Abstract
Uncertainty is a problem not only in human decision-making, but is a prevalent quality of natural environments and thus requires evolutionary response. Unpredictable natural selection is expected to result in the evolution of bet-hedging strategies, which are adaptations to long-term fluctuating selection. Despite a recent surge of interest in bet hedging, its study remains mired in conceptual and practical difficulties, compounded by confusion over what constitutes evidence for its existence. Here, I attempt to resolve misunderstandings about bet hedging and its relationship with other modes of response to environmental change, identify the challenges inherent to its study and assess the state of existing empirical evidence. The variety and distribution of plausible bet-hedging traits found across 16 phyla in over 100 studies suggest their ubiquity. Thus, bet hedging should be considered a specific mode of response to environmental change. However, the distribution of bet-hedging studies across evidence categories—defined according to potential strength—is heavily skewed towards weaker categories, underscoring the need for direct appraisals of the adaptive significance of putative bet-hedging traits in nature.
Keywords: bet-hedging strategy, bistability, climate change, diversification, fluctuating natural selection, risk aversion
1. Introduction
The ancient Greek philosopher Heraclitus claimed that change is the only constant in the universe. Human decision-making reflects this knowledge of the inevitability of change, and the theoretical underpinnings of decision-making under risk were first formalized by Bernoulli in the eighteenth century [1]. For example, insurance companies require clients that are willing to trade a small expected monetary loss in exchange for a reduced risk of financial ruin. Uncertainty is an elemental quality also of natural environments, and how organisms respond to unpredictable environments is increasingly being recognized as a fundamental question in biology.
Organisms may respond to change directly—through physiological or behavioural adjustment, periodic migration or range shifts; or evolutionarily—through adaptive tracking of change; or through combinations of direct and evolutionary responses. For example, short-term physiological response is shaped by evolutionary response to ecological conditions [2]. Changes in global climate pose real threats to biodiversity and ecosystem services [3–8], and a growing body of research is dedicated to evolutionary response to changing environments [2,9–16].
The evolutionary implications of environmental variance are vast. It may lead to variable selection, in that traits that are optimal at one time are disadvantageous at another [17–24]. Recent research has improved our understanding of two evolutionary modes of response—besides extinction—to changing environments: adaptive tracking and phenotypic plasticity, outlined below. However, a third mode known variously as bet hedging [25] or risk aversion exists, and is thought to be important in organisms spanning bacteria [26], invertebrates [27], vertebrates [28] and angiosperms [29–31], yet is under-represented in treatments of evolutionary response to environmental variance.
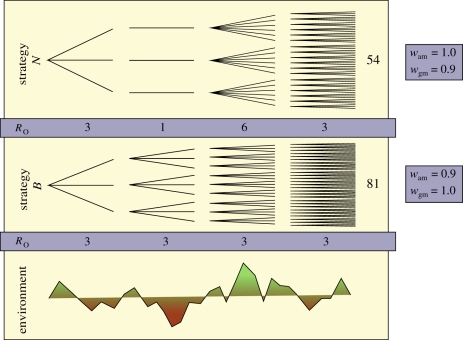
Bet-hedging theory is based on the idea that, because fitness is determined by the multiplicative process of reproduction, natural selection maximizes the long-run, or geometric-mean fitness (the nth root of the product of n fitness values) over generations (figure 1). The geometric mean declines with variance; thus, the ‘geometric-mean principle’ predicts the evolution of traits that have minimized fitness variance and the risk of failure [32–38] during past environmental fluctuations.
Figure 1.
The selective advantage of a bet-hedging strategy under environmental variance. Reproductive success associated with a non-bet-hedging (N) and a bet-hedging (B) strategy is represented under environmental unpredictability (‘bad’ conditions in red, ‘good’ in green). The expected, or relative arithmetic-mean fitness (wam) of the non-bet hedger is higher than that of the bet hedger yet, after four generations the non-bet hedger has produced fewer progeny. This illustrates that the arithmetic mean is not an appropriate measure of fitness. The comparative success of strategies N and B over the long term is accurately reflected by their relative geometric-mean fitnesses (wgm) because fitness is determined by an inherently multiplicative process. The advantage of the bet-hedging strategy (B) is that, although characterized by lower average fitness (RO), it shows reduced variance in fitness across generations under environmental unpredictability.
Bet-hedging traits are difficult to recognize because they are adaptive only over longer time scales. Appraisals of optimality are rare over a single generation, and are of course much rarer over longer time scales. The lack of attention to bet hedging reflects conceptual and practical challenges inherent to its study [27], and the absence of a framework for evaluating the strength of evidence where it does exist [23]. Thus, although bet hedging may be an important mode of response to changing environments, we simply do not know how common it is.
This review begins by providing an overview of the relationship among core modes of evolutionary response, with special attention to bet hedging. I outline the onerous requirements for tests of bet hedging, and construct a framework of evidence categories to evaluate the relative strength of empirical evidence from the literature. By clearly delineating what constitutes evidence for bet-hedging traits, and by exposing the dearth of strong evidence, it is hoped that this review might stimulate further empirical work on this neglected topic.
2. The modes of evolutionary response
Two evolutionary modes of response to environmental variance have been widely acknowledged—adaptive tracking and phenotypic plasticity—whereas bet hedging has received less attention. A coherent view of response to environmental change requires an understanding of each mode, as well as the complex relationships among modes.
(a). Adaptive tracking
In this mode of microevolution, optimal trait values change continually and natural selection eliminates suboptimal forms that may previously have been well adapted, increasing the frequency of alleles that result in phenotypes centred around the modified optimal trait values. Tracking thus depends on the availability of standing genetic variance and mutational variance for fitness with respect to environmental change [9,39–41]. The inevitable lag in adaptation [42] behind shifts in optimal trait values as environments change causes fitness depression, which may lead to extinction. Evolutionary rescue [43,44] is a particular form of tracking that occurs when a population experiences a decline towards extinction caused by rapid environmental change, followed by a recovery phase attributable to rapid evolution [40].
Adaptive tracking depends on the maximum rate of evolution, recently estimated to be in the order of 0.1–0.3 Haldanes at all time scales—higher than previously thought [45]. Phenotypic change under anthropogenic selection may be more rapid than under background selection [46], although these results have been challenged [45]. Whether, and to what extent these maximum rates apply to fluctuating selection is an area in need of further investigation.
The effectiveness of tracking under fluctuating selection is further constrained by several factors. Rate-determining genetic variance, or heritability, is environment-dependent, and predictions of evolutionary response must consider such environmental effects. For example, negative correlations measured between heritability and the strength of selection [47] suggest that response to selection will be impeded when environmental changes are most extreme. Environmental variance reduces heritabilities, both through an increase in the environmental component of variance and a reduction in the additive genetic variance [48], and a recent meta-analysis shows that heritabilities are generally reduced under unfavourable conditions [49]. Furthermore, response to selection may be concealed by a negative covariance between genetic and environmental influences on phenotypic expression when phenotypes are measured in an altered environment [15]. Finally, the effect of evolutionary dynamics on demography may have repercussive evolutionary effects [50,51], an area in the early stages of exploration. Although it is clear that the effectiveness of tracking under environmental variance is constrained, a more complete understanding is still needed.
(b). Adaptive phenotypic plasticity
Environmental conditions may affect physiology, behaviour or development such that a range of phenotypes is expressed over a range of environments—the phenotypic norm of reaction. Phenotypic plasticity may be a non-adaptive, direct result of an altered environment, or plasticity may be an adaptive response. The evolution of adaptive plasticity requires that the phenotype-fitness association be predictable across environments, and is thus a viable solution only under the range of environments normally encountered by the organism. For example, populations of the water flea, Daphnia magna, have evolved adaptive behavioural plasticity in response to the presence of predators' chemical cues, but populations that never encounter predators lack the plastic response [52].
Much recent attention to both evolutionary tracking and adaptive plasticity has been motivated by interest in the potential for evolutionary response to climate change [9–11,13,14,16,53–57]. Because plasticity is adaptive only if response to cues is appropriate, there is no a priori reason to expect norms of reaction to novel environmental change to be adaptive [57,58]. For example, early snowmelt in montane habitats leads to early flowering but reduced reproductive success because of high rates of frost kill [59]. Also, in insectivorous birds, plasticity has led to mismatches between timing of breeding—a key phenological event—and the emergence of their prey species—a time-sensitive resource [60]. Interestingly, plasticity to temperature in laying date in great tits is adaptive in a British population [53], but these norms of reaction are maladaptive and variable in a Dutch population [61], indicating that the effectiveness of plasticity under changing environments depends on the temporal stability of the particular mechanism underlying the relationship between phenotype and fitness; here, prey phenology [53].
Tracking and plasticity are not discrete categories of response. First, they may interact in the evolution of a single trait. For example, the advancement in breeding dates of red squirrels in the Yukon, Canada with warmer spring temperatures is attributed to both existing plasticity and microevolution [16]. Second, even non-adaptive plasticity may have adaptive consequences in that environment- or stress-induced phenotypic expression can facilitate microevolution [62] through genetic assimilation [63,64]. Third, the evolution of adaptive phenotypic plasticity, although interpreted as an alternative mode of response, itself evolves through tracking. Like any trait, adaptive plasticity requires heritable genetic variance (genotype-by-environment interaction) for the evolution of appropriate trait expression—i.e. norms of reaction [65].
Adaptive phenotypic plasticity is the ideal solution to environmental variance: to instantly assume the most appropriate phenotype for any conceivable environment. However, the evolution of plasticity is limited by available genetic variance in norms of reaction, by physiological, developmental and physical constraints, by the costs of plasticity, and is critically dependent on the availability of cues that reliably predict not only the environments in which the plastic trait will be expressed, but that predict the relationship between fitness and phenotype in these environments. Thus, like adaptive tracking, the evolution of adaptive plasticity is seriously constrained, and constraints are difficult to measure [66].
Survival under the broad array of circumstances that limit adaptive tracking and adaptive plasticity must depend on an alternative mode of response. Bet hedging provides such an alternative: rather than adaptation to specific or predictable environmental changes, bet hedging may be thought of as adaptation to unpredictability or to change itself [23].
(c). Bet hedging
Bet-hedging traits (defined above) are expected to evolve under conditions of unpredictable environmental variance (figure 1), and where adaptive tracking and the evolution of plasticity are partially impeded. Because bet-hedging traits maximize geometric-mean fitness of their carriers across generations but do not maximize the expected fitness within a generation [37], bet-hedging adaptations appear to be detrimental if observed over short periods of time—even under ‘normal’ or average conditions.
Bet-hedging traits are either conservative or diversifying [36,37]. Conservative bet hedging is often likened to an insurance policy, in that it is a character that is ‘safe’. For example, the copepod Diaptomus sanguineus practises conservative bet hedging by producing diapausing eggs earlier than expected as a hedge against seasonal, but unpredictable onset of catastrophic fish predation [67]. Diversification, by contrast, spreads risk among an array of phenotypes, ensuring non-zero survival overall. Diversification has received most attention because high trait variance is seen as requiring an explanation, whereas conservative traits are not readily identifiable.
Clearly defining bet hedging may help prevent confusion, but its relationship with the other modes of response is also a source of misinterpretation. The difference between evolutionary tracking and bet hedging lies in the distinction between response to specific changing parameters and response to variability itself. For example, annual plants experiencing multi-generational trends of lengthening then contracting growing seasons should show an evolutionary trend towards delayed, then earlier flowering. By contrast, unpredictable variance in season length each generation is expected to result in early reproduction that minimizes the risk of reproductive failure, a bet-hedging strategy [68].
The difference between adaptive plasticity and bet hedging is that plastic norms of reaction result in the expression of an optimal phenotype over a range of environments, whereas bet hedging expresses a single phenotype (that may be a fixed level of diversification) that is neither optimal nor a failure across all environments. Using the example of flowering time, adaptive plasticity would be characterized by environment-appropriate adjustment of flowering time, and its evolution would depend on the reliability of a season-length cue.
Diversification bet hedging and adaptive phenotypic plasticity evolve under different selective environments, but their developmental basis may be similar [69,70]. Developmental imprecision is responsible for a surprisingly high proportion of phenotypic variance [71]; thus, microplasticity—extreme non-adaptive plasticity to environmental cues—may be co-opted to generate diversification bet hedging [72,73]. Similarly, adaptive polyphenism—the expression of distinct phenotypes as a response to different environmental cues—may be co-opted for the expression of stochastic phenotype switching when bet hedging is favoured [70].
When both predictable and unpredictable environmental variance influence fitness, a blend of plasticity and bet hedging is expected to evolve [74,75]. Diversification bet hedging may thus occur around norms of reaction. For example, plasticity in seed germination has evolved in response to available cues, but the cues are imperfect indications of the environment–fitness relationship, resulting in the evolution of both adaptive plasticity and diversification [76,77].
Complicating matters even more is that, if the fitness consequences of norms of reaction are inconsistent over time, norms of reaction are themselves subject to the geometric-mean principle. Thus, norms of reaction may be conservative bet-hedging characters that differ in shape from those that maximize fitness over the short term. For example, norms of reaction influencing residence in freshwater versus sea water in sockeye salmon populations appear to be advantageous only over longer periods of fluctuating selection [20], indicative of conservative bet hedging.
Evolutionary tracking and phenotypic plasticity are best understood within the framework of the geometric-mean principle rather than the other way around. A reason for the inverted traditional perception of roles is the narrow focus on two specific scenarios—environmental constancy and perfect adaptation across all environments—under which the arithmetic- and geometric-mean fitnesses converge. The geometric-mean principle applies broadly to these, and cases of fluctuating selection. The prevalence of bet hedging depends entirely on whether the outcome of selection for the geometric-mean fitness generally differs from that of the arithmetic mean. The prevalence of bet hedging is thus an empirical issue, and is something we know astonishingly little about.
3. Strength of evidence in tests of bet hedging
The arduous requirements for strong tests at least partially accounts for the deficiency of empirical attention to bet hedging. The term ‘bet hedging’ is loaded in that it carries an inference of adaptation. A ‘regular’ (non-bet hedging) trait may be claimed as an adaptation for which natural selection is a sufficient cause only if the trait shows a quantitative fit to the expected (or optimal) trait value for a given environment, and only if the trait is shown to occur at the genotype level [78] or, in comparative studies, that population differentiation is as predicted. Claims of bet hedging are often not held to the same standard, because satisfying these demands would require a several-fold greater investment: performing a quantitative comparison of bet-hedging expression with that predicted for a given degree of fluctuating selection across a temporal sequence of environments.
Existing empirical support for bet hedging occurs in various forms, and the evidence may fall anywhere along an ordinal scale of six categories ranked by increasing potential strength of evidence (table 1). Categories are cumulative in that each also includes evidence types from all previous categories. Category I studies are those that propose a candidate bet-hedging (CBH) trait, and may point to relevant environmental variation. Category I studies are abundant but not enumerated here because they are difficult to search for, constitute very weak evidence for bet hedging, and would not be represented proportionately.
Table 1.
Categories of evidence for candidate bet-hedging (CBH) traits. Six levels of potential strength of evidence are described for each of three main types of study: within populations, comparative studies among populations or species and laboratory selection experiments, usually performed using microbial populations. Strength categories are cumulative in that each includes evidence types from previous categories.
| evidence category | type of study |
||
|---|---|---|---|
| within-population | comparative | laboratory | |
| I | CBH trait recognized | CBH trait recognized | CBH trait recognized |
| II (either) | unpredictable environmental factor observed | unpredictable environmental factor differs across habitats | environmental heterogeneity observed |
| III (both) | genotype-level CBH | CBH differs among populations | genotype-level CBH or genetic basis established |
| IV | demonstrated variable fitness consequences | demonstrated fitness consequences differ across environments | fitness consequences of CBH assayed under ≥2 environments |
| V | CBH advantageous under fluctuating selection | CBH advantageous in respective environments | CBH advantageous or evolves under fluctuating selection |
| VI | quantitative fit of CBH to degree of fluctuating selection | quantitative fit of population CBH and fitness effects | quantitative fit of evolved CBH to degree of fluctuating selection |
Category II evidence may take one of two distinct forms: if environmental unpredictability deemed relevant to the CBH is demonstrated directly—or, in a comparative study, sites differ in predictability—the study is placed in this category. Alternatively, category II evidence includes assessment of phenotypic expression of the CBH trait, and comprises a demonstration that it occurs at the level of the genotype, differs among populations or reveals the genetic basis of the CBH trait. For example, diversification in the timing of egg hatch was shown to occur within clutches in the ectoparasitic fish louse Argulus coregoni, and is proposed as an adaptation to uncertainty in host availability [79]. Category III studies provide stronger evidence because they include evidence of both environmental unpredictability and genotype-level (or population differentiation in) phenotypic expression. An example of category III evidence is a study of partial hatching delay within broods of 22 populations of mosquitoes, explained by differences in desiccation risk among local habitats [80]. Because much environmental variance is irrelevant to fitness, evidence directly linking this variance to fluctuating selection acting on the CBH provides stronger support for bet hedging, and fits the requirement for category IV. For example, individual Sinorhizobium meliloti bacteria produce both short- and long-term starvation-resistant daughter cells under carbon scarcity, and their fitness was shown to depend on starvation environment [81]. In the case of laboratory selection experiments, fitness assays performed over multiple environments also fall into category IV. Levels V and VI comprise direct tests of the adaptive significance of a CBH trait. If the geometric-mean fitness of the CBH trait is compared with that of a non-bet-hedging alternative, a qualitative conclusion may be drawn about whether the CBH trait is adaptive, which constitutes level V evidence. For example, diapause might be shown to be favoured over a non-diapausing strategy. Finally, if the degree of bet hedging is tested for optimality for an observed extent of fluctuating selection, then the test is level VI, and constitutes an ideal test of bet hedging. Phenotype switching, for example, might be shown to be adaptive but the rate of phenotype switching that maximizes fitness depends on the extent of fluctuating selection, and it is a test of this quantitative fit that is required for level VI evidence.
Our current grasp of the prevalence of bet hedging in nature may be gauged by determining how existing empirical tests from the literature fall into the ranked evidence categories.
4. The empirical evidence for bet hedging
A diversity of approaches has been used in the study of bet hedging, and a meta-analysis or a systematic review is precluded. Furthermore, an exhaustive search of the bet-hedging literature is hindered by the lack of common terminology and understanding of what bet-hedging is. Until recently [26,81–83], microbial studies have used terms that describe the mechanisms of putative bet hedging such as bistability, stochastic phenotype switching or developmental noise (for a review, see [84]) without explicit reference to bet hedging or risk aversion; plant and animal studies rarely refer to the underlying mechanism but use an assortment of terms to describe an adaptation to environmental uncertainty, thus explaining the low rate of cross citation between these areas. I searched the Web of Science using combinations of title and topic searches including ‘bet hedg* or risk avers* or risk spread*’ (excluding economics), ‘hedg* and bets’, ‘coin flipping’, ‘stochastic phenotype switching’, ‘bistability and microb* or bacteri*’ and ‘geometric-mean fitness’. The consistent use of ‘bet hedging’ in titles, abstracts or keywords would facilitate the identification of research common to this area. Some of the most sophisticated treatments of bet hedging [17,85] recognize implicitly that fluctuating selection will result in evolutionarily stable strategies that may differ from those that maximize average expected fitness, and contain no specific searchable reference to the field of bet hedging. The coverage of the empirical literature is thus as comprehensive as possible in spanning taxa and range of tests.
Over 100 empirical bet-hedging studies were found, and are tabulated by evidence category, synopsis of evidence, CBH trait, organism and citation (see the electronic supplementary material, table S1; this table constitutes the principal results of the survey; however, because of its size, space restrictions bar its inclusion in the main paper). This survey of empirical evidence reveals several remarkable tendencies. First is the sheer variety of proposed bet-hedging traits. These include the classic examples of diversification in the timing of seed germination [23,86,87] and egg hatch [67]—collectively known as germ banking—[88], and stochastic phenotype switching [82]. Intriguing examples too numerous to list here (see the electronic supplementary material, table S1) also include diversified offspring physiology [81], multiple mating [89], high ovule number per flower [90], iteroparity [91] and miscellaneous proposals such as population differences in fire dependence for flowering among sites differing in predictability of lightning strikes [92], and semelparity in males as a hedge against unpredictable and high post-mating female mortality in a polygynous marsupial [93].
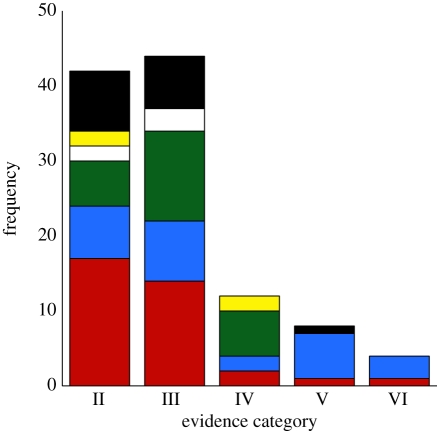
Second, conservative bet hedging is rare, with only 12 distinct proposals tabulated, mostly from class Aves (see the electronic supplementary material, table S1). For example, selection differentials indicate that earlier laying date would enhance average fitness in the coal tit Periparus ater, but strong interannual fluctuating selection seems to have resulted in a conservative late-laying strategy [94]. Third, proposals of bet hedging are taxonomically widespread (figure 2), with evidence accumulating in 114 study organisms across 29 classes within 16 phyla. Although the Magnoliophyta dominate evidence categories V and VI—with the most comprehensive studies of bet hedging in nature coming from semelparous plants (reviewed in Childs et al. [29])—studies of Arthropoda (especially insects; 20 studies) and Chordata are most prevalent in categories II through to IV. Finally, the frequency of studies diminishes sharply through higher evidential categories, with an almost complete lack of evidence in categories V and VI (figure 2).
Figure 2.
The distribution of existing empirical studies of bet hedging among strength-of-evidence categories (defined in table 1). Colours show the incidence of the five most commonly studied phyla among evidence categories. Yellow, Proteobacteria; white, Mollusca; green, Chordata; blue, Magnoliophyta; red, Arthropoda; black, other. ‘Other’ phyla are the subject of fewer than four studies, and include Annelida, Ascomycota, Bryozoa, Cnidaria, Echinodermata, Firmicutes, Nematoda, Platyhelminthes, Rotifera, Tardigrada and Vira. See the electronic supplementary material, table S1, for references and tabulated review of bet-hedging studies.
This review reveals that specific kinds of support are particularly needed. Namely, studies that (i) provide category IV evidence by directly linking the effect of environmental variance on the CBH trait to fitness variance, (ii) provide category V evidence that the trait confers a fitness advantage, and (iii) demonstrate that the degree of bet hedging observed maximizes fitness, given the degree of fluctuating selection in nature (category VI). This is not to say that studies corresponding to lower evidence categories are not important. For example, conservative strategies are under-represented across all evidence categories, and brings to light the need for further attention to this class of bet hedging. Because it is conservative bet hedging that may persist over longer time scales, proposals are especially needed.
It must be noted that a well-performed study in a lower evidence category may be of greater value than a weak study falling into a higher category. A case in point is a remarkable selection experiment [82] in which the de novo evolution of stochastic phenotype switching—a trait widely assumed to be bet hedging—was witnessed under microcosm conditions. Switching was directly selected for (sensu Sober [95, p. 100]) through an ‘exclusion rule’ and bottleneck; thus, this study does not aim to provide direct evidence that stochastic phenotype switching is a bet-hedging trait, but is a valuable contribution to knowledge of the mechanisms underlying a bet-hedging strategy. Placement of work into evidence categories thus indicates potential evidence and is not a judgement of the relative merits of individual studies. However, trends indicate a paucity of strong evidence for the existence of bet hedging.
5. Conclusions and implications
Studies of two modes of response to environmental variance—adaptive tracking and phenotypic plasticity—are being recognized for their importance to applied problems of climate change [9,16,53,54,57]. In parallel, there has been a recent surge of interest in bet hedging (see for example [23,81,82]). A fuller understanding of response to changing environments requires the inclusion of bet hedging as a core mode, and also requires an understanding of the complex relationship among the three modes of response. This review of the empirical evidence for bet hedging assesses our current grasp of the prevalence of bet hedging in nature and—through the framework of evidence categories—specifies what constitutes evidence to assist in the conceptual design of further studies.
Evidence for bet hedging has accumulated in over 100 studies and in a strikingly diverse range of taxa. However, a principal finding is that the distribution of bet-hedging studies among evidence categories is highly skewed, with most studies falling into categories of comparatively weak evidence. Only 12 studies provide evidence that the proposed bet-hedging traits are adaptive, and therefore fall into categories V and VI.
Because environmental variance is scale-independent [45,96,97], and increases indefinitely over time [98–101], the geometric-mean principle provides a hierarchical and internally consistent perspective of evolution, discussed fully elsewhere [38,102]. Trait persistence through all environmental variance encountered over extended periods of evolution demonstrates a consistent or reliable association with success. It is thus unreasonable to expect conserved traits (even those shared at deep phylogenetic levels) to maximize fitness under present, transient, conditions. Such evolutionarily conserved traits conform to the strict definition of bet hedging and, because they are expected to be maladaptive over the short term, acceptance of the geometric-mean principle forces an adjustment of our conception of optimality.
An important applied corollary to this argument is that the likelihood that populations will persist under climate change may depend on the evolution of bet hedging. For example, several winter-active Coleopteran species of northwestern Europe became cold-adapted during glaciation events, and escape from current effects of climate warming has been attributed to the evolution of diversification traits involved in this cold adaptation [56]. Specialist taxa such as arctic and alpine annual plants might be especially vulnerable to environmental change [103], whereas weedy and invasive species, and cosmopolitan species such as container-breeding mosquitoes [80] may be bet hedgers and less prone to climate-induced extinction.
This review has revealed that our knowledge of the prevalence of bet hedging in nature is at a formative stage. The challenge is to move from a broad base of anecdotal evidence to evidence for the importance of the geometric-mean principle based on the fitness effects of fluctuating selection in targeted tests of bet hedging. The paucity of direct tests together with the rich variety of candidate traits suggest both that the study of bet hedging is an area of high potential growth, and that bet hedging may be more common than previously believed.
Acknowledgements
I thank M. Forbes, W. Hughes, J. Graham and I. Wagner for discussion and comment, and two anonymous reviewers for constructive criticism. This work was supported by a Natural Sciences and Engineering Research Council of Canada Discovery grant to A.M.S.
References
- 1.Stearns S. C. 2000. Daniel Bernoulli (1738): evolution and economics under risk. J. Biosci. 25, 221–228 10.1007/BF02703928 (doi:10.1007/BF02703928) [DOI] [PubMed] [Google Scholar]
- 2.Chown S. L., Hoffmann A. A., Kristensen T. N., Angilletta M. J., Stenseth N. C., Pertoldi C. 2010. Adapting to climate change: a perspective from evolutionary physiology. Clim. Res. 43, 3–15 10.3354/cr00879 (doi:10.3354/cr00879) [DOI] [Google Scholar]
- 3.Cleland E. E., Chuine I., Menzel A., Mooney H. A., Schwartz M. D. 2007. Shifting plant phenology in response to global change. Trends Ecol. Evol. 22, 357–365 10.1016/j.tree.2007.04.003 (doi:10.1016/j.tree.2007.04.003) [DOI] [PubMed] [Google Scholar]
- 4.IPCC 2007. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press [Google Scholar]
- 5.Piao S. L., et al. 2008. Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 451, 49–52 10.1038/nature06444 (doi:10.1038/nature06444) [DOI] [PubMed] [Google Scholar]
- 6.Pimm S. L., Russell G. J., Gittleman J. L., Brooks T. M. 1995. The future of biodiversity. Science 269, 347–350 10.1126/science.269.5222.347 (doi:10.1126/science.269.5222.347) [DOI] [PubMed] [Google Scholar]
- 7.Root T. L., Price J. T., Hall K. R., Schneider S. H., Rosenzweig C., Pounds J. A. 2003. Fingerprints of global warming on wild animals and plants. Nature 421, 57–60 10.1038/nature01333 (doi:10.1038/nature01333) [DOI] [PubMed] [Google Scholar]
- 8.Ruokolainen L., Fowler M. S., Ranta E. 2007. Extinctions in competitive communities forced by coloured environmental variation. Oikos 116, 439–448 10.1111/j.2006.0030-1299.15586.x (doi:10.1111/j.2006.0030-1299.15586.x) [DOI] [Google Scholar]
- 9.Bell G., Collins S. 2008. Adaptation, extinction and global change. Evol. Appl. 1, 3–16 10.1111/j.1752-4571.2007.00011.x (doi:10.1111/j.1752-4571.2007.00011.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berteaux D., Reale D., McAdam A. G., Boutin S. 2004. Keeping pace with fast climate change: can arctic life count on evolution? Integr. Comp. Biol. 44, 140–151 10.1093/icb/44.2.140 (doi:10.1093/icb/44.2.140) [DOI] [PubMed] [Google Scholar]
- 11.Chevin L. M., Lande R., Mace G. M. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357. 10.1371/journal.pbio.1000357 (doi:10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins S., Bell G. 2006. Rewinding the tape: selection of algae adapted to high CO2 at current and pleistocene levels of CO2. Evolution 60, 1392–1401 10.1111/j.0014-3820.2006.tb01218.x (doi:10.1111/j.0014-3820.2006.tb01218.x) [DOI] [PubMed] [Google Scholar]
- 13.Crozier L. G., Hendry A. P., Lawson P. W., Quinn T. P., Mantua N. J., Battin J., Shaw R. G., Huey R. B. 2008. Potential responses to climate change in organisms with complex life histories: evolution and plasticity in Pacific salmon. Evol. Appl. 1, 252–270 10.1111/j.1752-4571.2008.00033.x (doi:10.1111/j.1752-4571.2008.00033.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franks S. J., Weis A. E. 2009. Climate change alters reproductive isolation and potential gene flow in an annual plant. Evol. Appl. 2, 481–488 10.1111/j.1752-4571.2009.00073.x (doi:10.1111/j.1752-4571.2009.00073.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garant D., Kruuk L. E. B., McCleery R. H., Sheldon B. C. 2004. Evolution in a changing environment: a case study with great tit fledging mass. Am. Nat. 164, E115–E129 10.1086/424764 (doi:10.1086/424764) [DOI] [PubMed] [Google Scholar]
- 16.Réale D., McAdam A. G., Boutin S., Berteaux D. 2003. Genetic and plastic responses of a northern mammal to climate change. Proc. R. Soc. Lond. B 270, 591–596 10.1098/rspb.2002.2224 (doi:10.1098/rspb.2002.2224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Childs D. Z., Rees M., Rose K. E., Grubb P. J., Ellner S. P. 2004. Evolution of size-dependent flowering in a variable environment: construction and analysis of a stochastic integral projection model. Proc. R. Soc. Lond. B 271, 425–434 10.1098/rspb.2003.2597 (doi:10.1098/rspb.2003.2597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellner S. P., Hairston N. G., Kearns C. M., Babai D. 1999. The roles of fluctuating selection and long-term diapause in microevolution of diapause timing in a freshwater copepod. Evolution 53, 111–122 10.2307/2640924 (doi:10.2307/2640924) [DOI] [PubMed] [Google Scholar]
- 19.Grant P. R., Grant B. R. 2002. Unpredictable evolution in a 30-year study of Darwin's finches. Science 296, 707–711 10.1126/science.1070315 (doi:10.1126/science.1070315) [DOI] [PubMed] [Google Scholar]
- 20.Greene C. M., Hall J. E., Guilbault K. R., Quinn T. P. 2010. Improved viability of populations with diverse life-history portfolios. Biol. Lett. 6, 382–386 10.1098/rsbl.2009.0780 (doi:10.1098/rsbl.2009.0780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson M. R., Pilkington J. G., Clutton-Brock T. H., Pemberton J. M., Kruuk L. E. B. 2008. Environmental heterogeneity generates fluctuating selection on a secondary sexual trait. Curr. Biol. 18, 751–757 10.1016/j.cub.2008.04.059 (doi:10.1016/j.cub.2008.04.059) [DOI] [PubMed] [Google Scholar]
- 22.Seamons T. R., Bentzen P., Quinn T. P. 2007. DNA parentage analysis reveals inter-annual variation in selection: results from 19 consecutive brood years in steelhead trout. Evol. Ecol. Res. 9, 409–431 [Google Scholar]
- 23.Simons A. M. 2009. Fluctuating natural selection accounts for the evolution of diversification bet hedging. Proc. R. Soc. B 276, 1987–1992 10.1098/rspb.2008.1920 (doi:10.1098/rspb.2008.1920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sletvold N., Grindeland J. M. 2007. Fluctuating selection on reproductive timing in Digitalis purpurea. Oikos 116, 473–481 10.1111/j.2006.0030-1299.15263.x (doi:10.1111/j.2006.0030-1299.15263.x) [DOI] [Google Scholar]
- 25.Slatkin M. 1974. Hedging ones evolutionary bets. Nature 250, 704–705 10.1038/250704b0 (doi:10.1038/250704b0) [DOI] [Google Scholar]
- 26.Veening J. W., Smits W. K., Kuipers O. P. 2008. Bistability, epigenetics, and bet-hedging in bacteria. Annu. Rev. Microbiol. 62, 193–210 10.1146/annurev.micro.62.081307.163002 (doi:10.1146/annurev.micro.62.081307.163002) [DOI] [PubMed] [Google Scholar]
- 27.Hopper K. R. 1999. Risk-spreading and bet-hedging in insect population biology. Annu. Rev. Entomol. 44, 535–560 10.1146/annurev.ento.44.1.535 (doi:10.1146/annurev.ento.44.1.535) [DOI] [PubMed] [Google Scholar]
- 28.Thumm K., Mahony M. 2002. Hatching dynamics and bet-hedging in a temperate frog, Pseudophryne australis (Anura: Myobatrachidae). Amphib.-Reptil. 23, 433–444 10.1163/15685380260462347 (doi:10.1163/15685380260462347) [DOI] [Google Scholar]
- 29.Childs D. Z., Metcalf C. J. E., Rees M. 2010. Evolutionary bet-hedging in the real world: empirical evidence and challenges revealed by plants. Proc. R. Soc. B 277, 3055–3064 10.1098/rspb.2010.0707 (doi:10.1098/rspb.2010.0707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imbert E. 2002. Ecological consequences and ontogeny of seed heteromorphism. Perspect. Plant Ecol. Evol. Syst. 5, 13–36 10.1078/1433-8319-00021 (doi:10.1078/1433-8319-00021) [DOI] [Google Scholar]
- 31.Venable D. L. 2007. Bet hedging in a guild of desert annuals. Ecology 88, 1086–1090 10.1890/06-1495 (doi:10.1890/06-1495) [DOI] [PubMed] [Google Scholar]
- 32.Cohen D. 1966. Optimizing reproduction in a randomly varying environment. J. Theor. Biol. 12, 119–129 10.1016/0022-5193(66)90188-3 (doi:10.1016/0022-5193(66)90188-3) [DOI] [PubMed] [Google Scholar]
- 33.Dempster E. R. 1955. Maintenance of genetic heterogeneity. Cold Spring Harb. Symp. Quant. Biol. 20, 25–32 [DOI] [PubMed] [Google Scholar]
- 34.Gillespie J. H. 1974. Natural-selection for within-generation variance in offspring number. Genetics 76, 601–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewontin R. C., Cohen D. 1969. On population growth in a randomly varying environment. Proc. Natl Acad. Sci. USA 62, 1056–1060 10.1073/pnas.62.4.1056 (doi:10.1073/pnas.62.4.1056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Philippi T., Seger J. 1989. Hedging ones evolutionary bets, revisited. Trends Ecol. Evol. 4, 41–44 10.1016/0169-5347(89)90138-9 (doi:10.1016/0169-5347(89)90138-9) [DOI] [PubMed] [Google Scholar]
- 37.Seger J., Brockmann H. J. 1987. What is bet-hedging? Oxford Surveys Evol. Biol. 4, 182–211 [Google Scholar]
- 38.Simons A. M. 2002. The continuity of microevolution and macroevolution. J. Evol. Biol. 15, 688–701 10.1046/j.1420-9101.2002.00437.x (doi:10.1046/j.1420-9101.2002.00437.x) [DOI] [Google Scholar]
- 39.Barrett R. D. H., Schluter D. 2008. Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44 10.1016/j.tree.2007.09.008 (doi:10.1016/j.tree.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 40.Bell G., Gonzalez A. 2009. Evolutionary rescue can prevent extinction following environmental change. Ecol. Lett. 12, 942–948 10.1111/j.1461-0248.2009.01350.x (doi:10.1111/j.1461-0248.2009.01350.x) [DOI] [PubMed] [Google Scholar]
- 41.Lynch M., Lande R. 1993. Evolution and extinction in response to environmental change. In Biotic interactions and global change (eds Kareiva P. M., Kingsolver J. G., Huey R. B.), pp. 234–250 Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- 42.Hendry A. P., Kinnison M. T. 1999. Perspective: the pace of modern life: measuring rates of contemporary microevolution. Evolution 53, 1637–1653 10.2307/2640428 (doi:10.2307/2640428) [DOI] [PubMed] [Google Scholar]
- 43.Gomulkiewicz R., Holt R. D. 1995. When does evolution by natural-selection prevent extinction. Evolution 49, 201–207 10.2307/2410305 (doi:10.2307/2410305) [DOI] [PubMed] [Google Scholar]
- 44.Heino M., Hanski I. 2001. Evolution of migration rate in a spatially realistic metapopulation model. Am. Nat. 157, 495–511 10.1086/319927 (doi:10.1086/319927) [DOI] [PubMed] [Google Scholar]
- 45.Gingerich P. D. 2009. Rates of evolution. Annu. Rev. Ecol. Evol. Syst. 40, 657–675 10.1146/annurev.ecolsys.39.110707.173457 (doi:10.1146/annurev.ecolsys.39.110707.173457) [DOI] [Google Scholar]
- 46.Hendry A. P., Farrugia T. J., Kinnison M. T. 2008. Human influences on rates of phenotypic change in wild animal populations. Mol. Ecol. 17, 20–29 10.1111/j.1365-294X.2007.03428.x (doi:10.1111/j.1365-294X.2007.03428.x) [DOI] [PubMed] [Google Scholar]
- 47.Wilson A. J., Pemberton J. M., Pilkington J. G., Coltman D. W., Mifsud D. V., Clutton-Brock T. H., Kruuk L. E. B. 2006. Environmental coupling of selection and heritability limits evolution. PLoS Biol. 4, 1270–1275 10.1371/journal.pbio.0040216 (doi:10.1371/journal.pbio.0040216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simons A. M., Roff D. A. 1994. The effect of environmental variability on the heritabilities of traits of a field cricket. Evolution 48, 1637–1649 10.2307/2410253 (doi:10.2307/2410253) [DOI] [PubMed] [Google Scholar]
- 49.Charmantier A., Garant D. 2005. Environmental quality and evolutionary potential: lessons from wild populations. Proc. R. Soc. B 272, 1415–1425 10.1098/rspb.2005.3117 (doi:10.1098/rspb.2005.3117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hairston N. G., Ellner S. P., Geber M. A., Yoshida T., Fox J. A. 2005. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 8, 1114–1127 10.1111/j.1461-0248.2005.00812.x (doi:10.1111/j.1461-0248.2005.00812.x) [DOI] [Google Scholar]
- 51.Pelletier F., Clutton-Brock T., Pemberton J., Tuljapurkar S., Coulson T. 2007. The evolutionary demography of ecological change: linking trait variation and population growth. Science 315, 1571–1574 10.1126/science.1139024 (doi:10.1126/science.1139024) [DOI] [PubMed] [Google Scholar]
- 52.de Meester L. 1996. Evolutionary potential and local genetic differentiation in a phenotypically plastic trait of a cyclical parthenogen, Daphnia magna. Evolution 50, 1293–1298 10.2307/2410669 (doi:10.2307/2410669) [DOI] [PubMed] [Google Scholar]
- 53.Charmantier A., McCleery R. H., Cole L. R., Perrins C., Kruuk L. E. B., Sheldon B. C. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803 10.1126/science.1157174 (doi:10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 54.Chown S. L., Slabber S., McGeoch M. A., Janion C., Leinaas H. P. 2007. Phenotypic plasticity mediates climate change responses among invasive and indigenous arthropods. Proc. R. Soc. B 274, 2531–2537 10.1098/rspb.2007.0772 (doi:10.1098/rspb.2007.0772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skelly D. K., Joseph L. N., Possingham H. P., Freidenburg L. K., Farrugia T. J., Kinnison M. T., Hendry A. P. 2007. Evolutionary responses to climate change. Conserv. Biol. 21, 1353–1355 10.1111/j.1523-1739.2007.00764.x (doi:10.1111/j.1523-1739.2007.00764.x) [DOI] [PubMed] [Google Scholar]
- 56.Topp W. 2003. Phenotypic plasticity and development of cold-season insects (Coleoptera: Leiodidae) and their response to climatic change. Eur. J. Entomol. 100, 233–243 [Google Scholar]
- 57.Visser M. E. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659 10.1098/rspb.2007.0997 (doi:10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reed T. E., Waples R. S., Schindler D. E., Hard J. J., Kinnison M. T. 2010. Phenotypic plasticity and population viability: the importance of environmental predictability. Proc. R. Soc. B 277, 3391–3400 10.1098/rspb.2010.0771 (doi:10.1098/rspb.2010.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inouye D. W. 2008. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89, 353–362 10.1890/06-2128.1 (doi:10.1890/06-2128.1) [DOI] [PubMed] [Google Scholar]
- 60.Visser M. E., Van Noordwijk A. J., Tinbergen J. M., Lessells C. M. 1998. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. Lond. B 265, 1867–1870 10.1098/rspb.1998.0514 (doi:10.1098/rspb.1998.0514) [DOI] [Google Scholar]
- 61.Nussey D. H., Postma E., Gienapp P., Visser M. E. 2005. Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306 10.1126/science.1117004 (doi:10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- 62.West-Eberhard M. J. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press [Google Scholar]
- 63.Badyaev A. V. 2005. Stress-induced variation in evolution: from behavioural plasticity to genetic assimilation. Proc. R. Soc. B 272, 877–886 10.1098/rspb.2004.3045 (doi:10.1098/rspb.2004.3045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Badyaev A. V., Foresman K. R. 2004. Evolution of morphological integration. I. Functional units channel stress-induced variation in shrew mandibles. Am. Nat. 163, 868–879 10.1086/386551 (doi:10.1086/386551) [DOI] [PubMed] [Google Scholar]
- 65.Via S., Lande R. 1985. Genotype–environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522 10.2307/2408649 (doi:10.2307/2408649) [DOI] [PubMed] [Google Scholar]
- 66.Auld J. R., Agrawal A. A., Relyea R. A. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511 10.1098/rspb.2009.1355 (doi:10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hairston N. G., Munns W. R. 1984. The timing of copepod diapause as an evolutionarily stable strategy. Am. Nat. 123, 733–751 10.1086/284236 (doi:10.1086/284236) [DOI] [Google Scholar]
- 68.Simons A. M., Johnston M. O. 2003. Suboptimal timing of reproduction in Lobelia inflata may be a conservative bet-hedging strategy. J. Evol. Biol. 16, 233–243 10.1046/j.1420-9101.2003.00530.x (doi:10.1046/j.1420-9101.2003.00530.x) [DOI] [PubMed] [Google Scholar]
- 69.Simons A. M., Johnston M. O. 1997. Developmental instability as a bet-hedging strategy. Oikos 80, 401–406 10.2307/3546608 (doi:10.2307/3546608) [DOI] [Google Scholar]
- 70.Van Dooren T. J. M. 2001. Reaction norms with bifurcations shaped by evolution. Proc. R. Soc. Lond. B 268, 279–287 10.1098/rspb.2000.1362 (doi:10.1098/rspb.2000.1362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hansen T. F., Carter A. J. R., Pelabon C. 2006. On adaptive accuracy and precision in natural populations. Am. Nat. 168, 168–181 10.1086/505768 (doi:10.1086/505768) [DOI] [PubMed] [Google Scholar]
- 72.Bull J. J. 1987. Evolution of phenotypic variance. Evolution 41, 303–315 10.2307/2409140 (doi:10.2307/2409140) [DOI] [PubMed] [Google Scholar]
- 73.Simons A. M., Johnston M. O. 2006. Environmental and genetic sources of diversification in the timing of seed germination: implications for the evolution of bet hedging. Evolution 60, 2280–2292 10.1111/j.00143820.2006.tb01865.x (doi:10.1111/j.00143820.2006.tb01865.x) [DOI] [PubMed] [Google Scholar]
- 74.Halkett F., Harrington R., Hulle M., Kindlmann P., Menu F., Rispe C., Plantegenest M. 2004. Dynamics of production of sexual forms in aphids: theoretical and experimental evidence for adaptive ‘coin-flipping’ plasticity. Am. Nat. 163, E112–E125 10.1086/383618 (doi:10.1086/383618) [DOI] [PubMed] [Google Scholar]
- 75.Wong T. G., Ackerly D. D. 2005. Optimal reproductive allocation in annuals and an informational constraint on plasticity. New Phytol. 166, 159–171 10.1111/j.1469-8137.2005.01375.x (doi:10.1111/j.1469-8137.2005.01375.x) [DOI] [PubMed] [Google Scholar]
- 76.Donohue K., Dorn L., Griffith C., Kim E., Aguilera A., Polisetty C. R., Schmitt J. 2005. The evolutionary ecology of seed germination of Arabidopsis thaliana: variable natural selection on germination timing. Evolution 59, 758–770 10.1111/j.0014-3820.2005.tb01751.x (doi:10.1111/j.0014-3820.2005.tb01751.x) [DOI] [PubMed] [Google Scholar]
- 77.Simons A. M., Wagner I. 2007. The characterization of complex continuous norms of reaction. Oikos 116, 986–994 10.1111/j.0030-1299.2007.15814.x (doi:10.1111/j.0030-1299.2007.15814.x) [DOI] [Google Scholar]
- 78.Orzack S. H., Sober E. 1994. Optimality models and the test of adaptationism. Am. Nat. 143, 361–380 10.1086/285608 (doi:10.1086/285608) [DOI] [Google Scholar]
- 79.Hakalahti T., Hakkinen H., Valtonen E. T. 2004. Ectoparasitic Argulus coregoni (Crustacea: Branchiura) hedge their bets: studies on egg hatching dynamics. Oikos 107, 295–302 10.1111/j.0030-1299.2004.13213.x (doi:10.1111/j.0030-1299.2004.13213.x) [DOI] [Google Scholar]
- 80.Khatchikian C. E., Dennehy J. J., Vitek C. J., Livdahl T. P. 2010. Environmental effects on bet hedging in Aedes mosquito egg hatch. Evol. Ecol. 24, 1159–1169 10.1007/s10682-010-9359-4 (doi:10.1007/s10682-010-9359-4) [DOI] [Google Scholar]
- 81.Ratcliff W. C., Denison R. F. 2010. Individual-level bet hedging in the bacterium Sinorhizobium meliloti. Curr. Biol. 20, 1740–1744 10.1016/j.cub.2010.08.036 (doi:10.1016/j.cub.2010.08.036) [DOI] [PubMed] [Google Scholar]
- 82.Beaumont H. J. E., Gallie J., Kost C., Ferguson G. C., Rainey P. B. 2009. Experimental evolution of bet hedging. Nature 462, 90–97 10.1038/nature08504 (doi:10.1038/nature08504) [DOI] [PubMed] [Google Scholar]
- 83.Kussell E., Leibler S. 2005. Phenotypic diversity, population growth, and information in fluctuating environments. Science 309, 2075–2078 10.1126/science.1114383 (doi:10.1126/science.1114383) [DOI] [PubMed] [Google Scholar]
- 84.Dubnau D., Losick R. 2006. Bistability in bacteria. Mol. Microbiol. 61, 564–572 10.1111/j.1365-2958.2006.05249.x (doi:10.1111/j.1365-2958.2006.05249.x) [DOI] [PubMed] [Google Scholar]
- 85.Rees M., Childs D. Z., Metcalf J. C., Rose K. E., Sheppard A. W., Grubb P. J. 2006. Seed dormancy and delayed flowering in monocarpic plants: selective interactions in a stochastic environment. Am. Nat. 168, E53–E71 10.1086/505762 (doi:10.1086/505762) [DOI] [PubMed] [Google Scholar]
- 86.Clauss M. J., Venable D. L. 2000. Seed germination in desert annuals: an empirical test of adaptive bet hedging. Am. Nat. 155, 168–186 10.1086/303314 (doi:10.1086/303314) [DOI] [PubMed] [Google Scholar]
- 87.Philippi T. 1993. Bet-hedging germination of desert annuals: beyond the 1st year. Am. Nat. 142, 474–487 10.1086/285550 (doi:10.1086/285550) [DOI] [PubMed] [Google Scholar]
- 88.Evans M. E. K., Ferriere R., Kane M. J., Venable D. L. 2007. Bet hedging via seed banking in desert evening primroses (Oenothera, Onagraceae): demographic evidence from natural populations. Am. Nat. 169, 184–194 10.1086/510599 (doi:10.1086/510599) [DOI] [PubMed] [Google Scholar]
- 89.Byrne P. G., Keogh J. S. 2009. Extreme sequential polyandry insures against nest failure in a frog. Proc. R. Soc. B 276, 115–120 10.1098/rspb.2008.0794 (doi:10.1098/rspb.2008.0794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burd M., et al. 2009. Ovule number per flower in a world of unpredictable pollination. Am. J. Bot. 96, 1159–1167 10.3732/ajb.0800183 (doi:10.3732/ajb.0800183) [DOI] [PubMed] [Google Scholar]
- 91.Cunnington D. C., Brooks R. J. 1996. Bet-hedging theory and eigenelasticity: a comparison of the life histories of loggerhead sea turtles (Caretta caretta) and snapping turtles (Chelydra serpentina). Can. J. Zool. (Revue Canadienne De Zoologie) 74, 291–296 10.1139/z96-036 (doi:10.1139/z96-036) [DOI] [Google Scholar]
- 92.Brewer J. S. 2008. Geographic variation in flowering responses to fire and season of clipping in a fire-adapted plant. Am. Midl. Nat. 160, 235–249 10.1674/0003-0031(2008)160[235:GVIFRT]2.0.CO;2 (doi:10.1674/0003-0031(2008)160[235:GVIFRT]2.0.CO;2) [DOI] [Google Scholar]
- 93.Kraaijeveld K., Kraaijeveld-Smit F. J. L., Adcock G. J. 2003. Does female mortality drive male semelparity in dasyurid marsupials? Proc. R. Soc. Lond. B 270, S251–S253 10.1098/rsbl.2003.0082 (doi:10.1098/rsbl.2003.0082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goodenough A. E., Hart A. G., Stafford R. 2010. Is adjustment of breeding phenology keeping pace with the need for change? Linking observed response in woodland birds to changes in temperature and selection pressure. Clim. Change 102, 687–697 10.1007/s10584-010-9932-4 (doi:10.1007/s10584-010-9932-4) [DOI] [Google Scholar]
- 95.Sober E. 1984. The nature of selection: evolutionary theory in philosophical focus. Chicago, IL: University of Chicago Press [Google Scholar]
- 96.Koscielny-Bunde E., Bunde A., Havlin S., Roman H. E., Goldreich Y., Schellnhuber H. J. 1998. Indication of a universal persistence law governing atmospheric variability. Phys. Rev. Lett. 81, 729–732 10.1103/PhysRevLett.81.729 (doi:10.1103/PhysRevLett.81.729) [DOI] [Google Scholar]
- 97.Sole R. V., Manrubia S. C., Benton M., Bak P. 1997. Self-similarity of extinction statistics in the fossil record. Nature 388, 764–767 10.1038/41996 (doi:10.1038/41996) [DOI] [Google Scholar]
- 98.Arino A., Pimm S. L. 1995. On the nature of population extremes. Evol. Ecol. 9, 429–443 10.1007/BF01237765 (doi:10.1007/BF01237765) [DOI] [Google Scholar]
- 99.Cyr H. 1997. Does inter-annual variability in population density increase with time? Oikos 79, 549–558 10.2307/3546899 (doi:10.2307/3546899) [DOI] [Google Scholar]
- 100.Inchausti P., Halley J. 2002. The long-term temporal variability and spectral colour of animal populations. Evol. Ecol. Res. 4, 1033–1048 [Google Scholar]
- 101.Pimm S. L., Redfearn A. 1988. The variability of population densities. Nature 334, 613–614 10.1038/334613a0 (doi:10.1038/334613a0) [DOI] [Google Scholar]
- 102.Lee M. S. Y., Doughty P. 2003. The geometric meaning of macroevolution. Trends Ecol. Evol. 18, 263–266 10.1016/S0169-5347(03)00103-4 (doi:10.1016/S0169-5347(03)00103-4) [DOI] [Google Scholar]
- 103.Wagner I., Simons A. M. 2009. Divergence among arctic and alpine populations of the annual, Koenigia islandica: morphology, life-history, and phenology. Ecography 32, 114–122 10.1111/j.1600-0587.2008.05497.x (doi:10.1111/j.1600-0587.2008.05497.x) [DOI] [Google Scholar]