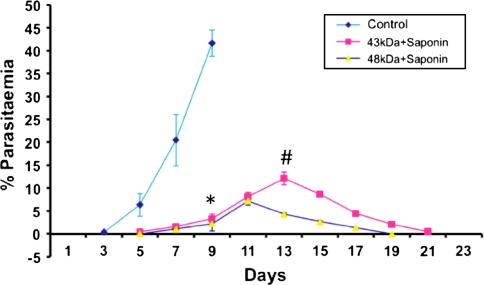
Six immunized mice from each group along with control animals were challenged by i.p. inoculation of 1 × 106P. berghei infected cells after 38 days of first immunization. In both the immunized groups, infection was observed on day 5-post challenge, which increased gradually up to day 13 post-challenge in mice immunized with 43 kDa (12.1 ± 1.3%) and up to day 11 in 48 kDa immunized mice (7.2 ± 1.6%). Parasite was cleared in 67% of 43 kDa immunized animals. Maximum parasitaemia observed in 43 kDa immunized group on day 13 was found to be significantly higher (P < 0.001) than that of 48 kDa group, in which out of six challenged mice, five survived the challenge and one mice died due to infection. Complete clearance of infection was observed after day 21 in surviving mice of both groups. In control mice, patency was observed on day 3 and maximum parasitaemia (41.6 ± 1.3%) was recorded on day 9-post inoculation, which was significantly higher (P < 0.001) than both the immunized groups on the same day. This was followed by death of all the mice of this group by day 10 (Fig. 3).
Fig. 3.
Course of parasitaemia in immunized mice after being challenged with 1 × 106P. berghei parasitized red blood cells. Parasitaemia is expressed as means ± standard deviations. Asterisk: Control versus vaccinated (43 kDa + Saponin, 48 kDa + Saponin mice) on day 9; Space: 43 kDa + Saponin versus 48 kDa + Saponin, P < 0.001 on day 13
Antibody detection by ELISA
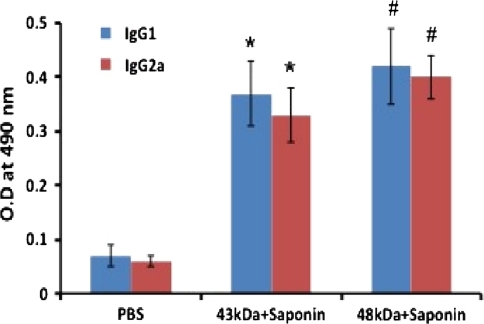
Sera samples from immunized groups were assayed for parasite specific antibodies by ELISA. The isotype analysis of immunized mice showed higher level of IgG1 in mice immunized with 48 kDa as compared to 43 kDa immunized mice. Besides IgG1, 48 kDa induced substantial level (P < 0.05) of IgG2a isotype antibody also. Sera from mice of both immunized groups contained significantly higher (P < 0.001) level of both the IgG1 and IgG2a isotypes as compared to controls (Fig. 4).
Fig. 4.
Antigen-specific IgG isotype responses in Balb/c mice immunized with 43 and 48 kDa antigens of P. berghei. The values expressed are the mean of the absorbance of the sera of the six different animals ± standard deviation. Asterisk: Control versus 43 kDa + Saponin, P < 0.001; Space: Control versus 48 kDa + Saponin, P < 0.001
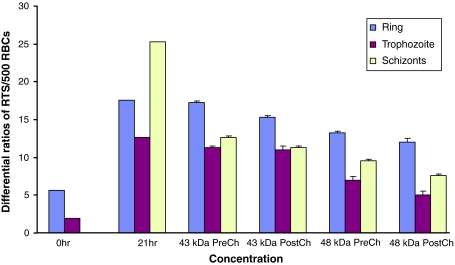
In vitro parasite invasion inhibition with the anti-43 kDa and anti-48 kDa sera
Immune sera (5%) were added to culture wells in duplicate at 0 h. After 21 h of incubation, 50.2 and 62.1% invasion inhibition was observed in pre-challenged sera of mice immunized with 43 and 48 kDa antigens respectively. Whereas, post-challenged sera of these antigens (43 and 48 kDa) resulted in 55.4 and 70.0% invasion inhibition respectively (Fig. 5).
Fig. 5.
In vitro merzoite invasion inhibition by pre and post challenge sera of 43 kDa immunized and 48 kDa immunized groups after 21 h incubation in culture as compared to controls
Discussion
Virulence of Plasmodium depends partly on strain of parasite and partly on host. Moreover, size of inoculation also influences the intensity of rodent malaria (Cox 1988). Morbidity and mortality due to Plasmodium result during the asexual development and replication of these protozoan parasites within erythrocytes (Miller et al. 2002). Preference of P. berghei and P. vivax for reticulocytes is also established (Janse et al. 1989; Galinski et al. 1992). Cell free parasite obtained by saponin lysis does contain some red cell contamination but it has been reported to be metabolically active (Kreier and Green 1977).
Successful vaccination depends not only on suitable antigen but also on other factors as mode and route of antigen administration, cytokine profile and use of suitable adjuvant (Sharma et al. 2007). Antigens are usually administered with adjuvants, which are known to play an important role in potentiating and inducing appropriate type of immune response (Gupta and Siber 1995). Saponin has been considered to be more potent adjuvant as compared to FCA and Al(OH)3 (Freeman and Holder 1983) and has been used in many research labs (Playfair and de Souza 1987). Efforts are ongoing to improve adjuvants that can be used safely for the immunization of human subjects within reasonable constraints of antigen dose and immunization frequency (Ockenhouse et al. 2006; Stoute et al. 2007).
Protection against malaria challenge in mice has been achieved upon vaccination with proteins and peptides derived from pre-erythrocytic stages of the parasite (Khusmith et al. 1991; Wang et al. 1996) as well as with DNA (Hoffman et al. 1994). In this study, protective efficacy of immunization was checked upon challenge with 1 × 106P. berghei parasites. In 43 kDa immunized group, 67% of immunized mice survived the challenge, whereas, in 48 kDa group, 83% of mice survived. Moreover, longer patency and higher infection was observed in mice immunized with 43 kDa as compared (P < 0.001) to that of 48 kDa group. These results point towards the higher efficacy of 48 kDa in providing protection against Plasmodium infection. All control mice died by day 10 post-inoculation because of high infection. Less parasitaemia in immunized mice points towards the activation of immune response upon vaccination.
Both antibody and cellular responses seem to be required for protective immunity against malaria (Mata et al. 2007). Antimalarial antibodies start appearing in the blood of infected mouse after a week of P. berghei infection, if mouse survives. It has been confirmed in present investigation too as level of anti-plasmodial antibodies (on day 45 post challenge) in post-challenged sera of both groups was almost same as that of unchallenged sera, in spite of involvement of antibodies in various protective immune mechanisms. CD4+ Th1 and Th2 subsets have crucial role in B cell differentiation, proliferation and isotype regulation (Ahmad et al. 2001). Specific IgG subclass particularly IgG2a has been reported to be involved in antibody mediated protection against NK-65 strain of P. berghei (Akanmori et al. 1994). Significantly higher levels of IgG1 isotype along with increased levels of IgG2a antibody in immunized groups before challenge in present study points towards a mixed Th1/Th2 cytokine profile. The study of isotypic distribution of the human antibodies against 48 kDa antigen of Plasmodium revealed that this protein is the target of the cytophilic antibodies (IgG1 or IgG3) from protected individuals and is recognized by non-cytophilic isotypes (IgG2 or/and IgM) in non-protected individuals (Oeuvray et al. 1994).
Disease is progressed by rupturing of infected erythrocytes and reinvasion of healthy cells by liberated merozoites. Merozoite invasion not only depends on MSP1 but also involves additional distinct receptor–ligand interactions (Shi et al. 2007). One of the most feasible strategies for blood-stage malaria is to immunize with subunit vaccines that induce high antibody titres that neutralize extracellular merozoites and prevent the invasion of erythrocytes (Mahanty et al. 2003). Reduced invasion of merozoites into red cells in vitro by pre- and post-challenge immune sera in present study is consistent with earlier studies (Cohen and Butcher 1970; Miller et al. 1975) demonstrating that merozoite agglutination by antibodies after their liberation from infected erythrocytes is associated with reduced reinvasion. More inhibition of merozoite invasion by sera of mice immunized with 48 kDa than that of 43 kDa immunized mice, points towards the better efficacy of 48 kDa in induction of protective response.
The investigation on these two proteins will further be extended to study the nature of cell-mediated immunity by checking cytokine profile and trafficking of immune cells. The multiple receptor–ligand interactions and alternate redundant pathways involved in the merozoite invasion of erythrocytes combined with the polymorphism of vaccine candidate antigens present a challenge for vaccine design (Shi et al. 2007). Availability of at least partially efficacious malaria vaccine is expected in the next decade, which will form the basis for the development of other effective vaccines against disease.
Acknowledgments
The authors hereby declare that the experiments comply with the IAEC rules in India. Mr. Anil Pawar is thankful to University Grant Commission, New Delhi for providing financial assistance in the form of RFSMS under UGC-CAS programme.
References
- Agrewala JN, Sumit S, Verma RK, Mishra GC. Differential effect of anti B7.1 anti-M150 antibodies in restricting the delivery of costimulatory signals from B-cells and macrophages. J Immunol. 1998;160:1067–1077. [PubMed] [Google Scholar]
- Ahmad N, Masood AK, Owais M. Fusogenic potential of prokaryotic membrane lipids: implication in vaccine development. Eur J Biochem. 2001;268:5667–5675. doi: 10.1046/j.0014-2956.2001.02507.x. [DOI] [PubMed] [Google Scholar]
- Akanmori BD, Waki S, Suzuki M. Immunoglobulin G2a isotope may have a protective role in Plasmodium berghei NK-65 infection in immunized mice. Parasitol Res. 1994;80:638–641. doi: 10.1007/BF00932945. [DOI] [PubMed] [Google Scholar]
- Banyal HS, Pandey VC, Dutta GP. Subcellular fractionation and localization of marker enzymes of erythrocytic stages of Plasmodium knowlesi. Indian J Parasitol. 1979;3:9–14. [Google Scholar]
- Burns JM, Dunn PD, Russo DM. Protective immunity against P. yoelii malaria induced by immunization with particulate blood-stage antigens. Infect Immun. 1997;65(8):3138–3145. doi: 10.1128/iai.65.8.3138-3145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Butcher GA. Properties of protective malarial antibody. Immunology. 1970;19:369–383. [PMC free article] [PubMed] [Google Scholar]
- Cox FEG. Major animal models in malaria research: rodents. In: Wernsdorfer WH, McGregor I, editors. Malaria: principles and practice of malariology. London: Churchill Livingstone; 1988. pp. 1503–1543. [Google Scholar]
- Dwivedi V, Vasco A, Vedi S, Dangi A, Arif K, Bhattacharya SM, Owais M. Adjuvanticity and protective immunity of Plasmodium yoelii nigeriensis blood-stage soluble antigens encapsulated in fusogenic liposome. Vaccine. 2009;27:473–482. doi: 10.1016/j.vaccine.2008.10.054. [DOI] [PubMed] [Google Scholar]
- Freeman RR, Holder AA. Characteristics of the protective response of Balb/c mice immunized with a purified P. yoelli schizont antigen. Clin Exp Immunol. 1983;54:609–616. [PMC free article] [PubMed] [Google Scholar]
- Galinski MR, Medina CC, Ingranallo P, Barnwell JW. A reticulocyte binding protein complex of P. vivax merozoites. Cell. 1992;69(7):1213–1226. doi: 10.1016/0092-8674(92)90642-P. [DOI] [PubMed] [Google Scholar]
- Gupta PK, Siber GR. Adjuvants for human vaccines; current status, problems and future prospects. Vaccine. 1995;13:1263–1276. doi: 10.1016/0264-410X(95)00011-O. [DOI] [PubMed] [Google Scholar]
- Helmby H, Cavelier L, Pettersson U, Wahlgren M. Rosetting P. falciparum-infected erythrocytes express unique strain specific antigens on their surface. Infect Immun. 1993;61(1):284–288. doi: 10.1128/iai.61.1.284-288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman SL, Sedegah M, Hedstrom RC. Protection against malaria by immunization with a Plasmodium yoelii circumsporozoite protein nucleic acid vaccine. Vaccine. 1994;12:1529–1533. doi: 10.1016/0264-410X(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Holder AA. Proteins on the surface of the malarial parasite and cell invasion. Parasitology. 1994;105:S5–S18. doi: 10.1017/S0031182000075673. [DOI] [PubMed] [Google Scholar]
- Huaman MC, Mullen GED, Long CA, Mahanty S. Plasmodium falciparum apical membrane antigen 1 vaccine elicits multifunctional CD4 cytokine-producing and memory T cells. Vaccine. 2009;27:5239–5246. doi: 10.1016/j.vaccine.2009.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui GS, Hashimoto A, Chang SP. Roles of conserved and allelic regions of the major merozoite surface protein (gp 195) in immunity against P. falciparum. Infect Immun. 1992;60(4):1422–1433. doi: 10.1128/iai.60.4.1422-1433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse CJ, Boorsma EG, Ramesar J, Grobbe MJ, Mas B. Host cell specificity and schizogony of P. berghei under different in vitro conditions. Int J Parasitology. 1989;19:509–514. doi: 10.1016/0020-7519(89)90080-5. [DOI] [PubMed] [Google Scholar]
- Khusmith S, Choronevit Y, Kumar S, Sedegah M, Beandoin RL, Hoffman SL. Protection against malaria by vaccination with sporozoite surface protein 2 plus CS protein. Science. 1991;252:715–718. doi: 10.1126/science.1827210. [DOI] [PubMed] [Google Scholar]
- Kreier JP, Green TJ. The isolation and fractionation of malaria infected cells. Bull WHO. 1977;55:317–331. [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rasebrough NJ, Farr AL, Randall RJ. Protein measurement with phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mahanty S, Saul A, Miller LH. Progress in the development of recombinant and synthetic blood-stage malaria vaccines. J Exp Biol. 2003;206:3781–3788. doi: 10.1242/jeb.00646. [DOI] [PubMed] [Google Scholar]
- Manuel EP, Patarroyo AM. Emerging rules for subunit based multi antigenic multistage chemically synthesized vaccines. Acc Chem Res. 2008;41:377–386. doi: 10.1021/ar700120t. [DOI] [PubMed] [Google Scholar]
- Mata E, Carcaboso AM, Hernandez RM, Igartua M, Corradin G, Pedraz JL. Adjuvant activity of polymer microparticles and montanide ISA 720 on immune responses to P. falciparum MSP2 long synthetic peptides in mice. Vaccine. 2007;25:877–885. doi: 10.1016/j.vaccine.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Miller LH, Aikawa M, Dvorak JA. Malaria Plasmodium knowlesi merozoites : immunity and the surface coat. J Immunol. 1975;114:1237–1242. [PubMed] [Google Scholar]
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- Ockenhouse CF, Angov E, Kester KE, Diggs C, et al. Phase 1 safety and immunogenicity trial of FMP1/AS02A, a P. falciparum MSP-1 asexual blood stage vaccine. Vaccine. 2006;24:3009–3017. doi: 10.1016/j.vaccine.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Oeuvray C, Bouharoun-Tayoun H, Grass-Masse H, Lepers JP, Ralamboranto L, Tartar A, Druilhe P. A novel merozoite surface antigen of P. falciparum (MSP-3) identified by cellular-antibody cooperative mechanism antigenicity and biological activity of antibodies. Mem Inst Oswaldo Cruz. 1994;89:77–80. doi: 10.1590/S0074-02761994000600018. [DOI] [PubMed] [Google Scholar]
- Playfair JHL, Souza JB. Vaccination of mice against malaria with soluble antigen I. The effect of detergent route of infection and adjuvant. Parasite Immunol. 1987;8:409–414. doi: 10.1111/j.1365-3024.1986.tb00857.x. [DOI] [PubMed] [Google Scholar]
- Santiyanont R (1985) Parasite identification, counting and staining. In: Application of genetic engineering techniques: diseases, pathogens with special reference to Plasmodia. A laboratory manual of selected techniques. UNDP/World Bank/WHO special programme for research and techniques in tropical diseases, Bangkok, Thailand 8.7. pp 413–418
- Sharma SK, Gupta C, Dwivedi V, Misra-Bhattacharya S, Owais M. Prophylactic potential of liposomized integral membrane protein of P. yoelii nigeriensis against blood stage infection in Balb/c mice. Vaccine. 2007;25:2103–2111. doi: 10.1016/j.vaccine.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Shi Q, Lynch MM, Romero M, Burns JM. Enhanced protection against malaria by a chimeric merozoite surface protein vaccine. Infect Immun. 2007;75:1349–1358. doi: 10.1128/IAI.01467-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoute JA, Gombe J, Withers MR, Siangla J, et al. Phase-1 randomized double-blind safety and immunogenicity trial of P. falciparum malaria merozoite surface protein FMP1 vaccine, adjuvanted with AS02A, in adults in western Kenya. Vaccine. 2007;25:176–184. doi: 10.1016/j.vaccine.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Tan TMC, Goh KL, Binh LN, Ting RCY, Kar UAK. Identification of a Plasmodium berghei antigen sharing common features with P. falciparum and P. chabaudi parasitophorous-vacoule membrane antigens. Parasitol Res. 1996;82:130–135. doi: 10.1007/s004360050083. [DOI] [PubMed] [Google Scholar]
- Upma, Agrewala J, Banyal HS. A 24, 000 g sediment of Plasmodium berghei induces IL-1 response in mice and exhibit protection against malaria infection. Parasit Hung. 1998;31:13–21. [Google Scholar]
- Wang R, Charoenvit Y, Corradin G. Protection against by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction by CD4 + T cell and IFN-γ dependent elimination affected by hepatocytes. J Immunol. 1996;157:4061–4067. [PubMed] [Google Scholar]