Abstract
There is growing evidence that the reproductive schedules of female mammals can be affected by conditions experienced during early development, with low parental investment leading to accelerated life-history strategies in the offspring. In humans, the relationships between early-life conditions and timing of puberty are well studied, but much less attention has been paid to reproductive behaviour. Here, we investigate associations between early-life conditions and age at first pregnancy (AFP) in a large, longitudinally studied cohort of British women (n = 4553). Low birthweight for gestational age, short duration of breastfeeding, separation from mother in childhood, frequent family residential moves and lack of paternal involvement are all independently associated with earlier first pregnancy. Apart from that of birthweight, the effects are robust to adjustment for family socioeconomic position (SEP) and the cohort member's mother's age at her birth. The association between childhood SEP and AFP is partially mediated by early-life conditions, and the association between early-life conditions and AFP is partially mediated by emotional and behavioural problems in childhood. The overall relationship between early-life adversities and AFP appears to be approximately additive.
Keywords: developmental plasticity, developmental programming, maternal effects, parental effects, life history, National Child Development Study
1. Introduction
There is growing evidence that the reproductive schedules of female mammals can be influenced by conditions experienced during early development. For example, female rats whose mothers are kept under caloric restriction during pregnancy reach puberty earlier than controls [1]. Similarly, rat pups that receive low levels of maternal licking and grooming post-birth reach sexual maturity earlier and copulate more indiscriminately than those receiving more maternal attention [2,3]. Female rhesus monkeys (Macaca mulatta) that experience poor maternal care develop an increased interest in infants during the juvenile period, suggestive of accelerated reproductive schedules [4]. Effects of this kind may have an adaptive basis, since low parental investment may either constrain the phenotype attainable as an adult, and thus alter the individual's optimal life-history strategy, or else carry information about the prevailing ecological conditions. Thus, natural selection may have favoured mechanisms for facultative acceleration of reproductive scheduling in response to early adversity (for a number of discussions along these lines with slightly different emphases, see [5–10]).
These mechanisms may be conserved in humans. Many studies have found associations between low birthweight in girls and subsequent accelerated maturation [11–16], or between low parental investment in the first five years post-birth and subsequent accelerated maturation [6,10,17–23]. These studies are by necessity correlational rather than experimental, and thus the main difficulty has been demonstrating that the effects of childhood conditions effects are causal, rather than, for example, there being some genetic correlation between parental investment behaviours and offspring reproductive schedules [24]. However, evidence from some genetically informative study designs [25] and natural experiments [26] suggests that the effects may be at least partly causal (though see [27–29]). Belsky et al. [6] suggested that the post-birth effects might be specifically mediated by childhood emotional symptoms: girls receiving low levels of parental investment in early life would develop more emotional and behavioural problems in childhood, and these problems would be associated with faster subsequent reproductive schedules.
Previous studies of the associations between early conditions and reproductive maturation in humans have almost all used age at puberty as the outcome variable, sometimes in conjunction with other measures such as age at first sexual activity. There are far fewer studies which go on to examine the actual schedule of childbearing itself. Ellis et al. [30] and our own earlier study [31] are partial exceptions, in that they examined effects of early conditions on the likelihood of teenage pregnancy, but they did not consider age at first pregnancy (AFP) across the full range of ages. Pesonen et al. [26] examined not only age at first reproduction, but also the total family size and inter-birth intervals in a Finnish cohort who were children during World War II, but their only measure of early-life conditions was whether the child had been evacuated away from parents or not. The other notable exception is a study by Chisholm et al. [32], which found that early-life stress was negatively associated with age at first childbearing. However, the study used a modest opportunity sample of Australian women, and the early-life measures were based on retrospective recall by the women themselves.
Another feature of the literature on post-birth effects on reproductive development in humans is that paternal effects have received much more attention than maternal effects. This reflects early theories giving fathers a special role in girls' development [33]. However, in non-human mammals, it is maternal effects that have been found to be particularly important [2,4], and they are likely to be so in humans too. A strong design should therefore measure both maternal and paternal influences [18,23].
In this paper, we use data from a large, longitudinally studied cohort of British women (the National Child Development Study, NCDS) to examine the associations between early-life conditions and AFP. We include all women who had ever been pregnant by age 33, and assess both pre-birth (birthweight for gestational age) and post-birth factors. The post-birth factors studied not only include paternal involvement, but also breastfeeding, separation of the child from mother during the first five years of life, and, as a measure of general family disruption, number of residential moves during early childhood. We will compare the relative strengths of effect of the different predictors, and also examine whether they operate independently and additively. Our dataset has the advantages of exceptional social representativeness (the NCDS cohort consists of all children born in the UK during a particular week in 1958, and two thirds were still in the study after 33 years), detailed socioeconomic indicators, and a genuinely prospective design (the childhood variables were collected during childhood, and have subsequently been linked with the later-collected data on AFP, thus avoiding any issues of recall bias).
We predict that low birthweight for gestational age (BGA), lack of breastfeeding, separation from mother, residential disruption and lack of paternal involvement will all be associated with earlier first pregnancy. We initially test this prediction with no other factors controlled for, and then, in a second model, add in childhood socioeconomic position (SEP) and the cohort member's mother's age at birth, as controls. Each of these is likely to covary with AFP, since there are strong socioeconomic gradients in age at reproduction in developed populations [34], and there are also suggestions in the literature of intergenerational transmission of age at childbearing [35,36]. This second analysis is conservative, since SEP and early-life conditions are not necessarily alternative explanatory factors for variation in reproductive timing. Rather, the effect of low childhood SEP on reproductive behaviour may be mediated by harsher early-life conditions [34]. We thus additionally use structural equation modelling to examine whether adverse early-life experiences mediate the relationship between SEP and AFP, and also to test the proposal of Belsky et al. [6] that the relationship between adverse early-life experiences and AFP will itself be mediated by childhood emotional and behavioural problems.
2. Methods
(a). Sample
The NCDS is an ongoing longitudinal study of all people born in the UK between 3rd and 9th March 1958 (initial n = 17 416), augmented by those who immigrated into the UK between 1958 and 1974 and had a birth date in the target week. The cohort has been repeatedly surveyed at irregular intervals in the years since 1958, and the data include parental and school reports, perinatal health records, physician assessments and interviews with the cohort members themselves. We consider here only women who were still in the study at age 33 (1991). There was considerable sample attrition between childhood and 1991, but the probability of attrition was unrelated to the early-life conditions we measure (see electronic supplementary material). Our sample of 4553 women includes only those who gave a valid year for their first pregnancy in NCDS variable n502 023, which is 78 per cent of women still in the study at 1991. The remainder had either not been pregnant by this age, or had not provided the relevant information.
Most of the variables studied contain around 10 per cent missing values, owing to non-response or coding as ‘other’. We dealt with these using the multiple imputation procedure of SPSS v. 17.0 [37]. In this procedure, multivariate regression is used to produce a parametric model for the missing values, and five complete versions of the dataset are created by Monte-Carlo draws from the conditional probability distribution of missing values given the observed ones [38]. The analyses presented here are based on the pooled results from the five versions of the dataset. Replacing missing values with means or medians instead of using multiple imputation produces almost identical results.
(b). Measures
We converted the year of the woman's first pregnancy into a chronological age, to give the AFP variable. Note that an AFP of 25 means that a woman was first pregnant in the calendar year of her 25th birthday, rather than in the interval beginning with her 25th birthday and ending with her next birthday. We considered the following measures of early-life conditions: birthweight for gestational age (BGA), breastfeeding received (BFD), longest period of separation from mother in the first seven years, involvement of father in management of the child in the first seven years (PAT) and number of family residential moves in the first seven years (MOV). BGA was calculated from the original birthweight and gestational age reports as outlined in Nettle et al. [31]. For the statistical analysis (though not for the creation of figure 1), the other early-life conditions variables were recoded into dichotomies, with 0 representing high investment or undisrupted conditions, and 1 representing low investment or disrupted conditions. The electronic supplementary material provides descriptive statistics, details of recoding and numbers of missing values for each of the variables.
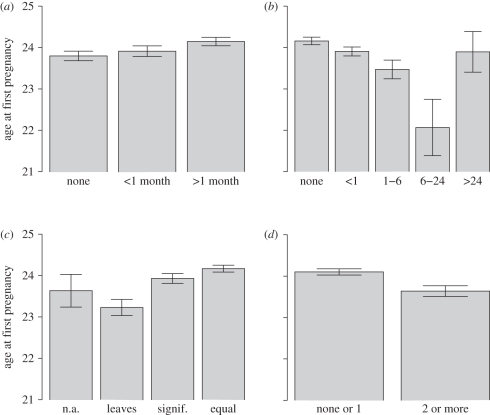
Figure 1.
Marginal means (±1 s.e.) of age at first pregnancy, adjusted for childhood socioeconomic position and mother's age at cohort member's birth (a) by breastfeeding received (none, less than one month, more than one month), (b) by longest separation from mother in first five years of life (none of more than one week, less than one month, 1–6 months, 6–24 months, more than 24 months), (c) by paternal involvement in first seven years (inapplicable, leaves to mother, significant, equal to mother), and (d) by number of family residential moves in the first seven years (none or one, two or more).
To measure childhood SEP, we used three variables: paternal social class in 1958, which was assessed using the registrar general's fivefold classification of occupations, cohort member's mother's age at leaving education and the proportion of fathers in the child's school class at age 7 who had non-manual occupations (defined as classes I, II and non-manual class III). These three variables covary strongly, so we used principal components analysis to extract a single underlying factor. This factor accounted for 55–56% of the total variation in all five imputations, with each of the three variables loading on it between 0.7 and 0.8. Scores were saved using the regression method, and these scores constitute the SEP variable for each woman. We also measured the cohort member's mother's age at the time of the cohort member's birth (MAG). Note that this variable is not identical to the cohort member's mother's age at her first birth, a datum, which is unfortunately not available for the whole sample. However, we also repeated key analyses for the subset of girls who were their mother's first pregnancy, and for whom, therefore, mother's age at her birth is mother's age at first birth (see electronic supplementary material).
Emotional and behavioural maladjustment in childhood (EBM) was assessed by schoolteachers when the girls were 11, using items from the Bristol Social Adjustment Guides [39]. These consist of ratings on 12 dimensions (unforthcomingness, withdrawal, depression, anxiety about acceptance by adults, hostility towards adults, writing off adults and standards, anxiety about acceptance by children, hostility towards children, restlessness, inconsequential behaviour, miscellaneous symptoms and miscellaneous nervous symptoms), which are here summed to give an overall score (higher score = more EBM). EBM scores were highly skewed, and so we square-root transformed them for analysis.
(c). Analysis
In a first analysis (model 1), we used a general linear model to investigate whether each of the five early-life conditions variables significantly predicted AFP, with BGA as a covariate and BFD, SFM, PAT and MOV as factors. Subsequently, we added SEP and MAG as covariates (model 2). If the early-life conditions variables continue to have significant predictive power, this suggests that the relationships are not spurious artefacts of differences in SEP. Finally, we fitted a structural equation model (using maximum-likelihood estimation) to test whether the early-life conditions variables mediate the relationships between childhood SEP and AFP, and whether EBM in childhood mediates between early-life conditions and AFP. All statistical analyses were performed in SPSS v. 17.0 [37], apart from the structural equation modelling, which used Amos v. 17.0 [40].
3. Results
The first analysis (model 1 in table 1) contained AFP as the outcome variable, and the early-life conditions variables as the predictors. As the table shows, all five predictors had significant effects in the predicted directions. The parameter estimates can be interpreted as the change in AFP associated with shifting from one value of the predictor to the other (or, in the case of BGA, increasing BGA by one standard deviation). Thus, for example, low paternal involvement (PAT) is associated with a reduction of 0.74 years in AFP, all else being equal and separated from mother under five is associated with a reduction of 0.64 years.
Table 1.
Results of general linear models predicting age at first pregnancy (AFP) from developmental conditions variables (BGA, birthweight for gestational age; BFD, short breastfeeding; SFM, separation from mother; PAT, low paternal involvement; MOV, residential moves in childhood), childhood socioeconomic position (SEP), and cohort member's mother's age at birth (MAG). Parameters estimates and their confidence intervals are pooled from across all five imputations of the dataset.
| model 1 |
model 2 |
|||
|---|---|---|---|---|
| predictor | parameter estimate | 95% CI | parameter estimate | 95% CI |
| BGA | 0.16* | (0.03 to −0.29) | 0.08 | (−0.05 to 0.21) |
| BFD | −0.50* | (−0.76 to −0.24) | −0.25a | (−0.52 to 0.02) |
| SFM | −0.64* | (−1.08 to −0.20) | −0.59* | (−1.00 to −0.18) |
| PAT | −0.74* | (−1.14 to −0.35) | −0.66* | (−0.99 to −0.32) |
| MOV | −0.54* | (−0.85 to −0.23) | −0.41* | (−0.72 to −0.10) |
| SEP | — | — | 0.89* | (0.76 to 1.02) |
| MAG | — | — | 0.06* | (0.04 to 0.08) |
*p < 0.05.
ap = 0.07.
The second analysis (model 2 in table 1) additionally included SEP and MAG as covariates. There is a large effect of childhood SEP, with every standard deviation of SEP associated with 0.89 years' delay in AFP. There is also an effect of cohort member's mother's age at cohort member's birth, with women whose mothers were older at their birth in turn having their children later. The effect of BGA becomes non-significant with the additional covariates entered, and that of BFD becomes borderline (p = 0.07). However, the effects of the other early-life conditions variables remain significant with their strengths only mildly attenuated. For example, the parameter estimate for PAT is reduced from 0.74 to 0.66 years. Figure 1 shows the marginal means for AFP, controlled for SEP and MAG, associated with each level of the early-life conditions variables (here, the variables have been left in their original coding rather than made into dichotomies in order to get a better visual sense of the nature of the associations).
To examine the potentially cumulative effect of early-life exposures, we computed an index of early-life adversity (ADV). This was simply the sum of BFD, SFM, PAT and MOV. Since each of these variables is coded so that 1 represents the low investment or high disruption situation, and 0 represents the alternative, the index is a measure of how many of the adverse conditions the cohort member was exposed to (0–4). We used a general linear model to calculate marginal means and 95% confidence intervals for AFP for each value of ADV, adjusted for SEP and MAG. The results are shown in figure 2. Each additional exposure is associated with an earlier AFP, in an apparently additive fashion, though the standard error for four exposures is large because of the small number of cases.
Figure 2.
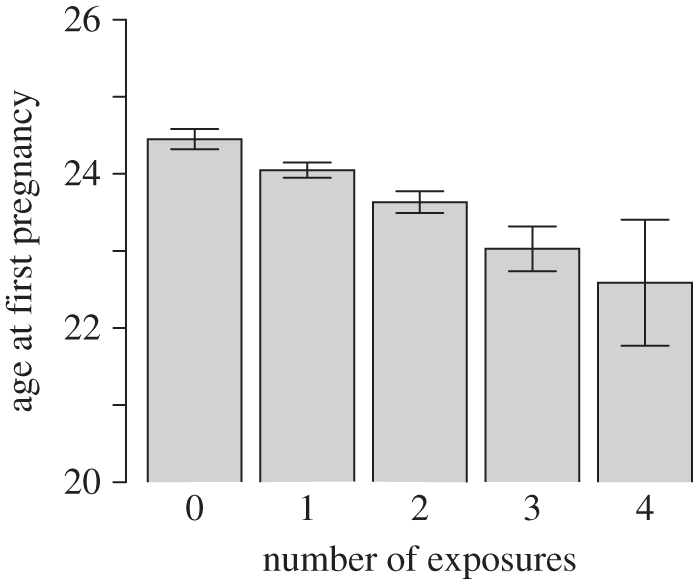
Marginal means (±1 s.e.) of AFP (adjusted for childhood SEP and mother's age at cohort member's birth) according to the number of early-life adversities the cohort member was exposed to, from short breastfeeding, separation from mother, lack of paternal involvement and frequent family residential moves.
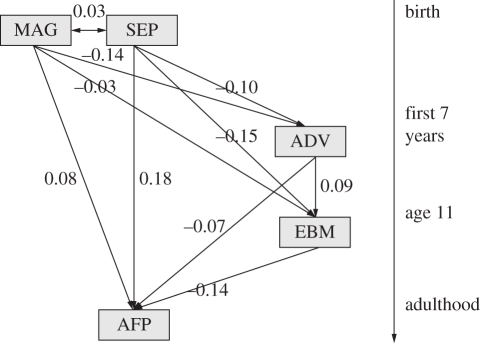
Finally, we fitted a structural equation model to the relationships among the study variables. The model reveals a plethora of significant associations between family background, ADV and emotional and behavioural problems in childhood (figure 3). EBM has a significant negative association with AFP. SEP has a direct association with AFP, and also indirect ones via ADV and EBM. ADV has a direct association with AFP, and also an indirect one via EBM. These mediation pathways are, however, weak (table 2). Thus, only a small proportion of the covariance of childhood socioeconomic status and younger AFP can be explained by children of lower SEP experiencing harsher early-life conditions, and only a small proportion of the covariance of early-life conditions and AFP can be explained by harsher early-life conditions being associated with greater EBM in childhood.
Figure 3.
Standardized parameter estimates from fitting a structural equation model to the possible relationships between the main study variables. SEP, childhood socioeconomic position; MAG, cohort member's mother's age at her birth; ADV, number of early-life adversities experienced; EBM, emotional and behavioural maladjustment at age 11; AFP, age at first pregnancy.
Table 2.
Summary of standardized direct and indirect effects of predictor variables on age at first pregnancy, from the structural equation model.
| variable | effects |
|---|---|
| socioeconomic position (SEP) | total 0.213 |
| direct 0.184 | |
| indirect (via ADV and EBM) 0.029 | |
| cohort member's mother's age (MAG) | total 0.092 |
| direct 0.077 | |
| indirect (via ADV and EBM) 0.015 | |
| early-life adversities (ADV) | total −0.082 |
| direct −0.071 | |
| indirect (via EBM) −0.012 | |
| emotional and behavioural maladjustment (EBM) | direct −0.137 |
4. Discussion
In this cohort of British women, the early-life factors low birthweight for gestational age, short duration of breastfeeding, separation from mother, lack of paternal involvement and frequent residential moves are all independently associated with earlier first pregnancy. Adjusting for childhood socioeconomic position and the cohort member's mother's age renders the effect of low birthweight for gestational age non-significant, but all the post-birth effects remain significant or borderline. The early-life exposures appear to operate approximately additively (figure 2). This echoes a finding by Coall & Chisholm that early-life stressors have an approximately additive negative relationship with age at menarche ([20], figure 1). The effect sizes are not trivial, with each additional exposure associated with first pregnancy nearly half a year earlier on average.
The findings with respect to lack of paternal involvement are consistent with several others that have found earlier menarche or sexual activity, or greater likelihood of teenage pregnancy, in girls from father-absent households (e.g. [17,25,30,41]), but extends these findings by showing that the whole distribution of AFP is shifted by paternal involvement. The association with separation from mother which we observed is comparable to the finding by Pesonen et al. [26] that evacuation away from parents during childhood was associated with earlier childbearing (though their effect was significant only in men), and also with recent findings that adoption from birth family is associated with increased likelihood of precocious puberty [42]. The effect of a separation of 6–24 months in the first five years was, unexpectedly, stronger than that of a separation of more than 24 months (figure 1). A possible explanation is that the group with a separation of more than 24 months includes many individuals who were permanently and stably adopted into a new family, whereas the 6–24 month group includes many girls who had ongoing but intermittent relationships with their birth mothers. In accordance with this possibility, the parent interview at age 7 was completed by the birth mother in 72.7 per cent of cases for the ‘6–24 month separation’ group, but only 5.4 per cent of cases for the ‘More than 24 month’ separation group, for whom the modal respondent was an adoptive or foster mother. Thus, it may be that stable adoption has similar consequences to the family being intact, whereas intermittent or insecure relationships are associated with accelerated reproductive schedules.
The association between low birthweight for gestational age and reproductive schedule is in the predicted direction, but weak, and does not survive control for SEP and maternal age. This concurs with our previous findings on teenage pregnancy, where birthweight effects were found but were small and not robust [31]. However, this does not mean that in utero conditions are not associated with later reproductive schedules. Rather, birthweight for gestational age is likely to be a relatively crude proxy for those aspects of foetal experience, which are associated with adult phenotype [43].
In addition to the associations that were our main focus, we observed powerful influences of childhood SEP and maternal age at cohort member's birth on the cohort members' ages at first pregnancy. The maternal age effect could suggest either heritable genetic influence on reproductive schedules [44], or else vertical cultural transmission of childbearing behaviour [45]. Alternatively, there may be unmeasured intergenerational continuities in the social environment that evoke similar behaviour in mothers and their daughters. The effect of SEP was predictable given the strong socioeconomic gradients in age at first childbearing in the UK [34]. However, we expected that there might be stronger mediation of the social gradient by early-life experiences. Although there is a significant mediation pathway from childhood SEP to AFP via early-life adversity, it is very weak relative to the direct one (figure 3 and table 2). It is possible that more sensitive and comprehensive measures of early-life conditions might produce a larger mediation effect. We also observed mediation of the relationship between early-life adversity and AFP by emotional and behavioural problems in childhood. This effect was predicted by Belsky et al. [6], and also accords with qualitative evidence of an association between unhappiness in childhood and early childbearing [46]. However, this mediation pathway was also weak, and accounted for only a fraction of the covariation of early-life conditions and reproductive schedule.
One limitation of the study is that not all women who would ever reproduce had begun to do so by age 33, when we measured AFP. However, reproductive onset was generally in the 20s for this generation of the British population, and since a large preponderance of the sample (79%) had been pregnant by 33, this is unlikely to strongly affect the results. A more serious limitation is that the study design does not allow us to separate genetic explanations for the associations (for example, a genetic correlation between maternal breastfeeding behaviour and offspring reproductive schedule), from the hypothesis that the early-life environmental inputs play a causal role. However, including maternal age at cohort member's birth is a partial, albeit imperfect, control for heritable aspects of reproductive strategy, and this does not abolish the early-life associations. (We also tested whether the association between early-life adversity and AFP is robust to control for maternal age in the first-borns only, and it is; see the electronic supplementary material). Moreover, it seems relatively unlikely that the detailed patterns shown for example in figures 1 and 2 could easily be explained by genetic correlations alone.
The idea that harsh conditions very early in life might cause acceleration of reproductive schedules as an adaptive response has developed somewhat independently in behavioural ecology, where these phenomena are described as maternal or parental effects [9,47], in developmental biology, where they are referred to as developmental programming or developmental induction [5,48] and in developmental psychology [6]. This study adds to the accumulating evidence that the mechanisms of developmental plasticity that have been studied experimentally in other species could work in similar ways in humans, and might exert powerful influences on reproductive behaviour, even in an industrialized, low-fertility population. Exactly how the early-life influences lead to earlier pregnancy, if they do, requires further investigation. Our previous study of this cohort showed that by age 16, girls can state personal ideal ages for parenthood, and that these goals correspond fairly well to their subsequent behaviour [31]. Early-life experiences may contribute to the setting of these goals, as well as to the tempo of physical maturation. If so, we need to unravel the exact epigenetic [48], physiological [43,49] and psychological mechanisms by which this happens.
Acknowledgements
The NCDS is coordinated by the Center for Longitudinal Studies, Institute of Education, London (www.cls.ioe.ac.uk). We would like to thank the generations of NCDS researchers whose endeavour has made studies like this one possible, and the CLS for making the data available.
References
- 1.Sloboda D. M., Howie G. J., Pleasants A., Gluckman P. D., Vickers M. H. 2009. Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PLoS ONE 4, e6744. 10.1371/journal.pone.0006744 (doi:10.1371/journal.pone.0006744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameron N. M., Fish E. W., Meaney M. J. 2008. Maternal influences on the sexual behavior and reproductive success of the female rat. Horm. Behav. 54, 178–184 10.1016/j.yhbeh.2008.02.013 (doi:10.1016/j.yhbeh.2008.02.013) [DOI] [PubMed] [Google Scholar]
- 3.Cameron N. M., Shahrokh D., Del Corpo A., Dhir S. K., Szyf M., Champagne F. A., Meaney M. J. 2008. Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. J. Neuroendocrinol. 20, 795–801 10.1111/j.1365-2826.2008.01725.x (doi:10.1111/j.1365-2826.2008.01725.x) [DOI] [PubMed] [Google Scholar]
- 4.Maestripieri D. 2005. Effects of early experience on female behavioural and reproductive development in rhesus macaques. Proc. R. Soc. B 272, 1243–1248 10.1098/rspb.2005.3059 (doi:10.1098/rspb.2005.3059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bateson P., et al. 2004. Developmental plasticity and human health. Nature 430, 419–421 10.1038/nature02725 (doi:10.1038/nature02725) [DOI] [PubMed] [Google Scholar]
- 6.Belsky J., Steinberg L., Draper P. 1991. Childhood experience, interpersonal development, and reproductive strategy—an evolutionary theory of socialization. Child Dev. 62, 647–670 10.2307/1131166 (doi:10.2307/1131166) [DOI] [PubMed] [Google Scholar]
- 7.Chisholm J. S. 1993. Death, hope, and sex: life-history theory and the development of reproductive strategies. Curr. Anthropol. 34, 1–24 10.1086/204131 (doi:10.1086/204131) [DOI] [Google Scholar]
- 8.Gluckman P. D., Hanson M. A., Beedle A. S. 2007. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am. J. Hum. Biol. 19, 1–19 10.1002/ajhb.20590 (doi:10.1002/ajhb.20590) [DOI] [PubMed] [Google Scholar]
- 9.Mousseau T. A., Fox C. W. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407 10.1016/S0169-5347(98)01472-4 (doi:10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 10.Quinlan R. J. 2003. Father absence, parental care, and female reproductive development. Evol. Hum. Behav. 24, 376–390 10.1016/S1090-5138(03)00039-4 (doi:10.1016/S1090-5138(03)00039-4) [DOI] [Google Scholar]
- 11.Adair L. S. 2001. Size at birth predicts age at menarche. Pediatrics 107, e59. 10.1542/peds.107.4.e59 (doi:10.1542/peds.107.4.e59) [DOI] [PubMed] [Google Scholar]
- 12.Cooper C., Kuh D., Egger P., Wadsworth M., Barker D. 1996. Childhood growth and age at menarche. Br. J. Obstet. Gynaecol. 103, 814–817 [DOI] [PubMed] [Google Scholar]
- 13.Ibanez L., Jimenez R., de Zegher F. 2006. Early puberty-menarche after precocious pubarche: relation to prenatal growth. Pediatrics 117, 117–121 10.1542/peds.2005-0664 (doi:10.1542/peds.2005-0664) [DOI] [PubMed] [Google Scholar]
- 14.Koziel S., Jankowska E. A. 2002. Effect of low versus normal birthweight on menarche in 14-year-old Polish girls. J. Paediatr. Child Health 38, 268–271 10.1046/j.1440-1754.2002.00793.x (doi:10.1046/j.1440-1754.2002.00793.x) [DOI] [PubMed] [Google Scholar]
- 15.Opdahl S., Nilsen T. I. L., Romundstad P. R., Vanky E., Carlsen S. M., Vatten L. J. 2008. Association of size at birth with adolescent hormone levels, body size and age at menarche: relevance for breast cancer risk. Br. J. Cancer 99, 201–206 10.1038/sj.bjc.6604449 (doi:10.1038/sj.bjc.6604449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sloboda D. M., Hart R., Doherty D. A., Pennell C. E., Hickey M. 2007. Rapid communication—age at menarche: influences of prenatal and postnatal growth. J. Clin. Endocrinol. Metab. 92, 46–50 10.1210/jc.2006-1378 (doi:10.1210/jc.2006-1378) [DOI] [PubMed] [Google Scholar]
- 17.Alvergne A., Faurie C., Raymond M. 2008. Developmental plasticity of human development: effects of early family environment in modern-day France. Physiol. Behav. 95, 625–632 10.1016/j.physbeh.2008.09.005 (doi:10.1016/j.physbeh.2008.09.005) [DOI] [PubMed] [Google Scholar]
- 18.Belsky J., Steinberg L. D., Houts R. M., Friedman S. L., DeHart G., Cauffman E., Roisman G. I., Halpern-Felsher B. L., Susman E. 2007. Family rearing antecedents of pubertal timing. Child Dev. 78, 1302–1321 10.1111/j.1467-8624.2007.01067.x (doi:10.1111/j.1467-8624.2007.01067.x) [DOI] [PubMed] [Google Scholar]
- 19.Bogaert A. F. 2008. Menarche and father absence in a national probability sample. J. Biosoc. Sci. 40, 623–636 10.1017/S0021932007002386 (doi:10.1017/S0021932007002386) [DOI] [PubMed] [Google Scholar]
- 20.Coall D. A., Chisholm J. S. 2010. Reproductive development and parental investment during pregnancy: moderating influence of mother's early environment. Am. J. Hum. Biol. 22, 143–153 [DOI] [PubMed] [Google Scholar]
- 21.Ellis B. J., Essex M. J. 2007. Family environments, adrenarche, and sexual maturation: a longitudinal test of a life history model. Child Dev. 78, 1799–1817 10.1111/j.1467-8624.2007.01092.x (doi:10.1111/j.1467-8624.2007.01092.x) [DOI] [PubMed] [Google Scholar]
- 22.Ellis B. J., Garber J. 2000. Psychosocial antecedents of variation in girls' pubertal timing: maternal depression, stepfather presence, and marital and family stress. Child Dev. 71, 485–501 10.1111/1467-8624.00159 (doi:10.1111/1467-8624.00159) [DOI] [PubMed] [Google Scholar]
- 23.Ellis B. J., McFadyen-Ketchum S., Dodge K. A., Pettit G. S., Bates J. E. 1999. Quality of early family relationships and individual differences in the timing of pubertal maturation in girls: a longitudinal test of an evolutionary model. J. Pers. Soc. Psychol. 77, 387–401 10.1037/0022-3514.77.2.387 (doi:10.1037/0022-3514.77.2.387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moffitt T. E., Caspi A., Belsky J., Silva P. A. 1992. Childhood experience and the onset of menarche—a test of a sociobiological model. Child Dev. 63, 47–58 10.2307/1130900 (doi:10.2307/1130900) [DOI] [PubMed] [Google Scholar]
- 25.Tither J. M., Ellis B. J. 2008. Impact of fathers on daughters' age at menarche: a genetically and environmentally controlled sibling study. Dev. Psychol. 44, 1409–1420 10.1037/a0013065 (doi:10.1037/a0013065) [DOI] [PubMed] [Google Scholar]
- 26.Pesonen A.-K., Räikkönen K., Heinonen K., Kajantie E., Forsén T., Eriksson J. G. 2008. Reproductive traits following a parent–child separation trauma during childhood: a natural experiment during World War II. Am. J. Hum. Biol. 20, 345–351 10.1002/ajhb.20735 (doi:10.1002/ajhb.20735) [DOI] [PubMed] [Google Scholar]
- 27.Comings D. E., Muhleman D., Johnson J. P., MacMurray J. P. 2002. Parent–daughter transmission of the androgen receptor gene as an explanation of the effect of father absence on age of menarche. Child Dev. 73, 1046–1051 10.1111/1467-8624.00456 (doi:10.1111/1467-8624.00456) [DOI] [PubMed] [Google Scholar]
- 28.Mendle J., Harden K. P., Turkheimer E., Van Hulle C. A., D'Onofrio B. M., Brooks-Gunn J., Rodgers J. L., Emery R. E., Lahey B. B. 2009. Associations between father absence and age of first sexual intercourse. Child Dev. 80, 1463–1480 10.1111/j.1467-8624.2009.01345.x (doi:10.1111/j.1467-8624.2009.01345.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendle J., Turkheimer E., D'Onofrio B. M., Lynch S. K., Emery R. E., Slutske W. S., Martin N. G. 2006. Family structure and age at menarche: a children-of-twins approach. Dev. Psychol. 42, 533–542 10.1037/0012-1649.42.3.533 (doi:10.1037/0012-1649.42.3.533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis B. J., Bates J. E., Dodge K. A., Fergusson D. M., Horwood L. J., Pettit G. S., Woodward L. 2003. Does father absence place daughters at special risk for early sexual activity and teenage pregnancy? Child Dev. 74, 801–821 10.1111/1467-8624.00569 (doi:10.1111/1467-8624.00569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nettle D., Coall D. A., Dickins T. E. 2010. Birthweight and paternal involvement predict early reproduction in British women: evidence from the National Child Development Study. Am. J. Hum. Biol. 22, 172–179 [DOI] [PubMed] [Google Scholar]
- 32.Chisholm J. S., Quinlivan J. A., Petersen R. W., Coall D. A. 2005. Early stress predicts age at menarche and first birth, adult attachment, and expected lifespan. Hum. Nat. 16, 233–265 10.1007/s12110-005-1009-0 (doi:10.1007/s12110-005-1009-0) [DOI] [PubMed] [Google Scholar]
- 33.Draper P., Harpending H. 1982. Father absence and reproductive strategy—an evolutionary perspective. J. Anthropol. Res. 38, 255–273 [Google Scholar]
- 34.Nettle D. 2010. Dying young and living fast: variation in life history across English neighborhoods. Behav. Ecol. 21, 387–395 10.1093/beheco/arp202 (doi:10.1093/beheco/arp202) [DOI] [Google Scholar]
- 35.Meade C. S., Kershaw T. S., Ickovics J. R. 2008. The intergenerational cycle of teenage motherhood: an ecological approach. Health Psychol. 27, 419–429 10.1037/0278-6133.27.4.419 (doi:10.1037/0278-6133.27.4.419) [DOI] [PubMed] [Google Scholar]
- 36.Seamark C. J., Pereira Gray D. J. 1997. Like mother, like daughter: a general practice study of maternal influences on teenage pregnancy. Br. J. Gen. Pract. 47, 175–176 [PMC free article] [PubMed] [Google Scholar]
- 37.SPSS 2008. SPSS statistics release 17.0.0. Chicago, IL: SPSS Inc [Google Scholar]
- 38.Schafer J. L. 1999. Multiple imputation: a primer. Stat. Methods Med. Res. 8, 3–15 10.1191/096228099671525676 (doi:10.1191/096228099671525676) [DOI] [PubMed] [Google Scholar]
- 39.Stott D. H. 1965. The social-adjustment of children: manual to the Bristol social adjustment guides. London, UK: University of London Press [Google Scholar]
- 40.Arbuckle J. L. 2008. Amos (Version 17.0.2). Chicago, IL: SPSS Inc [Google Scholar]
- 41.Vikat A., Rimpela A., Kosunen E., Rimpela M. 2002. Sociodemographic differences in the occurrence of teenage pregnancies in Finland in 1987–1998: a follow-up study. J. Epidemiol. Community Health 59, 223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teilmann G., Pedersen C. B., Skakkebaek N. E., Jensen T. K. 2006. Increased risk of precocious puberty in internationally adopted children in Denmark. Pediatrics 118, e391–e399 10.1542/peds.2005-2939 (doi:10.1542/peds.2005-2939) [DOI] [PubMed] [Google Scholar]
- 43.Seckl J. R., Cleasby M., Nyirenda M. J. 2000. Glucocorticoids, 11 beta-hydroxysteroid dehydrogenase, and fetal programming. Kidney Int. 57, 1412–1417 10.1046/j.1523-1755.2000.00984.x (doi:10.1046/j.1523-1755.2000.00984.x) [DOI] [PubMed] [Google Scholar]
- 44.Byars S. G., Ewbank D., Govindaraju D. R., Stearns S. C. 2010. Natural selection in a contemporary human population. Proc. Natl Acad. Sci. USA 107, 1787–1792 10.1073/pnas.0906199106 (doi:10.1073/pnas.0906199106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McElreath R., Strimling P. 2008. When natural selection favors imitation of parents. Curr. Anthropol. 49, 307–316 10.1086/524364 (doi:10.1086/524364) [DOI] [Google Scholar]
- 46.Harden A., Brunton G., Fletcher A., Oakley A. 2009. Teenage pregnancy and social disadvantage: systematic review integrating controlled trials and qualitative studies. Br. Med. J. 339, b4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uller T. 2008. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol. 23, 432–438 10.1016/j.tree.2008.04.005 (doi:10.1016/j.tree.2008.04.005) [DOI] [PubMed] [Google Scholar]
- 48.Weaver I. C. G., Cervoni N., Champagne F. A., D'Alessio A. C., Sharma S., Seckl J. R., Dymov S., Szyf M., Meaney M. J. 2004. Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854 10.1038/nn1276 (doi:10.1038/nn1276) [DOI] [PubMed] [Google Scholar]
- 49.Maestripieri D., Higley J. D., Lindell S. G., Newman T. K., McCormack K. M., Sanchez M. M. 2006. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques (Macaca mulatta). Behav. Neurosci. 120, 1017–1024 10.1037/0735-7044.120.5.1017 (doi:10.1037/0735-7044.120.5.1017) [DOI] [PubMed] [Google Scholar]