Abstract
Since the beginning of the AIDS pandemic, opportunistic infections have been recognized as common complications of HIV infection. Enteric protozoan parasitic infections are one of the leading causes of morbidity and mortality in HIV infected patients. The present study is, therefore, aimed to determine the prevalence of these parasites and study their association with immune status in HIV patients with emphasis on the correlation between various diagnostic techniques to give an accurate diagnosis to avoid empirical treatment. This prospective study, carried out between November 2009 and May 2010 included all HIV seropositive patients presenting with diarrhea to the ART center. A total of 64 stool samples were analyzed by wet mount examination, three different staining techniques, and antigen detection by ELISA for various enteric protozoan infections. Total prevalence of enteric protozoan parasites was 30%. Among the total cases, Cryptosporidium was seen in 12% cases followed by Giardia, E. histolytica and Isospora belli. The maximum diagnostic yield for coccidian parasites was with safranin–methylene blue staining technique. Parasitic burden contributes towards early morbidity in HIV infection. This study provides important information about prevalence of intestinal protozoan parasites in HIV infection. A combination of procedures should be carried out for the screening of stool specimens of HIV patients for better diagnosis and management.
Keywords: Prevalence, Enteric protozoan parasites, Staining, HIV/AIDS
Introduction
As the number of people living with human immunodeficiency virus (HIV) continues to increase, acquired immunodeficiency syndrome (AIDS) remains to be a major global health priority and among the leading causes of death (AIDS epidemic update 2009). Since the beginning of the AIDS pandemic, opportunistic infections (OIs) have been recognized as common complications of HIV infection. Diarrhea is one of the most common AIDS-related illnesses causing a significant morbidity and mortality in HIV infected patients (Siddiqui et al. 2007). Reports indicate that diarrhea occurs in 30–60% of AIDS patients in developed countries and in about 90% of AIDS patients in developing countries (Framm and Soave 1997). The etiologic spectrum of enteric pathogens causing diarrhea includes bacteria, parasites, fungi and viruses (Mitra et al. 2001), though that of parasitic origin is prominent in patients with AIDS in developing countries (Cimerman et al. 1999). Of these, protozoan parasitic infections are the most serious ones causing severe morbidity and mortality. More importantly, with emergence of AIDS, the epidemiology as well as outcome of diseases caused by protozoan parasites is significantly modified (Kelly et al. 2009). With the progressive introduction of HAART starting in 1996, modifications have been observed in the morbi-mortality profile among HIV/AIDS patients, reflected in the reduced occurrence of opportunistic infections, including those caused by enteroparasites (Willemot and Klein 2004).
There have been reports regarding frequency of various pathogens causing diarrhea in this group of patients from different parts of India. However, there is paucity of data on correlations of CD4 levels and HIV/AIDS status with prevalence of enteric protozoan parasites among the HIV patients of East Delhi, India. The present study is, therefore, aimed to determine the prevalence of enteric protozoal parasites and study their association with immune status in HIV patients with emphasis on the correlation between various diagnostic techniques to give an accurate diagnosis to avoid empirical treatment.
Materials and methods
This prospective study was carried out between November 2009 to May 2010 in the Department of Microbiology and Antiretroviral therapy (ART) center, University college of Medical Sciences (UCMS), Guru Teg Bahadur (GTB hospital), Delhi after approval by the Institutional Ethical Committee.
Study population included all HIV seropositive patients presenting with diarrhea to the ART center. Patients who had taken antibiotics, antiprotozoals or antimotility drugs in the preceding two weeks were excluded. The CD4 counts, clinical staging, co-existent opportunistic infections and other relevant epidemiological data of all the cases were recorded.
A total of 64 stool samples were obtained in clean, leak proof, screw-capped plastic containers and examined by the different techniques for various enteric protozoan infections.
Laboratory methods
Wet mount of stool Saline and iodine wet mounts were scanned for cysts and trophozoites of protozoa under low power (10×) of light microscope and all suspicious findings were confirmed under high power (40×). Stool examination was done after formalin-ether concentration method.
Permanent Staining Stool smears were prepared, air dried, heat fixed. The smears were subjected to three staining techniques as per standard procedures (Garcia 2001) (a) Hot modified acid fast stain; (b) Kinyoun carbol fuchsin stain; (c) Safranin-methylene blue stain. These smears were then examined under oil immersion objective (100×) of light microscope for oocysts of coccidian parasites.
ELISA for detection of Cryptosporidium antigen This was done by a commercially available kit for qualitative determination of Cryptosporidium antigen in feces based on double antibody sandwich ELISA format (DRG International Inc., USA), according to manufacturer’s instructions. The level of detection was approximately 30 ng/ml of Cryptosporidium antigen. Sensitivity and specificity were 93 and 98%, respectively.
ELISA for detection of Entamoeaba histolytica/dispar antigen This double antibody sandwich ELISA for antigen detection in stool was done by commercially available kit from DRG International Inc., USA according to manufacturer’s instructions. Sensitivity and specificity of the test were 88 and 100%, respectively.
ELISA for detection of Giardia antigen This was also a double antibody sandwich format ELISA available from DRG International Inc., USA. The test was carried according to manufacturer’s instructions. Sensitivity and specificity of the test were 85 and 95%, respectively. The detection limit was approximately 5–10 ng/ml of Giardia antigen.
Results
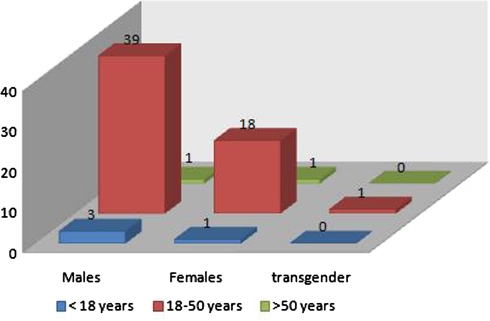
This study was done on 64 HIV seropositive patients presenting to the ART center with diarrhea between November 2009 and May 2010. We divided all the patients into three groups depending upon their CD4 counts at the time of their registration into the ART center. Thus we had 31 (48%), 26 (41%) and 7 (11%) patients in group I (CD4 <200), group II (CD4 = 200–500) and group III (CD4 >500) respectively. The age and sex distribution of the study population is depicted in Fig. 1.
Fig. 1.
Age and sex distribution of study population
Out of all the patients, only 4 (6.25%) were ART naïve while the rest 60 (93.75%) were on ART for variable periods of time. As regards the WHO clinical staging of the patients, maximum patients (48%) belonged to stage III followed by 25, 14 and 13% in stages II, I and IV, respectively. The presence of associated opportunistic infections (OIs) was seen in only 10 (16%) of the total patients. These opportunistic infections were found more in group I (8) as compared to the other groups (2 and 0, respectively in groups II and III). Tuberculosis was found in 7 (11%) patients (5 pulmonary and 2 extrapulmonary) and Pneumocystis jirovecii pneumonia was seen in 3 (4.7%) patients (Table 1).
Table 1.
Clinical profile of study population
| CD4 COUNT | <200 | 200–500 | >500 |
|---|---|---|---|
| Prevalence of enteric protozoal parasites | 12 | 6 | 1 |
| WHO clinical stage | |||
| I | 3 | 6 | 0 |
| II | 4 | 9 | 3 |
| III | 20 | 7 | 4 |
| IV | 4 | 4 | 0 |
| Presence of OI | |||
| TB | 5 | 2 | 0 |
| PCP | 3 | 0 | 0 |
| ART | |||
| ART naive | 2 | 2 | 0 |
| On ART | 29 | 24 | 7 |
OI Opportunistic illness, ART Anti retroviral therapy, TB tuberculosis, PCPPneumocystis jirovecii pneumonia
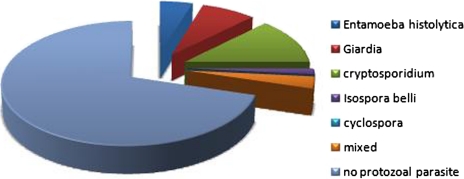
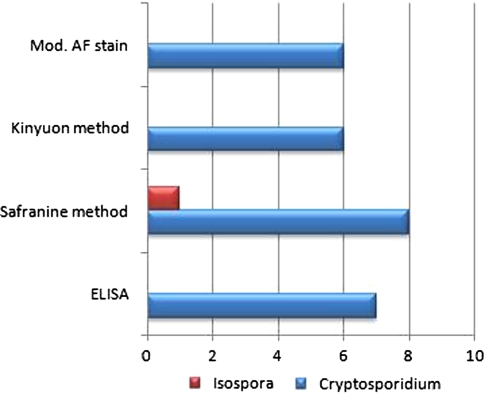
Out of the 64 patients included in the study population, a protozoal etiology of diarrhea could be seen in 19 (30%) patients (Fig. 2). Most common of all the protozoal pathogens was Cryptosporidium in 8 (42%) cases, followed by Giardia, E. histolytica, and Isospora belli in 5 (26%), 3 (16%), and 1(5%) respectively. Mixed infection was seen in 2 (11%) cases. In one of these cases a coinfection of Giardia and Cryptosporidium was present and in the other coinfection of Giardia and E. histolytica was seen. The positivity rate of these pathogens by different techniques is shown in Table 2. Figure 3 depicts the comparison of the three permanent staining techniques and Elisa for detection of coccidian parasites. Among all the techniques the Safranin–methylene blue method was found to be the most superior method in our study.
Fig. 2.
Distribution of enteric protozoal parasites among the study population (n = 64)
Table 2.
Detection of enteric protozoal parasites by various diagnostic methods
| Enteric protozoal parasite | Wet mount examination only | Staining technique only | ELISA for Ag detection in stool only | Both microscopy and ELISA | Total positives |
|---|---|---|---|---|---|
| Entamoeba histolytica | 1 | – | 0 | 3 | 4 |
| Giardia | 0 | – | 0 | 7 | 7 |
| Cryptosporidium | – | 2 | 1 | 6 | 9 |
| Isospora belli | – | 1 | Not done | – | 1 |
| Cyclospora | – | 0 | Not done | – | 0 |
Fig. 3.
Comparison of different staining techniques and ELISA for the diagnosis of coccidian parasites
Discussion
The GTB Hospital attached to the UCMS, Delhi, India, is a tertiary care hospital catering to a large population from eastern Delhi and adjoining states. The present study reports the prevalence of enteric protozoan parasites in HIV seropositive patients presenting with diarrhea to the ART center of this hospital, and investigates the correlation with immune status of the patients and different lab diagnostic techniques used.
Numerous opportunistic infections occur in HIV infected patients, due to down regulation of the immune system. Diarrhea is well recognized as an important component of HIV related morbidity. With the advent of AIDS, parasitic diarrhea has gained a lot of significance, especially due to protozoan parasites which are responsible for potentially severe diarrheic profiles with morbidity directly related to the degree of immune suppression.
In the present study, the majority of the study population belonged to the age group of 18–50 years corresponding to the most active period of life and among all the age groups males predominated with a male to female ratio of almost 2:1 reflecting a higher HIV prevalence in males in India as reported earlier (Suresh et al. 2006).
The highest proportion (48%) of our study population consisted of those with CD4 count <200 cells/mm3 and maximum patients (48%) belonged to WHO clinical stage III. Another recent Indian study had also reported almost 50% of their study population having CD4 count <200 cells/mm3 (Kulkarni et al. 2009).
The presence of enteric protozoan infections was seen in only 19 (30%) of our total patients. These pathogens were found more in group I (12) as compared to the other groups (6 and 1, respectively in groups II and III). Previous Indian studies (Kulkarni et al. 2009) have reported higher prevalence of opportunistic parasites in stool samples with proportion of these pathogens significantly higher in patients with CD4 count <200 cells/mm3. In fact another study (de Oliveira-Silva et al 2007) reported 78.6% patients who presented coccidian parasites in the faeces, had a CD4 + lymphocyte count less than or equal to 200 cells/mm3. The diagnostic yield of stool analysis our study was low in patients with higher CD4 cell counts as found in other studies. A possible explanation as given by Suresh et al. (2006) could be the fact that other gastrointestinal diseases which are common in young age group (inflammatory bowel disease, coeliac disease, irritable bowel syndrome and idiopathic steatorrhoea) are presently the leading cause of diarrhea, as effective HAART helps in eradicating the opportunistic protozoan infections. This can also explain the low prevalence of protozoan parasites in our study population as almost 96% of our patients were already on HAART before being enrolled into the study. A study from Brazil also showed significant reduction in the general prevalence of intestinal parasites among HIV positive patients between the pre- HAART and HAART eras (Bachur et al 2008).
Among the protozoan etiology of diarrhea in our study the most common pathogen found was Cryptosporidium (42%) followed by Giardia, Entamoeba histolytica, and Isospora belli in 26, 16 and 5%, respectively. Mixed infection was seen in 11% cases. The prevalence of cryptosporidiosis in our study is much higher than some of the earlier Indian reports; (Mohandas et al. 2002) however, a relatively higher prevalence of this pathogen has been reported form Delhi recently (Dwivedi et al. 2007). This can be explained in our study by the fact that East Delhi is one of the most crowded areas of Delhi where many slums with minimal level of hygiene exist. Moreover, use of three different staining techniques in addition to ELISA must also have contributed to the higher prevalence whereas most of the previous studies have reported their findings based only on a single method (modified acid fast staining technique). One study (Dwivedi et al. 2007) that reported a higher prevalence used direct IFA in addition to conventional techniques. Our findings of low prevalence for other coccidian parasites compared well with the studies carried out elsewhere. The combination sulfamethoxazole + trimethoprim prescribed usually as chemoprophylaxis or treatment of pneumocystosis, is active against Isospora belli, which could have contributed to the low prevalence of these coccidiosis detected in this era of improving comprehensive management of these patients especially after introduction of HAART. The prevalence of mixed protozoan infection in our study was much lower than previously reported (11 vs. 36%) (Dwivedi et al. 2007).
In our study we compared the conventional staining techniques with ELISA. For the diagnosis of cryptosporidiosis we found that taking Safranin method as gold standard, ELISA, modified acid fast staining and Kinyoun staining gave a sensitivity of 80% each and a specificity of 98, 100 and 100%, respectively. The positive predictive value (PPV) and the negative predictive value (NPV) of ELISA were 88.9 and 96.5%, respectively whereas the corresponding values for modified acid fast staining and Kinyoun staining were 100 and 96.5%, respectively. For Isospora, modified acid fast staining and Kinyoun staining gave a sensitivity and specificity of 50 and 100%, each respectively. The PPV and NPV of these staining techniques were 100 and 98.4%, respectively. So in our study we found safranin method of staining fast, reliable, easy to perform and superior to ELISA and other staining techniques both for Cryptosporidium and Isospora. Previous studies have found safranin staining superior for identification of Cyclospora spp. as the Cyclospora oocysts were variably stained with distorted and wrinkled appearance leading to misdiagnosis with Kinyoun’s staining. On the other hand Kinyoun’s staining was found better for Cryptosporidium spp. identification compared to safranin staining as the later required heating and structural details of Cryptosporidium oocysts were poorly defined (Tuli et al. 2010).
Though ELISA is a simple test which could be very useful in routine diagnosis and for screening a large number of specimens in short time our study shows a relatively lower sensitivity of ELISA, similar to some previous studies, (Ungar 1990) suggesting that staining holds importance due to its low cost in addition to having a comparable efficacy with the assay and therefore, besides direct microscopy a combination of procedures should be carried out for the screening of stool specimens of HIV patients.
An etiology of diarrhea could not be determined in 70 per cent of our study patients, suggesting a need for comprehensive etiological studies covering bacterial, fungal, viral, and parasitic causes of diarrhea among HIV infected patients in India which may help in better management of these patients. This proportion of patients with HIV and diarrhea and no identifiable pathogen is consistent with other studies in patients infected with HIV (Dwivedi et al. 2007). Parasitic burden accelerates HIV disease progression and contributes towards early morbidity. This study provides important information about the prevalence of important intestinal protozoan parasites in HIV infection. Continuation of this surveillance study is necessary to obtain an accurate understanding of the burden and cause of protozoan parasitic diarrhea in this area.
Acknowledgments
Conflict of interest None.
Contributor Information
Bineeta Kashyap, Phone: +919899583514, Email: dr_bineetakashyap@yahoo.co.in.
Sanchaita Sinha, Phone: +919958823183, Email: sonara81@gmail.com.
Shukla Das, Phone: +919810047204, Email: shukladas_123@yahoo.com.
Nitesh Rustagi, Phone: +919899656099, Email: nitesh.rustagi@yahoo.co.in.
Rajat Jhamb, Phone: +919868399556, Email: rajatjhamb@yahoo.com.
References
- Bachur TPR, Vale JM, Coêlho ICB, de Queiroz TRBS, de Souza Chaves C (2008) Enteric parasitic infections in HIV/AIDS patients before and after the highly active antiretroviral therapy. Brazilian J Infect Dis 12(2):115–122 [DOI] [PubMed]
- Cimerman S, Cimerman B, Lewi SD. Enteric parasites and AIDS. Sao Paulo Med J. 1999;117(6):266–273. doi: 10.1590/S1516-31801999000600007. [DOI] [PubMed] [Google Scholar]
- de Oliveira-Silva MB, de Oliveira LR, Resende JCP et al (2007) Seasonal profile and level of CD4+ lymphocytes in the occurrence of cryptosporidiosis and cystoisosporidiosis in HIV/AIDS patients in the Triângulo Mineiro region, Brazil. Revista da Sociedade Brasileira de Medicina Trop 40(5):512–515 [DOI] [PubMed]
- Dwivedi KK, Prasad G, Saini S, Mahajan S, Lal S, Baveja UK. Enteric opportunistic infections among HIV infected individuals: associated risk factors and immune status. Jpn J Infect dis. 2007;60:76–81. [PubMed] [Google Scholar]
- Framm SR, Soave R. Agents of diarrhea. Med Clin North Am. 1997;81:427–447. doi: 10.1016/S0025-7125(05)70525-3. [DOI] [PubMed] [Google Scholar]
- Garcia LS (2001) Macroscopic and microscopic examination of fecal specimens. In: Diagnostic medical parasitolgy 4th edn. American Society for Microbiology, Washington DC, pp 771–774
- Kelly P, Todd J, Sianongo S, et al. Susceptibility to intestinal infection and diarrhea in Zambian adults in relation to HIV status and CD4 count. BMC Gastroenterol. 2009;9:7. doi: 10.1186/1471-230X-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SV, Kairon R, Sane SS, et al. Opportunistic parasitic infections in HIV/AIDS patients presenting with diarrhoea by the level of immunosuppression. Indian J Med Res. 2009;130:63–66. [PubMed] [Google Scholar]
- Mitra AK, Hernandez CD, Hernandez CA, Siddiq Z. Management of diarrhea in HIV infected patients. Int J STD AIDS. 2001;12:630–639. doi: 10.1258/0956462011923840. [DOI] [PubMed] [Google Scholar]
- Mohandas K, Sehgal R, Sud A, Malla N. Prevalence of intestinal parasitic pathogens in HIV-seropositive individuals in Northern India. Jpn J Infect Dis. 2002;55:83–84. [PubMed] [Google Scholar]
- Siddiqui U, Bini EJ, Chandarana K, et al. Prevalence and impact of diarrhea on health-related quality of life in HIVinfected patients in the era of highly active antiretroviral therapy. J Clin Gastroenterol. 2007;41(5):484–490. doi: 10.1097/01.mcg.0000225694.46874.fc. [DOI] [PubMed] [Google Scholar]
- Suresh VSA, Gulati AK, Singh VP, Varma DV, Rai M, Sundar S (2006) Diarrhea, CD4 counts and enteric infections in a hospital––based cohort of HIV-infected patients around Varanasi, India. BMC Infectious Diseases 6:39 doi: 10.1186/1471-2334-6-39 [DOI] [PMC free article] [PubMed]
- The Joint United Nations Programme on HIV/AIDS and World Health Organization (2009) AIDS epidemic update 2009. (http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf)
- Tuli L, Singh DK, Gulati AK, Sundar S, Mohapatra TM. A multi attribute utility evaluation of different methods for the detection of enteric protozoa causing diarrhea in AIDS patients. BMC Microbiol. 2010;10:11. doi: 10.1186/1471-2180-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar BLP. Enzyme-linked immunoassay for detection of Cryptosporidium antigens in fecal specimens. J Clin Microbiol. 1990;28:2491–2495. doi: 10.1128/jcm.28.11.2491-2495.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemot P, Klein MB. Prevention of HIV-associated opportunistic infections and diseases in the age of highly active antiretroviral therapy. Expert Rev Anti Infect Ther. 2004;2:521–532. doi: 10.1586/14787210.2.4.521. [DOI] [PubMed] [Google Scholar]