Abstract
The greater ani (Crotophaga major), a Neotropical cuckoo, exhibits an unusual breeding system in which several socially monogamous pairs lay eggs in a single nest and contribute care to the communal clutch. Cooperative nesting is costly—females compete for reproduction by ejecting each other's eggs—but the potential direct or indirect fitness benefits that might accrue to group members have not been identified. In this study, I used molecular genotyping to quantify patterns of genetic relatedness and individual reproductive success within social groups in a single colour-banded population. Microsatellite analysis of 122 individuals in 49 groups revealed that group members are not genetic relatives. Group size was strongly correlated with individual reproductive success: solitary pairs were extremely rare and never successful, and nests attended by two pairs were significantly more likely to be depredated than were nests attended by three pairs. Egg loss, a consequence of reproductive competition, was greater in large groups and disproportionately affected females that initiated laying. However, early-laying females compensated for egg losses by laying larger clutches, and female group members switched positions in the laying order across nesting attempts. The greater ani, therefore, appears to be one of the few species in which cooperative breeding among unrelated individuals is favoured by direct, shared benefits that outweigh the substantial costs of reproductive competition.
Keywords: Barro Colorado Island, Crotophaginae, joint nesting, cooperation, microsatellite, nest predation
1. Introduction
In many cooperatively breeding animals, social groups form when offspring from one generation remain on their natal territory to help rear future generations of relatives [1,2]. This type of cooperation is favoured by kin selection, the process by which cooperating individuals gain indirect fitness benefits by helping to raise non-descendent kin [3]. However, indirect fitness alone may not be sufficient to maintain cooperative breeding, and recent research has increasingly emphasized the possibility that members of social groups may also gain substantial direct fitness benefits by cooperating with both related and unrelated individuals [4,5].
As a growing number of studies have confirmed the importance of direct benefits to cooperative breeders, it has become clear that high levels of within-group relatedness are not always necessary for the evolutionary stability of these societies. Recent molecular analyses have revealed that, in many classic examples of cooperative societies, genetic relatedness among group members is considerably lower than originally estimated (e.g. chimpanzees (Pan troglodytes), [6] mole-rats (Cryptomys damarensis), [7] and honeybees (Apis mellifera), [8]). In addition, molecular analyses of several species of tropical birds show that cooperative alliances among unrelated breeders are far more common than previously recognized ([9–11]; reviewed in [12]). Several recent reviews have proposed that these alliances may in fact represent intraspecific mutualisms, in which one or more cooperators suffer temporary fitness costs in order for all to gain net benefits [13–15]. However, few field studies have quantified the individual-level costs, direct benefits and indirect benefits that accrue to members of social groups, and there is little empirical evidence to suggest that common interest alone can maintain cooperation in the absence of kin selection.
In this paper, I consider the selective pressures that favour costly, highly developed cooperation in a social bird, the greater ani (Crotophaga major). This Neotropical cuckoo breeds in groups composed of up to four socially monogamous pairs; males and females are monomorphic. Groups form as coalitions of adult pairs rather than as family groups, so it has previously been assumed that group members are not related to one another [16]. Each group builds a communal nest in which all of the females lay their eggs. Although the division of labour is frequently unequal, all group members participate in territorial defence, incubation and food delivery to nestlings in the shared clutch. Adults are not capable of recognizing their own eggs or nestlings, so they cannot preferentially provide care to their own offspring within the clutch ([17,18]; C. Riehl 2007–2009, unpublished data). Social groups are highly stable during the breeding season, and group members participate in communal rallies (ritualized displays in which group members vocalize simultaneously) that may synchronize egg-laying and reinforce social bonds between group members [19].
However, reproductive competition among females is intense. Prior to laying her first egg, each female ejects any eggs that her fellow group members have already laid in the nest. Each female stops ejecting eggs once she has laid her first egg, presumably to avoid accidentally removing her own. As a result, the first female to begin laying in the communal nest invariably loses at least one egg—sometimes several—whereas the last female to enter the laying sequence loses none. Egg ejection ceases when all of the females in the group have laid at least one egg, thereby synchronizing reproduction. The number of eggs lost by early-laying females increases with group size, and the high cost of egg ejection appears to be the primary factor limiting group size. No advantages of communal nesting have yet been identified, and the social or ecological factors that maintain variation in group size are not known. Nevertheless, single pairs rarely nest alone and are never successful, suggesting that solitary nesting is under strong constraints [19].
The purpose of this four year study was to genetically confirm the observation that adult group members are unrelated to one another, and to test the hypothesis that communal breeding in this species is favoured by direct, shared benefits that outweigh the immediate costs of competition. To quantify the individual-level costs and benefits associated with communal nesting, I used microsatellite analysis to determine the reproductive success of individual females in a colour-banded study population in central Panama. I examined reproductive fitness in relation to the size of the breeding group, the location of the nest site, and the female's position in the laying order of the communal clutch. In addition, I examined whether individual females switch positions in the laying sequence of the group across nesting attempts, and whether breeding adults remain in the same group across nesting seasons. If breeding groups are stable across years and female group members consistently lay in the same order, then the costs and benefits of social nesting should vary significantly among individuals. Alternatively, if laying order and/or group composition change across nesting attempts, then these should equalize over time.
2. Material and methods
(a). Study area and nest monitoring
The greater ani ranges from Panama to northern Argentina [20]. Nests are built exclusively in riparian vegetation on the edges of ponds, lakes and rivers with partly inundated banks, and are typically placed less than or equal to 2 m above the water's surface. I studied a colour-banded population of greater anis on the shores of Lago Gatún, Panama, a man-made lake formed when the Chagres River was dammed to create the Panama Canal. Annual rainfall averages 265 cm with a marked dry season lasting from mid-December to mid-April [21]. Greater anis breed during the rainy season, typically between June and October.
In this study population, greater anis rarely nest as lone pairs. Groups typically contain between two and five socially monogamous pairs, but groups of two and three pairs are most common (ca 61% and 32% of groups in the population, respectively). Groups containing more than three pairs are uncommon and their nests are almost always abandoned during the laying period [19]. Nest predation is the most common cause of nest failure for groups of all sizes, and snakes and terrestrial mammals appear to be the most frequent nest predators [19]. Greater anis use two different types of nest sites, which have previously been shown to be associated with different levels of nest predation [22]. I followed the definitions of Lau et al. [22] to categorize nest sites as either ‘emergent’ (supporting vegetation completely surrounded by water, and no other vegetation within 1 m) or ‘shoreline’ (supporting vegetation not surrounded by water). Nests in emergent sites were typically located in Annona glabra, a small tree that grows in shallow water, whereas nests in shoreline sites were located in the overhanging branches of riparian trees.
Details of colour banding, genetic sampling and nest monitoring are given in Riehl & Jara [19]. Briefly, all nests within the study area were monitored yearly from 2007 to 2009 following preliminary work in 2006 (between 40 and 58 nests per year). Group size was determined by counting all adults present at each visit to the nest. Each egg was numbered with a permanent felt-tip marker to identify its position in the communal clutch, and the fate of each egg was recorded (ejected, depredated, unhatched and hatched). Nests were checked every 2–3 days during the 12 day incubation period and daily during the 6 day nestling period. Each nestling was marked with a temporary, expandable plastic leg band on the day of hatching and, in most cases, matched to the egg from which it hatched. A small blood sample (less than10 µl) was taken by puncture of the brachial vein at 2–3 days of age for molecular sexing and other genetic analyses. Each nestling was given a permanent combination of coloured and aluminium leg bands at 4–6 days of age (immediately prior to fledging). In addition, 25–50 adults were mist-netted, colour banded and genetically sampled each year.
(b). Genetic determination of egg and nestling maternity
In order to determine which female laid each egg in the communal clutch, maternal DNA was isolated from blood stains and shed cells on the external surface of the freshly laid egg [23]. Samples were then genotyped with a set of 12 highly variable microsatellite markers developed for the greater ani [24]. The accuracy of this method has been extensively cross-checked and validated in this study population [17]. After genotyping, egg maternity was assigned to females in communal clutches with the ‘identity check’ function in Cervus v. 3.0, a maximum-likelihood-based programme for parentage analyses that can also be used to identify repeat samples from the same individual [25,26]. In each nesting group, females were numbered according to the order in which they entered the laying sequence of the communal clutch. Two-pair groups, for example, contained one female that initiated laying (female ‘A’) and one female that began laying after the clutch had already been initiated (female ‘B’). Three-pair groups contained a first-laying A and second-laying B female, and a third ‘C’ female that was the last to enter the laying sequence. Therefore, this method allowed determination of the number of eggs that each female laid, the number that each female lost to ejection and the effect of her position in the laying sequence on her overall reproductive output.
(c). Sample sizes and statistical analyses
The study included 35 different two-pair groups and 22 different three-pair groups for which I had complete information on egg maternity, laying order, egg fate and nestling fate. Single pairs were observed nesting alone only twice in four years, so these data were excluded from the analyses. Of the 35 two-pair groups, I obtained 1 year of data for 26 groups, two years of data for seven groups, and three years of data for two groups (n = 46 group-years). Of the 22 three-pair groups, I obtained one year of data for 20 groups and two years of data for two groups (n = 24 group-years). Microsatellite genotypes were isolated from 584 eggs and assigned to 142 females (n = 164 female-years). Nest survival probabilities were calculated using an additional 38 nests for which I had complete information on group size and nest fate, but not on egg maternity and/or laying order. The Mayfield [27] method with the modifications recommended by Johnson [28] was used to compute nest survival probabilities since some nests (n = 12) were located after laying had begun.
All adult group members were trapped, colour banded and genetically sampled in 36 group-years; some but not all group members were captured at an additional 13 nests (n = 122 adults genotyped). Pairwise genetic relatedness of adults within breeding groups was calculated with the programme Kingroup ([29], derived from Queller & Goodnight [30]) and standard errors for pairwise estimations were obtained by jackknifing over loci [30]. In order to ensure that the set of loci was sufficiently robust to estimate relatedness, I used an online tool to perform a rarefaction analysis in which relatedness values were calculated for each successive inclusion of loci beginning with one locus (http://people.musc.edu/~schwaclh/). Calculated relatedness values did not change significantly after the inclusion of the eighth locus, indicating that the 12 loci used here were sufficiently polymorphic for relatedness calculations. Calculated relatedness values for known mother–offspring dyads were in accordance with expected values (n = 21 dyads, r = 0.48 ± 0.003 s.e.).
Average relatedness values were calculated for four types of relationships among group members (mated pairs, male group members, female group members and opposite-sex group members excluding mated pairs). Relatedness values for the entire group were calculated by averaging over all dyads. I then used permutation tests to determine whether group members were significantly more related to each other than to randomly chosen individuals in the rest of the population. Permutation tests were performed in Identix 1.1, a computer program that uses a Monte-Carlo resampling procedure to generate a null distribution of genotypes based on population-wide allele frequencies. Observed values of pairwise relatedness were compared with the mean of the null distribution generated by 1000 permutations. Average pairwise relatedness across the entire population is, by definition, set at zero. These analyses were performed at the level of group-years; therefore, not all data points were independent since some individuals remained in the same group across years and were sampled multiple times. However, this approach is unlikely to have biased the results since the sample sizes are relatively large (122 different adults) and the majority of groups experienced substantial turnover in membership over the 4 year study period (see §3).
I constructed mixed models in SAS to identify factors predicting individual reproductive success and to examine the effects of group size on different measures of reproductive fitness (SAS Institute, Cary, NC, USA). Group identity and/or individual female identity (nested in group identity) were included as random effects in all models in order to control for repeated observations of the same group or individual across years. Response variables with continuous normal distributions were assessed with linear mixed-effects models using a restricted/residual maximum-likelihood approach (PROC MIXED). Response variables with binary distributions (nest survival) were assessed with generalized linear mixed models using a restricted pseudo-likelihood approach, a binomial error structure, and a logit link function (PROC GLIMMIX). Initial models included all variables and their two-way interactions; final (minimum adequate) models were chosen by stepwise removal of non-significant (p > 0.05) terms. For datasets in which the individual identity was not a significant term, post hoc tests were done on the pooled data (not accounting for repeated measures).
In the first set of models, I examined the effects of group size (two-pair or three-pair) and nest site (shore or isolated) on the probability of nest success, and on the number of nestlings fledged per group member. The main purpose of these analyses was to determine whether the group size influenced the per capita reproductive output of group members. To investigate differences in reproductive fitness among individuals within groups, I constructed a second set of models examining the effects of group size and the female's position in the laying order on six measures of annual reproductive output (number of eggs laid, lost to ejection, incubated, hatched and fledged; and the number of nestlings fledged per egg laid). Group size was coded as a two-level factor (‘two-pair’ or ‘three-pair’) and female position in the laying order was coded as a three-level factor (first-laying, A second-laying, B or last-laying, C). The purpose of these analyses was to determine whether the costs of communal nesting (such as egg ejection, low hatching rates and post-hatching mortality) are equally distributed among all females in a group, and whether increased reproductive investment could compensate for these costs.
3. Results
(a). Group members are not genetic relatives
Dispersal patterns of colour-banded nestlings indicated that breeding groups are composed of unrelated adults. Of 341 nestlings, colour banded in 58 breeding groups, only four (all males) remained with their natal group the following year. Of those four, none was paired to a social mate at 1 year of age, and only one remained as a paired breeder with his natal group at 2 years of age. In one instance, a pair of brothers dispersed together and joined the same breeding group as adults, and in two instances male nestlings dispersed from the natal group and later joined a breeding group that contained second- or third-order male kin (uncles or cousins). Sixteen male nestlings eventually joined breeding groups in the study area that contained no known relatives, and the remaining 137 male nestlings either died or dispersed outside the study area. Colour-banded female nestlings were never observed to stay with their natal groups. Five joined groups within the study area, none of which contained known relatives. The remaining 175 female nestlings either died or dispersed outside the study area.
Pairwise genetic relatedness among adult group members, calculated from microsatellite genotypes, supported the hypothesis that group members are not significantly more related to one another than to randomly selected individuals in the study population (table 1). As expected from the observed dispersal patterns of colour-banded nestlings, male group members shared higher average coefficients of relatedness than did female group members (r = 0.012 versus −0.003, respectively), and the maximum observed relatedness between male group members was also greater (r = 0.47, indicating a first-order kin relationship; table 1). However, this difference was driven by a very few pairs of close relatives, and average pairwise relatedness of male group members was not significantly higher than the population-level average. Similarly, in three of 44 dyads, relatedness values of female group members indicated second-order kin relationships (r ≈ 0.25), suggesting that female relatives also breed together occasionally. Overall, within-group relatedness values (calculated by averaging the relatedness values of all dyads within the breeding group) were not significantly higher than expected by chance (n = 36 completely sampled group-years, r = 0.006 ± 0.024 s.e., p > 0.3).
Table 1.
Average pairwise genetic relatedness of adult greater anis within breeding groups, calculated from the complete genotypes of 122 adults at 12 microsatellite loci. Relatedness values are given as the coefficient of relatedness (r) ± s.e. Sample size (n) refers to the number of dyads sampled. p-values are given for permutation tests testing whether mean pairwise relatedness values for each category are significantly higher than for randomly selected dyads from the study population.
| n | r (± s.e.) | range | p | |
|---|---|---|---|---|
| mated pairs | 64 | −0.010 (±0.006) | −0.021 to 0.003 | 0.44 |
| male group members | 49 | 0.012 (±0.241) | −0.125 to 0.472 | 0.17 |
| female group members | 44 | −0.003 (±0.090) | −0.032 to 0.271 | 0.62 |
| opposite-sex group members (excluding mated pairs) | 90 | 0.024 (±0.081) | −0.122 to 0.117 | 0.17 |
(b). Nest predation risk decreases with group size
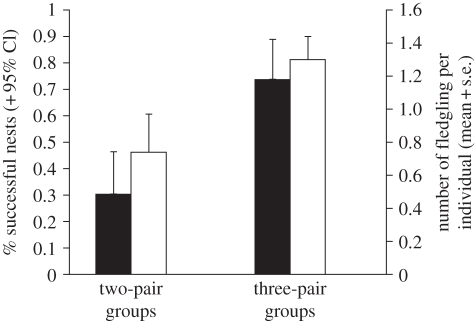
Single pairs were observed to nest alone only twice, and both attempts were depredated before clutch completion. Nests of three-pair groups were significantly less likely to be depredated than were nests of two-pair groups (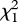 = 10.01, p < 0.002; figure 1), and the number of fledglings produced per group member was significantly higher (t57 = −2.08, p < 0.05; figure 1). A mixed model analysis (including group size, nest-site type, group ID, individual ID and all two-way interactions as explanatory variables) revealed that the probability of nest success was significantly influenced by group size, nest-site type and their interaction (table 2). Post hoc tests, which did not control for repeated measures, indicated that nests built in emergent vegetation were more likely to be successful than were nests in shoreline vegetation (
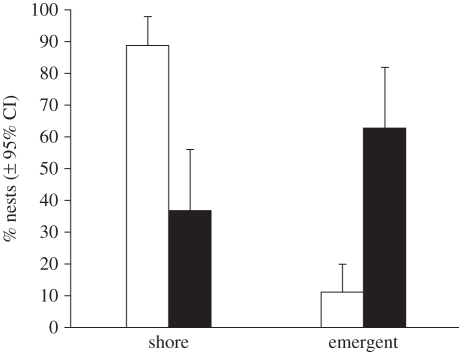
= 10.01, p < 0.002; figure 1), and the number of fledglings produced per group member was significantly higher (t57 = −2.08, p < 0.05; figure 1). A mixed model analysis (including group size, nest-site type, group ID, individual ID and all two-way interactions as explanatory variables) revealed that the probability of nest success was significantly influenced by group size, nest-site type and their interaction (table 2). Post hoc tests, which did not control for repeated measures, indicated that nests built in emergent vegetation were more likely to be successful than were nests in shoreline vegetation (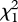 = 7.84, p = 0.005), and three-pair groups were more likely to occupy these sites than were two-pair groups (
= 7.84, p = 0.005), and three-pair groups were more likely to occupy these sites than were two-pair groups (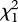 = 6.98, p = 0.008; figure 2). Group size influenced nest depredation rates even when controlling for nest-site type: three-pair groups nesting in high-risk shoreline sites were still more likely to fledge young than were two-pair groups in shoreline sites (
= 6.98, p = 0.008; figure 2). Group size influenced nest depredation rates even when controlling for nest-site type: three-pair groups nesting in high-risk shoreline sites were still more likely to fledge young than were two-pair groups in shoreline sites (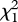 = 5.84, p = 0.016).
= 5.84, p = 0.016).
Figure 1.
Percentage (±95% CI) of successful nests (black bar, left axis) and average individual reproductive success (white bar, right axis) with respect to group size of communally breeding greater anis.
Table 2.
Final linear mixed models testing the effects of group size (two-pair versus three-pair) and nest-site type (shore versus emergent) on the probability of nest success and on the per capita number of young fledged. Other model terms and interactions were not significant (see §2 for details of full model).
| variable | probability of success |
number of fledglings (per capita) |
||||
|---|---|---|---|---|---|---|
| d.f. | χ2 | p | d.f. | F | p | |
| group size | 1 | 5.12 | 0.024 | 1,67 | 4.34 | 0.041 |
| nest site | 1 | 7.84 | 0.005 | 1,67 | 10.05 | 0.002 |
| group size × nest site | 1 | 4.62 | 0.032 | 1,67 | 6.23 | 0.015 |
Figure 2.
Percentage (±95% CI) of greater ani breeding groups that nest in shore or emergent vegetation. White bars, two-pair groups; black bars, three-pair groups.
(c). Group size and position in the laying order determine reproductive success
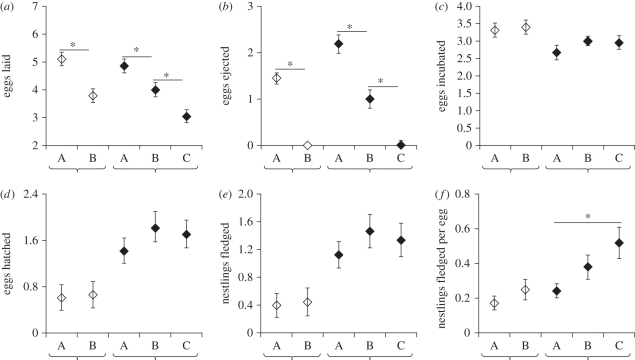
Female reproductive success was influenced by the size of the breeding group and by the female's position in the laying order of the communal clutch (figure 3). In both two-pair and three-pair groups, first-laying females laid more eggs than did females that subsequently entered the laying sequence (figure 3a; two-pair groups: F1,74 = 58.1, p < 0.001; three-pair groups: F1,40 = 5.7, p = 0.02), but they also lost more eggs to ejection (figure 3b; two-pair groups: F1,74 = 128.5, p < 0.001; three-pair groups: F1,40 = 19.1, p < 0.001). These effects were most extreme for early-laying females in large groups: since egg ejection did not cease until all group members had begun to lay, both group size and position in the laying order influenced ejection probability (figure 3b; female position × group size interaction, likelihood ratio χ2 = 4.21, p = 0.04). However, position in the laying order did not affect the final number of eggs that each female contributed to the incubated clutch (figure 3c; F2,161 = 1.44, p = 0.24). In the incubated clutch, therefore, reproductive skew among group members was very low in both two-pair groups (s = 0.031) and three-pair groups (s = 0.062).
Figure 3.
Six measures of reproductive fitness (mean ± s.e.) for female greater anis in communal breeding groups with respect to group size (two-pair, white diamonds, or three-pair, black diamonds) and position in the laying order (first-laying, A; second-laying, B or third-laying, C). (a) Total number of eggs laid, (b) number of eggs lost to ejection, (c) number of eggs incubated in the final clutch, (d) number of eggs that survived to hatching, (e) number of nestlings that survived to fledging and (f) number of fledglings produced per egg laid. Asterisks indicate statistically significant (p < 0.05) differences between females within groups of the same size. Comparisons between groups of different sizes, and details of statistical tests, are given in the text.
Regardless of position in the laying order, females in three-pair groups hatched and fledged more offspring than did their counterparts in two-pair groups (figure 3d,e; hatched: t61 = −2.23, p = 0.03; fledged: t57 = −2.08, p = 0.04). This difference was entirely attributable to higher predation at two-pair nests: when the comparison was restricted to successful nests only, eggs in three-pair nests had lower hatching probabilities (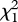 = 4.47, p = 0.03) and post-hatching survivorship (
= 4.47, p = 0.03) and post-hatching survivorship (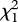 = 5.11, p = 0.02). Predation, not ejection, was the most important cause of egg loss for first-laying females in two-pair groups (eggs lost to ejection:
= 5.11, p = 0.02). Predation, not ejection, was the most important cause of egg loss for first-laying females in two-pair groups (eggs lost to ejection: 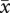 = 1.45 ± 0.12; eggs lost to predation:
= 1.45 ± 0.12; eggs lost to predation: 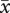 = 2.5 ± 0.28; matched-pairs: t37 = −3.27, p = 0.002). As a result, females in two-pair groups did not significantly differ in the number of offspring fledged per egg laid (figure 3f, F1,74 = 1.15, p = 0.29). By contrast, first-laying females in three-pair groups lost more eggs to ejection (
= 2.5 ± 0.28; matched-pairs: t37 = −3.27, p = 0.002). As a result, females in two-pair groups did not significantly differ in the number of offspring fledged per egg laid (figure 3f, F1,74 = 1.15, p = 0.29). By contrast, first-laying females in three-pair groups lost more eggs to ejection (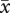 = 2.2 ± 0.76) than to predation (
= 2.2 ± 0.76) than to predation (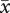 = 1.0 ± 1.44; matched-pairs: t20 = 3.59, p = 0.002). For females in three-pair groups, therefore, position in the laying order was a significant predictor of the number of offspring fledged per egg laid (F2,39 = 4.77, p = 0.014; figure 3f).
= 1.0 ± 1.44; matched-pairs: t20 = 3.59, p = 0.002). For females in three-pair groups, therefore, position in the laying order was a significant predictor of the number of offspring fledged per egg laid (F2,39 = 4.77, p = 0.014; figure 3f).
(d). Laying order and group membership change over time
Individual females did not consistently occupy the same position in the laying order of the communal clutch. An analysis of 22 females for which I had two consecutive years of data revealed that females are no more likely to retain the same position in the laying order (10/22, 45%) than to switch positions (12/22, 55%; one-tailed binomial test, p > 0.1). Nor did a female's position in one year influence her position in the subsequent year: first-laying (A) females were equally as likely to change positions as were later-laying (B and C) females (Fisher's exact test, p > 0.1). Laying order also changed across nesting attempts within the same season: the identity of the first-laying female changed in four of the six instances in which a breeding group laid a second clutch following the depredation of the first clutch. As a result, skew among group members in the number of eggs lost to ejection was significantly lower when both years of data were included (s = 0.33) than when each year was analysed separately (s = 0.71 and 0.83; Kruskal–Wallis H = 6.4, p < 0.05).
Group membership also changed across years, although the number of individuals in each group typically remained constant (electronic supplementary material, table S1). Adult females were significantly more likely to die or disperse than were adult males: only 58 per cent of colour-banded females remained in the same breeding group for at least two consecutive years, whereas 87 per cent of colour-banded males did (n = 19 females and 23 males; 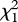 = 4.5, p = 0.03). Mated pairs occasionally dispersed together and joined a different breeding group the subsequent year (n = 4 of 22 colour-banded pairs), but the majority of replacements involved just one member of a mated pair (n = 15). Average yearly turnover, calculated as the per cent of group members that die or leave before the subsequent breeding season, was estimated to be between 21 and 40 per cent (electronic supplementary material, table S1; lower and upper limits calculated from 11 breeding groups in which at least 50% of group members were colour banded for multiple years).
= 4.5, p = 0.03). Mated pairs occasionally dispersed together and joined a different breeding group the subsequent year (n = 4 of 22 colour-banded pairs), but the majority of replacements involved just one member of a mated pair (n = 15). Average yearly turnover, calculated as the per cent of group members that die or leave before the subsequent breeding season, was estimated to be between 21 and 40 per cent (electronic supplementary material, table S1; lower and upper limits calculated from 11 breeding groups in which at least 50% of group members were colour banded for multiple years).
4. Discussion
(a). Costs and benefits of communal nesting
Field studies of cooperatively breeding vertebrates have largely focused on species that breed in kin groups, in which it is difficult to determine the relative importance of direct and indirect fitness benefits to individual group members. In this study, I found that greater anis typically nest in groups composed of unrelated, socially monogamous pairs. Levels of genetic relatedness within breeding groups were not significantly higher than background levels of relatedness in the study population, indicating that group members do not derive indirect fitness benefits by raising the offspring of relatives.
Instead, these results suggest that social nesting is favoured by direct fitness benefits, and that stable group size is constrained by a trade-off between predation avoidance and reproductive competition among group members. Nests attended by three pairs were significantly less likely to be depredated than were nests attended by two pairs, and individual reproductive success (measured as the number of fledglings produced per female) was correspondingly higher in three-pair groups. Not only were three-pair groups more likely to nest in isolated sites that were less vulnerable to terrestrial predators but three-pair groups were more likely to be successful even in riskier shoreline sites. Egg loss, a consequence of reproductive competition, was greater in three-pair groups than in two-pair groups, and disproportionately affected early-laying females. Early-laying females compensated for egg losses by laying larger clutches, and reproductive skew among females was low in both two-pair and three-pair groups.
(b). Nest-site limitation and cooperative nest defence
Many long-term studies of cooperatively breeding birds have documented the importance of nest-site availability in determining the reproductive decisions of individuals [31–33]. The data presented in this paper indicate that larger groups were more likely to nest in isolated, emergent sites that were less vulnerable to terrestrial predators. This pattern implies that, as in many other species of cooperative breeders, high-quality nest sites are scarce and larger groups are better able to acquire and defend them against neighbouring groups. Neither assumption has been tested in this population, although it is possible that neighbouring groups compete with one another for nest sites. Future work is needed to determine whether high-quality nest sites actually are limited in the study area, and whether the size of the displaying group predicts its ability to displace a neighbouring group.
This study also found that larger groups experience lower rates of nest predation than smaller groups even when nest-site quality is controlled for, suggesting that communal nest defence may be another important benefit of cooperation. Even in habitats where safe nest sites are not limited, it is possible that the benefits of cooperative nest defence alone may be sufficient to outweigh the costs of communal nesting, at least for some group members. Previous work has shown that all group members routinely mob predators at the nest, particularly after the eggs have hatched [19]. Data from motion-activated nest cameras show that mobbing can be effective at driving off predators that approach the nest—including snakes, which appear to account for the majority of predation events ([19]; C. Riehl 2008–2009, unpublished data). These results indicate that the relative pay-offs of belonging to a two-pair or three-pair group—or of nesting alone—are likely to depend on ecological factors, particularly on the risk of predation and availability of safe nest sites. Neither variable is amenable to experimental manipulation, but further insights may come from comparative studies of populations across the greater ani's range, or from longer term studies of this population. In the groove-billed ani (Crotophaga sulcirostris), for example, a related species with a similar breeding system, single pairs appear to be more common in the northern portion of its breeding range (s. Texas and n. Mexico) than in Central America [34]. Future comparative studies should attempt to document the occurrence and causes of similar variation across greater ani populations.
(c). Individual variation and position in the laying order
The number of eggs lost by each female was strongly influenced by her position in the laying order of the communal clutch, with early-laying females losing significantly more eggs than late-laying females. Although early-laying females fledged equivalent numbers of nestlings by laying more eggs, egg losses still impose a physiological cost. Greater ani eggs are unusually large relative to female body mass (approx. 18%; [19]), and early-laying females must lay the equivalent of less than or equal to 70 per cent of their body mass in a matter of days [17]. What factors, then, determine a female's position in the laying order of her group? In the groove-billed ani, Vehrencamp [35] found that female-laying order was correlated with behavioural dominance of the social mate, suggesting that a female's position in the laying order is determined by her mate's position in the dominance hierarchy of the group. Similarly, in meerkats (Suricata suricatta) and dwarf mongooses (Helogale parvula), communally breeding mammals in which several females give birth synchronously, dominant breeders commonly suppress the reproduction of younger subordinates through infanticide and eviction from the group [36,37].
However, no behavioural dominance hierarchy is apparent in greater anis. All group members participate in the establishment of the nest site and the construction of the nest, and eviction is exceedingly rare [19]. Furthermore, this study shows that a female's position in the laying order can change across subsequent nesting attempts within the same breeding season, as well as across years, suggesting that female ‘roles’ within the group are flexible rather than fixed by age or dominance status. In the guira cuckoo (Guira guira), another communally nesting crotophagine cuckoo, Macedo et al. [38] also found that females within the group switched position in the laying order across repeated nesting bouts. These studies indicate that the true costs of egg loss—and, by extension, the individual-level costs and benefits of communal nesting—cannot be accurately measured within a single breeding season. More information is needed on the behavioural mechanisms by which group members synchronize copulation and egg-laying, as well as the proximate physiological cues that trigger the onset of egg formation. Adult greater anis exhibit an extraordinary range of variation in both adult body mass and egg size [17], and it seems likely that this extreme physiological variation may influence the order of egg-laying within communal groups.
Acknowledgements
This work was approved by the Institutional Animal Care and Use Committees of the Smithsonian Tropical Research Institute and Princeton University. Export permits were granted by Panama's Autoridad Nacionál del Ambiente (ANAM).
I thank W. Webber and L. Jara for invaluable assistance in the field; T. Doak, S. M. Bogdanowicz and L. Stenzler for assistance with genetic analyses; and M. C. Wikelski, H. S. Horn and E. G. Leigh, Jr, for helpful advice on the project. Molecular analyses were performed at the Cornell Laboratory of Ornithology (Fuller Laboratory), at the Smithsonian Tropical Research Institute (Naos Molecular Laboratory) and at the core facility of Princeton University's Department of Ecology and Evolutionary Biology. Funding was provided by an NSF Graduate Research Fellowship, the Smithsonian Tropical Research Institute, Princeton University's Programme in Latin American Studies, the Max Planck Institute for Ornithology, the American Ornithologists' Union and the American Museum of Natural History.
References
- 1.Brown J. L. 1987. Helping and communal breeding in birds: ecology and evolution. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Emlen S. T. 1997. Predicting family dynamics in social vertebrates. In Behavioural ecology: an evolutionary approach (eds Krebs J. R., Davies N. B.), pp. 228–253, 4th edn. Oxford, UK: Blackwell Sciences [Google Scholar]
- 3.Hamilton W. D. 1964. The genetical evolution of social behaviour. I. II. J. Theor. Biol. 7, 1–52 10.1016/0022-5193(64)90038-4 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 4.Clutton-Brock T. H. 2002. Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296, 69–72 10.1126/science.296.5565.69 (doi:10.1126/science.296.5565.69) [DOI] [PubMed] [Google Scholar]
- 5.Bergmüller R., Johnstone R. A., Russell A. F., Bshary R. 2007. Integrating cooperative breeding into theoretical concepts of cooperation. Behav. Process. 76, 61–72 10.1016/j.beproc.2007.07.001 (doi:10.1016/j.beproc.2007.07.001) [DOI] [PubMed] [Google Scholar]
- 6.Vigilant L., Hofreiter M., Siedel H., Boesch C. 2001. Paternity and relatedness in wild chimpanzee communities. Proc. Natl Acad. Sci. USA 98, 12 890–12 895 10.1073/pnas.231320498 (doi:10.1073/pnas.231320498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burland T. M., Bennett N. C., Jarvis J. U. M., Faulkes C. G. 2002. Eusociality in African mole-rats: new insights from patterns of genetic relatedness in the Damaraland mole-rat (Cryptomys damarensis). Proc. R. Soc. Lond. B 269, 1025–1030 10.1098/rspb.2002.1978 (doi:10.1098/rspb.2002.1978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarpy D. R., Nielsen D. I. 2002. Sampling error, effective paternity, and estimating the genetic structure of honey bee colonies (Hymenoptera: Apoidae). Ann. Entomol. Soc. Am. 95, 513–528 10.1603/0013-8746(2002)095[0513:SEEPAE]2.0.CO;2 (doi:10.1603/0013-8746(2002)095[0513:SEEPAE]2.0.CO;2) [DOI] [Google Scholar]
- 9.Dunn P. O., Cockburn A., Mulder R. A. 1995. Fairy-wren helpers often care for young to which they are unrelated. Proc. R. Soc. Lond. B 259, 339–343 10.1098/rspb.1995.0050 (doi:10.1098/rspb.1995.0050) [DOI] [Google Scholar]
- 10.Sherman P. T. 1995. Social organization of cooperatively polyandrous white-winged trumpeters (Psophia leucoptera). Auk 112, 296–309 [Google Scholar]
- 11.Whittingham L. A., Dunn P. O., Magrath R. D. 1997. Relatedness, polyandry and extra-group paternity in the cooperatively breeding white-browed scrubwren (Sericornis frontalis). Behav. Ecol. Sociobiol. 40, 261–270 10.1007/s002650050341 (doi:10.1007/s002650050341) [DOI] [Google Scholar]
- 12.Cockburn A. 1998. Evolution of helping behavior in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 29, 141–177 10.1146/annurev.ecolsys.29.1.141 (doi:10.1146/annurev.ecolsys.29.1.141) [DOI] [Google Scholar]
- 13.Clutton-Brock T. H. 2009. Cooperation between non-kin in animal societies. Nature 462, 51–57 10.1038/nature08366 (doi:10.1038/nature08366) [DOI] [PubMed] [Google Scholar]
- 14.Connor R. 2010. Cooperation beyond the dyad: on simple models and a complex society. Phil. Trans. R. Soc. B 365, 2687–2697 10.1098/rstb.2010.0150 (doi:10.1098/rstb.2010.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leimar O., Hammerstein P. 2010. Cooperation for direct fitness benefits. Phil. Trans. R. Soc. B 365, 2619–2626 10.1098/rstb.2010.0116 (doi:10.1098/rstb.2010.0116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vehrencamp S. L., Quinn J. S. 2004. Avian joint laying systems. In Ecology and evolution of cooperative breeding in birds (eds Koenig W. D., Dickinson J.), pp. 177–196 Cambridge, UK: Cambridge University Press [Google Scholar]
- 17.Riehl C. 2010. Egg rejection risk and hatching asynchrony predict egg mass in a communally breeding cuckoo, the greater ani (Crotophaga major). Behav. Ecol. 21, 676–683 10.1093/beheco/arq038 (doi:10.1093/beheco/arq038) [DOI] [Google Scholar]
- 18.Riehl C. 2010. A simple rule reduces costs of extragroup parasitism in a communally breeding bird. Curr. Biol. 20, 1830–1833 10.1016/j.cub.2010.09.005 (doi:10.1016/j.cub.2010.09.005) [DOI] [PubMed] [Google Scholar]
- 19.Riehl C., Jara L. 2009. Natural history and reproductive biology of the communally breeding greater ani (Crotophaga major) at Gatún Lake, Panama. Wilson J. Ornithol. 121, 679–687 10.1676/09-017.1 (doi:10.1676/09-017.1) [DOI] [Google Scholar]
- 20.Payne R. B. 2005. The cuckoos. Oxford, UK: Oxford University Press [Google Scholar]
- 21.Rand A. S., Rand W. M. 1982. Variation in rainfall on Barro Colorado Island. In The ecology of a tropical forest: seasonal rhythms and long-term changes (eds Rand A. S., Windsor D. M., Leigh E. G., Jr), pp. 47–60 Washington, DC: Smithsonian Institute Press [Google Scholar]
- 22.Lau P., Bosque C., Strahl S. D. 1998. Nest predation in relation to nest placement in the greater ani (Crotophaga major). Ornitol. Neotrop. 9, 87–92 [Google Scholar]
- 23.Schmaltz G., Somers C. M., Sharma P., Quinn J. S. 2006. Non-destructive sampling of maternal DNA from the external shell of bird eggs. Conserv. Genet. 7, 543–549 10.1007/s10592-005-9065-x (doi:10.1007/s10592-005-9065-x) [DOI] [Google Scholar]
- 24.Riehl C., Bogdanowicz S. M. 2009. Isolation and characterization of microsatellite markers from the greater ani Crotophaga major (Aves: Cuculidae). Mol. Ecol. Res. See http://tomato.biol.trinity.edu/manuscripts/9-6/mer-09-0270.pdf
- 25.Marshall T. C., Slate J., Kruuk L., Pemberton J. M. 1998. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 7, 639–655 10.1046/j.1365-294x.1998.00374.x (doi:10.1046/j.1365-294x.1998.00374.x) [DOI] [PubMed] [Google Scholar]
- 26.Kalinowski S. T., Taper M. L., Marshall T. C. 2007. Revising how the computer program Cervus accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106 10.1111/j.1365-294X.2007.03089.x (doi:10.1111/j.1365-294X.2007.03089.x) [DOI] [PubMed] [Google Scholar]
- 27.Mayfield H. 1975. Suggestions for calculating nest success. Wilson Bull 87, 456–466 [Google Scholar]
- 28.Johnson D. H. 1979. Estimating nest success: the Mayfield method and an alternative. Auk 96, 651–661 [Google Scholar]
- 29.Konovalov D. A., Manning C., Henshaw M. T. 2004. Kingroup: a program for pedigree relationship reconstruction and kin group assignments using genetic markers. Mol. Ecol. Notes 4, 779–782 10.1111/j.1471-8286.2004.00796.x (doi:10.1111/j.1471-8286.2004.00796.x) [DOI] [Google Scholar]
- 30.Queller D. C., Goodnight K. F. 1989. Estimation of genetic relatedness using allozyme data. Evolution 43, 258–275 10.2307/2409206 (doi:10.2307/2409206) [DOI] [PubMed] [Google Scholar]
- 31.Woolfenden G. E., Fitzpatrick J. W. 1984. The Florida scrub jay: demography of a cooperative-breeding bird. Princeton, NJ: Princeton University Press [Google Scholar]
- 32.Koenig W. D., Pitelka F. A. 1981. Ecological factors and kin selection in the evolution of cooperative breeding in birds. In Natural selection and social behavior: recent research and new theory (eds Alexander R. D., Tinkle D. W.), pp. 261–280 New York, NY: Chiron Press [Google Scholar]
- 33.Koford R. R., Bowen B. S., Vehrencamp S. L. 1990. Groove-billed anis: joint-nesting in a tropical cuckoo. In Cooperative breeding in birds (eds Stacey P. B., Koenig W. D.), pp. 335–355 Cambridge, UK: Cambridge University Press [Google Scholar]
- 34.Bowen B. S. 2002. Groove-billed ani (Crotophaga sulcirostris). In The Birds of North America online (ed. Poole A.), Ithaca, NY: Cornell Lab of Ornithology; Retrieved from the Birds of North America Online See http://bna.birds.cornell.edu/bna/species/612 (doi:10.2173/bna.612) [Google Scholar]
- 35.Vehrencamp S. L. 1978. The adaptive significance of communal nesting in groove-billed anis (Crotophaga sulcirostris). Behav. Ecol. Sociobiol. 4, 1–33 10.1007/BF00302558 (doi:10.1007/BF00302558) [DOI] [Google Scholar]
- 36.Creel S. R., Waser P. M. 1997. Variation in reproductive suppression among dwarf mongooses: interplay between mechanisms and evolution. In Cooperative breeding in mammals (eds Solomon N. G., French J. A.), pp. 150–170 Cambridge, UK: Cambridge University Press [Google Scholar]
- 37.Clutton-Brock T. H., Brotherton P. N. M., Smith R., McIlrath G. M., Kansky R., Gaynor D., O'Riain M. J., Skinner J. D. 1998. Infanticide and expulsion of females in a cooperative mammal. Proc. R. Soc. Lond. B 265, 2291–2295 10.1098/rspb.1998.0573 (doi:10.1098/rspb.1998.0573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macedo R. H. F., Cariello M. O., Graves J., Schwabl H. 2004. Reproductive partitioning in communally breeding guira cuckoos, Guira guira. Behav. Ecol. Sociobiol. 55, 213–222 10.1007/S00265-003-0697-x (doi:10.1007/S00265-003-0697-x) [DOI] [Google Scholar]