Abstract
I apply evolutionary perspectives and conceptual tools to analyse central issues underlying child health, with emphases on the roles of human-specific adaptations and genomic conflicts in physical growth and development. Evidence from comparative primatology, anthropology, physiology and human disorders indicates that child health risks have evolved in the context of evolutionary changes, along the human lineage, affecting the timing, growth-differentiation phenotypes and adaptive significance of prenatal stages, infancy, childhood, juvenility and adolescence. The most striking evolutionary changes in humans are earlier weaning and prolonged subsequent pre-adult stages, which have structured and potentiated maladaptations related to growth and development. Data from human genetic and epigenetic studies, and mouse models, indicate that growth, development and behaviour during pre-adult stages are mediated to a notable degree by effects from genomic conflicts and imprinted genes. The incidence of cancer, the primary cause of non-infectious childhood mortality, mirrors child growth rates from birth to adolescence, with paediatric cancer development impacted by imprinted genes that control aspects of growth. Understanding the adaptive significance of child growth and development phenotypes, in the context of human-evolutionary changes and genomic conflicts, provides novel insights into the causes of disease in childhood.
Keywords: evolutionary medicine, child health, cancer, growth
1. Introduction
‘Our whole life is but a greater and longer childhood’
Benjamin Franklin
In this review, I develop and apply a conceptual framework for evolutionary-medical analyses of post-natal child physical growth and development. This framework is based predominantly on two axes: (i) analyses of the adaptive significance and evolutionary trajectories of the stages of human child growth and differentiation, in the context of health-related alterations, and (ii) elucidation of the arenas, causes and outcomes of genomic conflicts during these stages of development.
I first briefly describe evolutionary theory relevant to child development. Second, I review studies on the evolution of human childhood with regard to how pre-adult life-history stages have evolved along the human lineage, in conjunction with studies of genomic conflicts that mediate child health-related phenotypes. As in other areas of evolutionary medicine, discerning the adaptive significance of traits is a prerequisite for understanding the causes of health-related departures from adaptation [1].
2. Theory
A fundamental postulate of natural-selection theory for evolutionary medicine is that human disease risks have evolved in close conjunction with human phenotypes, genes and environments [2,3]. For non-infectious diseases that are not functions of recent phenotype-environment mismatches, deviations from health are therefore expected to reflect alterations to more or less adaptive, human-evolved systems. Such alterations may represent effects of under-development or over-development of adaptations, losses or gains of homeostatic control, changes in timing of development, or more complex changes that distort evolved development or function.
The evolution of human life history is expected to be modulated by trade-offs between components of growth, survival and reproduction, whereby the timing of different stages, and time-dependent investment in different physiological and morphological systems, are subject to selection in the contexts of ecological and social selective agents [4–6]. Key expectations from life-history theory applied to the human lineage are that evolutionary prolongation of pre-adult stages should engender lifetime-reproductive benefits sufficiently strong to counterbalance increased pre-adult survival costs [7], and that strong selection for survival and growth early in life should, owing to antagonistic pleiotropy, sustain considerable deleterious, more weakly selected effects later in life [2,3,8].
Inclusive fitness theory is central to human life-history evolution, with mother–offspring interactions most directly influencing patterns of child growth, development and behaviour owing to high levels of prenatal and postnatal maternal investment. Under parent–offspring conflict theory, offspring are under selection to solicit or extract more parental investment than parents (mainly mothers) are selected to provide. Outcomes of conflict—including health-related costs—should depend critically upon the phenotypic tactics available to each party (especially who controls the resources involved), the strengths of selection, and the costs of conflict to one or both parties [9]. For parent–child interactions, parents are often in control of resource allocation owing to their physical and social dominance [10], but offspring may impose costs on, or provide benefits to, parents that modulate their behaviour, or manipulate parents by physiological or psychological means. Parent–offspring conflict theory has accrued considerable empirical support across diverse animals including humans and other primates (e.g. [11]), yet thus far it has been comprehensively applied to child health primarily with regard to prenatal development [12], infant crying and feeding [13] and the timing of weaning [6,14].
Parent–offspring conflicts are intergenomic, mediated ultimately by condition-dependent, stage-specific gene expression. Under Haig's kinship theory, genomic imprinting has evolved in the context of intragenomic (here, within-offspring) conflict, such that fathers have been selected to silence genes, during spermatogenesis, that reduce demands on mothers when expressed in dependent offspring, and mothers have been selected to silence genes, during oogenesis, that increase demands of their children upon them [15]. A useful feature of imprinted genes is that their effects on human growth, development, physiology and behaviour can often be predicted from their functions and directions of imprinting, which provides for robust hypothesis generation and testing. Imprinted genes have recently been demonstrated to differentially interact in networks [16–19], which may represent generalized tugs of war involving mixtures of cooperation and conflict [20].
Mother–offspring conflict and genomic-imprinting conflict are expected to interact in that the outcome of imprinting conflicts modulate the ‘set-levels’ of resource demands or solicitation imposed upon the mother by the child. In turn, mothers are under selection to provide resources to the offspring so as to maximize their own inclusive fitness, even if doing so comes at some cost to the offspring. This simple model may apply in a variety of situations, including: (i) placentation and overall foetal growth [21], (ii) late-foetal stage fat deposition [22], (iii) infant crying in relation to lactational feeding by suckling [13], and (iv) early social development [20], in each case providing insight into potential causes of effects on health.
Developmental and growth systems underlaid by genomic-imprinting conflicts are expected to exert disproportionate effects on health, due to rates of epimutation (e.g. altered methylation) many times higher than rates of mutation, larger-magnitude changes in gene expression owing to altered imprinting, and expectations that the gene-expression changes will impact directly and strongly upon fitness-related traits [15,23]. Under genomic-imprinting conflict, if one party ‘wins’ (controls the resource, or the demand set-level), the other party may suffer health-related costs; tugs or ‘webs’ or war may impose physiological or behavioural costs on both parties; and the evolution of conflict systems in one or both parties, such as imprinting itself, potentiates new forms of maladaptive dysregulation [3,24]. In the context of child development, a child's paternal-gene phenotypic optimum is expected to be closer than its maternal-gene (and mother's) optimum to child individual fitness (and, generally, health). More generally, conflict systems appear non-intuitive from physiological and medical perspectives, and may commonly not be discerned unless explicitly postulated and sought.
3. A greater and longer childhood
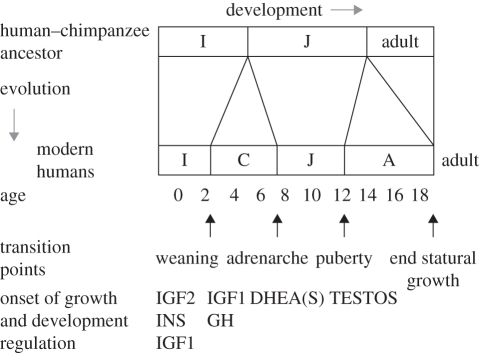
Human post-natal, pre-adult development can be divided into four stages, separated by physiological and behavioural transitions [4,25,26] (figure 1). Each stage is characterized by its own pattern of feeding (as reflected in development of teeth and other organs, and behaviour), growth and growth control (as reflected in endocrine secretion), and cognitive-affective, social and brain development (as reflected in behaviour). Given this overall structure to early human life history, each stage can be considered with regard to the adaptive significance of the phenotypes involved, evidence for the evolution of uniquely human or human-elaborated phenotypes, evidence regarding the presence and forms of genomic conflicts and trade-offs, and the implications of these phenotypes and processes for child health.
Figure 1.
The stages of human pre-adult development, including transition landmarks and endocrine factors mediating growth and development at different stages. IGF2 also strongly mediates prenatal growth. Adapted from Bogin [4,27,28] and Hochberg [29]. IGF2, insulin-like growth factor 2; INS, insulin; IGF1, insulin-like growth factor 1; GH, growth hormone; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulphate; TESTOS, testosterone.
(a). Infancy
Infancy can be defined as the stage between birth and weaning characterized by lactational feeding, rapid and decelerating growth, and the presence of deciduous teeth. Infancy represents the only postnatal human stage when growth is directly and strongly dependent upon nutrition (in conjunction with hormonal systems that mediate growth, mainly insulin-like growth factor 2 (IGF2), insulin (INS) and insulin-like growth factor 1 (IGF1) in the foetal and early-infancy periods), compared to later stages when growth rates (in stature) are canalized by the growth hormone (GH)-IGF1 axis [30,31]. The infant stage is about half as long in humans, at 3 to 4 years, compared to chimpanzees and gorillas at 6 to 7 years [6,32]. This early human age at weaning, which generates greatly reduced inter-birth intervals and higher female fertility rates, represents one of the most prominent life-history shifts along the human lineage [5].
Patterns of infant feeding, in conjunction with prenatal growth, potentiate trajectories of post-natal development. The primary relevance of evolutionary considerations to such trajectories involves: (i) analyses of the adaptive significance, for mothers and offspring, of alternative infant growth and development patterns [33,34], in the context of early weaning and early introduction of complementary foods [35–37]; (ii) survival advantages to early catch-up growth [38–40], which may potentiate, and be counterbalanced by, later-life heath costs [41–43]; and (iii) roles for mother–offspring and imprinted-gene conflicts in prenatal and postnatal growth and development [7,15,31]. The genetic and epigenetic bases of human birth weight and early growth are mediated in considerable part by imprinted genes, from studies of non-clinical populations (e.g. [44,45]) and the two clinical syndromes, Silver–Russell Syndrome and Beckwith–Wiedemann Syndrome, that involve general undergrowth and overgrowth, respectively, [46]. By contrast, the adaptive significance and selective contexts of foetal and infant physiological–developmental reaction norms related to early survival and growth remain largely unknown.
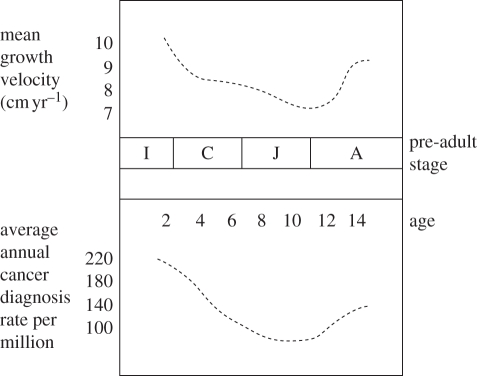
A form of local overgrowth, cancer, represents the second most common cause of childhood mortality (after accidents) in developed countries, with an overall incidence of about 1 in 600 before age 15 [47], and its highest rates of diagnosis among infants. The similarity between human child growth velocities, and overall cancer incidence with age (figure 2), suggests that the development of paediatric cancers is closely associated with rates of cell division and growth. This hypothesis is also supported by well-replicated positive associations of higher birth weight and accelerated foetal growth with higher rates of most of the major childhood cancers ([50–52], see also [53]).
Figure 2.
The pattern of changes in human growth velocity (adapted from [48]) over the pre-adult period, in comparison to age-specific incidence of all paediatric cancers (adapted from data in [49]).
Available evidence on the roles of different imprinted genes in the best studied childhood cancers (see the electronic supplementary material, table S2) demonstrates that such genes are often differentially dysregulated, with paternal biases tending to promote carcinogenesis. However, some paternally expressed genes function as tumour suppressors, and the importance and patterns of imprinted-gene effects in the stages of paediatric (or adult) carcinogenesis, compared with effects from non-imprinted genes, have yet to be systematically ascertained. Data from Rezvani et al. [54] showing that co-expressed, co-regulated imprinted genes, the expression of which is associated with childhood growth and increasing body size, also promote growth of the childhood cancers rhabdomyosarcoma and Wilm's tumour, convergently suggest strong functional linkages between childhood growth, imprinted gene expression, and risk of cancer. The adaptive significance of imprinted genes—in the context of intragenomic conflicts in early development—are directly relevant to the phenotypic effects of these genes when they become maladaptively dysregulated during carcinogenesis. For example, the non-coding RNA product of the maternally expressed H19 gene restricts placental and foetal growth during development, apparently, in part, via promoting differentiation of placental cytotrophoblast tissue in the hypoxic environment of early placental development, with direct effects on trophoblast cell phenotypes underlying cell migration and angiogenesis [55,56]. By contrast, in adult tissues, H19 is expressed in the hypoxic conditions found within tumours, where it exerts oncogenic effects under the pathological conditions of reduced p53 function, through promotion of metastasis and angiogenesis [57]. Such considerations suggest that evolved mechanisms of imprinted-gene-mediated growth restraint or differentiation induction (e.g. [58]) may be deployed therapeutically to help control childhood cancers.
Post-natal infant growth is orchestrated physiologically by INS and IGF pathways, but behaviourally by crying and suckling. Wells [13] and Soltis [59] provide comprehensive reviews of evidence regarding the adaptive significance of human crying, including considerations from mother–offspring conflict. Vocal begging has been linked with access to the nipple only among apes [13]; in other species, infant vocalizations appear to function primarily for maintaining proximity to the mother to solicit care and protection. Human-specific adaptations salient to human crying and feeding include: (i) highly developed maternal deposition of fat during gestation, to support the relatively high energetic demands of lactation; (ii) mother–infant proximity serving to facilitate the development of language and complex social interactions as well as feeding [60]; and (iii) early use of transitional, complementary foods in humans, to supplement breastfeeding and allow earlier weaning [36,37].
The clearest evidence for an adaptive function to crying as a signal of relative infant physiological ‘need’ is the observation that crying rates are highest when infant growth rates are at their maximum [13]. In this context, the combination of effective signalling of need with maternal sensitivity to interpret infant cues and efficient mechanics of latching and suckling in breast-feeding appears central to the process. Difficulty in establishing proper nutrition via breastfeeding early in infancy is indeed a primary cause of doctor visits during the post-natal period [13], and poor infant nutrition, especially combined with infections, represents the main cause of infant mortality in hunter–gatherer, horticultural, and developing societies (e.g. [61]). Infant failure to thrive appears to commonly involve some combination of: (i) mismatches of infant solicitation patterns and intensity with maternal sensitivity, and (ii) oral-motor dysfunction that reduces the effectiveness of suckling [13]. Such mismatches of solicitation with sensitivity are expected to be potentiated by mother–offspring conflicts, to the extent that time and energy investment in a particular infant may, in some circumstances, engender opportunity costs (especially with regard to older and future offspring) that could reduce a mother's inclusive fitness (e.g. [62]). During infancy, infants control patterns of crying, suckling and, to some degree, solicitation of physical engagement; crying may impose psychological costs on mothers via functional design of its acoustic properties (e.g. [63]), and social engagement via smiling and other movements may provide social–psychological benefits to mothers [64]. However, mothers control proximity and access to the breast, and this power asymmetry is likely to make conflicts difficult to detect because the mothers are much better-positioned to achieve their optima (e.g. [36,65]).
Given that crying and suckling impose strong demands on mothers, with direct impacts on infant growth, genomic imprinting conflicts are predicted in the context of these phenotypes, with regard to both the infant anatomical structures involved and the behaviours that solicit and extract breast milk and social–behavioural benefits from reciprocal engagement. In particular, paternally expressed imprinted genes in offspring are expected to exert phenotypic effects that increase demands on mothers, while maternally expressed genes should engender reduced demands [15]. Such effects should be embedded within the developmental systems that underly crying, suckling and early social interactions, with direct effects on child health flowing from genetic, epigenetic and environmental perturbations. Indeed, high heritability estimates (e.g. 0.84 [66]) of appetitive traits in human infants, including slowness of eating, in a human population, and the roles of imprinted genes in regulating appetite and feeding [67–69], attest to potential for genetic and epigenetic dysregulation of such phenotypes.
Data salient to this prediction, from imprinted-gene experimental knockouts in mice and natural alterations to imprinted genes in humans, are compiled in table S1 (see the electronic supplementary material). These data provide convergent support for the hypothesis that paternally expressed imprinted genes promote enhanced suckling in mouse neonates and human infants, through effects that include: (i) the development of oro-facial musculature involved in suckling, (ii) size of the tongue (which serves as a ‘suction pump’ for milk extraction), (iii) the intensity of ultrasonic vocalizations (in mice) or crying (in humans), and (iv) motivation to suckle. Proximate mechanisms underlying these effects include the presence of an enhancer for IGF2 expression with differential effects on growth of tongue and skeletal muscle in mice [70], and roles for the imprinted genes Peg3, Peg1, and Ndn—hub' genes in Varrault et al.'s [16] imprinted-gene network—in promoting development of hypothalamic neurons that secrete oxytocin, a peptide hormone that motivates early bidirectional social bonding [71].
These diverse data suggest that genomic imprinting effects expressed in infants modulate central aspects of crying, suckling and early mother–offspring motivation. To the extent that such effects are especially prone to fitness-related dysregulation (as described above), and infant–mother relationships are highly interactive systems of solicitation, sensitivity, and effectiveness, a considerable proportion of dysfunctionality in human infant–mother early interactions may derive from variation in infant traits mediated in part by imprinted-gene effects. Recognizing these predictions from evolutionary theory should shift the focus of infant–mother nutritional and psychological research towards elucidating causes of variation among infants in feeding musculature and behaviour, with rodent models of mother–pup serving as useful experimental systems (e.g. [72]).
(b). Childhood
The transition from infancy to childhood is defined by weaning, which takes place relatively early in humans (between 9 and 36 months) compared with other great apes (e.g. approx. 60 months in chimpanzees) and at lower weights (about 2.7 times birth weight) [5,28]. Humans are unique among primates in that the first permanent molar teeth do not erupt until about 3 years after weaning, such that human children are highly dependent upon parents and alloparents for feeding during this period, commonly with foods that are chosen and processed by carers for ease of ingestion [37].
Prenatal and infant growth rates are determined predominantly by food intake via placenta and breast, but in mid-to-late infancy and during childhood, growth becomes increasingly regulated by the GH-IGF1 axis, and less controlled by IGF2 and INS. Hochberg and Albertsson–Wikland [26] present a model for adaptive timing of the infant-to-child growth transition, whereby infants under poor nutritional conditions, or who exhibited low birth weight, conditionally delay activation of the GH-IGF1 axis. Such delays are associated with long-term costs from reduced adult height, but may increase early survivorship and ameliorate the negative long-term fitness impacts of deleterious nutritional conditions during infancy.
In the context of parent–offspring conflicts, offspring control their behaviour and the timing and patterns of endocrine-system regulation of growth, but parents control the time of weaning and the age for introduction of complementary foods. Earlier weaning in the human lineage has been postulated as an evolved adaptation of mothers, to increase their fecundity via reduced interbirth intervals [6,35,73]; it is also expected to generate higher offspring mortality rates. Sellen [36,37] describes the child-health implications for different patterns of weaning and complementary feeding in contemporary populations; elucidating the relationships of such patterns to the timing of the transition to GH-IGF1 control over growth should provide useful insights into causes of variation in infant and childhood growth.
The evolution of early weaning and reduced inter-birth intervals along the human lineage is expected to involve not just conflicts between mothers and offspring, but also genomic-imprinting conflicts. Thus, maternally expressed genes should favour easier, cheaper offspring who wean early and readily engage in complementary and self-feeding, whereas paternally expressed genes favour higher, extended levels of energetic and behavioural solicitation for food. Haig and Wharton [74] interpret the behavioural phenotypes of Prader–Willi syndome, which involve sleepiness, poor suckling, complacency and weak solicitation during infancy, followed by compulsive foraging and self-feeding, as extreme, maladaptive manifestations of such maternal gene biases (owing to loss of one or more paternal gene products) in this syndrome. The development of Prader–Willi syndrome phenotypes from a broad range of genetic and epigenetic alterations in humans (table 3 in [75]), and the opposite nature of some Angelman-syndrome phenotypes related to solicitation [76], may reflect a history of strong selection on the phenotypes involved.
The primary upshot of these considerations for infant and childhood health is that they imply the existence of a set of interacting imprinted genes, some of which have undergone selection along the human lineage, that differentially impact upon the intensity of infant behavioural solicitation from the mother, behaviour associated with weaning (such as night-time suckling, which maintains lactational amenorrhea), tendency to accept complementary foods and a diversity of foods during and after the weaning period, and early patterns of mother–child attachment [20].
(c). Juvenile stage
Childhood lasts from weaning until about age 6–8, when the onset of adrenal androgen secretion (adrenarche) marks the start of the juvenile stage [28,77,78]. Adrenarche represents an early stage in sexual maturation, distinct from hypothalamic–pituitary–gonadal maturation and function during adolescence, whereby the adrenal cortex secretes increased levels of the hormones dehydroepiandrosterone and dehydroepiandrosterone sulphate (DHEAS) in the absence of increased levels of cortisol. Initiation of the juvenile stage tends to coincide with eruption of the first permanent molar teeth, and so-called adiposity rebound (a relative increase in fat levels, which may mark reallocation of energy from brain growth to fat and muscle; [29]), but it involves little change in growth rate. At adrenarche, brain growth is nearly complete; behaviourally, the juvenile stage involves much greater independence from parents in feeding, and in survival ability, than earlier in life [28,79]. Whereas the childhood stage (defined by its immature dentition, and weaning but dependency on parents for food) appears unique to humans, adrenarche similar to that of humans has been described only for chimpanzees (where it also occurs notably earlier), and apparently also for gorillas [77].
The adaptive significance, in humans and other great apes, of a life-history stage that involves adrenal activation at least several years prior to the onset of puberty remains unclear. Campbell [77] suggests, based on the demonstrated role of DHEAS as a neurosteroid hormone, that the primary function of adrenarche involves psychological maturation in the context of increasing complexity and importance of social–environmental information during this period of increasing child independence. Del Giudice et al. [79] and Flinn et al. [80] extend this hypothesis, in suggesting that adrenarche serves as an endocrine mechanism for integrating social–environmental with genetic factors to conditionally adapt social–reproductive strategies based on experiences earlier in life. Examples of conditional variation in adrenarche timing and intensity include a positive association of earlier adrenarche with indicators of lower-quality parental investment ([80], in females), and with rapid weight gain during infancy [30]; similarly, adrenal androgen levels at age 8 are higher in boys and girls of lower birth weight who gain postnatal weight more rapidly [81]. Age at adrenarche shows substantial heritability (0.58; [82]) and strongly overlapping genetic effects with the age at gonadarche in females [83], but this latter study also reported environmental effects unique to the timing of each transition, which are consistent with at least the potential for independent adaptive modification of this life-history stage.
Parent–offspring conflicts, and genomic-imprinting conflicts, might be predicted to have mediated the timing of human adrenarche, given that this stage marks a notable acceleration of the transition to greater independence—especially social-behavioural independence—in children. As for the timing of puberty, discussed below, offspring are expected to control the expression of this phenotype (although parents may exert environmental impacts that affect the offspring optimum), and maternally expressed imprinted genes might be expected to favour a relatively early transition compared with paternally expressed genes [6]. The health-related correlates of the timing of adrenarche, which include increased risks of the metabolic syndrome and polycystic ovary disease [84,85], are difficult to assess in the context of human evolutionary history without evaluation of alternative hypotheses for the adaptive significance of this trait. Tests for the role of adrenal androgens in human social–emotional and social–cognitive development should, however, be feasible via longitudinal studies jointly tracking hormonal, psychological and behavioural changes, especially in societies not strongly impacted by westernization [78].
(d). Adolescence
The transition from the juvenile stage to adolescence represents a gradual process, initiated by pubertal activation of the hypothalmic–pituitary–gonadal axis and a dramatic increase in secretion of sex hormones [27,86]. The adolescent stage lasts about 5–10 years (approx. the teenage years), and its primary characteristics as a life-history stage include: (i) the pubertal growth spurt in height and weight, which is notably more rapid in humans than in chimpanzees and other related primates; (ii) development of secondary sexual traits, including sex-specific alterations to fat and muscle distribution [87]; (iii) completion of permanent tooth eruption; and (iv) psychological and behavioural changes related to sexual and social maturation [4,27]. The suite of differences in these and other traits between humans and chimpanzees suggest that an adolescent stage of gradual physiological, sexual and social maturation can be considered as unique to the human lineage [4]. However, the adaptive significance of the presence, timing of onset, and duration of the adolescent stage remains largely a matter of conjecture, mainly involving the acquisition of social, sexual and parenting skills that will be useful in adulthood [88,89].
Compared with other primates, humans have evolved a late age at puberty and sexual maturity [32], with the timing of puberty in females influenced by a large suite of factors including birth weight, patterns of growth, energetics during the juvenile stage, and social factors such as quality of parenting [89,90]. Early puberty in females engenders a broad range of negative physical and psychological health outcomes [91,92] in western societies, with a strong secular trend towards earlier puberty indicating impacts from environmental factors such as increased nutrition [93]. As for adrenarche, the negative effects from earlier life-history transitions are consistent with a selective history for elongation of these stages.
To the extent that puberty marks a major transition towards decreased energetic and social dependence on older kin (especially the mother), its timing should also be determined in part by imprinted-gene effects, with maternally expressed genes favouring earlier puberty and subsequent reduced demands; by contrast, paternally expressed genes should favour prolongation of this partially dependent stage [6]. Early puberty has indeed been reported as a feature of four imprinted-gene syndromes with maternal bias: Prader–Willi syndrome [94], Silver–Russell syndrome (table 1 in [95]; [96,97]), Temple syndrome [98,99], and McCune–Albright syndrome [100]. By contrast, among imprinted-gene syndromes involving paternal bias, pubertal timing has been reported as delayed in Albright's Hereditary Osteodystropy ([100] in females), delayed or normal in Angelman syndrome [101,102], and within normal limits in Beckwith–Wiedemann syndrome [103]. The primary caveat to interpretation of these results is that syndromes with maternal-gene bias also involve relatively low birth weight, which is independently associated with earlier puberty [90,104]. Studies that directly relate genetic and epigenetic variation in imprinted genes to birth weight and pubertal timing are required to partition these effects.
4. Conclusions
Human genetically based and epigenetically based health risks have evolved, in the contexts of human adaptations and conflicts that cause or potentiate maladaptations. Large-scale changes in child developmental trajectories along our lineage, high intensities of genomic conflicts at young ages, and the sequential, cascading nature of development, compel a focus on child health, and its evolutionary foundations, as a means to enhance human well-being throughout the lifespan. Evolutionary perspectives generate a wealth of testable hypotheses directly germane to the health of children, most of which can direct data collection along novel, promising paths. Currently, the primary impediments to progress towards this goal are limited understanding of the adaptive significance of human–child phenotypes, lack of data concerning the mechanisms whereby genomic conflicts can cause deviations from health, and a need for increased integration of evolutionary with proximate perspectives on the causes of disease.
Acknowledgements
I am grateful to NSERC for financial support, and to S. Frank, M. Greaves, C. Kuzawa, C. Maley, R. Nesse, D. Sellen and J. Wells for helpful insights and comments.
References
- 1.Nesse R. 2005. Maladaptation and natural selection. Q. Rev. Biol. 80, 62–70 10.1086/431026 (doi:10.1086/431026) [DOI] [PubMed] [Google Scholar]
- 2.Williams G. C., Nesse R. M. 1991. The dawn of Darwinian medicine. Q. Rev. Biol. 66, 1–22 10.1086/417048 (doi:10.1086/417048) [DOI] [PubMed] [Google Scholar]
- 3.Crespi B. J. 2010. The origins and evolution of genetic disease risk in modern humans. Ann. NY Acad. Sci. 1206, 80–109 10.1111/j.1749-6632.2010.05707.x (doi:10.1111/j.1749-6632.2010.05707.x) [DOI] [PubMed] [Google Scholar]
- 4.Bogin B. 2006. Modern human life history: the evolution of human childhood and adult fertility. In The evolution of human life history (eds Hawkes K., Paine R.), pp. 197–230 Santa Fe, NM: School of American Research Press [Google Scholar]
- 5.Hawkes K., Paine R. R. (eds) 2006. The evolution of human life history. Santa Fe, NM: School of American Research Press [Google Scholar]
- 6.Haig D. 2010. Transfers and transitions: parent–offspring conflict, genomic imprinting, and the evolution of human life history. Proc. Natl Acad. Sci. USA 107(Suppl. 1), 1731–1735 10.1073/pnas.0904111106 (doi:10.1073/pnas.0904111106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haig D. 2008. Intimate relations: evolutionary conflicts of pregnancy and childhood. In Evolution in health and disease, 2nd edn (eds Stearns S., Koella J.), pp. 65–77 Oxford, UK: Oxford University Press [Google Scholar]
- 8.Crespi B. J. In press The emergence of human-evolutionary medical genomics. Evol. Appl. (doi:10.1111/j.1752-4571.2010.00156.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beekman M., Komdeur J., Ratnieks F. L. W. 2003. Reproductive conflicts in social animals: who has power? Trends Ecol. Evol. 18, 277–282 10.1016/S0169-5347(03)00068-5 (doi:10.1016/S0169-5347(03)00068-5) [DOI] [Google Scholar]
- 10.Alexander R. D. 1974. The evolution of social behavior. Annu. Rev. Ecol. Syst. 5, 325–363 10.1146/annurev.es.05.110174.001545 (doi:10.1146/annurev.es.05.110174.001545) [DOI] [Google Scholar]
- 11.Maestripieri D. 2002. Parent–offspring conflict in primates. Int. J. Primatol. 23, 923–951 10.1023/A:1015537201184 (doi:10.1023/A:1015537201184) [DOI] [Google Scholar]
- 12.Haig D. 2003. Genetic conflicts in human pregnancy. Q. Rev. Biol. 68, 495–532 10.1086/418300 (doi:10.1086/418300) [DOI] [PubMed] [Google Scholar]
- 13.Wells J. C. 2003. Parent–offspring conflict theory, signaling of need, and weight gain in early life. Q. Rev. Biol. 78, 169–202 10.1086/374952 (doi:10.1086/374952) [DOI] [PubMed] [Google Scholar]
- 14.Humphrey L. T. 2010. Weaning behaviour in human evolution. Semin. Cell. Dev. Biol. 21, 453–461 10.1016/j.semcdb.2009.11.003 (doi:10.1016/j.semcdb.2009.11.003) [DOI] [PubMed] [Google Scholar]
- 15.Haig D. 2004. Genomic imprinting and kinship: how good is the evidence? Annu. Rev. Genet. 38, 553–585 10.1146/annurev.genet.37.110801.142741 (doi:10.1146/annurev.genet.37.110801.142741) [DOI] [PubMed] [Google Scholar]
- 16.Varrault A., et al. 2006. Zac1 regulates an imprinted gene network critically involved in the control of embryonic growth. Dev. Cell. 11, 711–722 10.1016/j.devcel.2006.09.003 (doi:10.1016/j.devcel.2006.09.003) [DOI] [PubMed] [Google Scholar]
- 17.Finkielstain G. P., et al. 2009. An extensive genetic program occurring during postnatal growth in multiple tissues. Endocrinology 150, 1791–1800 10.1210/en.2008-0868 (doi:10.1210/en.2008-0868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arima T., et al. 2005. ZAC, LIT1 (KCNQ1OT1) and p57KIP2 (CDKN1C) are in an imprinted gene network that may play a role in Beckwith–Wiedemann syndrome. Nucleic Acids Res. 33, 2650–2660 10.1093/nar/gki555 (doi:10.1093/nar/gki555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabory A., Jammes H., Dandolo L. 2010. The H19 locus: role of an imprinted non-coding RNA in growth and development. Bioessays 32, 473–480 10.1002/bies.200900170 (doi:10.1002/bies.200900170) [DOI] [PubMed] [Google Scholar]
- 20.Crespi B. J. In press The strategies of the genes: genomic conflicts, attachment theory and development of the social brain. In Brain, behavior and epigenetics (eds Petronis A., Mil J.), New York, NY: Springer [Google Scholar]
- 21.Reik W., Constância M., Fowden A., Anderson N., Dean W., Ferguson-Smith A., Tycko B., Sibley C. 2003. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J. Physiol. 547, 35–44 10.1113/jphysiol.2002.033274 (doi:10.1113/jphysiol.2002.033274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuzawa C. W. 1998. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am. J. Phys. Anthropol. Suppl. 27, 177–209 10.1002/(SICI)1096-8644(1998)107:27177::AID-AJPA73.0.CO2-B (doi:10.1002/(SICI)1096-8644(1998)107:27177::AID-AJPA73.0.CO2-B) [DOI] [PubMed] [Google Scholar]
- 23.Schneider E., et al. 2010. Spatial, temporal and interindividual epigenetic variation of functionally important DNA methylation patterns. Nucleic Acids Res. 38, 3880–3890 10.1093/nar/gkq126 (doi:10.1093/nar/gkq126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das R., Hampton D. D., Jirtle R. L. 2009. Imprinting, evolution and human health. Mamm. Genome 20, 563–572 10.1007/s00335-009-9229-y (doi:10.1007/s00335-009-9229-y) [DOI] [PubMed] [Google Scholar]
- 25.Bogin B. 1999. Evolutionary perspective on human growth. Annu. Rev. Anthropol. 28, 109–153 10.1146/annurev.anthro.28.1.109 (doi:10.1146/annurev.anthro.28.1.109) [DOI] [PubMed] [Google Scholar]
- 26.Hochberg Z., Albertsson-Wikland K. 2008. Evo–devo of infantile and childhood growth. Pediatr. Res. 64, 2–7 10.1203/PDR.0b013e318177590f (doi:10.1203/PDR.0b013e318177590f) [DOI] [PubMed] [Google Scholar]
- 27.Bogin B. 1994. Adolescence in evolutionary perspective. Acta Paediatr. Suppl. 406, 29–35 10.1111/j.1651-2227.1994.tb13418.x (doi:10.1111/j.1651-2227.1994.tb13418.x) [DOI] [PubMed] [Google Scholar]
- 28.Bogin B. 1997. Evolutionary hypotheses for human childhood. Am. J. Phys. Anthropol. 104, 63–89 10.1002/(SICI)1096-8644(1997)2563::AID-AJPA33.0.CO;2-8 (doi:10.1002/(SICI)1096-8644(1997)2563::AID-AJPA33.0.CO;2-8) [DOI] [Google Scholar]
- 29.Hochberg Z. 2010. Evo–devo of child growth III: premature juvenility as an evolutionary trade-off. Horm. Res. Paediatr. 73, 430–437 10.1159/000282109 (doi:10.1159/000282109) [DOI] [PubMed] [Google Scholar]
- 30.Dunger D. B., Ahmed M. L., Ong K. K. 2006. Early and late weight gain and the timing of puberty. Mol. Cell. Endocrinol. 254–255, 140–145 10.1016/j.mce.2006.04.003 (doi:10.1016/j.mce.2006.04.003) [DOI] [PubMed] [Google Scholar]
- 31.Smith F. M., Garfield A. S., Ward A. 2006. Regulation of growth and metabolism by imprinted genes. Cytogenet. Genome Res. 113, 279–291 10.1159/000090843 (doi:10.1159/000090843) [DOI] [PubMed] [Google Scholar]
- 32.Robson S. L., Van Schaik C. P., Hawkes K. 2006. The derived features of human life history. In The evolution of human life history (eds Hawkes K., Paine R.), pp. 17–44 Santa Fe, NM: School of American Research Press [Google Scholar]
- 33.Barker D. J. P. 2001. The malnourished baby and infant. Br. Med. Bull. 60, 69–88 10.1093/bmb/60.1.69 (doi:10.1093/bmb/60.1.69) [DOI] [PubMed] [Google Scholar]
- 34.Wells J. C. 2007. The thrifty phenotype as an adaptive maternal effect. Biol. Rev. 82, 143–172 10.1111/j.1469-185X.2006.00007.x (doi:10.1111/j.1469-185X.2006.00007.x) [DOI] [PubMed] [Google Scholar]
- 35.Hrdy S. 1999. Mother Nature: a history of mothers, infants and natural selection. New York, NY: Pantheon; [DOI] [PubMed] [Google Scholar]
- 36.Sellen D. W. 2006. Lactation, complementary feeding and human life history. In The evolution of human life history (eds Paine R., Hawkes K.), pp. 155–197 Santa Fe, NM: School of American Research Press [Google Scholar]
- 37.Sellen D. W. 2007. Evolution of infant and young child feeding: implications for contemporary public health. Annu. Rev. Nutr. 27, 123–148 10.1146/annurev.nutr.25.050304.092557 (doi:10.1146/annurev.nutr.25.050304.092557) [DOI] [PubMed] [Google Scholar]
- 38.Victora C. G., Barros F. C., Horta B. L., Martorell R. 2001. Short-term benefits of catch-up growth for small-for-gestational-age infants. Int. J. Epidemiol. 30, 1325–1330 10.1093/ije/30.6.1325 (doi:10.1093/ije/30.6.1325) [DOI] [PubMed] [Google Scholar]
- 39.Ibáñez L., Ong K., Dunger D. B., de Zegher F. 2006. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J. Clin. Endocrinol. Metab. 91, 2153–2158 10.1210/jc.2005-2778 (doi:10.1210/jc.2005-2778) [DOI] [PubMed] [Google Scholar]
- 40.Ong K. K. 2007. Catch-up growth in small for gestational age babies: good or bad? Curr. Opin. Endocrinol. Diabetes Obes. 14, 30–34 10.1097/MED.0b013e328013da6c (doi:10.1097/MED.0b013e328013da6c) [DOI] [PubMed] [Google Scholar]
- 41.Kuzawa C. W. 2010. Beyond feast-famine: brain evolution, human life history and the metabolic syndrome. In Human evolutionary biology (ed. Muehlenbein M.), pp. 518–527 Cambridge, UK: Cambridge University Press [Google Scholar]
- 42.Wells J. C. 2009. Ethnic variability in adiposity and cardiovascular risk: the variable disease selection hypothesis. Int. J. Epidemiol. 38, 63–71 10.1093/ije/dyn183 (doi:10.1093/ije/dyn183) [DOI] [PubMed] [Google Scholar]
- 43.Wells J. C. 2010. The evolutionary biology of human body fatness: thrift and control. Cambridge, UK: Cambridge Studies in Biological and Evolutionary Anthropology [Google Scholar]
- 44.Petry C. J. & ALSPAC Study Team 2005. Common polymorphism in H19 associated with birthweight and cord blood IGF-II levels in humans. BMC Genet. 6, 22. 10.1186/1471-2156-6-22 (doi:10.1186/1471-2156-6-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adkins R. M., Krushkal J., Klauser C. K., Magann E. F., Morrison J. C., Somes G. 2008. Association between small for gestational age and paternally inherited 5′ insulin haplotypes. Int. J. Obes. (Lond.) 32, 372–380 10.1038/sj.ijo.0803700 (doi:10.1038/sj.ijo.0803700) [DOI] [PubMed] [Google Scholar]
- 46.Eggermann T., Eggermann K., Schönherr N. 2008. Growth retardation versus overgrowth: Silver–Russell syndrome is genetically opposite to Beckwith–Wiedemann syndrome. Trends Genet. 24, 195–204 10.1016/j.tig.2008.01.003 (doi:10.1016/j.tig.2008.01.003) [DOI] [PubMed] [Google Scholar]
- 47.Roman E., Doyle P., Lightfoot T., Ansell P., Simpson J., Allan J. M., Kinsey S., Eden T. O. 2006. Molar pregnancy, childhood cancer and genomic imprinting: is there a link? Hum. Fertil. 9, 171–174 10.1080/14647270600636400 (doi:10.1080/14647270600636400) [DOI] [PubMed] [Google Scholar]
- 48.Hochberg Z. 2009. Evo–devo of child growth II: human life history and transition between its phases. Eur. J. Endocrinol. 160, 135–141 10.1530/EJE-08-0445 (doi:10.1530/EJE-08-0445) [DOI] [PubMed] [Google Scholar]
- 49.Ries L. A. G., Smith M. A., Gurney J. G., Linet M., Tamra T., Young J. L., Bunin G. R. 1999. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. Bethesda, MD: National Cancer Institute, SEER Program; edsNIH Pub. No. 99-4649 [Google Scholar]
- 50.Laurvick C. L., Milne E., Blair E., de Klerk N., Charles A. K., Bower C. 2008. Fetal growth and the risk of childhood non-CNS solid tumours in Western Australia. Br. J. Cancer 99, 179–181 10.1038/sj.bjc.6604424 (doi:10.1038/sj.bjc.6604424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Callan A. C., Milne E. 2009. Involvement of the IGF system in fetal growth and childhood cancer: an overview of potential mechanisms. Cancer Causes Cont. 20, 1783–1798 10.1007/s10552-009-9378-z (doi:10.1007/s10552-009-9378-z) [DOI] [PubMed] [Google Scholar]
- 52.Samuelsen S. O., Bakketeig L. S., Tretli S., Johannesen T. B., Magnus P. 2009. Birth weight and childhood cancer. Epidemiology 20, 484–487 10.1097/EDE.0b013e3181a7786d (doi:10.1097/EDE.0b013e3181a7786d) [DOI] [PubMed] [Google Scholar]
- 53.Leroi A. M., Koufopanou V., Burt A. 2003. Cancer selection. Nat. Rev. Cancer 3, 226–231 10.1038/nrc1016 (doi:10.1038/nrc1016) [DOI] [PubMed] [Google Scholar]
- 54.Rezvani G. J., Lui C. K., Barnes K. M., Baron J. 2010. Imprinted genes that affect body size promote growth of embryonal malignancies. Horm. Res. 72(Suppl. 3), 25 [Google Scholar]
- 55.Poirier F., Chan C. T., Timmons P. M., Robertson E. J., Evans M. J., Rigby P. W. 1991. The murine H19 gene is activated during embryonic stem cell differentiation in vitro and at the time of implantation in the developing embryo. Development 113, 1105–1114 [DOI] [PubMed] [Google Scholar]
- 56.Rachmilewitz J., Gileadi O., Eldar-Geva T., Schneider T., de-Groot N., Hochberg A. 1992. Transcription of the H19 gene in differentiating cytotrophoblasts from human placenta. Mol. Reprod. Dev. 32, 196–202 10.1002/mrd.1080320303 (doi:10.1002/mrd.1080320303) [DOI] [PubMed] [Google Scholar]
- 57.Matouk I. J., Mezan S., Mizrahi A., Ohana P., Abu-Lail R., Fellig Y., Degroot N., Galun E., Hochberg A. 2010. The oncofetal H19 RNA connection: hypoxia, p53 and cancer. Biochim. Biophys. Acta 1803, 443–451 10.1016/j.bbamcr.2010.01.010 (doi:10.1016/j.bbamcr.2010.01.010) [DOI] [PubMed] [Google Scholar]
- 58.Hedborg F., Fischer-Colbrie R., Östlin N., Sandstedt B., Tran M. G., Maxwell P. H. 2010. Differentiation in neuroblastoma: diffusion-limited hypoxia induces neuro-endocrine secretory protein 55 and other markers of a chromaffin phenotype. PLoS ONE 17, e12825. 10.1371/journal.pone.0012825 (doi:10.1371/journal.pone.0012825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soltis J. 2004. The signal functions of early infant crying. Behav. Brain Sci. 27, 443–458 [PubMed] [Google Scholar]
- 60.Locke J. L., Bogin B. 2006. Language and life history: a new perspective on the development and evolution of human language. Behav. Brain Sci. 29, 259–280 10.1017/S0140525X0600906X (doi:10.1017/S0140525X0600906X) [DOI] [PubMed] [Google Scholar]
- 61.Gurven M., Kaplan H. 2007. Hunter–gatherer longevity: cross-cultural perspectives. Popul. Dev. Rev. 33, 321–365 10.1111/j.1728-4457.2007.00171.x (doi:10.1111/j.1728-4457.2007.00171.x) [DOI] [Google Scholar]
- 62.Strassmann B. I., Gillespie B. 2002. Life-history theory, fertility and reproductive success in humans. Proc. R. Soc. Lond. B 269, 553–562 10.1098/rspb.2001.1912 (doi:10.1098/rspb.2001.1912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cecchini M., Lai C., Langher V. 2010. Dysphonic newborn cries allow prediction of their perceived meaning. Infant Behav. Dev. 33, 314–320 10.1016/j.infbeh.2010.03.006 (doi:10.1016/j.infbeh.2010.03.006) [DOI] [PubMed] [Google Scholar]
- 64.Strathearn L., Li J., Fonagy P., Montague P. R. 2008. What's in a smile? Maternal brain responses to infant facial cues. Pediatrics 122, 40–51 10.1542/peds.2007-1566 (doi:10.1542/peds.2007-1566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kushnick G. 2009. Parental supply and offspring demand amongst Karo Batak mothers and children. J. Biosoc. Sci. 41, 183–193 10.1017/S0021932008002988 (doi:10.1017/S0021932008002988) [DOI] [PubMed] [Google Scholar]
- 66.Llewellyn C. H., Van Jaarsveld C. H., Johnson L., Carnell S., Wardle J. 2010. Nature and nurture in infant appetite: analysis of the Gemini twin birth cohort. Am. J. Clin. Nutr. 91, 1172–1179 10.3945/ajcn.2009.28868 (doi:10.3945/ajcn.2009.28868) [DOI] [PubMed] [Google Scholar]
- 67.Gregg C., Zhang J., Weissbourd B., Luo S., Schroth G. P., Haig D., Dulac C. 2010. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science 329, 643–648 10.1126/science.1190830 (doi:10.1126/science.1190830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schaller F., Watrin F., Sturny R., Massacrier A., Szepetowski P., Muscatelli F. 2010. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum. Mol. Genet. 19, 4893–4905 10.1093/hmg/ddq424 (doi:10.1093/hmg/ddq424) [DOI] [PubMed] [Google Scholar]
- 69.Vrang N., et al. 2010. The imprinted gene neuronatin is regulated by metabolic status and associated with obesity. Obesity (Silver Spring) 18, 1289–1296 10.1038/oby.2009.361 (doi:10.1038/oby.2009.361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davies K., Bowden L., Smith P., Dean W., Hill D., Furuumi H., Sasaki H., Cattanach B., Reik W. 2002. Disruption of mesodermal enhancers for Igf2 in the minute mutant. Development 129, 1657–1668 [DOI] [PubMed] [Google Scholar]
- 71.Macdonald K., Macdonald T. M. 2010. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv. Rev. Psychiatry 18, 1–21 10.3109/10673220903523615 (doi:10.3109/10673220903523615) [DOI] [PubMed] [Google Scholar]
- 72.Jiang Y. H., et al. 2010. Altered ultrasonic vocalization and impaired learning and memory in Angelman syndrome mouse model with a large maternal deletion from Ube3a to Gabrb3. PLoS ONE (doi:10.1371/journal.pone.0012278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crespi B. J. 2009. Social conflict resolution, life history, and the reconstruction of skew. In Reproductive skew in vertebrates: proximate and ultimate causes (eds Hager R., Jones C. B.), pp. 480–507 Cambridge, UK: Cambridge University Press [Google Scholar]
- 74.Haig D., Wharton R. 2003. Prader–Willi syndrome and the evolution of human childhood. Am. J. Hum. Biol. 15, 320–329 10.1002/ajhb.10150 (doi:10.1002/ajhb.10150) [DOI] [PubMed] [Google Scholar]
- 75.Crespi B. J. 2008. Genomic imprinting in the development and evolution of psychotic spectrum conditions. Biol. Rev. 83, 441–493 [DOI] [PubMed] [Google Scholar]
- 76.Crespi B. J., Badcock C. 2008. Psychosis and autism as diametrical disorders of the social brain. Behav. Brain Sci. 31, 241–261 10.1017/S0140525X08004214 (doi:10.1017/S0140525X08004214) [DOI] [PubMed] [Google Scholar]
- 77.Campbell B. 2006. Adrenarche and the evolution of human life history. Am. J. Hum. Biol. 18, 569–589 10.1002/ajhb.20528 (doi:10.1002/ajhb.20528) [DOI] [PubMed] [Google Scholar]
- 78.Flinn M. V., Muehlenbein M. P., Ponzi D. 2009. Social endocrinology of the human child. Behav. Brain Sci. 32, 27–28 [Google Scholar]
- 79.Del Giudice M., Angeleri R., Manera V. 2009. The juvenile transition: a developmental switch point in human life history. Dev. Rev. 29, 1–31 10.1016/j.dr.2008.09.001 (doi:10.1016/j.dr.2008.09.001) [DOI] [Google Scholar]
- 80.Ellis B. J., Essex M. J. 2007. Family environments, adrenarche, and sexual maturation: a longitudinal test of a life history model. Child Dev. 78, 1799–1817 10.1111/j.1467-8624.2007.01092.x (doi:10.1111/j.1467-8624.2007.01092.x) [DOI] [PubMed] [Google Scholar]
- 81.Ong K. K., Potau N., Petry C. J., Jones R., Ness A. R., Honour J. W., de Zegher F., Ibáñez L., Dunger D. B. & Avon Longitudinal Study of Parents and Children Study Team 2004. Opposing influences of prenatal and postnatal weight gain on adrenarche in normal boys and girls. J. Clin. Endocrinol. Metab. 89, 2647–2651 10.1210/jc.2003-031848 (doi:10.1210/jc.2003-031848) [DOI] [PubMed] [Google Scholar]
- 82.Pratt J. H., Manatunga A. K., Li W. 1994. Familial influences on the adrenal androgen excretion rate during the adrenarche. Metabolism 43, 186–189 10.1016/0026-0495(94)90243-7 (doi:10.1016/0026-0495(94)90243-7) [DOI] [PubMed] [Google Scholar]
- 83.Van den Berg S. M., Setiawan A., Bartels M., Polderman T. J., Van der Vaart A. W., Boomsma D. I. 2006. Individual differences in puberty onset in girls: Bayesian estimation of heritabilities and genetic correlations. Behav. Genet. 36, 261–270 10.1007/s10519-005-9022-y (doi:10.1007/s10519-005-9022-y) [DOI] [PubMed] [Google Scholar]
- 84.Ibáñez L., Potau N., Carrascosa A. 1998. Insulin resistance, premature adrenarche, and a risk of the polycystic ovary syndrome (PCOS). Trends Endocrinol. Metab. 9, 72–77 10.1016/S1043-2760(98)00014-9 (doi:10.1016/S1043-2760(98)00014-9) [DOI] [PubMed] [Google Scholar]
- 85.Leung A. K., Robson W. L. 2008. Premature adrenarche. J. Pediatr. Health Care 22, 230–233 10.1016/j.pedhc.2007.07.002 (doi:10.1016/j.pedhc.2007.07.002) [DOI] [PubMed] [Google Scholar]
- 86.Bogin B. 2009. Childhood, adolescence, and longevity: a multilevel model of the evolution of reserve capacity in human life history. Am. J. Hum. Biol. 21, 567–577 10.1002/ajhb.20895 (doi:10.1002/ajhb.20895) [DOI] [PubMed] [Google Scholar]
- 87.Wells J. C. 2007. Sexual dimorphism of body composition. Best Practice Res. Clin. Endocrin. Metab. 21, 415–430 10.1016/j.beem.2007.04.007 (doi:10.1016/j.beem.2007.04.007) [DOI] [PubMed] [Google Scholar]
- 88.Surbey M. K. 1998. Parent and offspring strategies in the transition at adolescence. Hum. Nat. 9, 67–94 10.1007/s12110-998-1012-3 (doi:10.1007/s12110-998-1012-3) [DOI] [PubMed] [Google Scholar]
- 89.Ellis B. J. 2004. Timing of pubertal maturation in girls: an integrated life history approach. Psychol. Bull. 130, 920–958 10.1037/0033-2909.130.6.920 (doi:10.1037/0033-2909.130.6.920) [DOI] [PubMed] [Google Scholar]
- 90.Nettle D., Coall D. A., Dickins T. E. 2010. Birthweight and paternal involvement predict early reproduction in British women: evidence from the National Child Development Study. Am. J. Hum. Biol. 22, 172–179 [DOI] [PubMed] [Google Scholar]
- 91.Golub M. S., Collman G. W., Foster P. M., Kimmel C. A., Rajpert-De Meyts E., Reiter E. O., Sharpe R. M., Skakkebaek N. E., Toppari J. 2008. Public health implications of altered puberty timing. Pediatrics 121 (Suppl. 3), S218–S230 10.1542/peds.2007-1813G (doi:10.1542/peds.2007-1813G) [DOI] [PubMed] [Google Scholar]
- 92.Tither J. M., Ellis B. J. 2008. Impact of fathers on daughters' age at menarche: a genetically and environmentally controlled sibling study. Dev. Psychol. 44, 1020–1429 10.1037/a0013065 (doi:10.1037/a0013065) [DOI] [PubMed] [Google Scholar]
- 93.Gluckman P. D., Hanson M. A. 2006. Changing times: the evolution of puberty. Mol. Cell. Endocrinol. 254–255, 26–31 10.1016/j.mce.2006.04.005 (doi:10.1016/j.mce.2006.04.005) [DOI] [PubMed] [Google Scholar]
- 94.Unanue N., Bazaes R., Iñiguez G., Cortés F., Avila A., Mericq V. 2007. Adrenarche in Prader–Willi syndrome appears not related to insulin sensitivity and serum adiponectin. Horm. Res. 67, 152–158 10.1159/000096742 (doi:10.1159/000096742) [DOI] [PubMed] [Google Scholar]
- 95.Silver H. K. 1964. Asymmetry, short stature, and variations in sexual development. A syndrome of congenital malformations. Am. J. Dis. Child. 107, 495–515 [DOI] [PubMed] [Google Scholar]
- 96.Wollmann H. A., Kirchner T., Enders H., Preece M. A., Ranke M. B. 1995. Growth and symptoms in Silver–Russell syndrome: review on the basis of 386 patients. Eur. J. Pediatr. 154, 958–968 10.1007/BF01958638 (doi:10.1007/BF01958638) [DOI] [PubMed] [Google Scholar]
- 97.Lodish M. B., et al. 2010. Intrauterine growth retardation associated with precocious puberty and sertoli cell hyperplasia. Horm. Metab. Res. 42, 682–688 10.1055/s-0030-1252021 (doi:10.1055/s-0030-1252021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kotzot D. 2004. Maternal uniparental disomy 14 dissection of the phenotype with respect to rare autosomal recessively inherited traits, trisomy mosaicism, and genomic imprinting. Ann. Genet. 47, 251–260 10.1016/j.anngen.2004.03.006 (doi:10.1016/j.anngen.2004.03.006) [DOI] [PubMed] [Google Scholar]
- 99.Kotzot D. 2007. Growth parameters in maternal uniparental disomy 7 and 14. Eur. J. Pediatr. 166, 1143–1149 10.1007/s00431-006-0396-5 (doi:10.1007/s00431-006-0396-5) [DOI] [PubMed] [Google Scholar]
- 100.de Sanctis C., Lala R., Matarazzo P., Andreo M., de Sanctis L. 2003. Pubertal development in patients with McCune–Albright syndrome or pseudohypoparathyroidism. J. Pediatr. Endocrinol. Metab. 16(Suppl. 2), 293–296 [PubMed] [Google Scholar]
- 101.Clayton-Smith J., Laan L. 2003. Angelman syndrome: a review of the clinical and genetic aspects. J. Med. Genet. 40, 87–95 10.1136/jmg.40.2.87 (doi:10.1136/jmg.40.2.87) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Campos-Castello J.Angelman Syndrome. Orphanet Encyclopedia. 2004. See http://www.orpha.net/path/GB/uk-Angelman.pdf .
- 103.Sippell W. G., Partsch C. J., Wiedemann H. R. 1989. Growth, bone maturation and pubertal development in children with the EMG-syndrome. Clin. Genet. 35, 20–28 10.1111/j.1399-0004.1989.tb02901.x (doi:10.1111/j.1399-0004.1989.tb02901.x) [DOI] [PubMed] [Google Scholar]
- 104.Ibáñez L., Jiménez R., de Zegher F. 2006. Early puberty-menarche after precocious pubarche: relation to prenatal growth. Pediatrics 117, 117–121 10.1542/peds.2005-0664 (doi:10.1542/peds.2005-0664) [DOI] [PubMed] [Google Scholar]