Abstract
A dramatic rise in obesity has occurred among humans within the last several decades. Little is known about whether similar increases in obesity have occurred in animals inhabiting human-influenced environments. We examined samples collectively consisting of over 20 000 animals from 24 populations (12 divided separately into males and females) of animals representing eight species living with or around humans in industrialized societies. In all populations, the estimated coefficient for the trend of body weight over time was positive (i.e. increasing). The probability of all trends being in the same direction by chance is 1.2 × 10−7. Surprisingly, we find that over the past several decades, average mid-life body weights have risen among primates and rodents living in research colonies, as well as among feral rodents and domestic dogs and cats. The consistency of these findings among animals living in varying environments, suggests the intriguing possibility that the aetiology of increasing body weight may involve several as-of-yet unidentified and/or poorly understood factors (e.g. viral pathogens, epigenetic factors). This finding may eventually enhance the discovery and fuller elucidation of other factors that have contributed to the recent rise in obesity rates.
Keywords: obesity, animals, epigenetic
‘Like humans, domestic animals and fish and other wildlife are exposed to contaminants in air, soil, water, and food, and they can suffer acute and chronic health effects from such exposures. Animal sentinel systems—systems in which data on animals exposed to contaminants in the environment are regularly and systematically collected and analyzed—can be used to identify potential health hazards to other animals or humans.’
National Academy of Sciences (1991, p. 1).
1. Introduction
There is a well-documented human obesity epidemic [1]. Although the increase in obesity rates started over 100 years ago [2], there has been an acceleration in the last half-century, with reasons incompletely understood. Although there is a focus on a lack of physical activity and a poor diet as the principal contributors to this recent acceleration, there are apparently many causes beyond the conventional wisdom that contribute to body weight increase either by influencing physical activity or dietary intake, or through other means such as influencing nutrient partitioning or energy metabolism [3–7].
Model organisms have potential value as ‘canaries in the coalmines’ or ‘sentinels’ informing us about environmental factors potentially impacting humans [8]. In this light, we compiled data to assess time trends in body weight in mammalian species that live with or around humans in industrialized societies. Such observations might help identify environmental influences that might otherwise go undetected.
From 24 distinct populations (12 subdivided into separate male and female populations), representing eight species (see §2 for inclusion criteria), over 20 000 animals were studied. Time trends for mean per cent weight change and the odds of obesity (see the electronic supplementary material for definition) were tested for the samples from each population at an age period that corresponded roughly to early-middle adulthood (35 years) in human development (see the electronic supplementary material for calculation) because on a per cent basis, in United States adults, 30–39 years is the decade of human life in which obesity has increased at least as much as any age interval during the last several decades (http://www.cdc.gov/nchs/data/nhanes/overweight.pdf).
2. Methods
(a). Dataset inclusion criteria
We searched PubMed, Web of Science, Agricola and JSTOR online for leads to relevant data and contacted colleagues at primate centres, toxicology programmes, pet food companies, veterinary programmes and authors of promising articles. We sought datasets from (i) mammalian species; that (ii) lived with or around humans in industrialized societies (e.g. pets, laboratory animals); and (iii) contained data spanning at least one decade with at least one data point in the second half of the twentieth century.
(b). Exclusion criteria
We excluded datasets (i) consisting solely of terminal or late-life weights because weight loss often occurs towards the end of life [9], and presages death [10], and population differences in late-life weights are often not representative of population differences in weight during earlier adulthood [11]; (ii) consisting of animals that, during the period considered, were known or were likely to have been exposed to deliberate selection for phenotypes related to weight or adiposity (effectively ruling out livestock); (iii) consisting of animals that were calorically restricted or had their food intake titrated to maintain relatively constant body weights; and (iv) uniformly exposed to suspected toxins or drugs (e.g. the treatment groups from toxicology programmes).
(c). Datasets used
Macaques—Wisconsin. Our sample consisted of 65 (23 males, 42 females) rhesus macaques (Macaca mulatta—Indian origin) from the Wisconsin National Primate Research Center (WNPRC) measured between 1971 and 2006.
Macaques—Oregon. Our sample consisted of 46 (14 males, 32 females) rhesus macaques (Macaca Mulatta—Indian strain) from the Oregon National Primate Research Center (ONRPC), measured between 1981 and 1993.
Macaques—California. Our sample consisted of 77 (30 males, 47 females) rhesus monkeys (Macaca mulatta), primarily of Indian origin from the CNPRC (California National Primate Research Center), measured between 1979 and 1992.
Chimpanzees. Our sample consisted of 46 (16 males, 30 females) chimpanzees (Pan troglodytes) that had been born and lived their entire lives at the Yerkes National Primate Research Center (YNPRC). These animals were measured between the years 1985–2005.
Vervets. Our sample included a total of 117 (36 males, 81 females) vervet monkeys (Chlorocebus aethiops sabaeus) living in 18 captive social groups at the UCLA-VA Vervet Research Colony, measured between the years 1990 and 2006.
Marmosets. Our sample included a total of 143 (65 males, 78 females) common marmosets (Callitrichix jacchus jacchus) from the WNPRC, measured between the years 1991 and 2006.
Mice and rats (laboratory). Our sample consisted of animals from 106 rat and 93 mouse studies. There was some variation in sample size between studies. For both rats and mice, the majority of studies had sample sizes of 60 males and 60 females. However, some studies had fewer (i.e. 50, 49, etc.) or more (i.e. 70) animals. In calculating our sample size, we decided to use a conservative estimate of 50 animals per study. Body weights for only untreated control mice and rats used in National Toxicology Programme (NTP) studies between the years of 1982 and 2005 were analysed.
Domestic dogs and cats. Our sample of dogs included a total of 2806 (1366 males, 1440 females) animals measured between the years of 1990 and 2002. Our sample of cats included a total of 574 (265 males, 309 females) animals, measured between the years of 1989 to 2001.
Feral rats. Our sample consisted of 6115 (2886 males, 3229 females) wild Norway rats (Rattus norvegicus) that were captured in the central alleys of high-density residential neighbourhoods using single-capture live traps, while rural rat populations were sampled from parklands and agricultural areas in areas surrounding the city [12,13], between the years 1948 and 2006.
More details about each dataset can be found in the electronic supplementary material.
(d). Statistical analysis
Each population sample was analysed separately using the following steps.
For humans, 80 years (78 to be precise) corresponds to roughly the life expectancy at birth in the United States (http://www.cdc.gov/nchs/data/nvsr/nvsr55/nvsr55_19.pdf) and can be taken as an indicator of human ‘lifespan’. On a per cent basis, in United States adults (men and women combined) the decade of human life in which obesity has increased at least as much as any age interval during the last several decades was the interval of 30–39 years of age (http://www.cdc.gov/nchs/data/nhanes/overweight.pdf), suggesting that this would be a good developmental interval to examine for a first cross-species study and that human age 35 is a good midpoint to choose for an interval to study.
For each species, let the age interval studied be: L (35/80) ± 0.025L, where L is the estimated lifespan for the species under study. Lifespan values were obtained from published papers and consultation with expert zoologists, veterinarians and primatologists, and are displayed in the electronic supplementary material, table S2. This gave a roughly 5 per cent interval of the lifespan corresponding to early-middle adulthood for each species.
For each dataset, Yi,j denotes the weight of the ith animal at the jth point in time. Only weights taken at ages within the defined age interval of study for that species were used.
Exclude any value Yi,j if the ith animal died on or before 1 year after time j.
Let W50 denote the median of either (A) the one-third of the Yi,j values recorded earliest in calendar time if the data were obtained roughly continuously throughout the total period of time studied; or (B) during the first interval of data collection if discreet sampling periods were used (e.g. for the feral rats). The Yi,j values used in computing this median include only those recorded during the age interval L (35/80) ± 0.025L.
We let
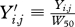 , such that this effectively scales the body weights to be comparable across species by having them represent species-specific ratio increases from median weight during the early period of the data collection.
, such that this effectively scales the body weights to be comparable across species by having them represent species-specific ratio increases from median weight during the early period of the data collection.For each
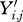 , let the age of the animal at the time of measurement be denoted by Ai,j and let
, let the age of the animal at the time of measurement be denoted by Ai,j and let 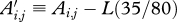 .
.Let Ti denote the calendar time (scaled in years/10, i.e. ‘decades’, for convenience) from the time of birth to the point at which
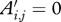 .
.Let
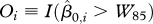 , where
, where 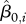 is the mean body weight of the ith animal, and W85 is defined as the 85th percentile of the sample distribution of the
is the mean body weight of the ith animal, and W85 is defined as the 85th percentile of the sample distribution of the 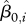 . The Oi are indicator variables of overweight or ‘obesity’ for animals where weight values have different meanings, following the approach used for human children, where body mass index values do not have equivalent meaning across ages.
. The Oi are indicator variables of overweight or ‘obesity’ for animals where weight values have different meanings, following the approach used for human children, where body mass index values do not have equivalent meaning across ages.Primary analysis of mean weight gain. To assess changes in weight over time, a relative weight gain-dependent variable was created in step 6 (
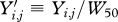 ). Even with restricted age intervals defined in steps 2–5, many animals had multiple measures in that time frame. To account for the dependency among these multiple observations and to capitalize on the power of repeated measurements, we used a linear mixed model using SAS PROC MIXED. An autogressive lag1 covariance structure was used for the residuals. The basic model used per cent weight gain (
). Even with restricted age intervals defined in steps 2–5, many animals had multiple measures in that time frame. To account for the dependency among these multiple observations and to capitalize on the power of repeated measurements, we used a linear mixed model using SAS PROC MIXED. An autogressive lag1 covariance structure was used for the residuals. The basic model used per cent weight gain (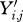 ) as the dependent variable. Age of the animal at the time of measurement (
) as the dependent variable. Age of the animal at the time of measurement (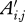 , see step (vii)) was used as a time-varying covariate to control for dependency among multiple measurements. Sex of the animal was used as a stratification factor. The main predictor of interests was Ti, which reflects the effect of time of birth of the animal and assesses whether animals born more recently have higher weights. We also investigate nonlinear trends in the decade variable and potential interactions (e.g. sex by decade).
, see step (vii)) was used as a time-varying covariate to control for dependency among multiple measurements. Sex of the animal was used as a stratification factor. The main predictor of interests was Ti, which reflects the effect of time of birth of the animal and assesses whether animals born more recently have higher weights. We also investigate nonlinear trends in the decade variable and potential interactions (e.g. sex by decade).Primary analysis on obesity prevalence. Since Oi is a dichotomous outcome variable, we used generalized estimating equations via SAS PROC GENMOD to account for the dependency among these multiple observations. As with the statistical analysis in step (x), we controlled for age at the time of measurement and sex of the animal. The main predictor of interest was again Ti. The effects of other relevant covariates and interactions were investigated. In cases where the data were sparse and produced unstable estimates or non-convergent results, we used penalized logistic regression to stabilize the estimates as previously described [14].
3. Results
For per cent weight change, 24 out of 24 time trends were positive (i.e. increasing). The probability of all out of 24 independent trend estimates being in the same direction by chance is 1.2 × 10−7. For the odds of obesity, 23 out of 24 cases were positive (p = 3.0 × 10−6; table 1 and figure 1). When we combine males and females of each species into a single analysis, we find that in all 12 populations, per cent weight change and odds of obesity time trends were positive (p = 4.9 × 10−5, for 12 out of 12 in the same direction). Given these overwhelmingly significant results at the ensemble or meta-analytical level, we describe the results below for samples from each individual population focusing on the magnitude of the coefficients. Standard errors, confidence intervals and p-values are shown in table 1 and figure 1.
Table 1.
Body weight changes per decade. (Per cent weight (PCT_WT) = 100 × (weight/norm), where norm is the sex-specific median of ‘baseline’ distribution and baseline distribution is distribution in first time interval if time intervals are discrete (e.g. feral rats) or distribution of earliest third of measurements. Obese = I(weight > τ85), where τ85 is the sex-specific 85th percentile of the baseline distribution.)
| females |
males |
combined |
||||
|---|---|---|---|---|---|---|
| dataset | OR per decade (CI; p-value) | PCT increase in weight per decade (CI; p-value) | OR per decade (CI; p-value) | PCT increase in weight per decade (CI; p-value) | OR per decade (CI; p-value) | PCT increase in weight per decade (CI; p-value) |
| macaques (WI) | 2.4 (0.91–6.34; p = 0.076) | 7.35 (−3.13 to 17.8; p = 0.17) | 1.02 (0.48–2.14; p = 0.97) | 1.56 (−13.1 to 16.3; p = 0.83) | 1.90 (0.89–4.05; p = 0.095) | 5.33 (−3.1 to 13.8; p = 0.213) |
| macaques (OR) | 3.76 (0.15–92.20; p = 0.417) | 6.8 (−23.9 to 37.5; p = 0.664) | 2.55 (0.16–39.42; p = 0.503) | 10.5 (−11.5 to 32.4; p = 0.345) | 2.94 (0.25–35.3; p = 0.394) | 9.61 (−8.1 to 27.4; p = 0.287) |
| macaques (CA) | 2.18 (0.26–18.38; p = 0.473) | 9.6 (−6.4 to 25.6; p = 0.240) | 5.44 (0.56–53.05; p = 0.145) | 15.2 (−2.9 to 33.2; p = 0.099) | 3.16 (0.65–15.5; p = 0.156) | 11.52 (−0.5 to 23.5; p = 0.059) |
| chimpanzee | 11.81 (1.88–74.10; p = 0.008) | 37.2 (7.3–67.2; p=.0152) | 18.78 (1.18–299.8; p = 0.038) | 33.2 (4.8–61.5; p = 0.022) | 14.54 (3.22–65.7; p < 0.001) | 33.60 (14.4–52.8; p < 0.001) |
| vervets | 1.83 (0.67–5.01; p = 0.238) | 9.4 (1.1–17.1; p = 0.026) | 9.34 (0.19–462.1; p = 0.262) | 2.9 (−21.4 to 27.2; p = 0.808) | 2.05 (0.77–5.49; p = 0.150) | 8.82 (1.1–16.5; p = 0.025) |
| marmosets (WI) | 2.73 (0.42–17.91; p = .295) | 9.7 (1.0–18.4; p = 0.028) | 1.64 (0.36–7.55; p = 0.526) | 9.2 (−0.9 to 19.3; p = 0.073) | 1.96 (0.58–6.64; p = 0.279) | 9.27 (2.7–15.8; p = 0.006) |
| mice (laboratory controls) | 2.89 (1.20–6.99; p = 0.019) | 11.8 (3.9–19.7; p = 0.004) | 7.17 (1.91–27.00; p = 0.003) | 10.5 (6.2–14.9; p < 0.001) | 3.84 (1.69–8.73; p = 0.001) | 12.46 (6.6–18.3; p < 0.001) |
| rats (laboratory controls) | 1.45 (0.42–5.03; p = 0.554) | 0.2 (−4.7 to 5.1; p = 0.936) | 2.25 (0.98–5.17; p = 0.055) | 6.0 (−0.5 to 12.6; p = 0.071) | 1.47 (0.76–2.83; p = 0.251) | 3.37 (−2.08 to 8.82; p = 0.220) |
| cats | 1.84 (0.64–5.30; p = 0.238) | 13.6 (3.5–23.8; p = 0.010) | 0.97 (0.29–3.23; p = 0.760) | 5.7 (−4.6 to 16.0; p = 0.276) | 1.38 (0.63–3.03; p = 0.426) | 9.72 (2.6–16.9; p = 0.008) |
| dog | 1.13 (0.67–1.90; p = 0.653) | 3.02 (−3.11 to 9.16; p = 0.333) | 1.07 (0.59–1.93; p = 0.826) | 2.20 (−4.15 to 8.54; p = 0.497) | 1.14 (0.77–1.69; p = .524) | 2.87 (−1.56 to 7.31; p = .204) |
| rats urban (1948, 2006) | 1.26 (1.18–1.34; p < 0.001) | 7.22 (5.3–9.2; p < 0.001) | 1.19 (1.12–1.27; p < 0.001) | 6.54 (4.7–8.5; p < 0.001) | 1.21 (1.16–1.26; p < 0.001) | 6.88 (5.5–8.2; p < 0.001) |
| rats rural (1948, 1986) | 1.19 (1.10–1.40; p = 0.035) | 5.16 (1.3–9.0; p = 0.008) | 1.11 (0.96–1.29; p = 0.171) | 4.46 (1.2–7.7; p = 0.007) | 1.15 (1.03–1.28; p = 0.016) | 4.81 (2.3–7.3; p < 0.001) |
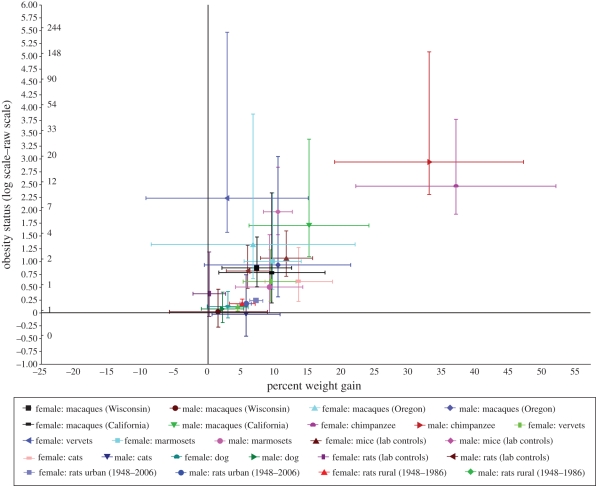
Figure 1.
Mean and ±1 s.e. of per cent weight gain and obesity status by decade. The left side of the y-axis refers to the raw scale of obesity status, and the right side refers to the log scale of obesity status.
We first examined primates living in highly controlled environments with nearly constant living conditions and diets. Across all three macaque populations, meta-analytically averaging the estimates weighted by the inverse of their variances yielded values of 7.7 per cent for the increase in body weight and a 86 per cent increase in the odds of obesity for males, and 7.9 per cent for the increase in body weight and a 144 per cent increase in the odds of obesity for females, on a per-decade basis. In the combined sex analysis, we find a 7.7 per cent increase in body weight and a 114 per cent increase in the odds of obesity. Among colonized chimpanzees, males and females, respectively, experienced a 33.2 and 37.2 per cent weight gain per decade, and a nearly 18-fold and 11-fold increase in the odds of obesity. In vervets, for females and males, respectively, there were 9.4 and 2.9 per cent increases in body weight per decade associated with 83 and 834 per cent increases in the odds of obesity. Among marmosets, females experienced a 9.7 per cent increase in body weight per decade, and a 1.73-fold increase in the odds of obesity. Among males, there was a 9.2 per cent increase in body weight per decade, and a 64 per cent increase in the odds of obesity.
Among mice in control groups in the National Toxicology Programme (NTP), there was a 11.8 per cent increase in body weight per decade from 1982 to 2003 in females coupled with a nearly twofold increase in the odds of obesity. In males there was a 10.5 per cent increase per decade. Among female rats in the NTP, there was a 0.2 per cent increase in body weight per decade, coupled with a 45 per cent increase in the odds of obesity, while among males there was a 6 per cent increase in body weight per decade coupled with a 1.25-fold increase in the odds of obesity.
Among animal species living in less-controlled environments, female cats experienced a 13.6 per cent increase in body weight per decade and an 84 per cent increase in the odds of obesity. Among male cats, there was a 5.7 per cent increase in body weight per decade, however a slight (not statistically significant) reduction in the odds of obesity. Male dogs experienced a 2.2 per cent increase in body weight per decade coupled with a 7 per cent increase in the odds of obesity per decade. Among female dogs, there was a 3 per cent increase in body weight per decade and a 13 per cent increase in obesity.
Finally, we examined a population of animals living close to people but not under their direct control. For the 1948–2006 time period, male rats trapped in urban Baltimore experienced a 5.7 per cent increase in body weight per decade from 1948 to 2006 and a nearly 20 per cent increase in the odds of obesity. Similarly, female rats trapped in urban Baltimore experienced a 7.22 per cent per decade increase in body weight, along with a 26 per cent increase in the odds of obesity. From 1948 to 1986, male rats trapped in the rural area gained 4.5 per cent in body weight, while females gained 5.2 per cent, and the increases in the odds of obesity were, respectively, 19 and 26 per cent. We did not find any evidence of nonlinear changes in weight increase and change in obesity prevalence that was statistically significant in any of the animals considered.
We next examined whether these body weight increases were different for male versus female animals. Recognizing that these analyses should be treated with some caution given that we are pooling across species, we compared the meta-analytically derived (i.e. averaged across all species and weighted by inverse of variances) point estimates, for males and females, and find that female animals experienced greater per cent weight gain and increase in the odds of obesity, but results are statistically significant only for the sex difference in the increase in odds of obesity (Z = 432, p < 0.0001).
Similarly, we examined whether body weight increases were greater for laboratory versus non-laboratory animals. The non-laboratory animals included urban rats, rural rats, and domestic cats and dogs. Again, we compared the meta-analytically derived estimates for each of these groups, and find that the laboratory animals show a greater increase in per cent weight gain and odds of obesity than non-laboratory animals (Z = 5.37, p = 0.003 and Z = 111, p < 0.0001, respectively).
4. Discussion
Our findings reveal that large and sustained population increases in body weights can occur in mammalian populations, just as they have occurred among human populations, even in the absence of those factors that are typically conceived of as the primary determinants of the human obesity epidemic via their influence on diet (e.g. access to vending machines) and physical activity (e.g. less physical education classes in schools). Though results were not statistically significant in every population (11 out of 24 are statistically significant for per cent increase in weight per decade, and 7 out of 24 are statistically significant for odds of obesity), viewed as an ensemble, the fact that nearly all independent time-trend coefficients were in the positive direction for both weight gain and for the odds of obesity, is overwhelmingly statistically significant.
That large population level changes in body weight distributions of mammalian populations can occur even when those populations are neither under obvious selection by predation nor are living with or among humans has been documented [15]. The particular upward trend we have observed towards obesity in multiple datasets of non-human animals has been suggested by anecdotal evidence for some time. A 2008 news report indicated that ‘trends in pet insurance are mirroring human healthcare. Obesity… is a growing problem for dogs and cats… (and 2007) saw a 19 per cent increase in claims related to obesity’ (http://www.petfirsthealthcare.com/2008/02/07/petfirst-pet-insurance-to-be-more-popular-in-2008/). According to a recent review by German [16], ‘Most investigators agree that, as in humans, the incidence of obesity in the pet population is increasing’. Despite this strong sentiment that obesity rates are increasing in pets (note that the United States Food and Drug Administration recently approved the first drug to treat obesity in dogs; Food and Drug Administration, 2007), we were unable to find previously published data actually showing this increase.
Others reported that 19 per cent of horses in a large cohort were obese, even among largely pasture-fed animals. Although a direct comparison with a similarly sampled earlier cohort was not available, the investigators remarked that the levels were higher than a 5 per cent rate observed in an earlier study [17]. Similarly, an increase in body weights was observed among rats used in carcinogenicity studies in France between 1979 and 1991, despite similar husbandry conditions [18]. The authors attributed the increase to the introduction of animals of the same substrain but raised under specific pathogen-free conditions, reinforcing the perspective that the presence of viral or other microbial pathogens [19,20] may affect body weight in populations either positively or negatively, depending on the pathogen. It is also noteworthy that the obesity epidemic has also occurred among children of six months of age and under [21], an age group for which explanations involving food marketing, less physical education is schools, and more labour-saving devices seem questionable.
There are multiple conceivable explanations for these observations. Feral rats could be increasing in weight because of selective predation on smaller animals [22,23] or because just as human real wealth and food consumption have increased in the United States, rats which presumably largely feed on our refuse, may also be essentially richer. But these factors cannot account for the findings in the laboratory animals that are on highly controlled diets, which have varied minimally over the last several decades. These animals are typically fed ad libitum, so if weight increases are attributable to increases in food consumption (which is possible), it is difficult to understand why animals in controlled environments on diets of constant composition are consuming more food today than in past decades. By contrast, one could hypothesize that better veterinary or husbandry care in laboratory and companion animals and better medical care in humans could be contributing to population level increases in body weight, but this cannot explain weight increases in feral rats. Our finding of greater weight gain among laboratory animals could also be explained by changes in animal husbandry standards, such as those imposed by the Animal Welfare Act, over the past 30 years. Though it is certainly not necessary that there be a single explanation for all of these population level increases nor even a single explanation for each individual population, it is intriguing to consider whether there are any factors that could conceivably account for weight increases in all of these populations.
One set of putative contributors to the human obesity epidemic is the collection of endocrine-disrupting chemicals (endocrine-disruptors), widely present in the environment [24]. Another conceivable explanation is obesity of infectious origin. Infection with adenovirus-36 (AD36) leads to obesity in multiple experimental models [7,25] and antibodies to AD36 are correlated with obesity in humans [26]. These observations suggest that AD36 and conceivably other infectious agents could be contributing to obesity within populations. Other explanations may include epigenetic-mediated programming of growth and energy-allocation patterns owing to any number of environmental cues such as stressors, resource availability, release from predation or climate change [27–31].
Increased body weights among laboratory animals have implications for the outcomes and design of the experiments that use these animals. Among several laboratory animals, it is known that calorically restricted individuals live longer and obese animals have shorter lifespans [32,33]. This has had implications for toxicology studies in which some researchers have shifted to controlling for reduced lifespan and increased body weight [34,35].
Our findings have implications for our understanding of the aetiological factors underlying human obesity, and we anticipate that they will lead to more research into the previously under-appreciated causes of the recent dramatic rise in obesity rates. Although dietary practices and physical activity levels are the most thoroughly studied risk factors for obesity, findings in humans and our findings in other animals add to the increasing evidence that other potential risk factors which may work through diet and physical activity or through other means (e.g. nutrient-partitioning, metabolic efficiency) should be incorporated into public health research and environmental medicine.
Acknowledgements
We thank the Red Bank Veterinary Hospital (Tinton Falls, NJ, USA), and Dr James Herndon at the Yerkes National Primate Research Center for providing data. We thank Dr Steve Kohama at the Oregon NPRC, and Dr John Capitanio at the California NPRC for their contributions to iPAD (The Internet Primate Ageing Database). We thank Dr Kyle Grimes for his helpful comments on earlier drafts of this paper, Vinodh Srinivasasainagendra for his help with the graphics, and Jelai Wang at UAB and Anand Paleja at the NTP for help with data management. We also thank Dr Mai Elobeid for her encouragement and suggestions on this manuscript. Supported in part by NIH grants T32HL007457 and P30DK056336. The opinions expressed are those of the authors and not necessarily those of the NIH or any other organization with which the authors are affiliated. D.B.A. has received grants, honoraria, consulting fees and donations from numerous food and pharmaceutical companies, litigators, and non-profit and government entities with interests in obesity-related matters. Supported partially by NIH grants P30DK056336, R01DK067487, P51RR000165, P51RR000167, P01AG026423 and T32HL007457.
References
- 1.Ogden C. L., Carroll M. D., Curtin L. R., McDowell M. A., Tabak C. J., Flegal K. M. 2006. Prevalence of overweight and obesity in the United States, 1999–2004. J. Am. Med. Assoc. 295, 1549–1555 10.1001/jama.295.13.1549 (doi:10.1001/jama.295.13.1549) [DOI] [PubMed] [Google Scholar]
- 2.Helmchen L. A., Henderson R. M. 2004. Changes in the distribution of body mass index of white US men, 1890–2000. Ann. Hum. Biol. 31, 174–181 10.1080/03014460410001663434 (doi:10.1080/03014460410001663434) [DOI] [PubMed] [Google Scholar]
- 3.Astrup A. V., Rossner S., Sorensen T. I. 2006. Alternative causes of obesity. Ugeskr Laeger 168, 135–137 [PubMed] [Google Scholar]
- 4.Bray G. A., Champagne C. M. 2005. Beyond energy balance: there is more to obesity than kilocalories. J. Am. Diet Assoc. 105(5 Suppl. 1), S17–S23 [DOI] [PubMed] [Google Scholar]
- 5.Eisenmann J. C. 2006. Insight into the causes of the recent secular trend in pediatric obesity: common sense does not always prevail for complex, multi-factorial phenotypes. Prev. Med. 42, 329–335 10.1016/j.ypmed.2006.02.002 (doi:10.1016/j.ypmed.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 6.Keith S. W., et al. 2006. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int. J. Obes. (Lond.) 30, 1585–1594 10.1038/sj.ijo.0803326 (doi:10.1038/sj.ijo.0803326) [DOI] [PubMed] [Google Scholar]
- 7.McAllister E. J., et al. 2009. Ten putative contributors to the obesity epidemic. Crit. Rev. Food Sci. Nutr. 49, 868–913 10.1080/10408390903372599 (doi:10.1080/10408390903372599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Schalie W. H., et al. 1999. Animals as sentinels of human health hazards of environmental chemicals. Environ. Health Perspect. 107, 309–315 10.1289/ehp.99107309 (doi:10.1289/ehp.99107309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasselli J. R., Weindruch R., Heymsfield S. B., Pi-Sunyer F. X., Boozer C. N., Yi N., Wang C., Pietrobelli A., Allison D. B. 2005. Intentional weight loss reduces mortality rate in a rodent model of dietary obesity. Obes. Res. 13, 693–702 10.1038/oby.2005.78 (doi:10.1038/oby.2005.78) [DOI] [PubMed] [Google Scholar]
- 10.Coffey C. S., Gadbury G. L., Fontaine K. R., Wang C., Weindruch R., Allison D. B. 2005. The effects of intentional weight loss as a latent variable problem. Stat. Med. 24, 941–954 10.1002/sim.1964 (doi:10.1002/sim.1964) [DOI] [PubMed] [Google Scholar]
- 11.Colman R. J., Beasley T. M., Allison D. B., Weindruch R. 2008. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J. Gerontol. A Biol. Sci. Med. Sci. 63, 556–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis D. E. 1949. A phenotypical difference in growth of wild rats. Growth 13, 1–6 [PubMed] [Google Scholar]
- 13.Glass G. E., Childs J. E., Korch G. W., LeDuc J. W. 1989. Comparative ecology and social interactions of Norway rats Rattus norvegicus. In Occasional Papers of the Museum of Natural History, vol. 130, pp. 1–33 Baltimore, MD: University of Kansas [Google Scholar]
- 14.Heinze G. 2006. A comparative investigation of methods for logistic regression with separated or nearly separated data. Stat. Med. 25, 4216–4226 10.1002/sim.2687 (doi:10.1002/sim.2687) [DOI] [PubMed] [Google Scholar]
- 15.Post E., Stenseth N. C., Langvatn R., Fromentin J. M. 1997. Global climate change and phenotypic variation among red deer cohorts. Proc. R. Soc. Lond. B 264, 1317–1324 10.1098/rspb.1997.0182 (doi:10.1098/rspb.1997.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.German A. J. 2006. The growing problem of obesity in dogs and cats. J. Nutr. 136(Suppl. 7), 1940S–1946S [DOI] [PubMed] [Google Scholar]
- 17.Thatcher C. D., Pleasant R. S., Geor R. J., Elvinger F., Negrin K. A., Franklin J., Gay L., Werre S. R. 2009. Prevalence of obesity in mature horses: an equine body condition study. J. Anim. Physiol. Anim. Nutr. 92, 222. 10.1111/j.1439-0396.2007.00789_8.x (doi:10.1111/j.1439-0396.2007.00789_8.x) [DOI] [Google Scholar]
- 18.Nohynek G. J., Longeart L., Geffray B., Provost J. P., Lodola A. 1993. Fat, frail and dying young: survival, body weight and pathology of the Charles River Sprague–Dawley-derived rat prior to and since the introduction of the VAFR variant in 1988. Hum. Exp. Toxicol. 12, 87–98 10.1177/096032719301200201 (doi:10.1177/096032719301200201) [DOI] [PubMed] [Google Scholar]
- 19.Ley R. E., Turnbaugh P. J., Klein S., Gordon J. I. 2006. Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 10.1038/4441022a (doi:10.1038/4441022a) [DOI] [PubMed] [Google Scholar]
- 20.Vijay-Kumar M., et al. 2010. Metabolic syndrome and altered gut microbiota in mice lacking toll-like receptor 5. Science 328, 228–231 10.1126/science.1179721 (doi:10.1126/science.1179721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J., Peterson K. E., Scanlon K. S., Fitzmaurice G. M., Must A., Oken E., Rifas-Shiman S. L., Rich-Edwards J. W., Gillman M. W. 2006. Trends in overweight from 1980 through 2001 among preschool-aged children enrolled in a health maintenance organization. Obesity (Silver Spring) 14, 1107–1112 10.1038/oby.2006.126 (doi:10.1038/oby.2006.126) [DOI] [PubMed] [Google Scholar]
- 22.Childs J. E. 1986. Size-dependent predation on rats (Rattus norvegicus) by house cats (Felis catus) in an urban setting. J. Mammal. 67, 196–199 10.2307/1381025 (doi:10.2307/1381025) [DOI] [Google Scholar]
- 23.Glass G. E., Gardner-Santana L. C., Holt R. D., Chen J., Shields T. M., Roy M., Schachterle S., Klein S. L. 2009. Trophic garnishes: cat–rat interactions in an urban environment. PLoS ONE 4, e5794. 10.1371/journal.pone.0005794 (doi:10.1371/journal.pone.0005794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elobeid M. A., Allison D. B. 2008. Putative environmental-endocrine disruptors and obesity: a review. Curr. Opin. Endocrinol. Diabetes Obes. 15, 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhurandhar N. V., et al. 2002. Human adenovirus Ad-36 promotes weight gain in male rhesus and marmoset monkeys. J. Nutr. 132, 3155–3160 [DOI] [PubMed] [Google Scholar]
- 26.Atkinson R. L., Dhurandhar N. V., Allison D. B., Bowen R. L., Israel B. A., Albu J. B., Augustus A. S. 2005. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int. J. Obes. (Lond.) 29, 281–286 10.1038/sj.ijo.0802830 (doi:10.1038/sj.ijo.0802830) [DOI] [PubMed] [Google Scholar]
- 27.Gluckman P. D., Hanson M. A. 2008. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int. J. Obes. (Lond.) 32(Suppl. 7), S62–S71 [DOI] [PubMed] [Google Scholar]
- 28.Waterland R. A., Michels K. B. 2007. Epigenetic epidemiology of the developmental origins hypothesis. Annu. Rev. Nutr. 27, 363–388 10.1146/annurev.nutr.27.061406.093705 (doi:10.1146/annurev.nutr.27.061406.093705) [DOI] [PubMed] [Google Scholar]
- 29.Speakman J. R. 2007. A nonadaptive scenario explaining the genetic predisposition to obesity: the ‘predation release’ hypothesis. Cell Metab. 6, 5–12 10.1016/j.cmet.2007.06.004 (doi:10.1016/j.cmet.2007.06.004) [DOI] [PubMed] [Google Scholar]
- 30.Ozgul A., Tuljapurkar S., Benton T. G., Pemberton J. M., Clutton-Brock T. H., Coulson T. 2009. The dynamics of phenotypic change and the shrinking sheep of St. Kilda. Science 325, 464–467 10.1126/science.1173668 (doi:10.1126/science.1173668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozgul A., Childs D. Z., Oli M. K., Armitage K. B., Blumstein D. T., Olson L. E., Tuljapurkar S., Coulson T. 2010. Coupled dynamics of body mass and population growth in response to environmental change. Nature 466, 482–485 10.1038/nature09210 (doi:10.1038/nature09210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keenan K. P. 1996. Commentary. The uncontrolled variable in risk assessment: ad libitum overfed rodents–fat, facts and fiction. Toxicol. Pathol. 24, 376–383 10.1177/019262339602400315 (doi:10.1177/019262339602400315) [DOI] [PubMed] [Google Scholar]
- 33.Keenan K. P., Laroque P., Ballam G. C., Soper K. A., Dixit R., Mattson B. A., Adams S. P., Coleman J. B. 1996. The effects of diet, ad libitum overfeeding, and moderate dietary restriction on the rodent bioassay: the uncontrolled variable in safety assessment. Toxicol. Pathol. 24, 757–768 10.1177/019262339602400620 (doi:10.1177/019262339602400620) [DOI] [PubMed] [Google Scholar]
- 34.Turturro A., Leakey J., Allaben W. T., Hart R. W. 1997. Fat (and thin) rats distort results. Nature 389, 326. 10.1038/38590 (doi:10.1038/38590) [DOI] [PubMed] [Google Scholar]
- 35.Keenan K. P., Laroque P., Dixit R. 1998. Need for dietary control by caloric restriction in rodent toxicology and carcinogenicity studies. J. Toxicol. Environ. Health B Crit. Rev. 1, 135–148 [DOI] [PubMed] [Google Scholar]