Abstract
Rising levels of ultraviolet radiation (UVR) secondary to ozone depletion are an issue of concern for public health. Skin cancers and intraepidermal dysplasia are increasingly observed in individuals that undergo chronic or excessive sun exposure. Such alterations of skin integrity and function are well established for humans and laboratory animals, but remain unexplored for mammalian wildlife. However, effects are unlikely to be negligible, particularly for species such as whales, whose anatomical or life-history traits force them to experience continuous sun exposure. We conducted photographic and histological surveys of three seasonally sympatric whale species to investigate sunburn and photoprotection. We find that lesions commonly associated with acute severe sun damage in humans are widespread and that individuals with fewer melanocytes have more lesions and less apoptotic cells. This suggests that the pathways used to limit and resolve UVR-induced damage in humans are shared by whales and that darker pigmentation is advantageous to them. Furthermore, lesions increased significantly in time, as would be expected under increasing UV irradiance. Apoptosis and melanocyte proliferation mirror this trend, suggesting that whales are capable of quick photoprotective responses. We conclude that the thinning ozone layer may pose a risk to the health of whales and other vulnerable wildlife.
Keywords: apoptosis, ozone depletion, photoprotection, pigmentation, skin lesions, whales
1. Introduction
Since the 20th century, worldwide mounting levels of solar ultraviolet radiation (UVR) consequential to ozone depletion [1–3] have generated concern owing to their health implications [4–5]. Across all latitudes, cases of skin cancers and intraepidermal dysplasia are being increasingly reported in humans who are chronically or excessively exposed to UVR [4–6]. Despite UVR-induced alterations of skin integrity and function being well established for humans and laboratory animals [4], similar studies on wild mammals are virtually non-existent [7]. However, effects are unlikely to be negligible, particularly for species such as cetaceans, which by anatomical (e.g. lack of fur, feathers or keratinized plates) or life-history constraints (e.g. obligate air-breathing physiology, lactation or socialization at the sea surface) are unable to avoid continuous exposure to UVR [8,9]. Interestingly, in recent years, reports of cetacean skin lesions have multiplied [10,11]. The aetiology of some of the skin conditions that show distinct patterns have already been characterized (e.g. lobomycosis, caused by the fungus Lacazia loboi [12], and poxvirus tattoo skin disease [13]), but many other types of lesion (e.g. blistering lesions) have not [14]. It is possible that these lesions are linked to mounting levels of UVR, since for each percentage of stratospheric ozone lost, erythema-inducing (skin-damaging) radiation increases by 1.2 per cent [1].
Studies in humans and laboratory animals have shown that lighter-skinned individuals are more sensitive to UVR than those with darker skin [15,16], whose risk of developing skin cancer is 10- to 100-fold lower [17]. When controlling for differences in skin pigmentation, longer periods of exposure to the sun influence severity of skin damage [4]. If this knowledge were extrapolated to cetaceans, one would expect cetaceans with paler skin pigmentation and those spending longer periods at the sea surface to be more severely exposed and develop more blistering skin lesions. In addition, as a result of mounting levels of UVR, an increment in skin lesions over time would also be expected.
We tested these predictions by examining skin lesions in three seasonally sympatric cetacean species (blue, fin and sperm whales) from the Gulf of California, a region situated near tropical latitudes, where skin cancer radiation dosages are five times higher than in mid-latitude zones [18]. Marked differences in skin pigmentation among these species, as well as distinct surface behaviours (figure 1a), made it possible to investigate the potential photoprotective role of cetacean skin pigmentation and the importance of sun exposure length for lesion development. We performed gross analysis of skin sections using high-quality photographs, and microscopic analysis that involved routine and specialized staining to detect apoptotic cells [17,19]. We find widespread evidence of epidermal damage commonly associated with acute and severe sunburn in the three species, and demonstrate that whales with more melanocytes have fewer lesions and more apoptotic cells. Interestingly, apoptosis and melanocyte proliferation increase in time, suggesting quick photoprotective responses.
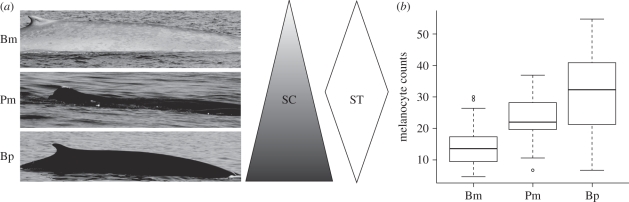
Figure 1.
(a) Differences in skin colour (SC) and time spent at the surface (ST) among blue (Bm), sperm (Pm) and fin whales (Bp). (b) Melanocyte counts in blue (Bm, n = 63), sperm (Pm, n = 17) and fin whales (Bp, n = 46).
2. Material and methods
(a). Sample collection
Cetacean surveys were conducted in the Gulf of California (Mexico) between January and June (2007–2009). High-quality photographs were obtained using a digital camera (Canon EOS20) and skin biopsies were collected with a 7 mm stainless steel dart from blue whales, Balaenoptera musculus (n = 98 and 71, respectively), fin whales, Balaenoptera physalus (n = 34 and 50), and sperm whales, Physeter macrocephalus (n = 24 and 21; details in electronic supplementary material, table S1). Epidermal samples were preserved in 10 per cent buffered formalin. Each whale was photo-identified and catalogued based on skin patterns and scars on the back and dorsal fin [20] and the ventral side of the flukes [21]. In each season recaptured individuals were excluded from the analyses, the first capture being the one included. Minimum age was calculated from the first year each whale was observed in the Gulf of California. We excluded new individuals (minimum age = 1) from the age-related analyses (n = 58).
(b). Analysis of gross lesions
Occurrence, prevalence (%) and intensity (number of lesions/individual) of gross lesions were determined in a previously defined area using high-quality photographs (see the electronic supplementary material). Photos were included only when the whale's flank was perpendicular to the camera and focus was sharp. To ensure consistency, all photographs were analysed by a single person at the end of the final sampling season.
(c). Histology
Skin sections were stained with haematoxylin and eosin (H&E). Accumulation of glycogen was confirmed with periodic acid schiff (PAS) and diastase-resistant (DPAS) stains [22]. Four categories were defined for cytoplasmic vacuolation, from zero (absence) to three (severe; see electronic supplementary material, figure S1a–c). Apoptosis was examined in a subset of 43 individuals using the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotine nick end labelling method (TUNEL) that detects DNA double-strand breaks typical of final stages of apoptosis [17,19,23]. A semi-quantitative measure of apoptotic cells was used as indicated above (level 0 to 2 = none to few localized apoptotic cells, level 3 = many and widely distributed apoptotic cells; see electronic supplementary material, figure S1d–f). Melanocytes (M = number of melanocytes per 100 arbitrary units), which were used as a surrogate measure of skin pigmentation, were counted within a standardized area (details in the electronic supplementary material). To avoid bias, all slides were examined at the end of the final sampling season.
(d). Statistical methods
Interspecies differences in lesion prevalence were examined with Fisher's exact tests. ANOVA and non-parametric Kruskall–Wallis tests were used to compare melanocyte counts between years and lesion intensity among species, respectively. Generalized linear models (GLMs) were constructed to investigate interspecies differences in melanocyte counts and epidermal lesions, and temporal trends in lesion prevalence. When explanatory variables were bimodal responses, error structure was defined accordingly. Analyses were conducted in R [24].
3. Results and discussion
Melanocyte counts varied significantly between species, being lowest for blue whales (14.1 M ± 0.77) and highest for fin whales (30.8 M ± 1.71; Kruskall–Wallis, χ2 = 54.1, d.f. = 2, p = 1.8 × 10−12; figure 1b). Gross lesions observed were predator bite marks (19% overall prevalence) and blister-type lesions (hereafter blisters; 28% overall prevalence). Histological analysis revealed a range of abnormalities, including intracellular oedema, cytoplasmic vacuolation, glycogen deposition, microvesicles and leucocyte infiltration (figure 2), all considered characteristic of sunburn and generally observed 24 h after UVR exposure [19,22]. Basal dendritic melanocytes and basal and suprabasal perinuclear melanin pigments (supranuclear caps) were common findings. These phenomena arise as protective responses following UVR exposure in humans [25,26].
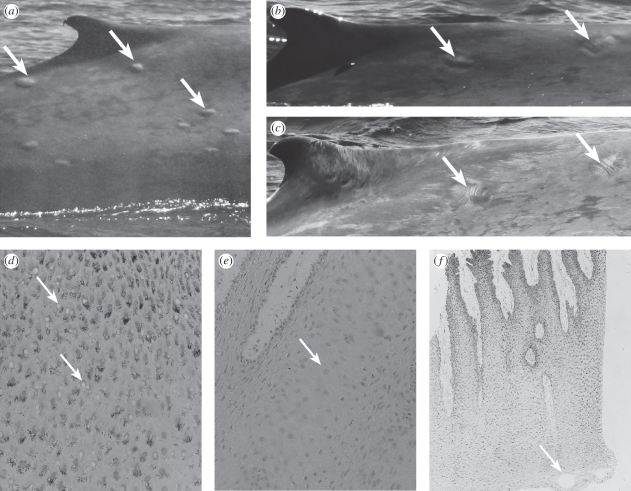
Figure 2.
Typical images of skin lesions. (a) Gross blistering on the dorsal surface of a blue whale, (b) bite marks on the dorsal surface of a blue whale seen as oval-shaped lesions with a sunken perimeter, (c) bite marks seen as parallel rakings, (d) cytoplasmic vacuolation (400×), (e) intracellular oedema (250×) and (f) microvesicles (50×). Lesions are indicated by arrows.
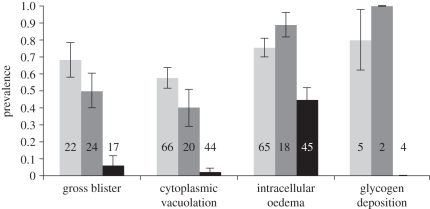
As predicted under the assumption that pigmentation plays a significant photoprotective role [16], the prevalence of blisters and microscopic abnormalities differed between species, being lowest for fin whales, the darkest species (figure 3). Moreover, for each species, melanocyte counts inversely predicted cytoplasmic vacuolation and intracellular oedema (p < 0.02 for all responses; full model details in electronic supplementary material, table S2), providing further evidence of photoprotection in cetaceans. Interestingly, despite having different average melanocyte counts, the prevalence of blisters and microscopic abnormalities was equal for blue and sperm whales (figure 3). This finding might reflect dissimilar sea-surfacing behaviours. Sperm whales spend approximately 7–10 min breathing at the surface between foraging dives, whereas both blue and fin whales tend to surface for less than 2 min at a time [27]. Moreover, although all species remain at the surface while resting, sperm whales also aggregate virtually all the daylight hours at the surface during socialization, in periods of up to 6 h at a time [21]. A fitted GLM showed that the length of sun exposure (i.e. surface time) predicted epidermal lesions, although skin pigmentation remained the most important explanatory factor for all lesions examined (full model details in electronic supplementary material, table S3a).
Figure 3.
Prevalence of gross blisters and microscopic epidermal abnormalities in blue whales (pale grey bars), sperm whales (dark grey bars) and fin whales (black bars). Sample sizes are indicated in the figure. Bars = ±s.e.m.
Another non-exclusive explanation for the higher prevalence of lesions observed in blue whales when compared with fin whales could relate to differences in migration patterns. This is because UVR (and consequently skin cancer radiation dosage) varies across latitudes, being five times higher at lower latitudes when compared with mid-latitudes [18]. Across Mexico, UVR is high during most of the year, and the UV index at clear sky values (a measure of the potential human exposure to UVR) is normally 6 (high) to 15 (extreme) [28]. Thus, blue whales from the northeast pacific population that migrate annually from the feeding areas between Alaska and California [29] to the Gulf of California, where some remain for at least two months (normally arriving in January/February and leaving in April/May) [30], are abruptly exposed to higher UVR. Conversely, fin whales are year-round residents of the Gulf of California [31] and thus constantly exposed to high UVR. If, as occurs in humans, sun-induced damage is most critical when first exposed to higher levels of UVR, it is possible that the observed variations in lesions, melanocytes and apoptotic cells between species reflect differences in migration. Interestingly, blue whales sampled at the beginning of each sampling season had a higher prevalence of microscopic lesions than those sampled at the end of each sampling season (LR = 37.99, d.f. = 1, p = 0.04; see electronic supplementary material, table S4 and figure S2), suggesting that some acclimatization might occur, as happens in humans [32].
Exposure to UVR produces numerous effects on keratinocytes, including the formation of ‘sunburn cells’: keratinocytes showing eosinophilic cytoplasm with or without remnants of shrunken and condensed nuclei [17,19]. These are apoptotic cells resulting from UV-induced DNA damage [17]. Highly pigmented skin is better able to prevent damage and remove potentially precancerous UVR-damaged cells via melanin-mediated apoptosis [15]. Thus, whales with more pigmentation would be expected to have higher epidermal apoptosis rates than less-pigmented whales. We found that sunburn cells were present in nearly all (95%) of the skin sections and in more than half (56%) of all whales; these cells were distributed throughout the epidermis (figure 4), including the basal layer. Such high levels and widespread distribution of apoptotic cells are uncommon in clinically healthy mouse skin, and are associated with acute responses to UVR exposure, which peak between 24 and 48 h [33]. When investigating the association between individual melanocyte counts and apoptosis, a positive relationship was found for all species (full GLM and model details in electronic supplementary material, table S3b), implying that darker pigmentation confers an advantage for the elimination of UVR-induced damage in whales. Geographical variation in pigmentation has been described for southern right whales, Eubalena australis [34], and humpback whales, Megaptera novaeangliae [35], and there is evidence that dorsal skin gradually darkens with age in right whales [34]. To our knowledge, the evolutionary significance of whale skin pigmentation patterns has not been discussed in terms of photoprotection, but it is tempting to speculate, based on our findings, that selection might operate at this level.
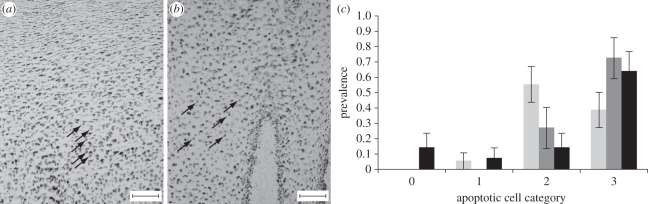
Figure 4.
Presence of apoptotic cells (AC) in skin sections. (a) TUNEL-stained fin whale skin showing AC (round-stained nucleus indicated by arrows) throughout the epidermis (category 3 AC; bar = 100 µm). (b) TUNEL-stained sperm whale skin with category 2 AC (bar = 100 µm). Melanin pigments are seen as black granular material. (c) Prevalence and categories of AC in blue whales (pale grey bars, n = 18), sperm whales (dark grey bars, n = 11) and fin whales (black bars, n = 14). Bars = ±s.e.m.
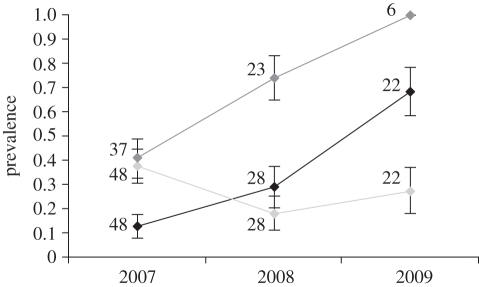
As a consequence of the global reduction in the stratospheric ozone layer, levels of UVR have augmented, leading to higher rates of acute lesions and skin cancer in humans [4]. If whale skin is affected by exposure to growing levels of UVR, a higher prevalence of lesions over time would be expected. When testing this hypothesis for blue whales, the species for which we had data and samples spanning a 3 year period, we found that while bite marks remained unchanged (GLM: LR = 110.33, d.f. = 2, p = 0.18), the prevalence of blisters rose significantly over time (GLM: LR = 90.50, d.f. = 2, p = 5.24 × 10−5; figure 5), being markedly higher in 2009. A similar (but statistically insignificant) trend was observed for cytoplasmic vacuolation (GLM: LR = 5.44, d.f. = 2, p = 0.07; figure 5). Despite the short time frame, our results would suggest that, as predicted, heightened exposure to UVR secondary to global [1–3] and regional [36,37] ozone depletion is leading to more skin damage in whales. It is worth mentioning that we found no evidence that the population is ageing (GLM: LR = 32.16, d.f. = 2, p = 0.31), suggesting that the observed results do not reflect an age-related decrease in repair mechanisms [38]. The obvious question to arise from our results is: if whales are historically adapted to daily UVR exposure, are their photoprotection and damage-repair mechanisms able to respond to increasing average radiation? When addressing this question we found that blue whale epidermal apoptotic cells and melanocytes also expanded in time (GLM: LR = 16.00, d.f. = 2, p = 0.04; ANOVA: F = 4.33, d.f. = 2, p = 0.02), a trend that also occurred in fin whales, the darkest species studied (GLM: LR = 5.00, d.f. = 1, p = 3.6 × 10−3; ANOVA: F = 11.20, d.f. = 2, p = 1.74 × 10−4). If, as occurs in humans and laboratory animals, exposure to UVR increases the number of melanocytes [25], stimulates the synthesis of melanin and leads to augmented apoptosis [17], it is possible that our results indicate quick responses to increasing irradiation. Testing this hypothesis in more depth was beyond the scope of our study, but quantifying the expression of genes involved in melanin production and DNA damage repair might help address this question in the future.
Figure 5.
Temporal changes in the prevalence of blue whale skin lesions (blisters, black line; bite marks, pale grey line; cytoplasmic vacuolation, dark grey line). Sample sizes are indicated in the figure. Bars = ±s.e.m.
Taken together, our results show that whales exhibit lesions typical of acute UVR exposure, suggesting that the thinning ozone layer poses a significant and rising threat to the health of our oceans' whales. Considering that UVR is expected to increase 4 per cent in the tropics and up to 20 per cent in the poles [39], more studies are needed to fully understand the consequences of UVR-induced damage and the evolutionary significance of cetacean pigmentation.
Acknowledgements
Members of the Cetacean Ecology Laboratory (CICIMAR) greatly assisted us during fieldwork. We are grateful to Hal Whitehead for allowing us to participate in his sperm whale research expedition, and to Prof. Cerio for his assistance with PAS/DPAS staining. Robert Brownell, William Amos and Paul Jepson provided valuable comments that greatly improved our manuscript. L.M. is funded by a NERC Studentship (NE/F00818X/1). This work was partially funded by a Royal Society Grant (2005/R2) awarded to K.A. and by the Instituto Politécnico Nacional. Samples were collected under permits SGPA/DGVS/00506/08, SGPA/DGVS/09760/08 and SGPA/DGVS/08021/06 issued by SEMARNAT.
References
- 1.McKenzie R. L., Aucamp P. J., Bais A. F., Bjorn L. O., Ilyas M. 2007. Changes in biologically-active ultraviolet radiation reaching the Earth's surface. Photochem. Photobiol. Sci. 6, 218–231 10.1039/B700017K (doi:10.1039/B700017K) [DOI] [PubMed] [Google Scholar]
- 2.Li F., Stolarski R. S., Newman P. A. 2009. Stratospheric ozone in the post-CFC era. Atmos. Chem. Phys. 9, 2207–2213 10.5194/acp-9-2207-2009 (doi:10.5194/acp-9-2207-2009) [DOI] [Google Scholar]
- 3.Dameris M. 2009. Depletion of the ozone layer in the 21st century. Angew. Chem. Int. Ed. 49, 489–491 10.1002/anie.200906334 (doi:10.1002/anie.200906334) [DOI] [PubMed] [Google Scholar]
- 4.De Gruijl F. R., Longstreth J., Norval M., Cullen A. P., Slaper H., Kripke M. L., Takizawa Y., Van der Leun J. C. 2003. Health effects from stratospheric ozone depletion and interactions with climate change. Photochem. Photobiol. Sci. 2, 16–28 10.1039/b211156j (doi:10.1039/b211156j) [DOI] [PubMed] [Google Scholar]
- 5.Gallagher R. P., Lee T. K. 2006. Adverse effects of ultraviolet radiation: a brief review. Prog. Biophys. Mol. Biol. 92, 119–131 10.1016/j.pbiomolbio.2006.02.011 (doi:10.1016/j.pbiomolbio.2006.02.011) [DOI] [PubMed] [Google Scholar]
- 6.Chang Y. M., et al. 2009. Sun exposure and melanoma risk at different latitudes: a pooled analysis of 5700 cases and 7216 controls. Int. J. Epidemiol. 38, 814–830 10.1093/ije/dyp166 (doi:10.1093/ije/dyp166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karentz D., Bosch I. 2001. Influence of ozone-related increases in ultraviolet radiation on antarctic marine organisms. Am. Zool. 41, 3–16 10.1093/icb/41.1.3 (doi:10.1093/icb/41.1.3) [DOI] [Google Scholar]
- 8.Geraci J. R., St Aubin D. J., Hicks B. D. 1986. Anatomy and physiology. The epidermis of odontocetes: a view from within. In Research on dolphins (eds Bryden M. M., Harrison R.), pp. 3–21 Oxford, UK: Clarendon Press [Google Scholar]
- 9.Acevedo-Whitehouse K., Duffus A. L. J. 2009. Effects of environmental change on wildlife health. Phil. Trans. R. Soc. B 364, 3429–3438 10.1098/rstb.2009.0128 (doi:10.1098/rstb.2009.0128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson B., et al. 1999. Epidermal diseases in bottlenose dolphins: impacts of natural and anthropogenic factors. Proc. R. Soc. Lond. B 266, 1077–1083 10.1098/rspb.1999.0746 (doi:10.1098/rspb.1999.0746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Bressem M. F., et al. 2009. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis. Aquat. Organ. 86, 143–157 10.3354/dao02101 (doi:10.3354/dao02101) [DOI] [PubMed] [Google Scholar]
- 12.Taborda P. R., Taborda V. A., McGinnis M. R. 1999. Lacazia loboi gen. nov., comb. nov., the etiologic agent of lobomycosis. J. Clin. Microbiol. 37, 2031–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Bressem M. F., et al. 2009. Epidemiological pattern of tattoo skin disease: a potential general health indicator for cetaceans. Dis. Aquat. Organ. 85, 225–237 10.3354/dao02080 (doi:10.3354/dao02080) [DOI] [PubMed] [Google Scholar]
- 14.Flach L., et al. 2008. Miscellaneous skin lesions of unknown aetiology in cetaceans from South America. Paper SC/60/DW4 presented to the International Whaling Commission Scientific Meeting, Santiago, Chile [Google Scholar]
- 15.Yamaguchi Y., Beer J., Hearing V. 2008. Melanin mediated apoptosis of epidermal cells damaged by ultraviolet radiation: factors influencing the incidence of skin cancer. Arch. Dermatol. Res. 300, 43–50 10.1007/s00403-007-0807-0 (doi:10.1007/s00403-007-0807-0) [DOI] [PubMed] [Google Scholar]
- 16.Lin J. Y., Fisher D. E. 2007. Melanocyte biology and skin pigmentation. Nature 445, 843–850 10.1038/nature05660 (doi:10.1038/nature05660) [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi S., Zhang W., Wakamatsu K., Ito S., Hearing V. J., Kraemer K. H., Brash D. E. 2004. Melanin acts as a potent UVB photosensitizer to cause an atypical mode of cell death in murine skin. Proc. Natl Acad. Sci. USA 101, 15 076–15 081 10.1073/pnas.0403994101 (doi:10.1073/pnas.0403994101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilyas M. 2007. Climate augmentation of erythemal UV-B radiation dose damage in the tropics and global change. Curr. Sci. 93, 1604–1608 [Google Scholar]
- 19.Nakaseko H., Kobayashi M., Akita Y., Tamada Y., Matsumoto Y. 2003. Histological changes and involvement of apoptosis after photodynamic therapy for actinic keratoses. Bri. J. Dermatol. 148, 122–127 10.1046/j.1365-2133.2003.04898.x (doi:10.1046/j.1365-2133.2003.04898.x) [DOI] [PubMed] [Google Scholar]
- 20.Hammond P. S. 1990. Capturing whales on film: estimating cetacean population parameters from individual recognition data. Mammal Rev. 20, 17–22 10.1111/j.1365-2907.1990.tb00099.x (doi:10.1111/j.1365-2907.1990.tb00099.x) [DOI] [Google Scholar]
- 21.Whitehead H. 2003. Sperm whale behavior and vocalizations. In Sperm whales: social evolution in the ocean, pp. 133–205 Chicago, IL: The University of Chicago Press [Google Scholar]
- 22.Ohkawara A., Halprin K., Levine V. 1972. Glycogen metabolism following ultraviolet irradiation. J. Invest. Dermatol. 59, 264–268 10.1111/1523-1747.ep12627285 (doi:10.1111/1523-1747.ep12627285) [DOI] [PubMed] [Google Scholar]
- 23.Kawagishi N., Hashimoto Y., Takahashi H., Ishida-Yamamoto A., Iizuka H. 1998. Epidermal cell kinetics of pig skin in vivo following UVB irradiation: apoptosis induced by UVB is enhanced in hyperproliferative skin condition. J. Dermatol. Sci. 18, 43–53 10.1016/S0923-1811(98)00024-3 (doi:10.1016/S0923-1811(98)00024-3) [DOI] [PubMed] [Google Scholar]
- 24.Ihaka R., Gentleman R. 1996. R: a language for data analysis and graphics. J. Comput. Graph Stat. 5, 299–314 10.1234/12345678 (doi:10.1234/12345678) [DOI] [Google Scholar]
- 25.Stierner U., Rosdahl I., Augustsson A., Kagedal B. 1989. UVB irradiation induces melanocyte increase in both exposed and shielded human skin. J. Invest. Dermatol. 92, 561–564 10.1111/1523-1747.ep12709572 (doi:10.1111/1523-1747.ep12709572) [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi N., Nakagawa A., Muramatsu T., Yamashina Y., Shirai T., Hashimoto M. W., Ishigaki Y., Ohnishi T., Mori T. 1998. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J. Invest. Dermatol. 110, 806–810 10.1046/j.1523-1747.1998.00178.x (doi:10.1046/j.1523-1747.1998.00178.x) [DOI] [PubMed] [Google Scholar]
- 27.Croll D. A., Acevedo-Gutiérrez A., Tershy B. R., Urbán-Ramírez J. 2001. The diving behavior of blue and fin whales: is dive duration shorter than expected based on oxygen stores? Comp. Biochem. Physiol. 129, 797–809 10.1016/S1095-6433(01)00348-8 (doi:10.1016/S1095-6433(01)00348-8) [DOI] [PubMed] [Google Scholar]
- 28.Lemus-Deschamps L., Galindo I., Solano R., Elizalde A. T., Fonseca J. 2002. Diagnosis of clear sky ultraviolet radiation for Mexico. Atmosfera 15, 165–171 [Google Scholar]
- 29.Calambokidis J., Barlow J., Ford J. K. B., Chandler T. E., Douglas A. B. 2009. Insights into the population structure of blue whales in the eastern North Pacific from recent sightings and photographic identification. Mar. Mammal Sci. 25, 816–832 10.1111/j.1748-7692.2009.00298 (doi:10.1111/j.1748-7692.2009.00298) [DOI] [Google Scholar]
- 30.Gendron D. 2002. Ecología poblacional de la ballena azul, Balaenoptera musculus, de la Península de Baja California. PhD thesis, Centro de Investigación Científica y Educación Superior de Ensenada, Mexico [Google Scholar]
- 31.Bérubé M., Urbán-Ramírez J., Dizon A. E., Brownell R. L., Palsbøll P. J. 2002. Genetic identification of a small and highly isolated population of fin whales (Balaenoptera physalus) in the Sea of Cortez, Mexico. Conserv. Genet. 3, 183–190 10.1234/12345678 (doi:10.1234/12345678) [DOI] [Google Scholar]
- 32.Sayre R. M., Desrochers D. L., Wilson C. J., Marlowe E. 1981. Skin type, minimal erythema dose (MED), and sunlight acclimatization. J. Am. Acad. Dermatol. 5, 439–443 10.1016/S0190-9622(81)70106-3 (doi:10.1016/S0190-9622(81)70106-3) [DOI] [PubMed] [Google Scholar]
- 33.De la Coba F., Aguilera J., De Galvez M. V., Alvarez M., Gallego E., Figueroa F. L., Herrera E. 2009. Prevention of the ultraviolet effects on clinical and histopathological changes, as well as the heat shock protein-70 expression in mouse skin by topical application of algal UV-absorbing compounds. J. Dermatol. Sci. 55, 161–169 10.1016/j.jdermsci.2009.06.004 (doi:10.1016/j.jdermsci.2009.06.004) [DOI] [PubMed] [Google Scholar]
- 34.Schaeff C. M., Best P. B., Rowntree V. J., Payne R., Jarvis C., Portway V. A. 1999. Dorsal skin color patterns among southern right whales (Eubalaena australis): genetic basis and evolutionary significance. J. Hered. 90, 464–471 10.1093/jhered/90.4.464 (doi:10.1093/jhered/90.4.464) [DOI] [PubMed] [Google Scholar]
- 35.Rosenbaum H. C., et al. 1995. Geographic variation in ventral fluke pigmentation of humpback whale (Megaptera novaeangliae) populations worldwide. Mar. Ecol. Prog. Ser. 124, 1–7 10.3354/meps124001 (doi:10.3354/meps124001) [DOI] [Google Scholar]
- 36.Kane R. P. 2009. Ozone depletion, worst not yet over. Indian J. Radio Space Phys. 38, 313–316 [Google Scholar]
- 37.Feng W., Chipperfield M. P., Davies S., von der Gathen P., Kyrö E., Volk C. M., Ulanovsky A., Belyaev G. 2007. Large chemical ozone loss in 2004/2005 Arctic winter/spring. Geophys. Res. Lett. 34, L09803. 10.1029/2006GL029098 (doi:10.1029/2006GL029098) [DOI] [Google Scholar]
- 38.Matts P. J., Fink B. 2010. Chronic sun damage and the perception of age, health and attractiveness. Photochem. Photobiol. Sci. 9, 421–431 10.1039/b9pp00166b (doi:10.1039/b9pp00166b) [DOI] [PubMed] [Google Scholar]
- 39.Hegglin M. I., Shepherd T. G. 2009. Large climate-induced changes in ultraviolet index and stratosphere-to-troposphere ozone flux. Nat. Geosci. 2, 687–691 10.1038/ngeo604 (doi:10.1038/ngeo604) [DOI] [Google Scholar]