Abstract
Members of social groups need to coordinate their behaviour when choosing between alternative activities. Consensus decisions enable group members to maintain group cohesion and one way to reach consensus is to rely on quorums. A quorum response is where the probability of an activity change sharply increases with the number of individuals supporting the new activity. Here, we investigated how meerkats (Suricata suricatta) use vocalizations in the context of movement decisions. Moving calls emitted by meerkats increased the speed of the group, with a sharp increase in the probability of changing foraging patch when the number of group members joining the chorus increased from two up to three. These calls had no apparent effect on the group's movement direction. When dominant individuals were involved in the chorus, the group's reaction was not stronger than when only subordinates called. Groups only increased speed in response to playbacks of moving calls from one individual when other group members emitted moving calls as well. The voting mechanism linked to a quorum probably allows meerkat groups to change foraging patches cohesively with increased speed. Such vocal coordination may reflect an aggregation rule linking individual assessment of foraging patch quality to group travel route.
Keywords: foraging patch, signal, vocalization, aggregation rule, quorum decision, meerkat
1. Introduction
Social species require decision-making processes in order to maintain their cohesiveness, allowing group members to benefit from advantages associated with group living. Signalling mechanisms that ensure group cohesion have been well studied in invertebrates and micro-organisms. Many of them rely on self-organization principles in which a pattern observed at the global level is the result of interactions among individuals ‘using only local information, without reference to the global pattern’ (p. 8 in [1]). For instance, individual amoebae of the slime mould Dictyostelium discoideum form multicellular slugs moving towards light. The cohesion of the slug during this phototaxis is mediated by a cascade of individual reactions to local changes induced by an external stimulus (the light) [2]. To maintain cohesion, some invertebrates use specific signals. For instance, individual army ants (Eciton burchelli) sigmoidally adjust their speed to the local concentration of a stimulus (the trail pheromone) produced by the ants themselves. The response to this signal allows army ants to display specific cohesion patterns under various environmental conditions [3]. In other taxa, honeybees (Apis mellifera) form a swarm and move towards their new nest, when only a small proportion (approx. 5%) of individuals know the final location. Nevertheless, the swarm remains cohesive because the informed scouts move faster than the naive bees, and naive bees are attracted by these fast streakers [4]. In vertebrates, empirical evidence shows that signals to maintain group cohesion are common in birds and mammals [5–13]. Yet the underlying mechanisms of these signals have not been thoroughly investigated.
Recently, cohesive collective movements have been considered as being the result of three different phases: the pre-departure, the departure itself and the post-departure [14]. The use of signals usually characterizes the pre-departure period. The transition between the pre-departure and the departure onset often relies on ‘quorums’ [15–17]. A quorum is the ‘minimum number of group members that need to take or favour a particular action for the whole group to adopt this action’ (p. 449 in [15]). As a consequence, ‘an individual's probability of selecting an option changes sharply when the number of like-minded conspecifics crosses a threshold’ (p. 745 in [17]). It is therefore similar to the ‘quorum-sensing’ mechanism described in micro-organisms; for example, to synchronize the production of light by bioluminescent bacteria [18]. However, Redfield [19] points out that quorum sensing in bacteria may in many cases be an artefact of ‘diffusion sensing’ studied under laboratory conditions. In all cases, these quorum processes describe the accumulation of a specific signal to a certain threshold. Once this threshold is reached, the collective entity expresses a new behaviour or a new metabolic pathway.
Quorum decisions ensure that a minimum number of individuals (the actual quorum number) are ready to shift from one behaviour to the next. As decisions taken by several individuals are generally more accurate than decisions made alone [20,21], quorum thresholds reduce the risk of relying on only one individual and can minimize errors in decisions. Group decisions mediated by a quorum of individuals have been described in honeybees [22], ants [16], fish [23] and humans [24]. Yet the communicative or signalling mechanism underlying the quorum decision has only been quantified in insects [16,22] and not in any vertebrate species besides humans [24].
Meerkats (Suricata suricatta) are cooperatively breeding mongooses, living across southern Africa in highly cohesive groups (a rare phenomenon in carnivores [25]) varying from 3 to 50 individuals [26]. They forage together but do not share their food or hunt cooperatively; therefore, the benefits of group foraging behaviour are probably due to other benefits, such as reduced predation risk [27]. Furthermore, while foraging for prey items living in the sand, meerkats often have their heads down or below ground, reducing the efficiency of visual communication [28]. Potentially owing to this constraint, meerkats have evolved a wide range of vocalizations used in various contexts [29]. Three types of spatial vocalizations in particular have been described in meerkats: the ‘close’ call, the ‘lead’ call and the ‘moving’ call. The close call is emitted by all group members of a meerkat group throughout their foraging activity, and is the most frequently used call [29,30]. Its most likely function is to maintain each individual's space relative to other group members while searching for food. The lead call is emitted by an individual while moving fast in a straight line. Lead calls are mainly produced in the morning when meerkats leave their sleeping burrow or after a predator alarm. Moving calls, on the other hand, are produced by meerkats while they are foraging. A meerkat starts to emit a moving call while foraging (i.e. before the individual has moved). Sometimes other foraging members join in what is called a ‘moving call chorus’.
We investigated the mechanisms underlying group decisions in meerkats while foraging. We focused on the onset of changes of foraging patches when moving calls were emitted prior to any group movement. We investigated whether moving calls were associated with a change of location by the group, either by an increase in speed or by a change in travel direction. We then tested with playback experiments the effect of moving calls emitted by a single individual. Based on our observations of naturally occurring events when moving calls were emitted, we expected these playbacks to elicit group movement only when meerkats responded to them with moving calls.
2. Material and methods
(a). Study site
We studied group coordination in meerkats at the Kalahari Meerkat Project, on ranchland in the South African Kalahari, near Van Zylsrus (26°58′ S, 21°49′ E). Data were collected during more than 100 group-hours, between August 2006 and November 2008. Description of habitat and climate are provided elsewhere [31,32]. All animals in the population could be individually identified by the use of unique dye mark combinations. Individuals were habituated to close observation (less than 1 m). The ages of almost all individuals (greater than 95%) were known precisely (±5 days), as well as most of their life-history events. We collected data on 12 habituated groups (group size varying from 6 to 19 individuals; mean group size: 10.8 ± 0.5), representing over 130 individuals. Owing to birth and death, group sizes of each focal group changed during the observation period, although within a small range.
(b). Observation of moving calls
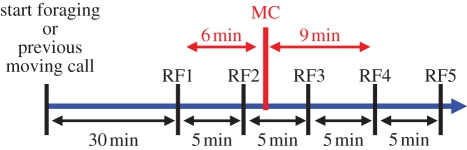
We analysed 48 naturally occurring events of meerkats emitting moving calls from 12 groups (range: 1–12 events per group, average ± s.e.: 4 ± 0.95 events per group) that we followed during foraging over 2–3 h in the morning. Every 5 min during these periods, we took GPS fixes of the location of the centre of the group (accuracy: 95% of fixes within 5 m; eTrex H, Garmin International Inc., Olathe, KS, USA). In addition, we recorded the location when moving calls were emitted by either a single individual or several individuals with an extra-GPS fix (figure 1). Thus, the duration between the previous regular GPS fix and the extra ‘moving call GPS fix’ could be any duration between 0 (moving call occurring during the regular GPS fix) and 4 min (moving call occurring 1 min before the next regular GPS fix).
Figure 1.
Overview of the protocol of GPS recordings. The thick blue arrow represents time. The first regular GPS fix (RF1) to be analysed was 30 min after the group started foraging or 30 min after the emission of a previous moving call event. Subsequent regular GPS fixes continued to be taken every 5 min (RF2 to RF5). A moving call's location was recorded by an extra-GPS fix (MC), which allowed the calculation of the average speed before the moving call (here from RF1 to MC) and of the average speed after the moving call (here from MC to RF4).
We decided to use the speed value over approximately 5 min to assess the immediate effects of moving calls. Further analysis showed that the results are qualitatively the same when we use approximately 10 or even 15 min (C. Bousquet 2010, unpublished data). To take into account the unpredictability of the moving call event, we calculated the average speeds in the following way: (i) ‘speed before the moving call’: GPS point of moving call event (MC) in comparison to previous regular GPS fix (≥5 and <10 min); and (ii) ‘speed after the moving call’: GPS point MC in relation to the following regular GPS fix (≥5 and <10 min; figure 1). Only calling events occurring 30 min after the group started foraging or 30 min apart from each other (to ensure independence of events) were taken into account. For each moving call event, we recorded the number of callers involved (and their identity whenever possible). We created four categories: one caller; two callers; three callers; and four or more callers. We did not further separate the latter category owing to difficulties in identifying all callers accurately when the group was spread over wide distances. For one group, we had no moving call chorus for the three-callers category. Thus, for statistical reasons, we had to merge the three-callers category with the four-or-more-callers category. The speed values for these two last categories were very similar. As a control for natural speed variations, we compared the speed 10 min before and 5 min before the calls occurred. As a further control, we assessed the group's speed difference owing to a naturally occurring close call by comparing the speed 5 min before a close call to the speed 5 min after that close call. Because of the high frequency of close call production, we always had close calls occurring at the same time (within a few seconds) as we took a regular GPS fix and therefore did not have to take an extra-GPS fix to coincide with close call emission.
Before and after moving call events, moving directions were measured from the previous regular GPS fix to the moving call GPS fix and from the moving call GPS fix to the next regular GPS fix, respectively. Afterwards, we calculated the angle of variation between the two moving directions.
(c). Quorum number estimation
Quorums are characterized by a sharp increase in the probability of exhibiting a behaviour, at a particular group size or quorum number. Such an increase can be mathematically approximated by fitting a sigmoidal logistic function to the observed data:
| 2.1 |
where pSI is the probability of a speed increase and n is the number of callers. The parameter T defines the quorum number at which the probability of a speed increase is 0.5, while β determines the steepness of the response. The logistic function is convenient for fitting data since we can rearrange equation (2.1) to give
allowing us to fit the relationship between pSI and n using linear regression. For observations, we defined pSI to be 1 if the change in speed was larger than that given by the 95th percentile of speed changes in the control observations; otherwise pSI was zero. The procedure was run in MatLab 7.7.0 (The MathWorks, Inc.).
(d). Playbacks of moving calls
To test whether the moving calls were the causal factor to initiate group movement, we performed playback experiments. We recorded moving and close calls of the group's dominant female by following her within 1–2 m with a Sennheiser ME66 directional microphone (Sennheiser Electronic Corp., Old Lyme, CT, USA), with windshield, connected to a solid-state recorder (Marantz PMD660, D & M Professional, Kanagawa, Japan; sampling frequency of 44.1 kHz). We edited the calls using Cool Edit 2000 (Syntrillium Software Corporation, Phoenix, AZ, USA). An edited sound file to be played back consisted of three different moving calls (test condition) or three different close calls (control condition), each separated by 2 s of silence (similar structure to a naturally occurring moving call bout), with an overall duration of 8 s.
Playbacks were conducted with the Marantz recorder connected to a portable loudspeaker (Hama AS-61 10W, Hama GmbH & Co KG, Monheim, Germany) at an amplitude similar to that in the wild (estimated by hearing). The loudspeaker was attached to the leg of the observer at the height of a foraging meerkat. All playbacks were made in the centre of the group, with no meerkats present within 5 m of the loudspeaker when the playback started (most of the group members were 5–10 m away from the loudspeaker). We video recorded (Everio GZ-MG150 digital video camera, JVC, Yokohama, Japan) the maximum visible number of meerkats to assess their first reaction. At the time of playback, all individuals were foraging and no sentinel had been on duty for at least 10 min. No natural moving calls had occurred in the previous 30 min. We took a GPS fix of the playback's location. If a disturbance (alarm call, intergroup encounter, presence of a car or another human) occurred within 5 min after the playback, the experiment was discarded (which was the case for two playbacks). We conducted moving call playbacks in six different meerkat groups until we had for each group at least one ‘vocal response’ and one ‘no vocal response’. Therefore, depending on groups, we conducted two or three playbacks. As a control, we played back close calls in five different groups. We ran two playbacks in each group, except for one group in which only one close call playback was possible owing to time constraints. We then compared the speed 5 min before the playback to the speed 5 min after the playback. Angles for movement direction changes were determined as described before. To avoid habituation, we waited at least 7 days between any two consecutive playbacks for a focal group.
(e). Statistical analysis
Statistical tests were done using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA). We compared meerkat group speed by using paired exact Wilcoxon signed-ranks tests, where the speed after the considered call was linked to the speed before the call. To test the influence of the number of callers, we conducted Friedman tests. For the test of dominance and number of callers, we used the Scheirer–Ray–Hare test, which is a non-parametric equivalent of a two-way ANOVA [33]. We conducted Watson–Williams tests to compare mean angles [34]. For the analysis of the playback experiments, we calculated the average speed per group for the playback experiments within the same condition—such as: (i) test condition, moving calls with ‘no vocal response’ (n = 6); (ii) moving calls with ‘vocal response’ (n = 6); and (iii) control condition, close calls (n = 5), and performed exact Wilcoxon signed-ranks tests.
3. Results
(a). Moving calls increase speed
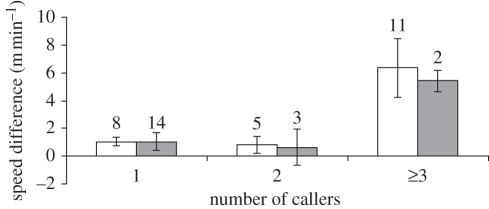
When meerkats emitted moving calls, the group's speed increased, but not when they emitted close calls. The group's speed in the 5 min before the naturally occurring moving calls was 3.31 ± 0.33 (mean ± s.e.) m min−1. The group's speed in the 5 min after the naturally occurring moving calls was 7.06 ± 0.85 m min−1 (exact Wilcoxon signed-ranks test: Z = −3.059, n = 12, p < 0.001). Therefore, meerkat groups travelled twice as fast after a moving call event versus before. By contrast, naturally occurring close calls did not affect group speed (average speed in the 5 min before a close call: 3.33 ± 0.52 m min−1; after a close call: 3.09 ± 0.35 m min−1; exact Wilcoxon signed-ranks test: Z = −0.524, n = 5, p = 0.69). When taking the social status of the callers into consideration, we found no effect of dominance on the movement of the group. Events with moving calls in which dominant individuals were involved did not affect the group speed more than moving call events in which only subordinate individuals were involved (figure 2 and table 1).
Figure 2.
Effect of social status on the speed difference from before emitting moving calls to the period afterwards. White bars indicate events involving at least one dominant individual calling, grey bars when only subordinate individuals called. Numbers above bars indicate the number of events for each category. Mean ± s.e.
Table 1.
Scheirer–Ray–Hare test output. SS, sum of square; d.f., degrees of freedom; MStot, mean sum of square of the total; SS/MStot, ratio sum of square of the factor by the mean sum of square of the total.
| SS | d.f. | MStot | SS/MStot | p | |
|---|---|---|---|---|---|
| dominance | 0.5 | 1 | 0.011 | 0.918 | |
| no. of callers | 580.0 | 2 | 12.608 | 0.002 | |
| interaction | 6.5 | 2 | 0.142 | 0.932 | |
| total | 1012.0 | 22 | 46 |
(b). Quorum of two or three individuals necessary to increase group speed
Moving calls dramatically affected the group speed when three or more callers joined the chorus (figure 2; Friedman test: χ2 = 9.333, n = 6, d.f. = 2, p = 0.006). When taken on their own the categories, ‘one caller’ and ‘two callers’ showed a small and non-significant increase in speed (one caller: +0.79 ± 0.61 m min−1, exact Wilcoxon signed-ranks test: Z = −1.363, n = 6, p = 0.22; two callers: +1.55 ± 0.64 m min−1, exact Wilcoxon signed-ranks test: Z = −1.782, n = 6, p = 0.09). However, when three or more callers were involved in the chorus, the group speed increased much more (+6.54 ± 1.82 m min−1, exact Wilcoxon signed-ranks test: Z = −2.201, n = 6, p = 0.03).
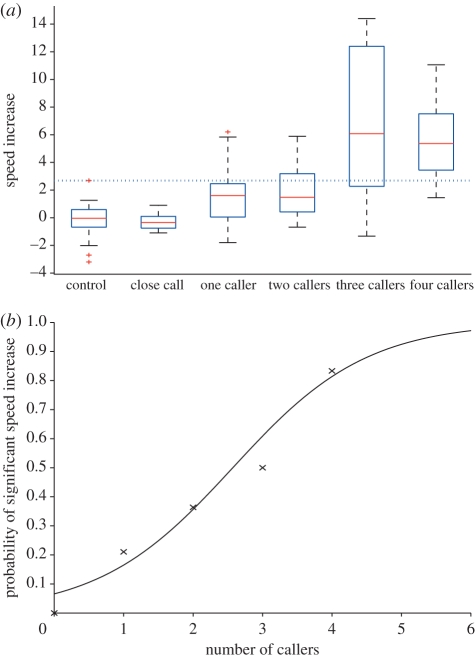
The importance of three calling individuals in increasing group speed is further clarified when the logistic function (equation (2.1)) is fitted to the probability of increasing speed. In the control observations, 95 per cent of changes in speed were less than 2.69 m min−1 (dotted line in figure 3a). Figure 3b shows the proportion of observations in which increase in speed was greater than 2.69 m min−1. Fitting to these observations gives an estimate of the quorum number of T = 2.57, suggesting that the switch from two to three callers marks the point at which a speed increase is highly probable. When only close calls were emitted, the group's speed increase never reached 2.69 m min−1.
Figure 3.
(a) Effect of the number of callers involved in the moving call chorus on group speed increase. The box plots give distribution of speed increases (minimum, first quartile, median, third quartile, maximum; asterisks represent outliers). The dotted line at 2.69 m min−1 indicates the 95 percentile of the distribution of speed changes in the control observations. (b) Identification of the quorum number required for an increase in speed. Crosses represent the proportion of moving calls inducing a speed increase higher than 2.69 m min−1. The dark line represents the fit of the sigmoidal logistic function (equation (2.1)) to the data. Parameter values determined by logistic regression are T = 2.57 and β = 1.03.
(c). Vocal response required for playbacks to increase group speed
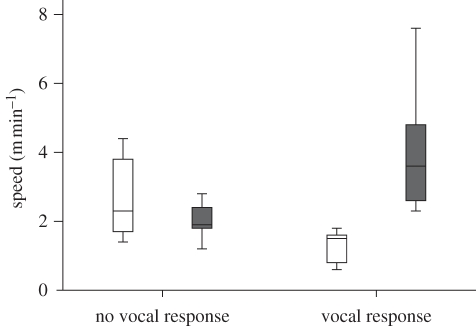
The vocal response to the playbacks of moving calls from the dominant female also had an impact on the increase in group speed (vocal response: +2.78 ± 0.82 m min−1, exact Wilcoxon signed-ranks test: Z = −2.201, n = 6, p = 0.028; figure 4). In contrast, playbacks of moving calls that did not elicit a vocal response did not affect group speed (no vocal response: −0.65 ± 0.39 m min−1, exact Wilcoxon signed-ranks test: Z = −1.483, n = 6, p = 0.138; figure 4). Close calls of the dominant female played back to the foraging group (n = 9 in five groups) did not influence the group speed (+0.38 ± 0.67 m min−1, exact Wilcoxon signed-ranks test: Z = −0.677, n = 5, p = 0.5).
Figure 4.
Effect of the presence or absence of a vocal response to the playback of moving calls on group speed. White bars, speed before a playback; grey bars, speed after a playback. For the box-plots, the bottom and top of the box represent the first and the third quartiles, respectively, and the line inside the box shows the median. Limits of the whiskers represent minimum and maximum values of the data.
(d). Moving calls do not influence travelling direction
Meerkats did not change their moving direction more after moving calls than after close calls. When meerkats emitted moving calls, the group's direction for the next 5 min changed by 49.1 ± 26.7° (mean angle ± angular deviation; n = 12) from the straight line (either on the left or on the right). The number of callers involved in the moving call chorus did not affect this turning angle. The change of direction after a moving call did not differ from the one following a close call (Watson–Williams test: F = 0.07, p > 0.25), which was 43.9 ± 46.1° (n = 6) from the straight line (either on the left or on the right). Playbacks of moving calls did not affect the direction change of the group when compared with playbacks of close calls (62.7 ± 32.3°, n = 6 and 70.2 ± 21.0°, n = 5, respectively; Watson–Williams test: F = 0.16, p > 0.25). Playbacks themselves did not have an effect on the group's direction as direction changes after playbacks did not differ from the direction changes after naturally occurring calls (61.7 ± 24.1°, n = 6 and 54.0 ± 30.6°, n = 12, respectively; Watson–Williams test: F = 0.25, p > 0.25).
4. Discussion
Meerkat groups remained cohesive during daily foraging, with groups only splitting up owing to external events such as predator approaches. Part of their group movements were initiated by specific vocalizations, the moving calls. Our results from natural observations, a mathematical model and playback experiments suggest that a quorum of at least two and usually three meerkats emitting moving calls are necessary for the whole group to move to a new foraging patch. If no other group member or only one joined the moving call chorus to support the initiator's motivation, then the group and the initiator usually continued to forage in the same patch. The initiator's signal became effective if at least two other meerkats supported its preference. In both cases, the group remained cohesive despite conflicting interests (or at least conflicting information) among group members. This cohesion is crucial for meerkats' survival, as single individuals outside their group have a higher mortality rate [31]. Our results therefore suggest that meerkats use a specific vocalization (the moving call) along with a quorum response mechanism as an efficient temporal coordination tool of group movement.
The effect of moving calls on group direction did not differ from the effect of close calls on group direction. This indicates that moving calls are not used as a directional coordination tool. Given that meerkats' prey are widely distributed and underground, it might be more relevant for meerkats to know when it is best for them to leave the current foraging patch rather than where to go next. However, once the quorum is reached in the group, some individuals might still choose the next direction. A closer look at the position of specific individuals (e.g. dominant pair, older individuals) might reveal that the choice of the next direction is not random.
Overall, the use of moving calls may function as a foraging-patch quality census system. A meerkat might emit a moving call when its immediate foraging patch is becoming food-depleted. If other meerkats, at a similar time, also find their foraging patch poor, then they might join the chorus. That a quorum of callers has been reached reflects an accumulation of evidence that a foraging patch is depleting. Such a system avoids errors where one unsuccessful individual wrongly concludes that food is depleted. In order for its call to be followed as a signal to leave, at least one and usually two other individuals have to emit similar calls. The fact that neither dominance status, sex nor age (disregarding pups and juveniles) of callers affected the success of moving calls further supports the idea of move calling as reflecting each individual's assessment of food patch quality. Such a quality census system on foraging patches fits well many of the observations described in primates [35,36] and birds [12], as well as theoretical models [37–40]. Thus, it provides a simple mechanism to coordinate group cohesion effectively with maximized foraging success for the majority of the group.
Moving calls are emitted before meerkats increase their speed, and are not just a by-product vocalization emitted by meerkats on the move. They act as a signal prior to group movement. This signal can still be used during group movement, potentially to reinforce its meaning. In quorum decisions, the signal eliciting the new behaviour does not necessarily have to stop being produced once the threshold is reached. For example, in quorum-sensing bacteria, the signal is even reinforced by the newly released metabolic pathway (fig. 1 in [18]). Additionally, in vertebrates, quorum thresholds have been described for which the signal used was the mere movement of individuals, without any vocalizations [23,41]. In this case, the signal used (the displacement itself) does not disappear once the threshold is reached as the group continues to move.
Another intriguing aspect of our findings is the absolute value of the quorum number: two to three individuals. Other studies in the field [7,42,43] also found similar results. For example, it takes more than two fish to make a decision in groups ranging up to 10 individuals [43]. In macaques, for two groups of 22 individuals, approximately three individuals were involved in pre-departure behaviour, which was linked to the departure success [42]. In horses, approximately three horses in a group of six individuals were involved, on average, in pre-departure behaviour [7]. It appears that two to three individuals acting as signallers is a common requirement in several species, at least for group sizes ranging from six to 22 individuals. Increasing the quorum number as group size increases could potentially increase the frequency of group splits, owing to the ‘strength in number’ effect [44]. However, there may also be a cognitive limitation in distinguishing among more than three individuals. Indeed, a quorum number does not need to be large to be effective since errors decrease exponentially with quorum size. If the probability that one meerkat wrongly concludes that it is time to leave a foraging patch is ɛ = 5 per cent, then the probability that two and three individuals will independently reach the same conclusion is ɛ2 = 0.25 per cent and ɛ3 = 0.0125 per cent, respectively [45].
The mechanism underlying the changing of foraging patches initiated by a single individual, but only successful with the support of additional group members, probably represents a common group coordination process in many vertebrate species (primates: [36]; fish: [23]), including humans [46]. This study, however, is a first step with wild animals towards understanding how individual decisions and group decisions are linked, and how a group's behaviour can result from the aggregation of individual behaviours, following a specific aggregation rule [47]. The aggregation rule of using calls allows a fast change in behaviour or direction, without relying on only one or two individual assessments. In essence, it reflects a so-called voting process [10,48], where the preference of several group members is expressed, and only then, depending on the support of enough individuals to reach the quorum needed, does the according alternative action follow. Previous studies have shown the importance of vocalizations in vertebrates to change foraging patches [11]. Here, we showed that the response of group members to the initiator's call determined the final group's response. This effect can be termed as ‘social feedback’, where followers responding to an initiator are important for the success or the failure of the initiator [14,36,49]. These approaches provide important insights into better understanding the transition from individual behaviour to group behaviour.
Acknowledgements
We are indebted to T. Clutton-Brock for allowing us to work on the meerkat population of the Kalahari Meerkat Project. R. Sutcliffe and D. Bell made every aspect of our research a lot easier. Thanks to the numerous project volunteers for maintaining habituation of the meerkats, and to M. Dewas for recording some of the moving calls used in our playbacks. We are also grateful to Family J. Koetze for the permission to work on their land. We would like to thank R. Furrer, J. Isden, D. Jansen, G. Kerth, J. Madden, S. Rands, S. Townsend and one anonymous referee for reading earlier versions of the manuscript and for useful discussions. This project was funded by a grant given to M.B.M. from the Swiss National Science Foundation, SNF-Förderprofessur 631-066129 and by the MNF of the University of Zurich.
References
- 1.Camazine S., Deneubourg J.-L., Franks N. R., Sneyd J., Theraulaz G., Bonabeau E. 2001. Self-organization in biological systems. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Marée A. F. M., Panfilov A. V., Hogeweg P. 1999. Phototaxis during the slug stage of Dictyostelium discoideum: a model study. Proc. R. Soc. Lond. B 266, 1351–1360 10.1098/rspb.1999.0787 (doi:10.1098/rspb.1999.0787) [DOI] [Google Scholar]
- 3.Franks N. R., Gomez N., Goss S., Deneubourg L. 1991. The blind leading the blind in army ant raid patterns: testing a model of self-organization (Hymenoptera: Formicidae). J. Insect Behav. 4, 583–607 10.1007/BF01048072 (doi:10.1007/BF01048072) [DOI] [Google Scholar]
- 4.Schultz K. M., Passino K. M., Seeley T. D. 2008. The mechanism of flight guidance in honeybee swarms: subtle guides or streaker bees? J. Exp. Biol. 211, 3287–3295 10.1242/jeb.018994 (doi:10.1242/jeb.018994) [DOI] [PubMed] [Google Scholar]
- 5.Black J. M. 1988. Preflight signalling in swans: a mechanism for group cohesion and flock formation. Ethology 79, 143–157 10.1111/j.1439-0310.1988.tb00707.x (doi:10.1111/j.1439-0310.1988.tb00707.x) [DOI] [Google Scholar]
- 6.Boinski S., Campbell A. F. 1995. Use of trill vocalizations to coordinate troop movement among white-faced capuchins: a second field test. Behaviour 132, 875–901 10.1163/156853995X00054 (doi:10.1163/156853995X00054) [DOI] [Google Scholar]
- 7.Bourjade M., Thierry B., Maumy M., Petit O. 2009. Decision-making in Przewalski horses (Equus ferus przewalskii) is driven by the ecological contexts of collective movements. Ethology 115, 321–330 10.1111/j.1439-0310.2009.01614.x (doi:10.1111/j.1439-0310.2009.01614.x) [DOI] [Google Scholar]
- 8.Byrne R. W. 2000. How monkeys find their way: leadership, coordination, and cognitive maps of African baboons. In On the move: how and why animals travel in groups (eds Boinski S., Garber P. A.), pp. 491–518 Chicago, IL: The University of Chicago Press [Google Scholar]
- 9.Lusseau D., Conradt L. 2009. The emergence of unshared consensus decisions in bottlenose dolphins. Behav. Ecol. Sociobiol. 63, 1067–1077 10.1007/s00265-009-0740-7 (doi:10.1007/s00265-009-0740-7) [DOI] [Google Scholar]
- 10.Prins H. H. T. 1996. Ecology and behaviour of the African buffalo: social inequality and decision making. Wildlife Ecology and Behaviour Series London, UK: Chapman & Hall [Google Scholar]
- 11.Radford A. N. 2004. Vocal coordination of group movement by green woodhoopoes (Phoeniculus purpureus). Ethology 110, 11–20 10.1046/j.1439-0310.2003.00943.x (doi:10.1046/j.1439-0310.2003.00943.x) [DOI] [Google Scholar]
- 12.Ramseyer A., Petit O., Thierry B. 2009. Decision-making in group departures of female domestic geese. Behaviour 146, 351–371 10.1163/156853909X410955 (doi:10.1163/156853909X410955) [DOI] [Google Scholar]
- 13.Sueur C., Petit O. 2010. Signal use by leaders in Macaca tonkeana and Macaca mulatta: group-mate recruitment and behaviour monitoring. Anim. Cogn. 13, 239–248 10.1007/s10071-009-0261-9 (doi:10.1007/s10071-009-0261-9) [DOI] [PubMed] [Google Scholar]
- 14.Petit O., Bon R. 2010. Decision-making processes: the case of collective movements. Behav. Process. 84, 635–647 10.1016/j.beproc.2010.04.009 (doi:10.1016/j.beproc.2010.04.009) [DOI] [PubMed] [Google Scholar]
- 15.Conradt L., Roper T. J. 2005. Consensus decision making in animals. Trends Ecol. Evol. 20, 449–456 10.1016/j.tree.2005.05.008 (doi:10.1016/j.tree.2005.05.008) [DOI] [PubMed] [Google Scholar]
- 16.Pratt S. C., Mallon E. B., Sumpter D. J. T., Franks N. R. 2002. Quorum sensing, recruitment, and collective decision-making during colony emigration by the ant Leptothorax albipennis. Behav. Ecol. Sociobiol. 52, 117–127 10.1007/s00265-002-0487-x (doi:10.1007/s00265-002-0487-x) [DOI] [Google Scholar]
- 17.Sumpter D. J. T., Pratt S. C. 2009. Quorum responses and consensus decision making. Phil. Trans. R. Soc. B 364, 743–753 10.1098/rstb.2008.0204 (doi:10.1098/rstb.2008.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waters C. M., Bassler B. L. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21, 319–346 10.1146/annurev.cellbio.21.012704.131001 (doi:10.1146/annurev.cellbio.21.012704.131001) [DOI] [PubMed] [Google Scholar]
- 19.Redfield R. J. 2002. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 10, 365–370 10.1016/S0966-842X(02)02400-9 (doi:10.1016/S0966-842X(02)02400-9) [DOI] [PubMed] [Google Scholar]
- 20.Codling E. A., Pitchford J. W., Simpson S. D. 2007. Group navigation and the ‘many-wrongs principle’ in models of animal movement. Ecology 88, 1864–1870 10.1890/06-0854.1 (doi:10.1890/06-0854.1) [DOI] [PubMed] [Google Scholar]
- 21.Kerr N. L., Tindale R. S. 2004. Group performance and decision making. Annu. Rev. Psychol. 55, 623–655 10.1146/annurev.psych.55.090902.142009 (doi:10.1146/annurev.psych.55.090902.142009) [DOI] [PubMed] [Google Scholar]
- 22.Seeley T. D., Visscher P. K. 2003. Choosing a home: how the scouts in a honey bee swarm perceive the completion of their group decision making. Behav. Ecol. Sociobiol. 54, 511–520 10.1007/s00265-003-0664-6 (doi:10.1007/s00265-003-0664-6) [DOI] [Google Scholar]
- 23.Ward A. J. W., Sumpter D. J. T., Couzin I. D., Hart P. J. B., Krause J. 2008. Quorum decision-making facilitates information transfer in fish shoals. Proc. Natl Acad. Sci. USA 105, 6948–6953 10.1073/pnas.0710344105 (doi:10.1073/pnas.0710344105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermeule A. 2005. Submajority rules: forcing accountability upon majorities. J. Pol. Phil. 13, 74–98 10.1111/j.1467-9760.2005.00214.x (doi:10.1111/j.1467-9760.2005.00214.x) [DOI] [Google Scholar]
- 25.Holekamp K. E., Boydston E. E., Smale L. 2000. Group travel in social carnivores. In On the move: how and why animals travel in groups (eds Boinski S., Garber P. A.), pp. 587–627 Chicago, IL: The University of Chicago Press [Google Scholar]
- 26.Clutton-Brock T. H., Hodge S. J., Spong G. F., Russell A. F., Jordan N. R., Bennett N. C., Sharpe L. L., Manser M. B. 2006. Intrasexual competition and sexual selection in cooperative mammals. Nature 444, 1065–1068 10.1038/nature05386 (doi:10.1038/nature05386) [DOI] [PubMed] [Google Scholar]
- 27.Krause J., Ruxton G. D. 2002. Living in groups. Oxford Series in Ecology and Evolution. New York, NY: Oxford University Press [Google Scholar]
- 28.Manser M. B. 1999. Response of foraging group members to sentinel calls in suricates, Suricata suricatta. Proc. R. Soc. Lond. B 266, 1013–1019 10.1098/rspb.1999.0737 (doi:10.1098/rspb.1999.0737) [DOI] [Google Scholar]
- 29.Manser M. B. 1998. The evolution of auditory communication in suricates (Suricata suricatta). PhD thesis, Cambridge University, Cambridge, UK [Google Scholar]
- 30.Townsend S. W., Hollén L. I., Manser M. B. 2010. Meerkat close calls encode group-specific signatures, but receivers fail to discriminate. Anim. Behav. 80, 133–138 10.1016/j.anbehav.2010.04.010 (doi:10.1016/j.anbehav.2010.04.010) [DOI] [Google Scholar]
- 31.Clutton-Brock T. H., Gaynor D., McIlrath G. M., MacColl A. D. C., Kansky R., Chadwick P., Manser M. B., Skinner J. D., Brotherton P. N. M. 1999. Predation, group size and mortality in a cooperative mongoose, Suricata suricatta. J. Anim. Ecol. 68, 672–683 10.1046/j.1365-2656.1999.00317.x (doi:10.1046/j.1365-2656.1999.00317.x) [DOI] [Google Scholar]
- 32.Russell A. F., Clutton-Brock T. H., Brotherton P. N. M., Sharpe L. L., McIlrath G. M., Dalerum F. D., Cameron E. Z., Barnard J. A. 2002. Factors affecting pup growth and survival in co-operatively breeding meerkats Suricata suricatta. J. Anim. Ecol. 71, 700–709 10.1046/j.1365-2656.2002.00636.x (doi:10.1046/j.1365-2656.2002.00636.x) [DOI] [Google Scholar]
- 33.Dytham C. 2003. Choosing and using statistics: a biologist's guide. Oxford, UK: Blackwell Publishing [Google Scholar]
- 34.Zar J. H. 1999. Biostatistical analysis. Upper Saddle River, NJ: Pearson Education, Inc [Google Scholar]
- 35.Boinski S. 2000. Social manipulation within and between troops mediates primate group movement. In On the move: how and why animals travel in groups (eds Boinski S., Garber P. A.), pp. 421–469 Chicago, IL: The University of Chicago Press [Google Scholar]
- 36.Petit O., Gautrais J., Leca J.-B., Theraulaz G., Deneubourg L. 2009. Collective decision-making in white-faced capuchin monkeys. Proc. R. Soc. B 276, 3495–3503 10.1098/rspb.2009.0983 (doi:10.1098/rspb.2009.0983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cant M. A., Shen F. 2006. Endogenous timing in competitive interactions among relatives. Proc. R. Soc. B 273, 171–178 10.1098/rspb.2005.3132 (doi:10.1098/rspb.2005.3132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rands S. A., Cowlishaw G., Pettifor R. A., Rowcliffe J. M., Johnstone R. A. 2003. Spontaneous emergence of leaders and followers in foraging pairs. Nature 423, 432–434 10.1038/nature01630 (doi:10.1038/nature01630) [DOI] [PubMed] [Google Scholar]
- 39.Rands S. A., Cowlishaw G., Pettifor R. A., Rowcliffe J. M., Johnstone R. A. 2008. The emergence of leaders and followers in foraging pairs when the qualities of individuals differ. BMC Evol. Biol. 8, 51. 10.1186/1471-2148-8-51 (doi:10.1186/1471-2148-8-51) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valone T. J. 1993. Patch information and estimation: a cost of group foraging. Oikos 68, 258–266 10.2307/3544838 (doi:10.2307/3544838) [DOI] [Google Scholar]
- 41.Sueur C., Deneubourg J.-L., Petit O. 2010. Sequence of quorums during collective decision making in macaques. Behav. Ecol. Sociobiol. 64, 1875–1885 10.1007/s00265-010-0999-8 (doi:10.1007/s00265-010-0999-8) [DOI] [Google Scholar]
- 42.Sueur C., Petit O. 2008. Shared or unshared consensus decision in macaques? Behav. Process. 78, 84–92 10.1016/j.beproc.2008.01.004 (doi:10.1016/j.beproc.2008.01.004) [DOI] [PubMed] [Google Scholar]
- 43.Sumpter D. J. T., Krause J., James R., Couzin I. D., Ward A. J. W. 2008. Consensus decision making by fish. Curr. Biol. 18, 1773–1777 10.1016/j.cub.2008.09.064 (doi:10.1016/j.cub.2008.09.064) [DOI] [PubMed] [Google Scholar]
- 44.Dyer J. R. G., Johansson A., Helbing D., Couzin I. D., Krause J. 2009. Leadership, consensus decision making and collective behaviour in humans. Phil. Trans. R. Soc. B 364, 781–789 10.1098/rstb.2008.0233 (doi:10.1098/rstb.2008.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumpter D. J. T. 2010. Collective animal behavior. Princeton, NJ: Princeton University Press [Google Scholar]
- 46.Faria J. J., Dyer J. R. G., Tosh C. R., Krause J. 2010. Leadership and social information use in human crowds. Anim. Behav. 79, 895–901 10.1016/j.anbehav.2009.12.039 (doi:10.1016/j.anbehav.2009.12.039) [DOI] [Google Scholar]
- 47.Conradt L., List C. 2009. Group decisions in humans and animals: a survey. Phil. Trans. R. Soc. B 364, 719–742 10.1098/rstb.2008.0276 (doi:10.1098/rstb.2008.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sellers W. I., Hill R. A., Logan B. S. 2007. An agent-based model of group decision making in baboons. Phil. Trans. R. Soc. B 362, 1699–1710 10.1098/rstb.2007.2064 (doi:10.1098/rstb.2007.2064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harcourt J. L., Ang T. Z., Sweetman G., Johnstone R. A., Manica A. 2009. Social feedback and the emergence of leaders and followers. Curr. Biol. 19, 248–252 10.1016/j.cub.2008.12.051 (doi:10.1016/j.cub.2008.12.051) [DOI] [PubMed] [Google Scholar]