Abstract
Fossils of a marsupial mole (Marsupialia, Notoryctemorphia, Notoryctidae) are described from early Miocene deposits in the Riversleigh World Heritage Area, northwestern Queensland, Australia. These represent the first unequivocal fossil record of the order Notoryctemorphia, the two living species of which are among the world's most specialized and bizarre mammals, but which are also convergent on certain fossorial placental mammals (most notably chrysochlorid golden moles). The fossil remains are genuinely ‘transitional', documenting an intermediate stage in the acquisition of a number of specializations and showing that one of these—the dental morphology known as zalambdodonty—was acquired via a different evolutionary pathway than in placentals. They, thus, document a clear case of evolutionary convergence (rather than parallelism) between only distantly related and geographically isolated mammalian lineages—marsupial moles on the island continent of Australia and placental moles on most other, at least intermittently connected continents. In contrast to earlier presumptions about a relationship between the highly specialized body form of the blind, earless, burrowing marsupial moles and desert habitats, it is now clear that archaic burrowing marsupial moles were adapted to and probably originated in wet forest palaeoenvironments, preadapting them to movement through drier soils in the xeric environments of Australia that developed during the Neogene.
Keywords: Australian marsupial moles, Riversleigh Miocene fossil, evolutionary convergence, zalambdodonty, fossorial adaptations, rainforest palaeohabitat
1. Introduction
Notoryctemorphia, the marsupial moles, is the least diverse but most extraordinarily distinct of the four orders of living Australian marsupials. It currently comprises one family (Notoryctidae), one genus (Notoryctes) and two species (Notoryctes typhlops, Notoryctes caurinus). Until the discovery of the extinct taxon described here, no fossil notoryctemorphians were known [1]. In part because of this lack of a fossil record, the origins and relationships of the group have long been the subject of speculation and debate, with some authors even questioning their marsupial status [2–4]. Most recent studies have supported a close relationship between Notoryctemorphia and Dasyuromorphia (Australian carnivorous marsupials [5]), Peramelemorphia (bandicoots and bilbies [6,7]) or both [8–13].
Among living marsupials, Notoryctes is unique in exhibiting a distinctive molar morphology termed zalambdodonty [14,15]. Zalambdodonty is characterized by upper molars having a single, central cusp homologous with either the paracone or the metacone [15,16]. Two crests extend buccally from this cusp, resulting in a v-shaped crown (figure 1). In addition, the protocones (upper molars) and talonids (lower) are usually reduced or lost. Besides Notoryctes, several living and fossil therian groups have zalambdodont molars (figure 2); these include extant solenodontids, chrysochlorids and tenrecids (all placentals) and fossil apternodontids (which also appear to be placentals [17]), Yalkaparidon (probable australidelphian marsupial [11,18]) and Necrolestes (probable metatherian [19,20]). Without a fossil record to reveal intermediate conditions, there has been uncertainty about which of the upper molar cusps Notoryctes has lost in comparison to other zalambdodont taxa. The question is interesting in terms of evolutionary process: do mammals evolving zalambdodonty (or any other comparably specialized dental morphology) become increasingly ‘channelled’ by an underlying morphological/genetic developmental constraint as they move down this pathway, or can these highly specialized patterns be achieved in completely different ways? That is, do they arise by parallelism (independent acquisition via the same evolutionary pathway) or convergence (independent acquisition via a different pathway)? While most placental mammals have achieved zalambdodonty via hypertrophy of the paracone and suppression of the metacone [15–17], until now there has been no hard (fossil) evidence for how this pattern has been achieved in marsupials.
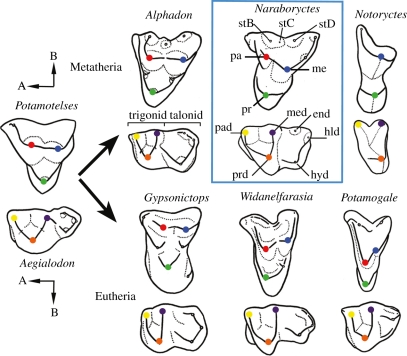
Figure 1.
Cusp evolution from tribosphenic to zalambdodont patterns in representative metatherians (top row) and eutherians (bottom row), and dental terminology used in text. Naraboryctes philcreaseri n. gen. and sp. indicates that in zalambdodont metatherians the paracone (red) is suppressed; in eutherians it is the metacone (blue) that is reduced. Upper and lower molars redrawn from: [63] (Gypsonictops); [64] (Potamotelses, Aegialodon, Alphadon); [65] (Widanelfarasia lower); [66] (Potamogale); [67] (Widanelfarasia upper). Abbreviations: A, anterior; B, buccal; end, entoconid; hld, hypoconulid; hyd, hypoconid; me, metacone; med, metaconid; pa, paracone; pad, paraconid; pr, protocone; prd, protoconid; stB, stylar cusp B; stC, stylar cusp C; stD, stylar cusp D.
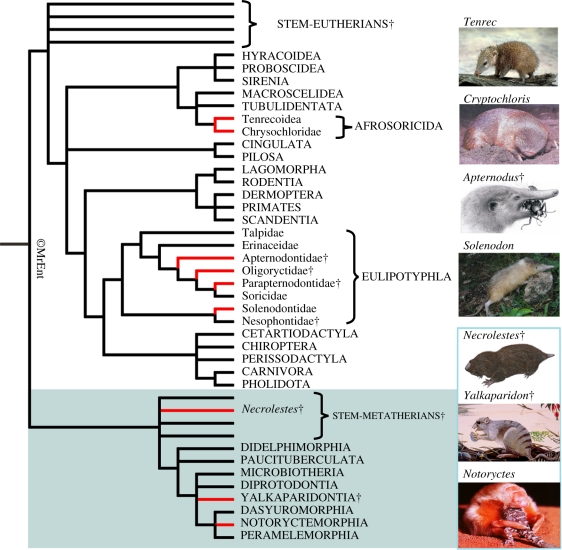
Figure 2.
Composite phylogeny of therian mammals illustrating the occurrence of dental zalambdodonty within Theria. The topology is based on recent studies including [11–13,15,19,20,68–72]. Taxa that include at least one fully zalambdodont member are indicated by red branches. Extinct taxa are indicated by a dagger. Images (from top to bottom) are: the tenrecoid Tenrec ecaudatus (J. F. Eisenberg; AMS Image Library); an unidentified chrysochlorid Cryptochloris sp. (Wikimedia Commons image; Killer18); the apternodontid Apternodus baladontus (frontispiece [17]: p. 2); the solenodontid Solenodon paradoxus (J. Nuñez-Miño; www.thelastsurvivors.org); the necrolestid Necrolestes patagonensis (N. P. Archer); the yalkaparidontian Yalkaparidon coheni (D. Dunphy); the notoryctid Notoryctes typhlops (M. Gillam). The phylogeny was created using the phylogenetic drawing tool MrEnt 2.0 [73].
Modern notoryctids are confined to deserts in Australia and exhibit numerous anatomical specializations that appear exceptionally well suited to burrowing through desert sands. These include a conical skull, extreme modifications of the axial and appendicular skeleton (in particular, enormous enlargement of the muscle attachment sites on the fore- and hindlimbs) and soft tissue features such as a ‘nasal shield’, a lack of eyes and external ears, and a tubular body shape [21]. As a result, it has long been assumed that notoryctids evolved these specializations in a desert environment. This led to speculation (e.g. [22]) that a desert palaeoenvironment must have existed somewhere in Australia throughout much of the Cenozoic to allow for the evolution of the highly modified, autapomorphic notoryctid body form, despite no direct evidence for sandy deserts in Australia prior to the Pleistocene [23,24]. Notoryctids have been used as text-book examples of convergence between themselves and the phylogenetically unrelated but morphologically very similar placental golden moles (chrysochlorids; e.g. [25,26]). As well as zalambdodont molars, Notoryctes and chrysochlorids share similar fossorial specializations of the skeleton [19,26] and closely resemble each other in terms of external appearance (figure 2). Like Notoryctes, some (but not all) chrysochlorids occupy sandy desert environments [27]. This fact contributed to earlier presumptions (e.g. [22]) that the similar overall morphology of both groups is the result of long-term adaptation to desert environments. Until now it has not been possible to test this presumption on the basis of palaeoenvironmental data.
Higher level systematic nomenclature used in this paper follows [28]. Dental terminology follows [18,29] for crown morphology (figure 1) and [30] for molar number. Case denotes upper (e.g. M2) and lower (e.g. m2) teeth.
2. Systematic palaeontology
Mammalia Linnaeus, 1758
Marsupialia Illiger, 1811
Notoryctemorphia Aplin & Archer, 1987
Notoryctidae Ogilby, 1892
Naraboryctes philcreaseri new genus and species.
(a). Etymology
From naraba ‘to drink’ (Garrawa and Waanyi languages of northwestern Queensland; [31]), in reference to its rainforest palaeohabitat, and oryctes meaning ‘digger’ (Greek), in reference to its fossorial specializations and close relationship to Notoryctes. The species name honours Phil Creaser for many contributions to Riversleigh and other palaeontological research at the University of New South Wales including establishment of the Coalition for Research into Australian Terrestrial Ecosystems (CREATE) Fund.
(b). Holotype
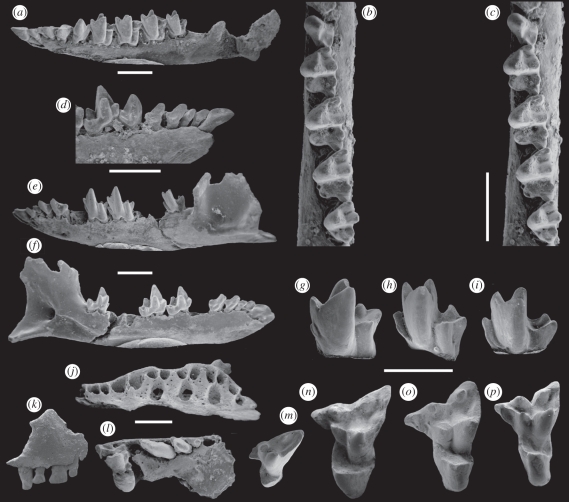
QM F23717 (Queensland Museum) from Upper Site, left dentary with i1-3 (i1 root only) c1 p1-3 m1-4, missing mandibular condyle and medially inflected portion of angular process (figure 3a–d).
Figure 3.
Naraboryctes philcreaseri new genus and species Riversleigh World Heritage Area, northwestern Queensland, Australia; early Miocene. (a–d) QM F23717, holotype, left dentary with i2-m4; (a) buccal view; (b,c) stereopair occlusal view; (d) lingual view of anterior dentition. (e,f) QM F23719, left dentary with i1-p2 m1-2 m4; (e) buccal view; (f) lingual view. (g) QM F23718, left m1. (h) QM F51329, left m3. (i) QM F51330, right m4 (reversed image). (j) QM F54502, left edentulous maxilla. (k) QM F51322, paratype, left premaxilla with I1-4. (l) QM F23716, paratype, partial right maxilla with P1-3 M1. (m) QM F51323, right dP3 (reversed image). (n) QM F51324, left M1. (o) QM F51325, right M2 (reversed image). (p) QM F51327, left M3 (scale bar, 2 mm).
(c). Paratypes and referred specimens
Paratypes that are also topotypes: QM F51322, partial left premaxilla with I1-4 (figure 3k); QM F23716, partial right maxilla with P1-3 M1 (figure 3l). Referred specimens: from Upper Site, QM F54502 (edentulous maxilla; figure 3j); QM F51323 (dP3; figure 3m), QM F51324 (LM1; figure 3n), QM F51325 (RM2; figure 3o), QM F51327 (LM3; figure 3p), QM F23718 (Lm1; figure 3g), QM F51329 (Lm3; figure 3h), QM F51330 (Rm4; figure 3i), QM F54559 (left humerus; figure 4b,e), QM F54560 (left ulna; figure 4h,k); from Wayne's Wok Site, QM F23719 (left dentary; figure 3e–f), QM F51328 (left dentary).
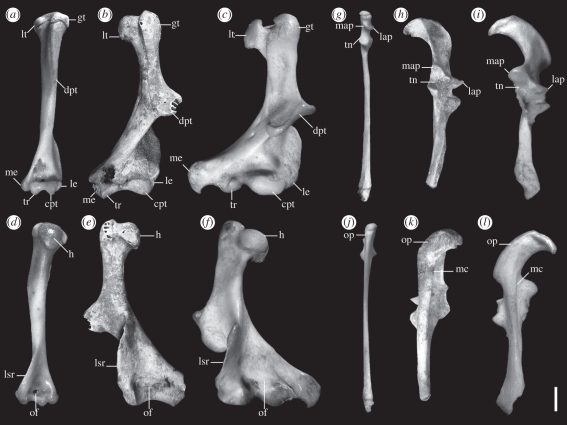
Figure 4.
Comparison of humeri and ulnae of Naraboryctes philcreaseri gen. et sp. nov., Notoryctes typhlops (SAM637) and Antechinus stuartii (UNSWZ465). (a–c) left humerus, anterior view; (a) A. stuartii; (b) N. philcreaseri (QM F54559); (c) N. typhlops. (d–f) left humerus, posterior view; (d) A. stuartii; (e) N. philcreaseri (QM F54559); (f) N. typhlops. (g–i) left ulna, anterior view; (g) A. stuartii; (h) N. philcreaseri (QM F54560); (i) N. typhlops. (j–l) left ulna, posterior view; (j) A. stuartii; (k) N. philcreaseri (QM F54560); (l) N. typhlops (scale bar, 2 mm). Mirror image of right humerus and ulna of A. stuartii and N. typhlops used for comparison. Abbreviations: cpt, capitulum; dpt, delto-pectoral tuberosity; gt, greater tuberosity; h, head; lap, lateral anconeal process; le, lateral epicondyle; lsr, lateral supracondylar ridge; lt, lesser tuberosity; map, medial anconeal process; mc, medial crest; me, medial epicondyle; of, olecranon fossa; op, olecranon process; tn, trochlear notch; tr, trochlea.
(d). Locality and horizon
The Type Locality is Upper Site, Godthelp Hill, Riversleigh World Heritage Area, Boodjamulla National Park, northwestern Queensland (19°0′42″ S, 138°40′24″ E). Wayne's Wok Site is on Hal's Hill and 250 m from Upper Site. Both have been interpreted as Faunal Zone B sites [32–35] and as such early Miocene in age based on biocorrelation and preliminary radiometric dating (U/Pb) of encasing limestone. All Riversleigh specimens have been recovered by acetic acid-processing of limestone.
(e). Diagnosis and description
The only known species of Naraboryctes differs from Notoryctes spp. in the following combination of features: retention of small paracone; postmetacrista much longer than premetacrista; medial cusp on postmetacrista variably present; well-developed three-cusped talonid which is slightly smaller than trigonid on m2-4; anterior cingulid present; complete adult dental formula I1-5/1-3, C1/1, P1-3/1-3, M1-4/1-4; proportionately larger coronoid process of dentary; medial epicondyle of humerus less enlarged; capitulum and trochlea continuous; supracondyloid foramen present; elongated olecranon process of ulna less hooked; flat (rather than concave) medial anconeal process of ulna; anconeal sides of capitular notch and trochlear notch of ulna continuous (rather than separated by a ridge).
Rediagnosis of family Notoryctidae: small-bodied (<100 g) marsupials; complete adult dental formula I1-5/1-3, C1/1, P1-3/1-3, M1-4/1-4; upper molars sub- or fully zalambdodont with paracone much smaller than metacone or missing altogether but with large protocone; lower molars with reduced or absent talonids; postcranium with fossorial adaptations. A detailed description of the dentition, cranial and postcranial elements of this new taxon is provided in the electronic supplementary material, appendix S1.
3. Palaeoenvironment
Previous authors [33,36–40] have concluded, on the basis of taxic representation, community structure, species morphology and geology that the early Miocene palaeocommunities of Riversleigh represent closed forest environments. Reasons include representation in these assemblages of taxa all living species of which exist only in rainforests (e.g. species of Menura, Orthonyx, Hypsiprymnodon, dactylopsiline petaurids and phalangerin phalangerids; e.g. [41–43]). Among chiropterans, the very diverse and abundant hipposiderids as well as mystacinids present at Riversleigh during the early Miocene are typical of modern closed forest environments [44,45]. Riversleigh frog assemblages of this age resemble those that today occupy permanently wet rather than dry or even seasonally dry forest environments (M. Tyler 2009, personal communication). Marsupial and bat diversity in Riversleigh assemblages is by modern Australian standards extraordinarily high. Diversity of these groups in Australasia today is highest in rainforest environments such as those in mid-montane New Guinea, where complex guilds based on, for example, size and feeding strategies enable many species to coexist.
4. Discussion
We refer Naraboryctes philcreaseri to Notoryctemorphia because of the extreme reduction of the paracone, anteroposterior compression of its upper molars and reduction of the talonid on its lower molars. Collectively, these apomorphies represent an incipiently zalambdodont morphology that anticipates the fully zalambdodont condition in species of Notoryctes. This familial attribution is further supported by cranial and isolated postcranial specimens referable to N. philcreaseri in addition to the premaxilla, humerus and ulna noted above [7,46]. These exhibit numerous adaptations for fossoriality very similar to but less well developed than those seen in species of Notoryctes [7,46]. The humerus (figure 4) exhibits enlargement of many sites for muscle attachment, in particular expansion of the medial epicondyle for enlarged flexor muscles that facilitate strong flexion in the wrist during digging by fossorial mammals. As in both species of Notoryctes, the delto-pectoral ridge is also markedly displaced distally, resulting in increased mechanical advantage of the musculature, and the enlarged lateral supracondylar ridge provides for increased musculature in the lower arm. Massively expanded articular surfaces help strengthen the joints during digging by spreading high mechanical forces over a greater surface area [7]. The hypertrophied olecranon process of the ulna, which enables powerful extension of the forelimb during digging, while decidedly hypertrophied and notoryctid-like in N. philcreaseri (figure 4) and hence a synapomorphy for this family, is not quite as large nor as strongly curved medially as it is in species of Notoryctes. Hypertrophy of the olecranon process is present in many fossorial mammals [7,19,26] but is unique to notoryctids within Marsupialia. Preliminary phylogenetic analyses by one of us (R.M.B.D.) indicate that Naraboryctes and Notoryctes form a clade.
Naraboryctes philcreaseri is dentally more plesiomorphic than either species of Notoryctes, notably in its apparent retention of five upper incisors, three upper and lower premolars, distinct paracone on the upper molars and three-cusped talonid on the lower molars. As noted above, the dental formula of N. philcreaseri appears to be I1-5 (or possibly I1-4; see the electronic supplementary material)/1-3; C1/1; P1-3/1-3; M1-4/1-4. This is the same as the plesiomorphic dental formula for Australian marsupials and, except for the loss of one lower incisor, the same as the most plesiomorphic dental formula known for any marsupial. All known Australian marsupials have lost i4 and all except some peramelemorphians and probably N. philcreaseri have lost I5. The dental formula for species of Notoryctes is controversial because of considerable polymorphism in tooth number, both between specimens and within the same specimen. While Archer [22] stated that the maximum dental formula of N. typhlops is I1-4/1-3 C1/1 P1-3/1-3 M1-4/1-4, loss of an upper incisor and at least one upper and one lower premolar is not uncommon. Thomas [47] gave the dental formula of N. caurinus as I1-3/1-2; C1/1; P1-2/1-2; M1-4/1-4. The tiny size of P1 in N. philcreaseri compared with P2-3 suggests that it is P1 rather than P3 that is lost in N. caurinus and some specimens of N. typhlops. An apparent autapomorphy of N. philcreaseri is the presence of a cuspule near the middle of the postmetacrista, although it is possible that this cuspule has been secondarily lost in species of Notoryctes.
The dental material of N. philcreaseri unequivocally demonstrates that zalambdodonty evolved in notoryctemorphians from a tribosphenic precursor by loss of the paracone (figure 1). Asher et al. [19] recently hypothesized that zalambdodonty in the probable metatherian Necrolestes was also acquired by suppression of the paracone. This hypothesis was based on occlusal relations in a fully zalambdodont taxon rather than discovery of an annectent taxon of the kind we describe here. Conversely, although the molars of the Eocene ?metatherian Kiruwamaq chisu [48] and some living dasyurids have greatly reduced paracones [49], they are not fully zalambdodont. The fossils of N. philcreaseri described here are therefore, to our knowledge, the first direct evidence that full zalambdodonty can be achieved by loss of the paracone rather than the metacone (the cusp lost by zalambdodont placentals). This demonstrates that highly specialized morphological patterns, such as zalambdodonty, can be achieved in very different ways in different mammalian clades and can result from convergent rather than parallel evolution (figure 1).
It is not clear exactly why notoryctemorphians evolved zalambdodonty by suppressing the paracone, whereas zalambdodont placentals (e.g. apternodontids, solenodontids, chrysochlorids, tenrecids) have suppressed the metacone. However, perhaps the simplest explanation is that the metacone is typically larger than the paracone in marsupials and other metatherians, whereas the reverse is usually true for placentals and other eutherians [50], and it is the smaller of the two cusps that is lost in zalambdodont forms. Within placentals, bats are unusual in that the metacone is usually larger than the paracone; it is therefore noteworthy that the bat Harpiocephalus has evolved an incipiently zalambdodont morphology by reduction of the paracone rather than the metacone [15].
The functional significance of zalambdodonty and hence the probable feeding ecology of N. philcreaseri remain unclear. However, based on the mechanical properties of food items (see [51]), Beck [52] suggested that zalambdodonty may represent a specialization for feeding on soft-bodied invertebrates such as worms or insect larvae. A tribosphenic dentition with a fully functional protocone–talonid complex, by contrast, may be better adapted for feeding on harder food items including adult insects. The natural diet of both species of Notoryctes is poorly known [53], although captive specimens appeared to prefer larvae over adult insects [54]. Within placentals, the tenrecid Hemicentetes, which shows extreme zalambdodonty ([55], fig. 41D), feeds almost exclusively on earthworms [56] whereas Potamogale, which feeds predominantly on hard-shelled crustaceans [27], retains a small metacone as well as a large protocone and relatively well-developed talonids ([15,55], fig. 41A). The presence of a small paracone and a functional talonid in N. philcreaseri may therefore indicate that its diet included a greater proportion of harder food items (such as hard-bodied invertebrates) than those of species of Notoryctes. Insect larvae and worms are common below ground; if zalambdodonty is indeed an adaptation for feeding on soft-bodied invertebrates, this may explain why many zalambdodont mammals are fossorial (species of Notoryctes, Necrolestes, chrysochlorids and the tenrecid Orizoryctes) or semi-fossorial (species of Solenodon, Hemicentetes and possibly apternodontids [57]). The coronoid process of the mandible of N. philcreaseri is higher than that of either species of Notoryctes, which suggests the presence of more powerfully developed temporalis musculature. The coronoid process is very low in the vermivorous species of Hemicentetes compared with tenrecids which feed on harder-bodied invertebrates [58]. In bats the coronoid process is smaller in those species that feed on softer-bodied prey such as moths (e.g. [59,60]). The morphology of the coronoid process may therefore be further evidence that N. philcreaseri fed on a wider range of food items including some harder prey than do species of Notoryctes.
Collectively, the dentition and mandible of N. philcreaseri appear to be less-specialized for feeding on soft-bodied invertebrates and hence they may have spent relatively less time feeding underground. However, because powerful digging in this species, as indicated by the hypertrophied, notoryctid-like olecranon process, is combined with incipient zalambdodonty, burrowing in notoryctids probably preceded evolution of fully developed zalambdodonty as well as a concomitant shift in preference for softer subterranean foods.
Recent dated molecular phylogenies suggest that Notoryctemorphia originated in either the Palaeocene or Eocene (e.g. [9,11,13]) but these studies do not shed any light on when the morphological specializations seen in extant notoryctids evolved. The specimens of N. philcreaseri described here demonstrate that notoryctids were incipiently zalambdodont and at least semi-fossorial by the early Miocene. The early Miocene Riversleigh Faunal Zone B faunal assemblages containing N. philcreaseri appear to represent rainforest biotas for reasons discussed above. This led some of us (e.g. [1,33,61]) to suggest, in contrast to our earlier presumption (e.g. [22]), that notoryctids, despite being confined today to Australia's sandy deserts [53], may actually have evolved burrowing adaptations in soft rainforest floors. We suggest that as a consequence, burrowing notoryctids were serendipitously preadapted in terms of strategies for avoiding physiological stresses, to the drier environments that developed in central Australia from the late Miocene onwards as rainforests gradually retreated to coastal margins and aridity gripped Australia's heart with the first sandy deserts developing around 1 million years ago [23,24,62].
Acknowledgements
The Riversleigh Fossil Project is supported by the Australian Research Council (LP0989969, LP100200486, DP1094569), Xstrata Copper Community Partnership Program North Queensland, Outback at Isa, Mount Isa City Council, Queensland Museum, University of New South Wales, Environment Australia, Queensland Department of Environment and Heritage, and Phil Creaser and the CREATE fund at UNSW. Additional financial support for R.B. was provided by NSF grant DEB-0743039 (in collaboration with R. Voss at the American Museum of Natural History). We thank K. Aplin and A. Gillespie for discussion, technical advice and photography; K. Kemper and L. Gibson kindly allowed access to comparative specimens in, respectively, the South Australian Museum, Adelaide and Australian Museum, Sydney. We thank the American Museum of Natural History for permission to reproduce the reconstruction of Apternodus baladontus shown in figure 2, M. Gillam and S. Tahourdin, Auscape International for the photograph of Notoryctes typhlops and J. Nuñez-Miño (www.thelastsurvivors.org) for the photograph of Solenodon paradoxus.
References
- 1.Long J. A., Archer M., Flannery T. F., Hand S. J. 2002. Prehistoric mammals of Australia and New Guinea: one hundred million years of evolution. Sydney, Australia: UNSW Press [Google Scholar]
- 2.Stirling E. C. 1888. Preliminary notes on a new Australian mammal. Trans. R. Soc. South Aust. 11, 21–24 [Google Scholar]
- 3.Cope E. D. 1892. On the habits and affinities of the new Australian mammal, Notoryctes typhlops. Am. Nat. 26, pp. 121–128 [Google Scholar]
- 4.Turnbull W. D. 1971. The Trinity therians: their bearing on evolution in marsupials and other therians. In Dental morphology and evolution (ed. Dahlberg A. A.), pp. 151–179 Chicago, IL: University of Chicago Press [Google Scholar]
- 5.Asher R. J., Horovitz I., Sánchez-Villagra M. R. 2004. First combined cladistic analysis of marsupial mammal interrelationships. Mol. Phylogenet. Evol. 33, 240–250 10.1016/j.ympev.2004.05.004 (doi:10.1016/j.ympev.2004.05.004) [DOI] [PubMed] [Google Scholar]
- 6.Horovitz I., Sánchez-Villagra M. R. 2003. A morphological analysis of marsupial mammal higher-level phylogenetic relationships. Cladistics 19, 181–212 10.1111/j.1096-0031.2003.tb00363.x (doi:10.1111/j.1096-0031.2003.tb00363.x) [DOI] [Google Scholar]
- 7.Warburton N. M. 2003. Functional morphology and evolution of marsupial moles (Marsupialia; Notoryctemorphia). Unpublished PhD thesis. School of Animal Biology, University of Western Australia [Google Scholar]
- 8.Amrine-Madsen H., Scally M., Westerman M., Stanhope M. J., Krajewski C. W., Springer M. S. 2003. Nuclear gene sequences provide evidence for the monophyly of australidelphian marsupials. Mol. Phylogenet. Evol. 28, 186–196 10.1016/S1055-7903(03)00122-2 (doi:10.1016/S1055-7903(03)00122-2) [DOI] [PubMed] [Google Scholar]
- 9.Nilsson M. A., Arnason U., Spencer P. B. S., Janke A. 2004. Marsupial relationships and a timeline for marsupial radiation in South Gondwana. Gene 340, 189–196 10.1016/j.gene.2004.07.040 (doi:10.1016/j.gene.2004.07.040) [DOI] [PubMed] [Google Scholar]
- 10.Phillips M. J., McLenachan P. A., Down C., Gibb G. C., Penny D. 2006. Combined mitochondrial and nuclear DNA sequences resolve the interrelations of the major Australasian marsupial radiations. Syst. Biol. 55, 122–137 10.1080/10635150500481614 (doi:10.1080/10635150500481614) [DOI] [PubMed] [Google Scholar]
- 11.Beck R. M. D. 2008. A dated phylogeny of marsupials using a molecular supermatrix and multiple fossil constraints. J. Mamm. 89, 175–189 10.1644/06-MAMM-A-437.1 (doi:10.1644/06-MAMM-A-437.1) [DOI] [Google Scholar]
- 12.Beck R. M. D., Godthelp H., Weisbecker V., Archer M., Hand S. J. 2008. Australia's oldest marsupial fossils and their biogeographical implications. PLoS ONE 3, e1858. 10.1371/journal.pone.0001858 (doi:10.1371/journal.pone.0001858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meredith R. W., Westerman M., Case J. A., Springer M. S. 2008. A phylogeny and timescale for marsupial evolution based on sequences for five nuclear genes. J. Mamm. Evol. 15, 1–36 10.1007/s10914-007-9062-6 (doi:10.1007/s10914-007-9062-6) [DOI] [Google Scholar]
- 14.Gill T. 1883. On the classification of the insectivorous mammals. Bull. Phil. Soc. Wash. 5, 118–120 [Google Scholar]
- 15.Asher R. J., Sánchez-Villagra M. R. 2005. Locking yourself out: diversity among dentally zalambdodont therian mammals. J. Mamm. Evol. 12, 265–282 10.1007/s10914-005-5725-3 (doi:10.1007/s10914-005-5725-3) [DOI] [Google Scholar]
- 16.Seiffert E. R., Simons E. L., Ryan T. M., Bown T. M., Attia Y. 2007. New remains of Eocene and Oligocene Afrosoricida (Afrotheria) from Egypt, with implications for the origin(s) of afrosoricid zalambdodonty. J. Vert. Pal. 27, 963–972 10.1671/0272-4634(2007)27[963:NROEAO]2.0.CO;2 (doi:10.1671/0272-4634(2007)27[963:NROEAO]2.0.CO;2) [DOI] [Google Scholar]
- 17.Asher R. J., McKenna M. C., Emry R. J., Tabrum A. R., Kron D. G. 2002. Morphology and relationships of Apternodus and other extinct, zalambdodont, placental mammals. Bull. Am. Mus. Nat. Hist. 273, 1–117 (doi:10.1206/0003-0090(2002)273<0001:MAROAA>2.0.CO;2) [DOI] [Google Scholar]
- 18.Archer M., Hand S. J., Godthelp H. 1988. A new order of Tertiary zalambdodont marsupials. Science 239, 1528–1531 10.1126/science.239.4847.1528 (doi:10.1126/science.239.4847.1528) [DOI] [PubMed] [Google Scholar]
- 19.Asher R. J., Horovitz I., Martin T., Sánchez-Villagra M. R. 2007. Neither a rodent nor a platypus: a reexamination of Necrolestes patagonensis Ameghino. Am. Mus. Nov. 3546, 1–40 10.1206/0003-0082(2007)3546[1:NARNAP]2.0.CO;2 (doi:10.1206/0003-0082(2007)3546[1:NARNAP]2.0.CO;2) [DOI] [Google Scholar]
- 20.Ladevèze S., Asher R. J., Sánchez-Villagra M. R. 2008. Petrosal anatomy in the fossil mammal Necrolestes: evidence for metatherian affinities and comparisons with the extant marsupial mole. J. Anat. 213, 686–697 10.1111/j.1469-7580.2008.00985.x (doi:10.1111/j.1469-7580.2008.00985.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warburton N. M. 2006. Functional morphology of marsupial moles (Marsupialia; Notoryctidae). Verh. Naturwiss. Ver. Hamburg 42, 39–149 [Google Scholar]
- 22.Archer M. 1984. The Australian marsupial radiation. In Vertebrate zoogeography and evolution in Australasia (eds Archer M., Clayton G.), pp. 633–808 Perth, Australia: Hesperian Press [Google Scholar]
- 23.Rhodes E., Chappell J., Fujioka T., Fitzsimmons K., Magee J., Aubert M., Hewitt D. 2005. The history of aridity in Australia: chronological developments. In Regolith 2005—ten years of CRC LEME (ed. Roach I.), pp. 265–268 Canberra, Australia: CRC LEME [Google Scholar]
- 24.Martin H. A. 2006. Cenozoic climatic change and the development of the arid vegetation in Australia. J. Arid Environ. 66, 533–563 10.1016/j.jaridenv.2006.01.009 (doi:10.1016/j.jaridenv.2006.01.009) [DOI] [Google Scholar]
- 25.Nevo E. 1999. Mosaic evolution of subterranean mammals: regression, progression and global convergence. Oxford, UK: Oxford University Press [Google Scholar]
- 26.Rose K. D., Emry R. J. 1983. Extraordinary fossorial adaptations in the Oligocene palaeanodonts Epoicotherium and Xenocranium (Mammalia). J. Morph. 175, 33–56 10.1002/jmor.1051750105 (doi:10.1002/jmor.1051750105) [DOI] [PubMed] [Google Scholar]
- 27.Nowak R. M. 1999. Walker's mammals of the world. Baltimore, MD: John Hopkins University Press [Google Scholar]
- 28.Aplin K., Archer M. 1987. Recent advances in marsupial systematics with a new syncretic classification. In Possums and opossums: studies in evolution, vol. 1 (ed. Archer M.), pp. xv–lxxii Sydney, Australia: Surrey Beatty and Sons and the Royal Zoological Society of New South Wales [Google Scholar]
- 29.Archer M. 1976. The dasyurid dentition and its relationships to that of didelphids, thylacinids, borhyaenids (Marsupicarnivora) and peramelids (Peramelina: Marsupialia). Aust. J. Zool., Suppl. Ser. S39, 1–34 [Google Scholar]
- 30.Luckett W. P. 1993. An ontogenetic assessment of dental homologies in therian mammals. In Mammal phylogeny, volume 1: Mesozoic differentiation, multituberculates, monotremes, early therians, and marsupials (eds Szalay F. S., Novacek M. J., McKenna M. C.), pp. 182–204 New York, NY: Springer [Google Scholar]
- 31.Breen G. 1985. Waanyi dialect. Darwin, Australia: Darwin Community College [Google Scholar]
- 32.Archer M., et al. 2006. Current status of species-level representation in faunas from selected fossil localities in the Riversleigh World Heritage Area, northwestern Queensland. Alcheringa (Special Issue 1: Proceedings of CAVEPS 2005), 1–17 [Google Scholar]
- 33.Archer M., Hand S. J., Godthelp H. 1994. Riversleigh: the story of animals in ancient rainforests of inland Australia. Sydney, Australia: Reed Books [Google Scholar]
- 34.Archer M., Hand S. J., Godthelp H., Creaser P. 1997. Correlation of the Cainozoic sediments of the Riversleigh World Heritage fossil property, Queensland, Australia. In Actes du Congrès BiochroM'97 (eds Aguilar J.-P., Legendre S., Michauz J.), pp. 131–152 Montpellier, France: École Pratique des Hautes Études, Institut de Montpellier [Google Scholar]
- 35.Travouillon K. J., Archer M., Hand S. J., Godthelp H. 2006. Multivariate analyses of Cenozoic mammalian faunas from Riversleigh, northwestern Queensland. Alcheringa (Special Issue 1: Proceedings of CAVEPS 2005), 323–349 [Google Scholar]
- 36.Archer M., Hand S. J., Godhelp H. 1994. Patterns in the history of Australia's mammals and inferences about palaeohabitats. In History of the Australian vegetation: Cretaceous to Recent (ed. Hill R. S.), pp. 80–196 Cambridge, UK: Cambridge University Press [Google Scholar]
- 37.Archer M., Hand S. J., Godthelp H. 1995. Tertiary environmental and biotic change in Australia. In Paleoclimate and evolution, with emphasis on human origins (eds Vrba E., Denton G. H., Partridge T. C., Burckle L. H.), pp. 77–90 New Haven, CT: Yale University Press [Google Scholar]
- 38.Bassarova M. 2005. Taphonomic and palaeoecological investigations of Riversleigh Oligo-Miocene fossil sites. Unpublished PhD thesis. School of Biological, Earth and Environmental Sciences, University of New South Wales [Google Scholar]
- 39.Myers T. J. 2002. Palaeoecology of Oligo-Miocene local faunas from Riversleigh. Unpublished PhD thesis. School of Biological, Earth and Environmental Sciences, University of New South Wales [Google Scholar]
- 40.Travouillon K. J., Legendre S., Archer M., Hand S. J. 2009. Palaeoecological analyses of Riversleigh's Oligo-Miocene sites: implications for Oligo-Miocene climate change in Australia. Palaeogeogr. Palaeoclimat., Palaeoecol. 276, 24–37 10.1016/j.palaeo.2009.02.025 (doi:10.1016/j.palaeo.2009.02.025) [DOI] [Google Scholar]
- 41.Boles W. E. 1993. A logrunner, Orthonyx (Passeriformes: Orthonychidae) from the Miocene of Riversleigh, north-western Queensland. Emu 93, 44–49 10.1071/MU9930044 (doi:10.1071/MU9930044) [DOI] [Google Scholar]
- 42.Boles W. E. 1995. A preliminary analysis of the Passeriformes from Riversleigh, northwestern Queensland, Australia, with the description of a new species of lyrebird. Courier Forchungsinstitut Senckenberg 181, 163–170 [Google Scholar]
- 43.Boles W. E. 1997. Riversleigh birds as palaeoenvironmental indicators. Mem. Qd Mus. 41, 241–246 [Google Scholar]
- 44.Hand S. J., Archer M. 2005. A new hipposiderid genus (Microchiroptera) from an early Miocene bat community in Australia. Palaeontology 48, 1–13 10.1111/j.1475-4983.2005.00444.x (doi:10.1111/j.1475-4983.2005.00444.x) [DOI] [Google Scholar]
- 45.Hand S., Archer M., Godthelp H. 2005. Australian Oligo-Miocene mystacinids (Microchiroptera): upper dentition, new taxa and divergence of New Zealand species. Geobios 38, 339–352 10.1016/j.geobios.2003.11.005 (doi:10.1016/j.geobios.2003.11.005) [DOI] [Google Scholar]
- 46.Gott M. 1988. A Tertiary marsupial mole (Marsupialia: Notoryctidae) from Riversleigh, northeastern Australia and its bearing on notoryctemorphian phylogenetic systematics. Unpublished Honours thesis. University of New South Wales [Google Scholar]
- 47.Thomas O. 1920. Notoryctes of north-west Australia. Ann. Mag. Nat. Hist. 6, 111–113 [Google Scholar]
- 48.Goin F. J., Candela A. M. 2004. New Paleogene marsupials from the Amazon Basin of Eastern Peru. In The Paleogene mammalian fauna of Santa Rosa, Amazonian Peru (ed. Campbell K. E., Jr), Science Series 40, pp. 15–60 Los Angeles, CA: Natural History Museum of Los Angeles County. [Google Scholar]
- 49.Archer M. 1981. Results of the Archbold Expeditions. No. 104. Systematic revision of the marsupial dasyurid genus Sminthopsis Thomas. Bull. Am. Mus. Nat. Hist. 168, 61–224 [Google Scholar]
- 50.Archer M. 1984. Origins and early radiations of marsupials. In Vertebrate zoogeography and evolution in Australasia (eds Archer M., Clayton G.), pp. 585–631 Perth, Australia: Hesperian Press [Google Scholar]
- 51.Lucas P. W. 2004. Dental functional morphology: how teeth work. Cambridge, UK: Cambridge University Press [Google Scholar]
- 52.Beck R. M. D. 2009. Was the Oligo-Miocene Australian metatherian Yalkaparidon a ‘mammalian woodpecker'? Biol. J. Linn. Soc. 97, 1–17 10.1111/j.1095-8312.2009.01171.x (doi:10.1111/j.1095-8312.2009.01171.x) [DOI] [Google Scholar]
- 53.Benshemesh J., Johnson K. 2003. Biology and conservation of marsupial moles (Notoryctes). In Predators with pouches: the biology of carnivorous marsupials (eds Jones M., Dickman C. R., Archer M.), pp. 464–474 Melbourne, Australia: CSIRO Publishing [Google Scholar]
- 54.Johnson K. A., Walton D. W. 1989. 23. Notoryctidae. In Fauna of Australia: volume 1B Mammalia (eds Walton D. W., Richardson B. J.), pp. 1–24 Canberra, Australia: AGPS [Google Scholar]
- 55.McDowell S. B. 1958. The Greater Antillean insectivores. Bull. Am. Mus. Nat. Hist. 115, 113–214 [Google Scholar]
- 56.Eisenberg J. F., Gould E. 1970. The tenrecs: a study in mammalian behavior and evolution. Washington, DC: Smithsonian Institution Press [Google Scholar]
- 57.Rose K. D. 2006. The beginning of the age of mammals. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 58.Salton J. A. 2005. Evolutionary morphology of the postcranial skeleton in Afro-Malagasy Tenrecoidea (Mammalia), Unpublished PhD thesis. Graduate Faculty in Biology, City University of New York [Google Scholar]
- 59.Freeman P. W. 1979. Specialized insectivory: beetle-eating and moth-eating molossid bats. J. Mamm. 60, 467–479 10.2307/1380088 (doi:10.2307/1380088) [DOI] [Google Scholar]
- 60.Hand S. J. 1997. Riversleigha williamsi n. gen. et n. sp., a large Miocene hipposiderid from Riversleigh, Queensland. Alcheringa 22, 259–276 10.1080/03115519808619204 (doi:10.1080/03115519808619204) [DOI] [Google Scholar]
- 61.Archer M., Hand S. J., Godthelp H. 1988. Green cradle: the rainforest origins of Australia's marsupials. Abstracts 7th International Palynological Congress, Brisbane, p. 2 [Google Scholar]
- 62.Chen X. Y., Barton C. E. 1991. Onset of aridity and dune-building in Central Australia: sedimentological and magnetostratigraphic evidence from Lake Amadeus. Palaeogeogr. Palaeoclimat. Palaeoecol. 84, 55–71 10.1016/0031-0182(91)90035-P (doi:10.1016/0031-0182(91)90035-P) [DOI] [Google Scholar]
- 63.Butler P. M. 1977. Evolutionary radiation of the cheek teeth of Cretaceous placentals. Acta Pal. Pol. 22, 241–271 [Google Scholar]
- 64.Cifelli R. L. 1993. Theria of metatherian-eutherian grade and the origin of marsupials. In Mammal phylogeny: Mesozoic differentiation, multituberculates, monotremes, early therians, and marsupials (eds Szalay F. S., Novacek M. J., McKenna M. C.), pp. 205–215 New York, NY: Springer [Google Scholar]
- 65.Seiffert E. R., Simons E. L. 2000. Widanelfarasia, a diminutive placental from the late Eocene of Egypt. Proc. Natl Acad. Sci. USA 97, 2646–2651 10.1073/pnas.040549797 (doi:10.1073/pnas.040549797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopatin A. 2006. Early Paleogene insectivore mammals of Asia and establishment of the major groups of Insectivora. Paleont. J. 40(Suppl. 3), S205–S405 [Google Scholar]
- 67.Seiffert E. R. In press The oldest and youngest records of Afrosoricida (Placentalia, Afrotheria) from the Fayum Depression of northern Egypt. Acta Pal. Pol. 10.4202/app.2010.0023 (doi:10.4202/app.2010.0023) [DOI] [Google Scholar]
- 68.Beck R. M. D., Bininda-Emonds O. R. P., Cardillo M., Liu F.-G. R., Purvis A. 2006. A higher-level MRP supertree of placental mammals. BMC Evol. Biol. 6, 93. 10.1186/1471-2148-6-93 (doi:10.1186/1471-2148-6-93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Janecka J. E., Miller W., Pringle T. H., Wiens F., Zitzmann A., Helgen K. M., Springer M. S., Murphy W. J. 2007. Molecular and genomic data identify the closest living relative of primates. Science 318, 792–794 10.1126/science.1147555 (doi:10.1126/science.1147555) [DOI] [PubMed] [Google Scholar]
- 70.Murphy W. J., Pringle T. H., Crider T. A., Springer M. S., Miller W. 2007. Using genomic data to unravel the root of the placental mammal phylogeny. Genome Res. 17, 413–421 10.1101/gr.5918807 (doi:10.1101/gr.5918807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nishihara H., Maruyama S., Okada N. 2009. Retroposon analysis and recent geological data suggest near-simultaneous divergence of the three superorders of mammals. Proc. Natl Acad. Sci. USA 106, 5235–5240 10.1073/pnas.0809297106 (doi:10.1073/pnas.0809297106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roca A. L., Bar-Gal G. K., Eizirik E., Helgen K. M., Maria R., Springer M. S., O'Brien S. J., Murphy W. J. 2004. Mesozoic origin for West Indian insectivores. Nature 429, 649–651 10.1038/nature02597 (doi:10.1038/nature02597) [DOI] [PubMed] [Google Scholar]
- 73.Zuccon A., Zuccon D. 2008. MrEnt v.2.0. Program distributed by the authors. See http://www.mrent.org