Abstract
There is growing evidence that maternal experience influences offspring via non-genetic mechanisms. When female three-spined sticklebacks (Gasterosteus aculeatus) were exposed to the threat of predation, they produced larger eggs with higher cortisol content, which consumed more oxygen shortly after fertilization compared with a control group. As juveniles, the offspring of predator-exposed mothers exhibited tighter shoaling behaviour, an antipredator defence. We did not detect an effect of maternal exposure to predation risk on the somatic growth of fry. Altogether, we found that exposure to an ecologically relevant stressor during egg formation had several long-lasting consequences for offspring, some of which might be mediated by exposure to maternally derived cortisol. These results support the hypothesis that female sticklebacks might influence the development, growth and behaviour of their offspring via eggs to match their future environment.
Keywords: maternal effects, egg size, antipredator behaviour, metabolic rate, stickleback, cortisol
1. Introduction
Maternal experience with stressors in the environment, such as predation risk, influences the growth and development of offspring, sometimes in adaptive ways. For example, exposure to predation risk caused female fall field crickets (Gryllus pennsylvanicus) to produce offspring that exhibited more antipredator behaviours [1]. Similarly, prenatally stressed female monkeys produced offspring that clinged together during challenging conditions [2], and the offspring of prenatally stressed rats avoided open locations [3]. A recent study suggested that intergenerational transmission of antipredator behaviour might occur via a hormonal mechanism: snowshoe hare females that were exposed to predation risk by lynx exhibited increased concentrations of glucocorticoids, and produced offspring with a more reactive stress response system [4].
Indeed, there is accumulating evidence for hormonally mediated maternal effects in vertebrates. For example, female birds deposit steroids in the yolk of their eggs, and those steroids influence the behaviour, physiology, growth and life history of their offspring [5]. In mammals, including humans, stressors during pregnancy can influence the development of offspring in utero via steroids transferred from mother to foetus [6].
However, less is known about hormonally mediated maternal effects in fishes. Important studies have shown that cortisol is present in freshly ovulated eggs [7,8], and egg cortisol is likely to be maternally derived [9,10] because unlike mammalian embryos which produce their own endocrine response prior to birth [11,12], in fishes, the hypothalamus–pituitary–interrenal (HPI) axis is not fully responsive until after hatching [8,13]. There is a strong positive relationship between maternal glucocorticoids and egg glucocorticoid concentrations in fishes [7,9,14,15]. Moreover, hormonally mediated maternal effects are likely to influence a wide variety of traits in offspring because fish embryos are sensitive to the organizational effects of cortisol in early development [16]. Previous studies have shown that early exposure to cortisol influences egg size and embryo survival [14,17,18], growth and development [18,19], and behaviour [20]. Early exposure to cortisol might also influence egg metabolic rate because cortisol has a strong positive effect on metabolic rates in adult fishes [21–23].
The goal of this study was to quantify the consequences of maternal exposure to predation risk on offspring in three-spined sticklebacks (Gasterosteus aculeatus). In sticklebacks, fathers provide all of the parental care. However, maternal effects might also be important in this system because female sticklebacks are exposed to predation risk during the breeding season, and previous studies have shown that sticklebacks exposed to predation risk have elevated concentrations of cortisol [24]. For this reason, we regularly exposed gravid female sticklebacks to a model predator (experimental) prior to and during egg formation, while other females were undisturbed (control). The resulting offspring from the two treatments were compared for egg characteristics (egg size, egg number, egg cortisol concentration, metabolic rate) and post-hatching characteristics (growth and shoaling behaviour, an antipredator behaviour).
2. Material and methods
Adult sticklebacks used in this experiment were F1 generation offspring from a natural population in Putah Creek, CA. Fish were maintained on a natural photoperiod at 20°C and fed a mixture of frozen bloodworms, mysis shrimp, cyclopleze and brine shrimp ad libitum.
In August 2009, female sticklebacks were randomly assigned to one of nine (n = 3 control, n = 6 experimental tanks) different 26.5 l tanks (36 L × 33 W × 24 H cm) with five fish per tank. We assigned more tanks to the experimental group because a pilot study detected greater variation in egg cortisol among mothers exposed to a predator (mean = 194.20 ng ml−1, s.e. = 51.50, n = 7) compared with the eggs of mothers not exposed to a predator (mean = 89.72 ng ml−1, s.e. = 7.76, n = 7). Each fish within a tank was given a unique spine clip to determine the duration of time each female was in the experimental or control treatment. The tanks were covered with opaque plastic with a small window to allow an observer to monitor the gravidity of females with minimal disturbance to fish.
(a). Disturbance protocol
Fish in the experimental group were chased for approximately 30 s once a day at a randomly chosen time with a clay model of a natural predator, the northern pike (Esox lucius, 23 L × 1 W × 6 H cm, painted to match natural markings). This protocol of unpredictable, repeated exposure to cues of predators was designed to mimic natural conditions where sticklebacks in high predation populations are regularly confronted by the threat of predation [25,26]. Fish in the control treatment remained undisturbed. On average, females spent approximately 25 days in the experimental or control group before being stripped for eggs (experimental: mean = 24 days (range = 2–57); control: mean = 28 days (range = 7–46)).
When a female from either the control or experimental group became gravid, she was measured for standard length and weight while still bearing eggs. She was then stripped of eggs and the mass of the entire clutch was weighed. Egg size (mg) was calculated for each clutch by batch-weighing a subset of eggs and dividing the subset mass by the number of eggs in the subset. The total number of eggs within a female's clutch was estimated by dividing her entire clutch mass by egg size. We observed little variation in egg size within clutches (A. M. Bell 2010, personal observation). Each female contributed one clutch of eggs to the experiment, and a subset of approximately 10–15 eggs were stored in 1.5 ml microcentrifuge tubes at −80°C for later cortisol quantification.
To control for paternal effects [27], we artificially fertilized a subset (n = 30–40) of eggs from each female. Sperm was collected from sacrificed males by dissecting out the testes; one testis from each male was used to fertilize an experimental clutch, the other fertilized a control clutch. Successful fertilization was confirmed by visual inspection for eye spots, and unfertilized eggs were removed. Subsets (n = 19–23) of fertilized eggs were measured for metabolic rate, while the remaining eggs (n = 7–21) were artificially incubated and reared in the laboratory for quantification of post-hatching characteristics. After a female was stripped of eggs, she was replaced by a new marked female. We continued sampling for 10 weeks until clutches from 30 different experimental mothers and 15 different control mothers were collected.
Unfertilized eggs were measured for cortisol content using an enzyme-linked immunosorbent assay (ELISA) (Assay Designs, Ann Arbor, MI, no. 900-071, Catalog no. 25-0359). Such ELISA kits have been used successfully to measure cortisol from plasma or whole body extracts from several species of fishes [28]. The antibody used in this assay is reported to have 27.7 per cent cross-reactivity with corticosterone, approximately 4 per cent with 11-deoxycortisol and progesterone, and 0.1 per cent or less with testosterone, androstenedione, cortisone and oestradiol. No data are currently available for cross-reactivity with conjugated steroids. Samples were run in duplicate without extraction following homogenization of 15–25 eggs from each clutch in 200 µl of assay buffer as per manufacturer instruction. Cortisol concentration is reported as ng ml−1. Two clutches were removed from the analysis because they failed to form a pellet during centrifugation. The intra-assay coefficient of variation was 2 per cent, and the inter-assay coefficient of variation was 11 per cent.
(b). Metabolic rate
The oxygen consumption rate (MO2 in mg O2 g−1 h−1) of six batches of 19–23 fertilized eggs from each treatment group were measured using intermittent flow respirometry (Loligo Systems, Hobro, Denmark [29]) following methods adapted from Gingerich et al. [30]. We used a respirometry system with four (725 µl) glass chambers outfitted with fibre optic oxygen probes connected to a peristaltic pump. The chambers were placed in a 120 l cooler of water at 20°C (±0.5°C) and outfitted with several air stones. Temperature was kept constant and a temperature probe was calibrated throughout the experiment. One of the four chambers was used as a blank to correct for background O2 and three chambers were used for measuring oxygen consumption of embryos. The peristaltic pump re-circulated water through the chambers across the oxygen probes to ensure proper mixing, and the fibre optic oxygen probes were calibrated with both sodium sulphite (oxygen-free) water and 100 per cent oxygen saturated water throughout the experiment.
Oxygen consumption of embryos in each chamber was determined with a 28 min cycle, which consisted of a 13 min measurement period, a 15 min flush period to re-oxygenate water, and a 2 s waiting period following the flush until the measurements were resumed. Oxygen consumption in each chamber was recorded every second, but only coefficient of determination (r2) values of more than 0.93 were analysed. We evaluated the oxygen consumption of embryos at different days since fertilization (average time to hatching = 6 days) in order to evaluate how oxygen consumption varies with embryo development. On average, 22 measurements were taken from each clutch (range = 5–35). De-oxygenation of the water in these chambers did not occur, and early measurements did not impact later measurements. We did not evaluate oxygen consumption after hatching. All data were recorded using AutoResp software (v. 1.4).
(c). Post-hatching characteristics
Fertilized eggs from each clutch were incubated separately in a cup with a mesh screen bottom. Between four to six newly hatched fry from each clutch were mixed and placed in one of four 83.28 l tanks (107 L × 33 W × 24 H cm, n = 2 experimental and n = 2 control tanks), and were fed newly hatched Artermia once a day. When the fry were approximately 2.5 cm standard length and safe to handle (1–3 months of age), we transferred them to 26.50 l tanks (36 L × 33W × 24 H cm, n = 9 experimental and n = 6 control tanks) at a density of approximately five fish per tank (mean = 4.86 fish, s.d. = 0.663). Fry were matched for size within each tank to minimize dominance interactions over access to food. A grid with cell size 3.7 × 3.7 cm was placed on the bottom of each tank for reference.
Because handling could influence the development of the HPI axis [16], we measured the standard length of fry non-invasively by photographing each tank from above. The first photograph of each tank was taken on 7 December 2009, when the fry were two to four months of age. We took photographs of each tank on six different occasions over the course of 12 weeks, with one to two weeks between measurements. Owing to difficulty locating all fry within a tank using the photographing protocol, it was necessary to take three pictures of each tank at each age. The first photograph (termed the ‘before’ photograph) was of the undisturbed tank. Then, we mildly disturbed the fish by gently moving fish that were not visible with a 26 mm piece of plastic tubing, and took a second photograph immediately following this disturbance (‘immediately following’). We took one more photograph 2 min following the mild disturbance (‘after’).
The photographs ‘before’, ‘immediately following’ and ‘after’ the mild disturbance allowed us to address two issues. First, some of the fish were not visible in the first picture, and by gently moving the tubing around the tank, more fish became visible (number of fish visible ‘before’ = 2.3 experimental, n = 2.0 control; number of fish visible ‘immediately following’ = 3.6 experimental, n = 3.5 control). Second, by comparing the average nearest-neighbour distance of control and experimental juveniles after the mild disturbance, we were able to determine whether maternal exposure to predation risk influenced juveniles' response to a mild disturbance.
Using ImageJ software (v. 1.38) and the grid on the bottom of the tank as reference, we calculated the average standard length of fish in each tank in the ‘immediately following’ photographs. On average, the fry in the experimental tanks were older than the fry in the control tanks, therefore growth was analysed as a function of age rather than a function of the day or measurement.
The photographs taken to monitor growth rates were also used to measure shoaling behaviour (nearest-neighbour distance) of the developing fry. Nearest-neighbour distance is often used as a measure of shoaling in fishes [31,32]. Nearest-neighbour distance was measured ‘before’, ‘immediately following’ and ‘after’ the mild disturbance on all six occasions. Nearest-neighbour distance was estimated from the centre of the head of each fish in the tank to the centre of the head of the nearest fish in each picture. A reference line between two fish was used to correct for distortion. Nearest-neighbour distance was measured on each fish in each tank and the average nearest-neighbour distance was calculated for each tank.
(d). Data analysis
Cortisol concentrations were not normally distributed and were therefore ln-transformed. We first fit a generalized linear model (GLM) with treatment (fixed), tank (nested within treatment, random), female length and time in treatment as covariates on egg cortisol content, egg size, and the number of eggs. We then reduced the full model by sequentially removing the least important covariates and then re-fitting the model.
Change in oxygen concentration (α) for each chamber was calculated as the slope (ΔO2saturation/Δt), and oxygen consumption rate (MO2, mg O2 kg−1 h−1) for each clutch of eggs was calculated by MO2 = αVrespβMb−1, where Vresp is the volume of each chamber minus number of eggs by volume of eggs in chamber (μl), β the oxygen solubility (adjusted for temperature and barometric pressure) and Mb−1 the clutch mass (kg) before placing in chamber [30]. We compared differences in the oxygen consumption rate in the eggs from experimental and control mothers using a GLM with treatment as a fixed factor, clutch nested within treatment as a random factor, time since fertilization as a covariate and the interaction between treatment and time since fertilization.
To avoid pseudoreplication [33], we analysed the average growth rate of each tank using a GLM with treatment as a fixed factor, tank nested within treatment as a random factor, age and density (number of fish per tank) as covariates, and the interaction between age and treatment. We then reduced the full model by sequentially removing non-significant terms, and then re-fitting the model. Similarly, we analysed the average nearest-neighbour distance of each picture of each tank because the nearest-neighbour distance among individuals within a tank was not independent. Average nearest-neighbour distance was square-root transformed to meet normality assumptions. Because the average nearest-neighbour distance differed ‘before’, ‘immediately following’ and ‘after’ the mild disturbance (see §3), we analysed the ‘before’, ‘immediately following’ and ‘after’ data separately. Sample size differed ‘before’, ‘immediately following’ and ‘after’ because fewer fish were visible in the ‘before’ picture. We tested for the effect of treatment as a fixed factor, tank nested within each treatment as a random effect, with density, average standard length of fish in each tank and age as covariates. All statistical analyses were two-tailed and were performed using SPSS v. 17.
3. Results
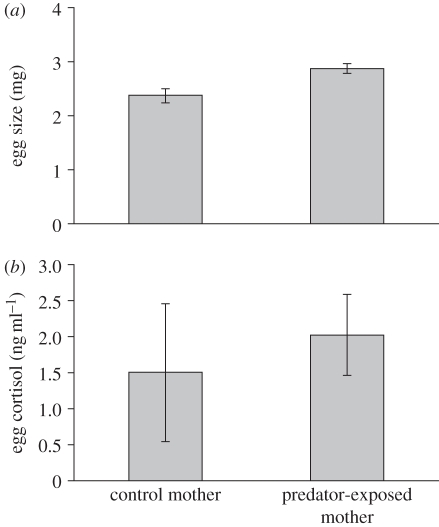
Females exposed to a predator during egg formation produced larger eggs (F1,11 = 12.63, p = 0.005; figure 1a), but there was no effect of treatment on the number of eggs (F1,14 = 0.005, p > 0.05). There was no effect of time spent in treatment or tank on egg size or number of eggs (all p > 0.05). Larger females produced more eggs (F1,34 = 6.822, p = 0.013).
Figure 1.
(a) Egg size (mg) from mothers exposed to a predator (experimental, n = 30) was significantly greater than egg size from clutches of mothers not exposed to a predator (control, n = 15). (b) Egg cortisol concentration (ng ml−1) from mothers exposed to a predator (experimental, n = 30) was significantly higher than eggs from mothers not exposed to a predator stressor (control, n = 13). Bars show means ± s.e. Statistical tests were carried out on ln-transformed cortisol values.
The eggs of mothers exposed to a predator had significantly higher concentrations of cortisol compared with the control group (F1,9.6 = 5.18, p = 0.04; figure 1b; experimental: 2.006 ng ml−1 ± 0.558, n = 30; control: 1.489 ± 0.951 ng ml−1, n = 13). Egg cortisol content was not influenced by the mother's standard length, days spent in the treatment or tank (all p > 0.05).
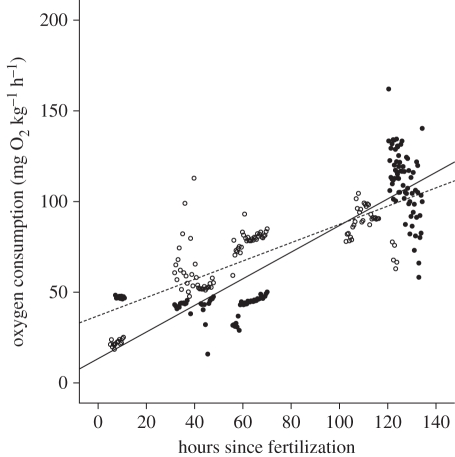
In both treatments, the metabolic rate of eggs increased with time since fertilization (time: F1.241 = 9.7, p = 0.002; figure 2). Eggs from mothers exposed to a predator consumed more oxygen than control clutches soon after fertilization, but the difference between treatments attenuated over time (treatment: F1,163 = 19.2, p < 0.0001; time × treatment: F1,241 = 10.4, p = 0.001; figure 2). There was significant variation among clutches in oxygen consumption (clutch(tank(treatment))) F4,241 = 30.4, p < 0.0001), and no effect of maternal tank on oxygen consumption (tank(treatment): F6,3.95 = 0.59, p > 0.05).
Figure 2.
Oxygen consumption (mg O2 kg−1 h−1) per egg increased over time regardless of treatment. Eggs from mothers exposed to a stressor (experimental: n = 6 clutches, open circles, dashed line) consumed more oxygen than eggs from mothers not exposed to a predator stressor (control: n = 6 clutches, closed circles, solid line) soon after fertilization, but the difference attenuated close to hatching.
Standard length of fry from both treatment groups increased over the course of the experiment (age: F1,69 = 618.4, p < 0.0001). We did not detect an effect of treatment on growth during the period when fry were measured (treatment: F1,34.19 = 1.235, p = 0.274; treatment × age: F1,69 = 1.508, p > 0.05).
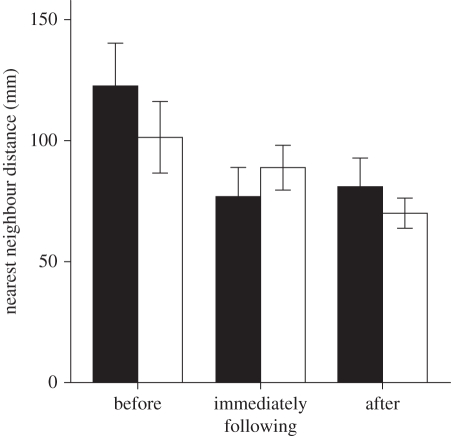
Prior to the mild disturbance, the fish in the experimental group were closer together compared with fish in the control group (treatment: F1,51 = 4.5, p = 0.038; figure 3). There was not an effect of density, age, standard length or tank on nearest-neighbour distance (all p > 0.05). An equal number of fish were visible in the control (mean number of fish visible = 2.00, s.e. = 0.264, n = 36) and the experimental (mean number of fish visible = 2.25, s.e. = 0.212, n = 53) tanks, so the closer nearest-neighbour distance in the experimental tanks was not owing to higher density. Control and experimental tanks did not differ in average nearest-neighbour distance ‘immediately following’ or ‘after’ the mild disturbance (all p > 0.05, figure 3).
Figure 3.
‘Before’ a mild disturbance, the nearest-neighbour distance was significantly shorter between fry from mothers exposed to a predator than from mothers not exposed to a predator. There was no difference in nearest-neighbour distance between control and experimental fry ‘immediately following’ or ‘after’ a mild disturbance. Each bar represents the mean ± s.e. of n = 9 experimental and n = 6 control tanks across all six measurements (filled bar, offspring of control mothers; open bar, offspring of predator-exposed mothers). Statistical tests were carried out on square-root transformed values.
4. Discussion
An outstanding question about maternal effects is when and whether they might be adaptive. On the one hand, maternal stress might have deleterious effects for her offspring. For example, human females exposed to famine during pregnancy produced smaller offspring that were more susceptible to disease and health problems, and this effect was passed down for generations [34]. On the other hand, developing embryos are highly sensitive to endocrine perturbations; therefore, we might expect embryos to possess mechanisms that buffer the embryo against hormonal fluctuations in utero. Moreover, the conditions experienced by a mother during oogenesis or pregnancy might be predictive of the future environment of her offspring [35]. Therefore, it might be adaptive for mothers to hormonally programme [36] their offspring to be suited for particular environments [37–39].
In this experiment, we found maternal effects on several offspring phenotypes, some of which might be adaptive. In particular, contrary to the hypothesis that maternal stress causes females to produce small, low-quality eggs [14,17,40–42], we found that exposure to predation risk caused females to produce larger eggs. Other studies on fishes have found positive effects of egg size on feeding [43], swimming abilities [44] and survival [45–48]. Therefore, this result suggests that increased egg size might give fry an advantage in a high predation environment [49,50] and is consistent with other studies [51–54] which have found that mothers can plastically adjust egg size in response to the environment.
In addition, we found long-term consequences of maternal exposure to simulated predation risk on the behaviour of their offspring: juveniles from experimental mothers were closer together prior to a mild disturbance. Shoaling behaviour is an effective antipredator strategy in fishes [55,56], therefore this result suggests that maternal experience might cause intergenerational transmission of information about predation risk.
We measured higher concentrations of cortisol in eggs of mothers exposed to a predator than in eggs from mothers not exposed to a predator. This parallels the results of a similar study on coho salmon where daily chasing during egg formation caused elevated concentrations of cortisol in both maternal plasma and eggs [10]. In our study, the amount of time that a female was exposed to predator-induced stress had no effect on measured egg cortisol concentrations, suggesting that females did not acclimate to repeated chasing by the predator model. We hypothesize that the positive effect of treatment on respiration rate was mediated by elevated concentrations of maternally derived cortisol rather than increased egg size because other studies have found positive effects of cortisol on metabolic rate [21–23], and mass-specific metabolic rate decreases with increasing body mass [57]. One hypothesis to explain the attenuating difference in oxygen consumption between control and experimental clutches is that the embryo has mechanisms to buffer any effects of cortisol that develop just prior to hatching [58]. Future studies should use high performance liquid chromatography to confirm that the antibody used specifically interacts with cortisol and should assess the accuracy of the assay for sticklebacks [59].
However, we do not know whether the effects of maternal experience on juvenile behaviour was owing to the organizational effects of early exposure to cortisol, increased egg size or other egg qualities such as protein content [60], lipid content [61] or other hormones. It is also unknown for how long maternal effects on offspring behaviour will persist, whether they are specific to antipredator behaviour or extend to other behaviours, and the mechanisms such as DNA methylation [36,62] that produce long-term, irreversible effects on behavioural development.
Acknowledgements
All of the procedures were carried out in accordance with the University of Illinois policies and were approved in Protocol no. 09 204.
We thank Rachel Vinsel for technical help and Katie McGhee for comments on the manuscript.
References
- 1.Storm J. J., Lima S. L. 2010. Mothers forewarn offspring about predators: a transgenerational maternal effect on behaviour. Am. Nat. 175, 382–390 10.1086/650443 (doi:10.1086/650443) [DOI] [PubMed] [Google Scholar]
- 2.Clarke A. S., Schneider M. L. 1993. Prenatal stress has long-term effects on behavioural responses to stress in juvenile rhesus monkeys. Dev. Psychobiol. 26, 293–304 10.1002/dev.420260506 (doi:10.1002/dev.420260506) [DOI] [PubMed] [Google Scholar]
- 3.Nishio H., Kasuga S., Ushijima M., Harada Y. 2001. Prenatal stress and postnatal development of neonatal rats: sex dependent effects on emotional behavior and learning ability of neonatal rats. Int. J. Dev. Neurosci. 19, 37–45 10.1016/S0736-5748(00)00070-8 (doi:10.1016/S0736-5748(00)00070-8) [DOI] [PubMed] [Google Scholar]
- 4.Sheriff M. J., Krebs C. J., Boonstra R. 2010. The ghosts of predators past: population cycles and the role of maternal programming under fluctuating predation risk. Ecology 91, 2983–2994 10.1890/09-1108.1 (doi:10.1890/09-1108.1) [DOI] [PubMed] [Google Scholar]
- 5.Gil D. 2008. Hormones in avian eggs: physiology, ecology and behaviour. Adv. Stud. Behav. 38, 337–398 10.1016/S0065-3454(08)00007-7 (doi:10.1016/S0065-3454(08)00007-7) [DOI] [Google Scholar]
- 6.Talge N. M., Neal C., Glover V. 2007. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J. Child Psychol. Psychiatr. 48, 245–261 10.1111/j.1469-7610.2006.01714.x (doi:10.1111/j.1469-7610.2006.01714.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang P., Wu S., Lin J., Wu L. 1992. Cortisol content of eggs and larvae of teleosts. Gen. Comp. Endocrinol. 86, 189–196 10.1016/0016-6480(92)90101-O (doi:10.1016/0016-6480(92)90101-O) [DOI] [PubMed] [Google Scholar]
- 8.Barry T. P., Malison J. A., Held J. A., Parrish J. J. 1995. Ontogeny of the cortisol stress response in larval rainbow trout. Gen. Comp. Endocrinol. 97, 57–65 10.1006/gcen.1995.1006 (doi:10.1006/gcen.1995.1006) [DOI] [PubMed] [Google Scholar]
- 9.De Jesus E. G., Hirano T., Inui Y. 1991. Changes in cortisol and thyroid hormone concentrations during early development and metamorphosis in Japanese flounder, Paralichthys olivaceus. Gen. Comp. Endocrinol. 82, 369–376 [DOI] [PubMed] [Google Scholar]
- 10.Stratholt M. L., Donaldson E. M., Liley N. R. 1997. Stress induced elevation of plasma cortisol in adult female coho salmon (Oncorhynchus kisutch), is reflected in egg cortisol content, but does not appear to affect early development. Aquaculture 158, 141–153 10.1016/S0044-8486(97)00165-8 (doi:10.1016/S0044-8486(97)00165-8) [DOI] [Google Scholar]
- 11.Sapolsky R. M., Meaney M. J. 1986. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain. Res. Rev. 11, 65–76 10.1016/0165-0173(86)90010-X (doi:10.1016/0165-0173(86)90010-X) [DOI] [PubMed] [Google Scholar]
- 12.Matthews S. G., Challis J. R. G. 1996. Regulation of the hypothalamo-pituitary-adrenocortical axis in fetal sheep. Trends Endocrinol. Metabol. 7, 239–246 10.1016/S1043-2760(96)00126-9 (doi:10.1016/S1043-2760(96)00126-9) [DOI] [PubMed] [Google Scholar]
- 13.Feist G., Schreck C. B. 2001. Ontogeny of the stress response in chinook salmon, Oncorhynchus tshawytscha. Fish. Physiol. Biochem. 25, 31–40 10.1023/A:1019709323520 (doi:10.1023/A:1019709323520) [DOI] [Google Scholar]
- 14.McCormick M. I. 1998. Behaviourally induced maternal stress in a fish influences progeny quality by a hormonal mechanism. Ecology 79, 1873–1883 10.1890/0012-9658(1998)079[1873:BIMSIA]2.0.CO;2 (doi:10.1890/0012-9658(1998)079[1873:BIMSIA]2.0.CO;2) [DOI] [Google Scholar]
- 15.Schreck C. B., Contreras-Sanchez W., Fitzpatrick M. S. 2001. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture 197, 3–24 10.1016/S0044-8486(01)00580-4 (doi:10.1016/S0044-8486(01)00580-4) [DOI] [Google Scholar]
- 16.Auperin B., Geslin M. 2008. Plasma cortisol response to stress in juvenile rainbow trout is influenced by their life history during early development and by egg cortisol content. Gen. Comp. Endocrinol. 158, 234–239 10.1016/j.ygcen.2008.07.002 (doi:10.1016/j.ygcen.2008.07.002) [DOI] [PubMed] [Google Scholar]
- 17.Campbell P. M., Pottinger T. G., Sumpter J. P. 1994. Preliminary evidence that chronic confinement stress reduces the quality of gametes produced by brown and rainbow trout. Aquaculture 120, 151–169 10.1016/0044-8486(94)90230-5 (doi:10.1016/0044-8486(94)90230-5) [DOI] [Google Scholar]
- 18.Eriksen M. S., Bakken M., Espmark A., Braastad B. O., Salte R. 2006. Pre-spawning stress in farmed Atlantic salmon, Salmo salar: maternal cortisol exposure and hyperthermia during embryonic development affect offspring survival, growth and incidence of malformations. J. Fish Biol. 69, 114–129 10.1111/j.1095-8649.2006.01071.x (doi:10.1111/j.1095-8649.2006.01071.x) [DOI] [Google Scholar]
- 19.Eriksen M. S., Espmark A., Braastad B. O., Salte R., Bakken M. 2007. Long-term effects of maternal cortisol exposure and mild hyperthermia during embryogeny on survival, growth and morphological anomalies in farmed Atlantic salmon Salmo salar offspring. J. Fish Biol. 70, 462–473 10.1111/j.1095-8649.2007.01317.x (doi:10.1111/j.1095-8649.2007.01317.x) [DOI] [Google Scholar]
- 20.Espmark A. M., Eriksen M. S., Salte R., Braastad B. O., Bakken M. 2008. A note on pre-spawning maternal cortisol exposure in farmed Atlantic salmon and its impact on the behaviour of offspring in response to a novel environment. Appl. Anim. Behav. Sci. 110, 404–409 10.1016/j.applanim.2007.04.003 (doi:10.1016/j.applanim.2007.04.003) [DOI] [Google Scholar]
- 21.Chan D. K. O., Woo N. Y. S. 1978. Effect of cortisol on metabolism of eel, Anguilla japonica. Gen. Comp. Endocrinol. 35, 205–215 10.1016/0016-6480(78)90064-3 (doi:10.1016/0016-6480(78)90064-3) [DOI] [PubMed] [Google Scholar]
- 22.Barton B. A., Schreck C. B. 1987. Metabolic cost of acute physical stress in juvenile steelhead. Trans. Am. Fish. Soc. 116, 257–263 (doi:10.1577/1548-8659(1987)116<257:MCOAPS>2.0.CO;2) [DOI] [Google Scholar]
- 23.Wendelaar Bonga S. E. 1997. The stress response in fish. Physiol. Rev. 77, 591–625 [DOI] [PubMed] [Google Scholar]
- 24.Bell A. M., Backstrom T., Huntingford F. A., Pottinger T. G., Winberg S. 2007. Variable neuroendocrine responses to ecologically-relevant challenges in sticklebacks. Physiol. Behav. 91, 15–25 10.1016/j.physbeh.2007.01.012 (doi:10.1016/j.physbeh.2007.01.012) [DOI] [PubMed] [Google Scholar]
- 25.Giles N., Huntingford F. A. 1984. Predation risk and inter-population variation in anti-predator behaviour in the 3-spined stickleback, Gasterosteus aculeatus. Anim. Behav. 32, 264–275 10.1016/S0003-3472(84)80346-2 (doi:10.1016/S0003-3472(84)80346-2) [DOI] [Google Scholar]
- 26.Bell A. M., Henderson L., Huntingford F. A. 2010. Behavioural and respiratory responses to stressors in multiple populations of three-spined sticklebacks that differ in predation pressure. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 180, 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tulley J. J., Huntingford F. A. 1987. Paternal care and the development of adaptive variation in anti-predator responses in sticklebacks. Anim. Behav. 35, 1570–1572 10.1016/S0003-3472(87)80034-9 (doi:10.1016/S0003-3472(87)80034-9) [DOI] [Google Scholar]
- 28.Sink T. D., Lochmann R. T., Fecteau K. A. 2008. Validation, use and disadvantages of enzyme-linked immunosorbent assay kits for detection of cortisol in channel catfish, largemouth bass, red pacu and golden shiners. Fish Physiol. Biochem. 34, 95–101 10.1007/s10695-007-9150-9 (doi:10.1007/s10695-007-9150-9) [DOI] [PubMed] [Google Scholar]
- 29.Steffensen J. F. 1989. Some errors in respirometry of aquatic breathers: how to avoid and correct them. Fish. Physiol. Biochem. 6, 49–59 10.1007/BF02995809 (doi:10.1007/BF02995809) [DOI] [PubMed] [Google Scholar]
- 30.Gingerich A. J., Philipp D. P., Suski C. D. 2010. Effects of nutritional status on metabolic rate, exercise and recovery in a freshwater fish. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 180, 371–384 [DOI] [PubMed] [Google Scholar]
- 31.Azuma T., Iwata M. 1994. Influences of illumination intensity on the nearest neighbor distance in coho salmon Oncorhynchus kisutch. J. Fish. Biol. 45, 1113–1118 10.1111/j.1095-8649.1994.tb01077.x (doi:10.1111/j.1095-8649.1994.tb01077.x) [DOI] [Google Scholar]
- 32.Masuda R., Shoji J., Nakayama S., Tanaka M. 2003. Development of schooling behaviour in Spanish mackerel, Scomberomorus niphonius, during early ontogeny. Fish. Sci. 69, 772–776 10.1046/j.1444-2906.2003.00685.x (doi:10.1046/j.1444-2906.2003.00685.x) [DOI] [Google Scholar]
- 33.Alvarez D., Metcalfe N. B. 2005. Catch-up growth and swimming performance in threespine sticklebacks (Gasterosteus aculeatus): seasonal changes in the cost of compensation. Can. J. Fish. Aquat. Sci. 62, 2169–2176 10.1139/f05-130 (doi:10.1139/f05-130) [DOI] [Google Scholar]
- 34.Painter R. C., Roseboom T. J., Bleker O. P. 2005. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod. Toxicol. 20, 345–352 10.1016/j.reprotox.2005.04.005 (doi:10.1016/j.reprotox.2005.04.005) [DOI] [PubMed] [Google Scholar]
- 35.Gluckman P. D., Hanson M. A., Spencer H. G., Bateson P. 2005. Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies. Proc. R. Soc. B 272, 671–677 10.1098/rspb.2004.3001 (doi:10.1098/rspb.2004.3001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weaver I. C. G., Cervoni N., Champagne F. A., D'Alessio A. C., Sharma S., Seckl J. R., Dymov S., Szyf M., Meaney M. J. 2004. Epigenetic programming by maternal behaviour. Nat. Neurosci. 7, 847–854 10.1038/nn1276 (doi:10.1038/nn1276) [DOI] [PubMed] [Google Scholar]
- 37.Mousseau T. A., Dingle H. 1991. Maternal effects in insect life histories. Annu. Rev. Entomol. 36, 511–534 10.1146/annurev.en.36.010191.002455 (doi:10.1146/annurev.en.36.010191.002455) [DOI] [Google Scholar]
- 38.Galloway L. F. 1995. Response to natural environmental heterogeneity: maternal effects and selection on life-history characters and plasticities in Mimulus guttatus. Evolution 49, 1095–1107 10.2307/2410434 (doi:10.2307/2410434) [DOI] [PubMed] [Google Scholar]
- 39.Agrawal A. A. 2002. Herbivory and maternal effects: mechanisms and consequences of transgenerational induced plant resistance. Ecology 83, 3408–3415 10.1890/0012-9658(2002)083[3408:HAMEMA]2.0.CO;2 (doi:10.1890/0012-9658(2002)083[3408:HAMEMA]2.0.CO;2) [DOI] [Google Scholar]
- 40.Campbell P. M., Pottinger T. G., Sumpter J. P. 1992. Stress reduces the quality of gametes produced by rainbow trout. Biol. Reprod. 47, 1140–1150 10.1095/biolreprod47.6.1140 (doi:10.1095/biolreprod47.6.1140) [DOI] [PubMed] [Google Scholar]
- 41.Contreras-Sanchez W. M., Schreck C. B., Fitzpatrick M. S., Pereira C. B. 1998. Effects of stress on the reproductive performance of rainbow trout (Oncorhynchus mykiss). Biol. Reprod. 58, 439–447 10.1095/biolreprod58.2.439 (doi:10.1095/biolreprod58.2.439) [DOI] [PubMed] [Google Scholar]
- 42.McCormick M. I. 1999. Experimental test of the effect of maternal hormones on larval quality of a coral reef fish. Oecologia 118, 412–422 10.1007/s004420050743 (doi:10.1007/s004420050743) [DOI] [PubMed] [Google Scholar]
- 43.Knutsen G. M., Tilseth S. 1985. Growth, development, and feeding success of Atlantic cod larvae, Gadus morhua, related to egg size. Trans. Am. Fish. Soc. 114, 507–511 (doi:10.1577/1548-8659(1985)114<507:GDAFSO>2.0.CO;2) [DOI] [Google Scholar]
- 44.Ojanguren A. F., ReyesGavilan F. G., Brana F. 1996. Effects of egg size on offspring development and fitness in brown trout, Salmo trutta. Aquaculture 147, 9–20 10.1016/S0044-8486(96)01398-1 (doi:10.1016/S0044-8486(96)01398-1) [DOI] [Google Scholar]
- 45.Lillelund K., Lasker R. 1971. Laboratory studies of predation by marine copepods on fish larvae. Fish. Bull. US 69, 655–667 [Google Scholar]
- 46.Blaxter J. H. S. 1988. Pattern and variety of development. In Fish physiology. (eds Hoar W. S., Randall D. J.), pp. 177–252 New York, NY: Academic Press [Google Scholar]
- 47.Henrich S. 1988. Variation in offspring sizes of the poeciliid fish, Heterandria Formosa, in relation to fitness. Oikos 51, 13–18 10.2307/3565801 (doi:10.2307/3565801) [DOI] [Google Scholar]
- 48.Leggett W. C., Deblois E. 1994. Recruitment in marine fishes: is it regulated by starvation and predation in the egg and larval stages? Neth. J. Sea Res. 32, 119–134 10.1016/0077-7579(94)90036-1 (doi:10.1016/0077-7579(94)90036-1) [DOI] [Google Scholar]
- 49.Marshall D. J., Uller T. 2007. When is a maternal effect adaptive? Oikos 116, 1957–1963 [Google Scholar]
- 50.Taborsky B., Skubic E., Bruintjes R. 2007. Mothers adjust egg size to helper number in a cooperatively breeding cichlid. Behav. Ecol. 18, 652–657 10.1093/beheco/arm026 (doi:10.1093/beheco/arm026) [DOI] [Google Scholar]
- 51.Bernardo J. 1996. Maternal effects in animal ecology. Am. Zool. 36, 83–105 [Google Scholar]
- 52.Mousseau T. A., Fox C. W. 1998. The adaptive significance of maternal effects. Trends. Ecol. Evol. 13, 403–407 10.1016/S0169-5347(98)01472-4 (doi:10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 53.Taborsky B. 2006. Mothers determine offspring size in response to own juvenile growth conditions. Biol. Lett. 2, 225–228 10.1098/rsbl.2005.0422 (doi:10.1098/rsbl.2005.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donelson J. M., McCormick M. I., Munday P. L. 2008. Parental condition affects early life-history of a coral reef fish. J. Exp. Mar. Biol. Ecol. 360, 109–116 10.1016/j.jembe.2008.04.007 (doi:10.1016/j.jembe.2008.04.007) [DOI] [Google Scholar]
- 55.Magurran A. E. 1990. The inheritance and development of minnow anti-predator behaviour. Anim. Behav. 39, 834–842 10.1016/S0003-3472(05)80947-9 (doi:10.1016/S0003-3472(05)80947-9) [DOI] [Google Scholar]
- 56.Queiroz H., Magurran A. E. 2005. Safety in numbers? Shoaling behaviour of the Amazonian red-bellied piranha. Biol. Lett. 1, 155–157 10.1098/rsbl.2004.0267 (doi:10.1098/rsbl.2004.0267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Makarieva A. M., Gorshkov V. G., Li B. L. 2005. Energetics of the smallest: do bacteria breathe at the same rate as whales? Proc. R. Soc. B 272, 2219–2224 10.1098/rspb.2005.3225 (doi:10.1098/rspb.2005.3225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paitz R. T., Bowden R. M. 2009. Rapid decline in the concentrations of three yolk steroids during development: is it embryonic regulation? Gen. Comp. Endocrinol. 161, 246–251 10.1016/j.ygcen.2009.01.018 (doi:10.1016/j.ygcen.2009.01.018) [DOI] [PubMed] [Google Scholar]
- 59.Rettenbacher S., Mostl E., Groothuis T. G. G. 2009. Gestagens and glucocorticoids in chicken eggs. Gen. Comp. Endocrinol. 164, 125–129 10.1016/j.ygcen.2009.05.019 (doi:10.1016/j.ygcen.2009.05.019) [DOI] [PubMed] [Google Scholar]
- 60.Goncalves I. B., Mobley K. B., Ahnesjö I., Sagebakken G., Jones A. G., Kvarnemo C. 2010. Reproductive compensation in broad-nosed pipefish females. Proc. R. Soc. B 277, 1581–1587 10.1098/rspb.2009.2290 (doi:10.1098/rspb.2009.2290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brooks S., Tyler C. R., Sumpter J. P. 1997. Quality in fish: what makes a good egg? Rev. Fish. Biol. Fish. 7, 387–416 10.1023/A:1018400130692 (doi:10.1023/A:1018400130692) [DOI] [Google Scholar]
- 62.Meaney M. J. 2001. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 24, 1161–1192 10.1146/annurev.neuro.24.1.1161 (doi:10.1146/annurev.neuro.24.1.1161) [DOI] [PubMed] [Google Scholar]