Abstract
Studies of social birds and mammals have produced extensive theory regarding the formation and dynamics of kin-based social groups in vertebrates. However, comparing kin dynamics in birds and mammals to social reptiles provides the opportunity to identify selective factors that promote independent origins of kin sociality across vertebrates. We combined a 5-year mark-recapture study with a DNA microsatellite analysis of relatedness in a social lizard (Xantusia vigilis) to examine the formation and stability of kin groups. We found that these lizards are highly sedentary and that groups often form through the delayed dispersal of offspring. Groups containing juveniles had higher relatedness than adult-only groups, as juveniles were commonly found in aggregations with at least one parent and/or sibling. Groups containing nuclear family members were more stable than groups of less-related lizards, as predicted by social theory. We conclude that X. vigilis aggregations conform to patterns of kin sociality observed in avian and mammalian systems and represent an example of convergent evolution in social systems. We suggest that kin-based sociality in this and other lizards may be a by-product of viviparity, which can promote delayed juvenile dispersal by allowing prolonged interaction between a neonate and its mother.
Keywords: sociality, kin, convergent evolution, dispersal, group stability, Xantusia vigilis
1. Introduction
Although general forms of sociality among unrelated individuals (e.g. herding, schooling) are widespread among vertebrates, many studies have focused on the role of kin in the organization and dynamics of social groups [1–4]. Vertebrate kin groups have been well-studied because their high levels of relatedness generate remarkably complex and interesting social behaviours [5], including cooperative breeding in birds [6,7] and mammals [8], alarm calling in rodents [9,10] and coalition formation in primates [11]. Notably, comparisons of kin group dynamics within and among social taxa can identify selective factors that promote independent origins of kin sociality across vertebrates [12–14].
Since Hamilton's [15] initial theory of the evolution of social behaviour, synthetic work across taxa has revealed that closely related individuals can assemble into groups, cooperate among individuals, and form semi-stable units based on group composition and resource availability (reviewed in [13,14,16,17]). While much of this theory was developed for cooperatively breeding birds, recent work to integrate this field with general social theory suggests that these hypotheses can and should be applied to other social systems as well [18,19]. Because some level of parental care (and thus obligate social interaction among kin) is ubiquitous among birds and mammals, our understanding of the early transitional stages to sociality from an asocial predecessor may be especially enhanced by studying species with simple, facultative social systems and no direct parental care, such as lizards.
Although patterns of kin sociality in birds and mammals are well-resolved, the mechanisms driving the dynamics of these kin groups remain contentious. While many studies support the hypothesis that kin groups form specifically through the delayed dispersal of offspring (reviewed in [13]), others suggest that alternative mechanisms (e.g. emigration by single or multiple individuals) might also be prevalent in social group formation, at least among mammals (reviewed in [20]). This distinction is important because delayed dispersal is often considered critical in the evolutionary origin of social behaviour [15,19]. A second important mechanism involves the role of relatedness in group stability, broadly defined as the strength of cohesion among group members [21]. Although theory predicts that groups of high relatedness will be more stable than groups of less-related individuals, this prediction has rarely been rigorously tested in vertebrates and is supported mainly by circumstantial evidence ([13], but see [22]). Examining this role of kin in group stability is important because it can inform how initial kin associations may then persist over time.
We used a social lizard (Xantusia vigilis, the Desert Night Lizard) to test these two hypotheses about the origin and stability of social groups. To test the first hypothesis that family groups form through the delayed dispersal of offspring, we examined the origin of social groups by quantifying natural dispersal and aggregation patterns in a mark-recapture study over a 5-year period. We then characterized the degree of kin sociality by estimating genetic relatedness and examining the frequency of nuclear family relationships within natural groups. To test the second hypothesis that kin groups are more stable than groups of lower relatedness, we used these movement and genetic data to quantify group stability and examine its relationship to relatedness. Finally, we examined other social lizards, compared patterns in lizards to mammals and birds and proposed that viviparity may have contributed to the evolution of kin sociality in reptiles by allowing prolonged interactions between parents and offspring.
2. Study system
Xantusia vigilis is a very small (adult mass = 1.5 g) lizard that is common throughout the deserts of the southwestern United States and Mexico [23]. It lives in high densities in Joshua tree (Yucca brevifolia) forests, depending on the shelter of fallen, decaying logs and rocks for cover [24]. This species is viviparous, giving birth to litters of one or two juveniles in August–October [25,26]. Individuals can live 8–10 years and have easily quantifiable levels of dispersal (less than 300 m; [24]).
From November through to February each year, these lizards actively aggregate underneath and inside fallen Joshua tree logs (figure 1). Winter aggregations can be as large as 20 lizards, but most commonly contain between two and six individuals [24]. Although this study does not seek to determine the cause of aggregation formation, it is of note that these winter groups appear to form independently of external resource distribution (A. Davis 2008, unpublished data), and mating does not occur until summer [27]. Summer collections rarely yield more than one lizard per log, and the few lizards found sharing a log in the summer were never in physical contact.
Figure 1.
In situ group of three adult lizards and one juvenile lizard (tail and hind legs extend perpendicular to and beneath the pelvis of the adult furthest to the right) in a winter aggregation underneath a Joshua tree log (Yucca brevifolia). The photograph was taken immediately after lifting the log under which the lizards were dwelling. Scale bar, 1 cm.
3. Material and methods
(a). Field collection
To quantify natural dispersal and aggregation patterns, we conducted a capture-mark-recapture study from August 2003 to January 2008 on a 36 ha plot in the western Mojave Desert near Llano, CA (Universal Transverse Mercator (UTM) 34°29.468′ N, 117°42.779′ W). We hand-captured and pitfall-trapped lizards underneath fallen logs every summer (late August to early September) and winter (late December to early January) during the course of the study (see [28] for extended details). Each log on the plot was sampled only once per field season, but traps were checked up to three times before closing them between seasons.
Upon capture, we measured the latitude–longitude coordinates of the capture location using Magellan eXplorist 300 and SporTrak handheld GPS units, and our measured error was less than the 3 m limit listed by the manufacturer (data not shown). We also marked the log of capture so that each lizard could be returned to the exact capture location following off-site processing after no more than 3 days. During winter collection, we designated any lizards found within 0.3 m of each other as ‘aggregated’, but most lizards were found in direct physical contact with the other members of an aggregation.
At each capture, we measured the mass and snout-vent length (SVL) of each lizard. We also sexed each lizard by shining a light through the base of the tail to visualize hemipenes in males [29]. We then toe-clipped each newly captured individual with a unique combination for future identification and took a small piece of tail tissue (stored in 95% ethanol) for genetic analyses of relatedness.
(b). Calculating home range and dispersal
To estimate home range size and dispersal, we first converted the latitude–longitude coordinates of all capture locations to UTM coordinates using Proj.4 v. 4.4.9 (http://trac.osgeo.org/proj/). We used Arcgis v. 9.2 extension XTOOLS PRO v. 5.2 to construct and calculate the area of the minimum convex polygon for each lizard with a minimum of three captures (n = 54). We excluded two of these lizards as statistical outliers because they moved more than five times the distance of any other lizard in the home range estimation (more than 50 m) and remained at their new locations during subsequent captures, clearly identifying them as dispersers (see below). Nine lizards caught exactly three times were captured twice at locations with identical GPS coordinates, so for each, we adjusted one of their coordinates by 1 cm to allow the construction of a polygon. We first tested for effects of lizard SVL (a proxy for age), sex and recapture time interval on the home range area using Spearman rank correlation and Wilcoxon signed-rank tests.
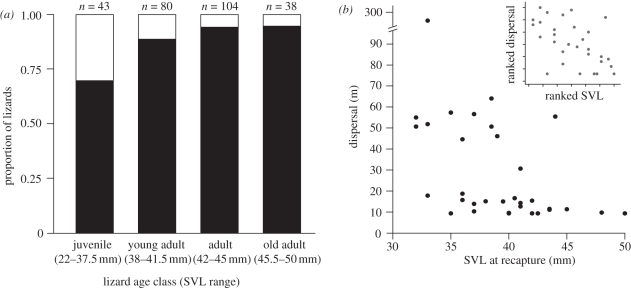
To quantify dispersal distance, we calculated the greatest straight-line distance between the two capture points of all recaptured lizards (n = 265). We then identified dispersers as any lizard that moved further than 1.65 times the average diameter of a home range. This value delineated the 90% quantile of the distribution of the greatest straight-line distances between capture points for each lizard in the home-range estimation (see [30] for similar designation). We binned dispersers into four age classes by SVL (juvenile, young adult, adult and old adult) modified from Zweifel & Lowe [24] and used nominal logistic models to test for age and sex effects on dispersal frequency. We then tested for age, sex and time interval effects on dispersal distance using Spearman rank correlation and Wilcoxon signed-rank tests.
(c). Molecular analyses of relatedness
To assess relatedness (r) of individuals, we estimated values of within-group r for each aggregation based on DNA microsatellite variation in a subset of field-caught lizards (n = 220 aggregations of 687 lizards). We extracted DNA from tail tissue using a standard Chelex protocol [31] and amplified eight microsatellite loci using the Qiagen Multiplex PCR Kit (see electronic supplementary material, appendix S1 and table S1). We visualized fluorescently labelled PCR products using an ABI 3730xl fragment analyser, and we scored genotypes using the GeneMapper v. 4.0 software package. We found that these highly polymorphic loci (averaging 19.3 alleles/locus; electronic supplementary material, table S1) showed no evidence of linkage disequilibrium between loci and that seven of the eight loci did not deviate from Hardy–Weinberg equilibrium after Bonferroni correction (the eighth locus was excluded from further analysis; see electronic supplementary material, appendix S1). Only individuals that we confidently genotyped for at least four loci were used in analysis.
To estimate within-group relatedness, we calculated pairwise values of r for all individuals and by group using Relatedness v. 5.0.8 [32]. Because of the presence of a large aqueduct bisecting the study site, we considered lizards caught on the north side of this barrier to be a separate breeding population from the south side and constructed separate allele frequency tables for each population. These tables included all adult lizards genotyped at each site, irrespective of aggregation participation (n = 628 and 624 lizards from north and south, respectively). Because there was no difference in allele frequencies among years (Fstat v. 2.9.3.2; p > 0.05 for all comparisons before Bonferroni correction), we pooled genotypes from all years into one allele frequency table per population. We then tested whether groups containing juveniles had higher within-group relatedness than adult-only groups by conducting four Wilcoxon signed-rank tests between groups with and without juveniles for groups of two, three, four and five individuals (no groups lacking juveniles contained more than five individuals) and combining p-values across these independent tests using the unweighted Z-method [33].
To assign nuclear family relationships, we used Kinship v. 1.3.1 [34] to calculate pairwise likelihood ratios for full sibling and parent–offspring relationships within aggregations. We conservatively assigned such relationships only to pairs with ratios that corresponded to p-values less than 0.001 to minimize erroneous assignments of relationship to unrelated pairs. We then assigned each aggregation to one of the three categories based on these kinship estimates: groups with nuclear family relationships, extended family relationships (r = 0.1–0.35), or no genetic relationships.
(d). Assessing group stability
To quantify group stability, we examined each of the groups for which we also had recapture data on one or more participants in consecutive winters and tallied the number of members of the original aggregation that were found at the same location the following year. If a lizard was the only remaining participant from the previous winter's aggregation at that locality, then we scored the group as ‘1’. Accordingly, other scores (2–4) also correspond to the number of stable participants found in the same location in consecutive years. We then divided this value by the total number of lizards present in the original aggregation to determine a proportion of stable participants for each social group. To ensure independence of data, groups that maintained stability over multiple years were only analysed for the first and consecutive winter of capture (although these ‘dynasties’ are subsequently discussed). Because of the differences in kin structure that we found between groups with and without juveniles, we analysed the stability of adult-only groups and groups containing juveniles separately.
We then assessed the relationship between group stability and relatedness in two ways. First, we used a nominal logistic model to test the recapture likelihood of lizards from groups of differing relatedness (nuclear family, extended family, unrelated) with group size as a covariate. Second, we regressed the log-transformed proportion of stable participants against group size, and used a Wilcoxon signed-rank test on the residuals of this regression (owing to unequal variance among groups) to determine whether groups with nuclear family relationships were more stable than groups of lower relatedness. We conservatively excluded unrelated groups with juveniles from this analysis owing to low sample size for group stability (n = 2), and again analysed adult-only groups separately.
(e). Statistical analyses
We performed all statistical tests with JMP v. 7 and assessed significance at p ≤ 0.05. All linear and logistic regressions were performed with forward and backward stepwise removal of non-significant terms and all appropriate interaction effects. Normality of residuals was assessed using Shapiro–Wilk tests, linearity by visual assessment of residual by predicted plots, autocorrelation with Durbin–Watson tests, and homogeneity of variance with Levene's test for all relevant analyses. Non-parametric tests chosen were described above for data that violated assumptions of parametric analyses. Unless otherwise noted, only significant effects are reported.
4. Results
(a). Field collection
We marked 2120 unique lizards and recaptured 265 of them for a total of 349 times. Incidentally, this low recapture rate (12.5%) was owing, in greater part, to low probability of detecting all individuals present during a census than to movement of individuals out of the study site or high mortality. This conclusion is supported by both demographic estimates of low catchability and high survival and by evidence of high genetic structure within populations (data not shown). We caught 67–125 aggregations of lizards each winter (n = 437 groups total), with group size ranging from 2 to 18 lizards. Depending on the year, we found 43–77% of the lizards participating in winter aggregations, yielding a global average of 65 per cent aggregation participation.
(b). Home range and dispersal
Fifty-two lizards (including 43 adults) caught three or more times were used in the calculation of home range area. Home range size varied from 0.1 to 53 m2, with a mean of 6.05 m2 and a median of 4.25 m2, and the size did not differ between the sexes (Wilcoxon signed-rank, Z = −0.24, p = 0.80). The mean diameter of a home range was 5.59 m, with a range from 2.39 to 20.04 m.
Of the 265 recaptured lizards, we classified 30 (11.3%) as dispersers moving more than 9.22 m. Despite these globally low levels of dispersal, we found more dispersing lizards in younger age classes than in older, larger classes (χ23 = 16.65, p = 0.001; figure 2a). We also found that smaller lizards dispersed further than larger lizards (Spearman rank correlation, ρ = −0.55, p = 0.002; inset figure 2b). There was no effect of sex (Wilcoxon signed-rank, Z = 0.29, p = 0.77), as mean male dispersal was 25.7 m and mean female dispersal was 26.1 m (excluding the one long distance disperser, a female, figure 2b), and dispersal distance was unaffected by the time span between the two captures (Spearman rank correlation, ρ = 0.24, p = 0.21). Despite finding higher levels of dispersal among young lizards, the vast majority of juveniles delayed their dispersal at least a year. In at least five cases, lizards followed since birth still had not dispersed from the natal location after more than 3 years.
Figure 2.
(a) Relative proportion of dispersing (open bars) and non-dispersing (filled bars) lizards by age class shows that lizards move more frequently when young (χ23 = 16.65, p = 0.001). Sample sizes above the bars indicate total number of recaptured lizards of each age class (n = 265 lizards total). (b) Lizard dispersal distance as a function of body size (n = 30). Inset graph displays the Spearman-rank correlation, showing that dispersal distance decreases with increasing body size (ρ = −0.552, p = 0.002).
(c). Kin structure of groups
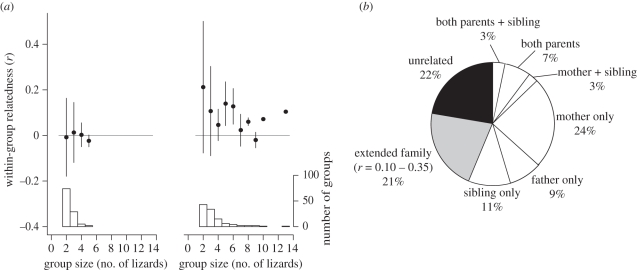
Mean within-group relatedness (r) was higher for groups with juveniles than adult-only groups (combined Wilcoxon signed-rank tests for groups of two to five lizards, Z = −4.41, p < 0.001; figure 3a), indicating a highly significant effect of juvenile presence on relatedness independent of group size. Remarkably, the global average of within-group r for all groups containing juveniles was 0.137 (the equivalent of cousin or half-sibling relatedness values), while groups with only adults averaged −0.002, as expected of groups not composed of kin.
Figure 3.
(a) Within-group relatedness of different group sizes by juvenile presence (right) or absence (left) shows that groups with juveniles have higher relatedness than adult-only groups (combined Wilcoxon signed-rank tests for groups of two to five lizards, Z = −4.41, p < 0.001). Horizontal grey reference lines correspond to a relatedness value of 0, expected if groups are composed of unrelated individuals. Histograms show the number of groups of each size, and error bars represent ±1 s.d. (b) Per cent of social juveniles (n = 117) in aggregations of differing kin relationship classes. White wedges denote different categories of nuclear family relationships (57% total). White, nuclear family; grey, extended family; black, unrelated.
At least 57 per cent of aggregating juveniles (n = 117 total) were found in groups with members of their nuclear family (parents and/or siblings; figure 3b). Another 21 per cent of juveniles were found in relationships categorized as ‘extended’ family, which had pairwise r-values between 0.1 and 0.35. Only 22 per cent of aggregating juveniles were found in groups of all unrelated individuals.
(d). Group stability
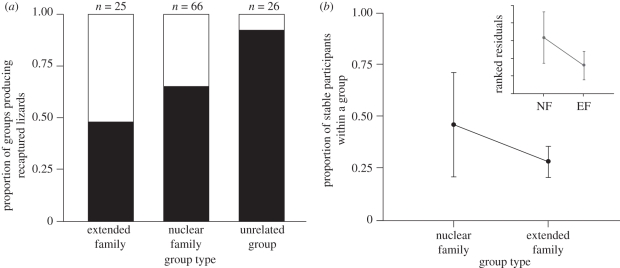
We had recapture data in consecutive winters for one or more group participants in 38 of the 117 groups containing juveniles and 21 of 109 groups with only adults. In groups with juveniles, lizard recapture success depended on group relatedness. We recaptured only two individuals from groups with no kin relationships, while lizards from groups with nuclear or extended family relationships were recaptured more successfully (from 40 to 50% of groups; whole model: χ23 = 19.72, p < 0.001; group size effect: χ21 = 6.26, p = 0.012; group type effect: χ22 = 10.45, p = 0.005; no interaction effect: p = 0.136; figure 4a). We found no effect of relatedness on recapture success in groups of only adults (χ23 = 4.26, p = 0.234).
Figure 4.
(a) Relative proportion of groups with juveniles by aggregation type shows higher recapture probability of lizards from groups containing kin than from unrelated groups (group type effect: χ22 = 10.45, p = 0.005; see text for full model details). Sample sizes above the bars indicate the total number of groups of each type (n = 117 groups total). White bar, recapture; black bar, no recapture. (b) Proportion of stable group participants by group type, in groups with both recapture data and juveniles (n = 24 groups). Inset graph displays the Wilcoxon signed-rank test using ranked residuals to remove a group size effect, showing that group stability is higher in groups with nuclear family members (χ21 = 7.38, p = 0.007). Error bars represent ±1 s.d.
In groups with juveniles, we found up to four group members aggregated in the same location in consecutive years, and high levels of group stability (two or more individuals) were detected in 29 per cent (11 of 38) of the groups. All of these aggregations with two or more stable participants were groups with nuclear family relationships, and such stability was absent from either extended family or unrelated groups. Additionally, the proportion of stable group participants (stability index) was almost twice as high in groups with nuclear family members than in groups with extended family relationships (figure 4b). Nearly half of the lizards in nuclear family groups containing juveniles rejoined their original aggregation the following year.
To verify that this effect was not driven by group size, we analysed the ranked residuals from the regression of stability index (log-transformed, see §3) on group size and found higher group stability in nuclear family groups (Wilcoxon signed-rank test, χ21 = 7.38, p = 0.007; inset figure 4b). This relationship is significant even after removing the two nuclear family groups with high (1.0) stability values (χ21 = 5.43, p = 0.02). Because there is a negative relationship between group size and stability index (F1,23 = 4.79, p = 0.039), it is unlikely that group size is driving the relationship between high relatedness and group stability because nuclear family groups are slightly (although not significantly) larger than extended family groups (mean group size of 5.2 versus 4.0 individuals, respectively; F1,23 = 1.07 p = 0.312).
Although we only considered the first and consecutive capture years of each group for this analysis to ensure independence of data, we found that four of these nuclear families (termed ‘dynasties’) continued to reform stable aggregations, with multiple cohorts of related juveniles and a single breeding pair, in the same location each winter for up to 4 years. However, relatedness had no effect on stability in groups with only adults (Wilcoxon signed-rank test, χ22 = 0.007, p = 0.99).
5. Discussion
We found that the dynamics of social groups in the lizard X. vigilis are strikingly similar to patterns of kin sociality observed in avian and mammalian systems, representing a clear example of convergent evolution in social systems. In the ensuing discussion, we briefly summarize our major conclusions and explore their implications in the study of sociality.
We found that individuals of X. vigilis are highly sedentary, with extremely small home ranges stable over multiple years and with very low dispersal (corroborating patterns inferred from molecular data [35]). This long-lived, sedentary life history is rare among small lizards [36], although common in bird and mammal species with kin sociality [37]. Almost three-quarters of juveniles delayed dispersal for at least 1 and up to 3 years, and more than half chose to aggregate during that time with nuclear family members. While the stability of kin groups depended on both high relatedness and presence of juveniles, we also found evidence of dynasties with one breeding pair and multiple cohorts of offspring stable over several years of capture. To the extent that kin groups form through delayed dispersal of juveniles, our study supports the conclusion that kin sociality can and does evolve in remarkably similar ways across vertebrate taxa (and, notably, among some social invertebrates [38,39]) despite vast differences in ecology, physiology and evolutionary history.
This study contributes to the understanding of vertebrate kin sociality in three major ways. First, our tests of kin group formation and especially stability in a third major radiation of terrestrial vertebrates extend the generality of predictions about kin groups to squamate reptiles, a group in which kin sociality has not historically been appreciated [40]. Second, our study helps clarify the mechanisms by which kin groups form and operate. Specifically, the delayed dispersal of juveniles has been under scrutiny as the fundamental mechanism by which kin groups form [20], and our data support the importance of juvenile philopatry in kin group formation [13,14]. Finally, comparisons among vertebrate social systems help identify complex traits that promote the evolution of sociality.
Kin group formation through juvenile philopatry must be explained by both the initiation and extension of interaction between parents and offspring. In avian and mammalian kin groups, this interaction is initiated through obligate parental care (provisioning of altricial young) and then extended through a variety of behaviours and environmental factors (such as cooperatively breeding when resources are scarce [37]). This progression is harder to explain in lizards, with no need for direct parental care. However, comparing social systems within lizards can identify factors that trigger sociality through delayed dispersal in autonomous young.
Complex, nuclear family-based sociality has been suggested in at least 18 other lizard species (of approximately 5000 total species) across at least seven different families (see electronic supplementary material, table S2; reviewed in [40,41]). Although much of this evidence is strictly anecdotal, extensive studies in the Australian skink, genus Egernia in particular show clear kin structure within social groups of several species, including some species with stable nuclear family groups strikingly similar to those we described here in the unrelated X. vigilis [40–50]. The rarity (<0.5% of all species) and broad taxonomic distribution of lizard kin sociality suggest a derived trait with multiple independent origins within squamates ([40, 41]; see also electronic supplementary material, table S2).
When all social lizard species are evaluated concurrently, an intriguing correlation appears between the mode of reproduction and the formation of kin groups. Seventeen of the 19 species suggested to form family groups are also viviparous (see electronic supplementary material, table S2), a mode of reproduction found only in about 20 per cent of squamate species and hypothesized to have evolved strictly as a physiological adaptation (generally to extreme or variable environments [51]). Although any in-depth analysis would have to account for phylogeny, this preliminary association between sociality and viviparity could be important for understanding the origins of long-term, kin-based social structure in lizards. In contrast to oviparity, viviparity ensures contact between a juvenile and its mother and siblings at birth [52]. This contact could then be extended through time, potentially for very long periods, thus resulting in family-based social structure.
We propose that viviparity in lizards can provide a mechanism for the prolonged parent–offspring contact that leads to extended kin sociality, analogous to that suggested in birds and mammals (e.g. cooperative breeding is rarer in clades with precocial young compared to taxa with altricial young [12]). In viviparous lizards, initial parent–offspring contact can then be extended either through passive build-up of offspring mediated by low juvenile dispersal or actively promoted through forms of indirect parental care (like reduced parental aggression [41,52,53]), the classic dichotomy in the evolution of sociality described by Hamilton [15]. Because viviparity fundamentally ensures parent–offspring contact in a way that oviparity does not, its relative rarity among lizards may explain why kin sociality is not more common in this taxon.
The importance of this and other comparisons of convergent systems lies in revealing both similarities and differences in how traits evolve. In studies of social lizards, convergence has revealed that direct parental care, a shared trait in kin social mammals and birds, is not necessary for the evolution of kin sociality. Although the details of the mechanisms that create and extend parent–offspring interaction may vary across species, studies like these suggest that this contact is fundamental to the evolution of kin sociality among diverse organisms.
Acknowledgements
All methods were approved by the Chancellor's Animal Research Committee (Sine0002-1) at the University of California, Santa Cruz.
This project was funded by an American Museum of Natural History Theodore Roosevelt Grant, an American Society of Ichthyologists and Herpetologists Gaige Fund Award, a US Department of Education GAANN Award and an NSF Postdoctoral Fellowship in Biology (DBI-09060346) to A.R.D. We also thank R. S. Thorpe for hosting the development of the DNA microsatellite library and the California Department of Fish and Game for issuing scientific collecting permits to A.R.D. Additionally, we thank S. Adolph, D. Leavitt, C. Hipsley, K. Moeller, M. Mulks, H. Liwanag, E. Bastiaans, D. Crain and R. Davies for assisting with field collection and molecular work. We especially thank D. Rabosky for statistical assistance and A. Lyubimov for language translation work. We thank B. Lyon, P. Raimondi, L. Lancaster, A. Rogers, two anonymous reviewers, and especially S. Kuchta and C. Cox for constructive comments in the preparation of this manuscript.
References
- 1.Aviles L., Fletcher J. A., Cutter A. D. 2004. The kin composition of social groups: trading group size for degree of altruism. Am. Nat. 164, 132–144 10.1086/422263 (doi:10.1086/422263) [DOI] [PubMed] [Google Scholar]
- 2.Hughes C. 1998. Integrating molecular techniques with field methods in studies of social behavior: a revolution results. Ecology 79, 383–399 10.1890/0012-9658(1998)079[0383:IMTWFM]2.0.CO;2 (doi:10.1890/0012-9658(1998)079[0383:IMTWFM]2.0.CO;2) [DOI] [Google Scholar]
- 3.Lacey E. A., Wieczorek J. R. 2004. Kinship in colonial tuco-tucos: evidence from group composition and population structure. Behav. Ecol. 15, 988–996 10.1093/beheco/arh104 (doi:10.1093/beheco/arh104) [DOI] [Google Scholar]
- 4.West-Eberhard M. J. 1975. The evolution of social behavior by kin selection. Q. Rev. Biol. 50, 1–33 [Google Scholar]
- 5.Axelrod R., Hamilton W. D. 1981. The evolution of cooperation. Science 211, 1390–1396 10.1126/science.7466396 (doi:10.1126/science.7466396) [DOI] [PubMed] [Google Scholar]
- 6.Emlen S. T., Wrege P. H. 1988. The role of kinship in helping decisions among white-fronted bee-eaters. Behav. Ecol. Sociobiol. 23, 305–315 10.1007/BF00300577 (doi:10.1007/BF00300577) [DOI] [Google Scholar]
- 7.Komdeur J. 1994. The effect of kinship on helping in the cooperative breeding Seychelles warbler (Acrocephalus sechellensis). Proc. R. Soc. Lond. B 256, 47–52 10.1098/rspb.1994.0047 (doi:10.1098/rspb.1994.0047) [DOI] [Google Scholar]
- 8.Jarvis J. U. M., Oriain M. J., Bennett N. C., Sherman P. W. 1994. Mammalian eusociality: a family affair. Trends Ecol. Evol. 9, 47–51 10.1016/0169-5347(94)90267-4 (doi:10.1016/0169-5347(94)90267-4) [DOI] [PubMed] [Google Scholar]
- 9.Hoogland J. L. 1996. Why do Gunnison's prairie dogs give anti-predator calls? Anim. Behav. 51, 871–880 10.1006/anbe.1996.0091 (doi:10.1006/anbe.1996.0091) [DOI] [Google Scholar]
- 10.Sherman P. W. 1977. Nepotism and evolution of alarm calls. Science 197, 1246–1253 10.1126/science.197.4310.1246 (doi:10.1126/science.197.4310.1246) [DOI] [PubMed] [Google Scholar]
- 11.Silk J. B. 2007. The adaptive value of sociality in mammalian groups. Phil. Trans. R. Soc. B 362, 539–559 10.1098/rstb.2006.1994 (doi:10.1098/rstb.2006.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cockburn A. 2006. Prevalence of different modes of parental care in birds. Proc. R. Soc. B 273, 1375–1383 10.1098/rspb.2005.3458 (doi:10.1098/rspb.2005.3458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emlen S. T. 1995. An evolutionary theory of the family. Proc. Natl Acad. Sci. USA 92, 8092–8099 10.1073/pnas.92.18.8092 (doi:10.1073/pnas.92.18.8092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatchwell B. J. 2009. The evolution of cooperative breeding in birds: kinship, dispersal and life history. Phil. Trans. R. Soc. B 364, 3217–3227 10.1098/rstb.2009.0109 (doi:10.1098/rstb.2009.0109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton W. D. 1964. Genetical evolution of social behaviour I. J. Theor. Biol. 7, 1–16 10.1016/0022-5193(64)90038-4 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 16.Clutton-Brock T. 2009. Structure and function in mammalian societies. Phil. Trans. R. Soc. B 364, 3229–3242 10.1098/rstb.2009.0120 (doi:10.1098/rstb.2009.0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cockburn A. 1998. Evolution of helping behavior in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 29, 141–177 10.1146/annurev.ecolsys.29.1.141 (doi:10.1146/annurev.ecolsys.29.1.141) [DOI] [Google Scholar]
- 18.Bergmuller R., Johnstone R. A., Russell A. F., Bshary R. 2007. Integrating cooperative breeding into theoretical concepts of cooperation. Behav. Process. 76, 61–72 10.1016/j.beproc.2007.07.001 (doi:10.1016/j.beproc.2007.07.001) [DOI] [PubMed] [Google Scholar]
- 19.Doerr E. D., Doerr V. A., Safran R. J. 2007. Integrating delayed dispersal into broader concepts of social group formation. Behav. Process. 76, 114–117 10.1016/j.beproc.2006.12.015 (doi:10.1016/j.beproc.2006.12.015) [DOI] [PubMed] [Google Scholar]
- 20.Ebensperger L. A., Hayes L. D. 2008. On the dynamics of rodent social groups. Behav. Process. 79, 85–92 10.1016/j.beproc.2008.05.006 (doi:10.1016/j.beproc.2008.05.006) [DOI] [PubMed] [Google Scholar]
- 21.Baglione V., Canestrari D., Marcos J. M., Ekman J. 2006. Experimentally increased food resources in the natal territory promote offspring philopatry and helping in cooperatively breeding carrion crows. Proc. R. Soc. B 273, 1529–1535 10.1098/rspb.2006.3481 (10.1098/rspb.2006.3481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lillandt B. G., Bensch S., von Schantz T. 2003. Family structure in the Siberian Jay as revealed by microsatellite analyses. Condor 105, 505–514 10.1650/7117 (doi:10.1650/7117) [DOI] [Google Scholar]
- 23.Stebbins R. C. 2003. A field guide to western reptiles and amphibians, 3rd edn. New York, NY: Houghton Mifflin Publishing Company [Google Scholar]
- 24.Zweifel R. G., Lowe C. H. 1966. The ecology of a population of Xantusia vigilis, the Desert Night Lizard. Am. Mus. Novit. 2247, 1–57 [Google Scholar]
- 25.Miller M. R. 1951. Some aspects of the life history of the Yucca Night Lizard, Xantusia vigilis. Copeia 1951, 114–120 10.2307/1437539 (doi:10.2307/1437539) [DOI] [Google Scholar]
- 26.Miller M. R. 1954. Further observations on reproduction in the lizard, Xantusia vigilis. Copeia 1954, 38–40 10.2307/1440634 (doi:10.2307/1440634) [DOI] [Google Scholar]
- 27.Miller M. R. 1948. The seasonal histological changes occurring in the ovary, corpus luteum, and testis of the viviparous lizard, Xantusia vigilis. U. Cal. Pub. Zool. 47, 197–224 [Google Scholar]
- 28.Davis A. R. 2009. Kin dynamics and adaptive benefits of social aggregation in the Desert Night Lizard, Xantusia vigilis. PhD dissertation, University of California, Santa Cruz [Google Scholar]
- 29.Davis A. R., Leavitt D. H. 2007. Candlelight vigilis: a noninvasive method for sexing small, sexually monomorphic lizards. Herpetol. Rev. 38, 402–404 [Google Scholar]
- 30.Clobert J., Massot M., Lecomte J., Sorci G., de Fraipont M., Barbault R. 1994. Determinants of dispersal behavior: the common lizard as a case study. In Lizard ecology: historical and experimental perspectives (eds Vitt L. J., Pianka E. R.), pp. 183–206 Princeton, NJ: Princeton University Press [Google Scholar]
- 31.Estoup A., Largiader C. R., Perrot E., Chourrout D. 1996. Rapid one-tube extraction for reliable PCR detection of fish polymorphic markers and transgenes. Mar. Biotechnol. 20, 295–298 [Google Scholar]
- 32.Queller D. C., Goodnight K. F. 1989. Estimating relatedness using genetic markers. Evolution 43, 258–275 10.2307/2409206 (doi:10.2307/2409206) [DOI] [PubMed] [Google Scholar]
- 33.Whitlock M. C. 2005. Combining probability from independent tests: the weighted Z-method is superior to Fisher's approach. J. Evol. Biol. 18, 1368–1373 10.1111/j.1420-9101.2005.00917.x (doi:10.1111/j.1420-9101.2005.00917.x) [DOI] [PubMed] [Google Scholar]
- 34.Goodnight K. F., Queller D. C. 1999. Computer software for performing likelihood tests of pedigree relationship using genetic markers. Mol. Ecol. 8, 1231–1234 10.1046/j.1365-294x.1999.00664.x (doi:10.1046/j.1365-294x.1999.00664.x) [DOI] [PubMed] [Google Scholar]
- 35.Sinclair E. A., Bezy R. L., Bolles K., Camarillo J. L., Crandall K. A., Sites J. W. 2004. Testing species boundaries in an ancient species complex with deep phylogeographic history: genus Xantusia (Squamata: Xantusiidae). Am. Nat. 164, 396–414 10.1086/381404 (doi:10.1086/381404) [DOI] [PubMed] [Google Scholar]
- 36.Dunham A. E., Miles D. B., Reznick D. N. 1988. Life history patterns in squamate reptiles. In Biology of the Reptilia, vol. 16 (eds Gans C., Huey R. B.), pp. 441–522 New York, NY: Alan R. Liss, Inc [Google Scholar]
- 37.Eikenaar C., Richardson D. S., Brouwer L., Komdeur J. 2007. Parent presence, delayed dispersal, and territory acquisition in the Seychelles warbler. Behav. Ecol. 18, 874–879 10.1093/beheco/arm047 (doi:10.1093/beheco/arm047) [DOI] [Google Scholar]
- 38.Jones T. C., Parker P. G. 2002. Delayed juvenile dispersal benefits both mother and offspring in the cooperative spider Anelosimus studiosus (Araneae: Theridiidae). Behav. Ecol. 13, 142–148 10.1093/beheco/13.1.142 (doi:10.1093/beheco/13.1.142) [DOI] [Google Scholar]
- 39.Roisin Y. 1999. Philopatric reproduction, a prime mover in the evolution of termite sociality? Insect. Soc. 46, 297–305 10.1007/s000400050149 (doi:10.1007/s000400050149) [DOI] [Google Scholar]
- 40.O'Connor D., Shine R. 2003. Lizards in ‘nuclear families’: a novel reptilian social system in Egernia saxatilis (Scincidae). Mol. Ecol. 12, 743–752 10.1046/j.1365-294X.2003.01777.x (doi:10.1046/j.1365-294X.2003.01777.x) [DOI] [PubMed] [Google Scholar]
- 41.Chapple D. G. 2003. Ecology, life-history, and behavior in the Australian Scincid genus Egernia, with comments on the evolution of complex sociality in lizards. Herpetol. Monogr. 17, 145–180 10.1655/0733-1347(2003)017[0145:ELABIT]2.0.CO;2 (doi:10.1655/0733-1347(2003)017[0145:ELABIT]2.0.CO;2) [DOI] [Google Scholar]
- 42.Chapple D. G., Keogh J. S. 2005. Complex mating system and dispersal patterns in a social lizard, Egernia whitii. Mol. Ecol. 14, 1215–1227 10.1111/j.1365-294X.2005.02486.x (doi:10.1111/j.1365-294X.2005.02486.x) [DOI] [PubMed] [Google Scholar]
- 43.Chapple D. G., Keogh J. S. 2006. Group structure and stability in social aggregations of White's skink, Egernia whitii. Ethology 112, 247–257 10.1111/j.1439-0310.2006.01153.x (doi:10.1111/j.1439-0310.2006.01153.x) [DOI] [Google Scholar]
- 44.Duffield G. A., Bull C. M. 2002. Stable social aggregations in an Australian lizard, Egernia stokesii. Naturwissenschaften 89, 424–427 10.1007/s00114-002-0346-7 (doi:10.1007/s00114-002-0346-7) [DOI] [PubMed] [Google Scholar]
- 45.Gardner M. G., Bull C. M., Cooper S. J. B., Duffield G. A. 2001. Genetic evidence for a family structure in stable social aggregations of the Australian lizard Egernia stokesii. Mol. Ecol. 10, 175–183 10.1046/j.1365-294X.2001.01171.x (doi:10.1046/j.1365-294X.2001.01171.x) [DOI] [PubMed] [Google Scholar]
- 46.Gardner M. G., Bull C. M., Fenner A., Murray K., Donnellan S. C. 2007. Consistent social structure within aggregations of the Australian lizard, Egernia stokesii, across seven disconnected rocky outcrops. J. Ethol. 25, 263–270 10.1007/s10164-006-0022-z (doi:10.1007/s10164-006-0022-z) [DOI] [Google Scholar]
- 47.Masters C., Shine R. 2003. Sociality in lizards: family structure in free-living King's skinks (Egernia kingii) from southwestern Australia. Aust. Zool. 32, 377–380 [Google Scholar]
- 48.Stow A. J., Sunnucks P. 2004. High mate and site fidelity in Cunningham's skinks (Egernia cunninghami) in natural and fragmented habitat. Mol. Ecol. 13, 419–430 10.1046/j.1365-294X.2003.02061.x (doi:10.1046/j.1365-294X.2003.02061.x) [DOI] [PubMed] [Google Scholar]
- 49.Stow A. J., Sunnucks P. 2004. Inbreeding avoidance in Cunningham's skinks (Egernia cunninghami) in natural and fragmented habitat. Mol. Ecol. 13, 443–447 10.1046/j.1365-294X.2003.02060.x (doi:10.1046/j.1365-294X.2003.02060.x) [DOI] [PubMed] [Google Scholar]
- 50.Stow A. J., Sunnucks P., Briscoe D. A., Gardner M. G. 2001. The impact of habitat fragmentation on dispersal of Cunningham's skink (Egernia cunninghami): evidence from allelic and genotypic analyses of microsatellites. Mol. Ecol. 10, 867–878 10.1046/j.1365-294X.2001.01253.x (doi:10.1046/j.1365-294X.2001.01253.x) [DOI] [PubMed] [Google Scholar]
- 51.Shine R. 1985. The evolution of viviparity in reptiles: an ecological analysis. In Biology of the Reptilia, vol. 15 (eds Gans C., Billet F.), pp. 605–694 New York, NY: John Wiley and Sons [Google Scholar]
- 52.Shine R. 1988. Parental care in reptiles. In Biology of the Reptilia, vol. 16 (eds Gans C., Huey R. B.), pp. 275–330 New York, NY: Alan R. Liss, Inc [Google Scholar]
- 53.Sinn D. L., While G. M., Wapstra E. 2008. Maternal care in a social lizard: links between female aggression and offspring fitness. Anim. Behav. 76, 1249–1257 10.1016/j.anbehav.2008.06.009 (doi:10.1016/j.anbehav.2008.06.009) [DOI] [Google Scholar]