Abstract
Chronic postnatal hyperoxia blunts the hypoxic ventilatory response (HVR) in rats, an effect that persists for months after return to normoxia. To determine whether decreased carotid body O2 sensitivity contributes to this lasting impairment, single-unit chemoafferent nerve and glomus cell calcium responses to hypoxia were recorded from rats reared in 60% O2 through 7 d of age (P7) and then returned to normoxia. Single-unit nerve responses were attenuated by P4 and remained low through P7. After return to normoxia, hypoxic responses were partially recovered within 3 d and fully recovered within 7–8 d (i.e., at P14-15). Glomus cell calcium responses recovered with a similar time course. Hyperoxia altered carotid body mRNA for O2-sensitive K+ channels TASK-1, TASK-3, and BKCa, but only TASK-1 mRNA paralleled changes in chemosensitivity (i.e., downregulation by P7, partial recovery by P14). Collectively, these data do not support a role for reduced O2 sensitivity of individual chemoreceptor cells in long-lasting reduction of the HVR after developmental hyperoxia.
Keywords: control of breathing, developmental plasticity, chemoreceptor, glomus cell, mRNA expression, K+ channel
1. Introduction
The carotid body is the primary O2 sensor for the cardiorespiratory system. It responds to a decrease in arterial O2 tension by increasing action potential activity on the carotid sinus nerve (CSN) which, in turn, stimulates breathing, increases sympathetic activity, and initiates arousal from sleep. The mechanism of hypoxia transduction within the carotid body is not completely resolved but it appears to be initiated by the glomus cell, a secretory cell apposed to the terminals of CSN axons. Hypoxia causes an increase in glomus cell calcium and the secretion of dense cored granules, which is postulated to mediate the increase in afferent nerve activity (Gonzalez et al., 1994; Kumar, 2007; López-Barneo et al., 2008). At least two types of O2-sensitive channels are expressed by rat glomus cells. One is a calcium- and voltage-dependent potassium channel (BKCa) that is inhibited by hypoxia and the other consists of a group of non-voltage-dependent ‘leak’ channels (TASK) that are also selective for K+ and inhibited by hypoxia (Gonzalez et al., 2009; Peers et al., 2010). Both types of channels are postulated to play a role in initiating the carotid body response to hypoxia.
The carotid body response to hypoxia is weak at birth and increases to adult levels over the first several weeks of life. During this period of postnatal maturation, the morphological and functional development of the carotid body is critically dependent on prevailing oxygen levels (Carroll, 2003; Bavis, 2005). Neonatal rats reared in moderate hyperoxia (60% O2) exhibit reduced numbers of carotid body glomus cells, smaller carotid body volumes, and primary afferent neuron degeneration within the first week of hyperoxia exposure (Erickson et al., 1998; Wang & Bisgard, 2005). These morphological changes are accompanied by a reduction or loss of carotid body chemoreceptor O2 sensitivity (Donnelly et al., 2005, 2009). In a recent study, neonatal rats were exposed to 60% O2 for up to two weeks beginning at 7 days of age (P7) (Donnelly et al., 2009). Single-unit carotid chemoafferent neuron responses and glomus cell intracellular calcium responses to hypoxia were significantly reduced by P12 (i.e., after 5 d in hyperoxia) and remained low throughout the remainder of the hyperoxic exposure.
The impairment of respiratory responses to hypoxia produced by postnatal exposure to hyperoxia persists despite a prolonged return to normoxia (Ling et al., 1996; 1997a,b; Fuller et al., 2002). This likely reflects loss of peripheral chemoreceptor input since whole-nerve CSN responses to asphyxia, cyanide, and isocapnic hypoxia are reduced into adulthood after 1-, 2-, or 4-week exposures to 60% O2 (Bisgard et al., 2003), and CSN responses still may be absent more than a year after return to room air (Fuller et al., 2002). However, it is presently unclear whether these reduced responses are due to loss of chemoafferent axons, reduced activity of individual axons, or some combination of these factors. This question was partially addressed by Prieto-Lloret et al. (2004) who measured spiking activity in rat chemoreceptor axons following a 2.5–3.5 mo return to normoxia. This group observed relatively normal spiking levels in the subset of preparations that responded to hypoxia, which suggests some level of functional recovery; however, the numbers of axons contributing to a recording were not identified (i.e., a mixture of single- and few-fiber recordings), and the timeframe for this recovery was not considered. Therefore, the primary objective of the present study was to explore functional changes in peripheral chemoreceptors following a return to the normoxic environment to determine whether recovery occurs at an individual axonal level and to establish the time course for this recovery.
To begin to understand the cellular basis of recovery, we also examined the time course for changes in the calcium response of glomus cells to hypoxia and for the expression of mRNA for the O2-sensitive K+ channels specifically linked to O2 transduction in the rat carotid body: TASK channels (TASK-1 and TASK-3) and the BKCa channel (Gonzalez et al., 2009; Peers et al., 2010). These K+ channels are functional in neonatal rat glomus cells, and their relative expression and/or O2 sensitivity appears to increase with age in parallel with glomus cell O2 sensitivity (Hatton et al. 1997; Wasicko et al., 2006; Kim et al., 2009). While the effect of hyperoxia on BKCa expression has not been studied, Kim et al. (2006) previously reported that TASK mRNA expression was reduced after 14 d in hyperoxia. If TASK-1, TASK-3, and/or BKCa channels are responsible for hyperoxia-induced changes in O2 sensitivity, we reasoned that expression of carotid body mRNA for these channels should parallel the impairment of chemoreceptor function produced by hyperoxia and (potential) recovery upon return to normoxia.
2. Methods
2.1. Experimental animals
Experiments were conducted on Sprague-Dawley rat pups of both sexes. Timed-pregnant rats were obtained from a commercial supplier (Charles River Laboratories; Portage, MI, USA) and placed into environmental chambers approximately one day before giving birth. Chambers were flushed with gases at sufficient flow rates to maintain 60% O2 and <0.4% CO2. The resulting litters (“Hyperoxia”) were raised in 60% O2 with their mothers. Individual Hyperoxia pups were removed from the chamber at specific ages (P1–P7 or P14) and sacrificed for study (see below; typically 5–15 min in normoxia prior to sacrifice). Some Hyperoxia litters were removed from the chamber when pups reached P7 and permitted to recover in room air prior to study at P10 or P14-15 (i.e., after 3 or 7–8 d recovery). Additional litters were raised in 21% O2 to serve as age-matched control groups (“Control”). Rats were maintained on a 12:12 light cycle throughout the study and provided food and water ad libitum.
All experimental procedures were approved by the Institutional Animal Care and Use Committees (IACUC) at Bates College (chemoafferent nerve and mRNA expression studies) and the University of Arkansas for Medical Sciences (intracellular calcium studies).
2.2. Single-unit carotid chemoafferent nerve recordings
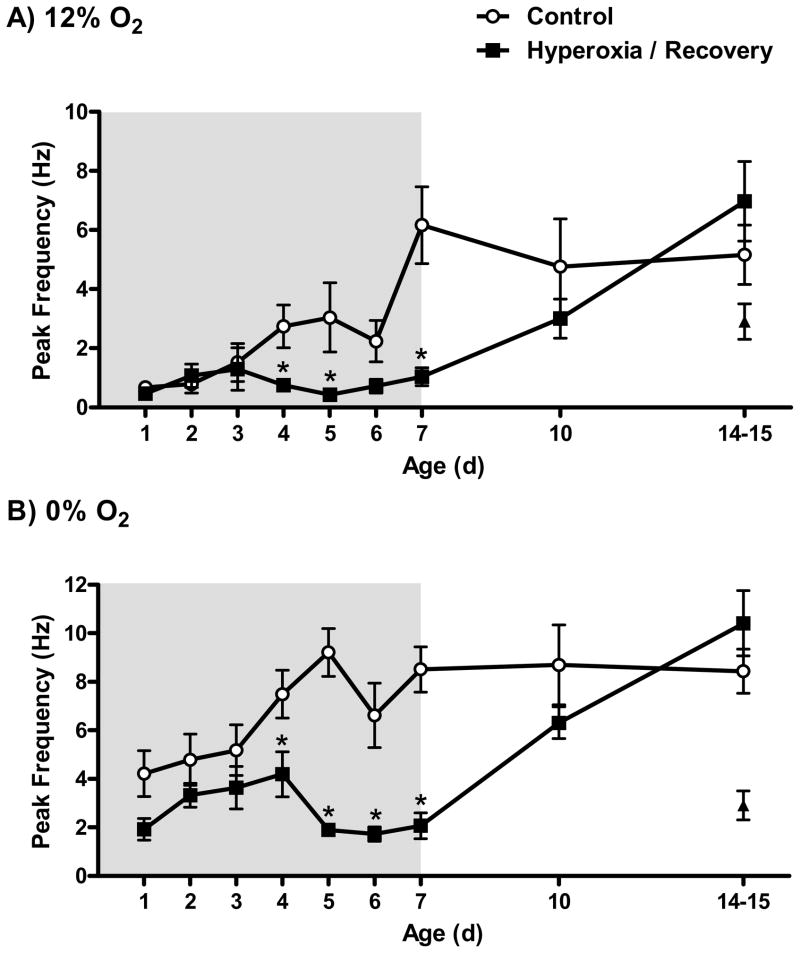
Carotid chemoafferent nerve recordings were made at P1-P7, P10, or P14-15 using the same methods as in our earlier studies (Donnelly et al., 2005, 2009). At each age, recordings were made from pups derived from 3–6 different litters per treatment group; the number of chemoreceptor units studied at each age is reported in Figure 2.
Fig. 2.
Single-unit carotid chemoafferent nerve responses to (A) 12% O2 and (B) 0% O2 in rats exposed to 21% O2 (Control) or 60% O2 (Hyperoxia) beginning 1 d prior to birth and studied at P1-P7; the shaded region represents the period of hyperoxic exposure. To assess potential recovery of chemoafferent responses, additional Control and Hyperoxia rats were returned to room air at P7 and studied after 3 or 7–8 d recovery (i.e., P10 or P14-15). Values are mean±SEM. Number of observations: Control: P1 (n=12), P2 (n=8), P3 (n=11), P4 (n=9), P5 (n=8), P6 (n= 11), P7 (n=16), P10 (n=11), P14-15 (n=19); Hyperoxia: P1 (n=8), P2 (n=10), P3 (n=10), P4 (n=16), P5 (n=8), P6 (n=6), P7 (n=14), P10 (n=14), P14-15 (n=13). *P<0.05 vs. Control at same age. For comparison, the triangles represent units recorded from P14 rats maintained in 60% O2 from birth (i.e., no normoxic recovery) in an earlier study (Donnelly et al., 2005).
Rat pups were killed by decapitation prior to tissue harvest; P7 and older pups were deeply anesthetized by exposure to 100% CO2 prior to decapitation. The carotid body, carotid sinus nerve (CSN), glossopharyngeal nerve, and petrosal ganglion were isolated en bloc and placed into an ice-cold, oxygenated (95% O2/5% CO2) bicarbonate-buffered balanced salt solution (BSS) (in mM: 125 NaCl, 5 KCl, 2 CaCl2, 1 Na2HPO4, 1 MgSO4, 26 NaHCO3, and 5 dextrose). The vagus nerve and carotid arteries were dissected free from the glossopharyngeal nerve and carotid body. To aid in tissue cleaning, the remaining complex (carotid body, CSN, glossopharyngeal nerve, and ganglion) was then transferred to oxygenated (95% O2/5% CO2) BSS containing 0.1–0.2% collagenase (type P; Roche Diagnostics, Indianapolis, IN USA) and 0.01–0.02% protease (type XIV; Sigma, St. Louis, MO) at 37 °C for 30 min with gentle agitation. The complex was further cleaned and transferred to a ~140 μL perfusion chamber (RC-22C; Warner Instrument Corp., Hamden, CT USA) mounted on the stage of an inverted microscope equipped with Hoffman contrast optics (Nikon Eclipse TE2000-U). The complex was superfused (~3 ml min−1) with oxygenated (21% O2/5% CO2/balance N2) BSS controlled at 37 °C with a water bath (while in reservoir) and by an in-line heater (SH-27B heater and TC344-B controller; Warner Instrument Corp.).
Single-unit activity was recorded using a suction electrode advanced into the petrosal ganglion. Electrode tip size was approximately 30 μm in diameter which allowed individual ganglion cells to enter the tip. The pipette potential was amplified 2,000–5,000× with an extracellular amplifier (EX-1; Dagan Instruments, Minneapolis, MN USA), passband-filtered (0.1–2 kHz), digitized (10 kHz sample rate; Powerlab 8/30 and Chart 5.2 software; ADInstruments, Colorado Springs, CO USA), and stored to a computer. To facilitate identification of nerve fibers projecting to the carotid body, a stimulus electrode (pipette filled with 1 M NaCl) was advanced into the carotid body. A constant-current stimulus (~200 μA × 0.05 ms pulse duration) was delivered at 0.5–1 Hz (Isostim A320; World Precision Instruments, Sarasota, FL USA) and the success of the stimulus in initiating an orthodromic action potential was used to identify chemoreceptor units.
Once a single-unit afferent neuron was identified, stimulation of the carotid body was halted and spontaneous action potentials were recorded for 2 min under baseline conditions (21% O2/5% CO2/balance N2). The superfusate was then switched to hypoxia (12% O2/5% CO2/balance N2) for a period of 2 min followed by a recovery in normoxia for 10 min. After the recovery period, the superfusate was switched to severe hypoxia (0% O2/5% CO2/balance N2) for a period of 2 min followed by a return to normoxia. This sequence was repeated (at least 15 min later) if a second afferent neuron was identified; no more than two recordings were made per preparation.
Two levels of oxygen were chosen to facilitate comparison to the previous studies on which the present study is based (Donnelly et al., 2005, 2009). Severe hypoxia (0% O2) evokes a maximal stimulation of chemoreceptor discharge; however, strong hypoxia often elicits a transient nerve response with considerable adaptation (e.g., Donnelly et al., 2005, 2009; Peng et al., 2010). The transient nature of the stimulation may be due to a high release of inhibitory neuromodulators (e.g., dopamine) or a non-specific anoxic depression of neuronal function. Thus, a moderate level of hypoxia (12% O2) which produces a sustained increase in chemoreceptor activity was also employed to study chemoreceptor function across a range of O2 values. Chamber oxygen tension adjacent to the carotid body was measured periodically using a fiberoptic probe with phosphorescence quenching (Oxy-Micro with PST-1 probe, World Precision Instruments). Average chamber O2 tensions were 131 mmHg at 21% O2, 78 mmHg at 12% O2, and 9 mmHg at 0% O2.
Chemoreceptor activity was discriminated off-line for height and timing (Spike Histogram Module v.1; ADInstruments). Only cells that exhibited spontaneous activity during the 16-min protocol were accepted as viable units for analysis. Baseline discharge rate was calculated from the number of action potentials over the final 60 s of baseline. For hypoxic challenges, the number of individual spikes per second was calculated and peak discharge frequency was determined after applying a 3-s moving average.
2.3. Glomus cell isolation and measurement of intracellular calcium
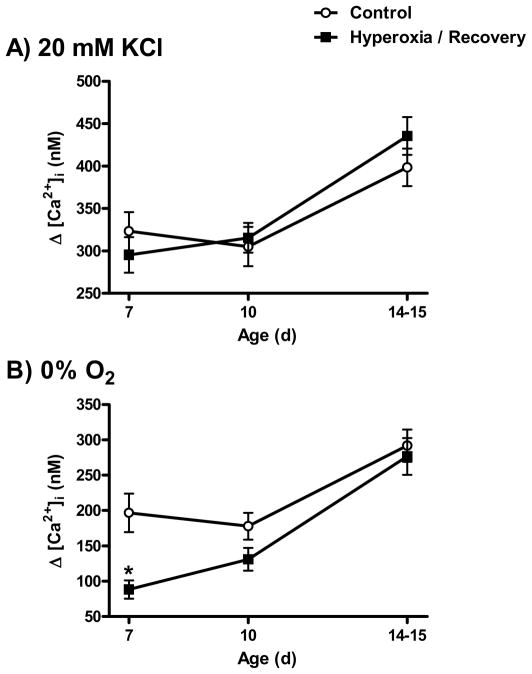
Intracellular calcium responses to elevated extracellular K+ and hypoxia were measured at P7, P10, or P14-15 using the same methods as in our earlier study (Donnelly et al., 2009). At each age, carotid bodies were harvested from pups derived from at least four different litters per treatment group; the numbers of observations at each age are reported in Figure 4.
Fig. 4.
Glomus cell calcium responses to (A) 20 mM KCl and (B) 0% O2 in rats exposed to 21% O2 (Control) or 60% O2 (Hyperoxia) through P7 and after 3 or 7–8 d recovery in room air (i.e., P10 or P14-15). Values are mean±SEM. Number of observations: Control: P7 (n=35), P10 (n=53), P14-15 (n=64); Hyperoxia: P7 (n=59), P10 (n=58), P14-15 (n=68). *P<0.05 vs. Control at same age.
Rat pups were anesthetized with isoflurane, the preferred method of the University of Arkansas IACUC, and decapitated. The carotid bifurcations were rapidly removed and placed into ice-cold, low-Ca2+-Mg2+ phosphate buffered saline (PBS). The carotid bodies were dissected from the bifurcations and placed into a solution comprised of trypsin (10,000–12,000 units ml−1, Sigma) and type-I collagenase (200–240 units ml−1, Worthington Biochemical, Lakewood, NJ USA) in low Ca2+-Mg2+ PBS, and incubated with the enzyme solution for ~25 min at 37°C in 21% O2/5% CO2 (cell culture incubator). Enzyme-treated carotid bodies were dispersed by trituration with a fire polished glass pipette. After adding carotid body culture medium (Ham’s F12 (GIBCO) with 10% fetal calf serum) to the dissociated carotid body cells, the cells were collected by centrifugation at 6000×g for 4–6 min. The cells were washed with fresh culture medium and centrifuged again. Pelleted carotid body cells were resuspended in culture medium. Dissociated cells were then plated on poly-D-lysine coated glass coverslip and incubated in growth media composed of Ham’s F12 (GIBCO) with 10% fetal calf serum, 33 mM glucose, 2 mM L-glutamine, 100 units ml−1 penicillin, 100 mg ml−1 streptomycin, and 0.08 units ml−1 insulin at 37°C in 21% O2/5% CO2 incubator until use.
Cells were loaded with the calcium-sensitive dye, FURA-2 by incubation with 4 mM FURA-2 acetoxymethyl ester for 30 min at 37 °C in saline equilibrated with 21% O2/5% CO2/balance N2. After loading with FURA-2, the coverslip was placed in a closed microscope chamber (0.1 ml total volume) and superfused with a bicarbonate-buffered balanced salt solution (BSS) (in mM: 118 NaCl, 23 NaHCO3, 3 KCl, 2 KH2PO4, 1.2 CaCl2, 1 MgCl2, and 10 glucose). The BSS was initially bubbled in a heated reservoir with 21% O2/5% CO2/balance N2. The response to increased K+ was elicited by switching the perfusate for 30 s to a reservoir containing 20 mM K+ (equimolar substitution of Na+) (e.g., Donnelly et al., 2009). Cells were allowed to recover in normal BSS for 5 min and then challenged with hypoxia by switching the perfusate to BSS equilibrated with 5% CO2/balance N2 (i.e., 0% O2) for 2 min.
FURA-2 fluorescent emission was measured every 8 s at ~510 nm in response to alternating excitation at 340 and 380 nm. Images were acquired and stored using a Nikon TE300 inverted microscope and cooled CCD camera (Photometrics, Tucson, AZ USA) under computer control (Metafluor, Universal Imaging, West Chester, PA, USA). For calcium measurements, areas of interest were selected over cells that demonstrated a rapid rise in intracellular calcium during superfusion with increased K+; only cells that responded to K+ were considered viable glomus cells and included in the analysis. Results from cells in a cluster were averaged and considered as a single observation, and both single cells and clusters were included in the analysis; preliminary analysis revealed no differences in the calcium responses of single or clustered cells.
2.4. mRNA expression for TASK-1, TASK-3, and BKCa
To assess the effects of chronic hyperoxia on mRNA expression of TASK-1, TASK-3, and BKCa, carotid bodies were collected from P7 and P14 rats that had been exposed continuously to hyperoxia or room air since birth, and from P14 rats that had been exposed to hyperoxia through P7 and then returned to room air (i.e., 7 d hyperoxia + 7 d recovery). Following decapitation, carotid bodies were rapidly harvested, frozen on dry ice, and stored at −80°C. Due to the small size of the neonatal carotid body, each sample consisted of carotid bodies pooled from one full litter of rats (i.e., 12–24 carotid bodies per sample). Carotid bodies were collected from a total of 6 litters for each group, except the P14 Hyperoxia group for which carotid bodies were collected from a total of 4 litters; thus, n= 4–6 independent samples per treatment group.
Pooled carotid bodies were homogenized for 20 s in RLT lysis buffer (Qiagen, Valencia, CA, USA). RNA was extracted from the homogenates using an RNeasy Micro RNA Isolation Kit (Qiagen) according to the manufacturer’s protocol with the exception of doubling the DNase volume and incubation time. RNA quantity and quality were assessed using an RNA 6000 Nano Chip in a Bioanalyzer (Agilent, Santa Clara, CA, USA). Total RNA yield averaged 0.57 μg per sample (30 ng per carotid body).
cDNA was prepared separately for each of three planned comparisons: P7 Control vs. P7 Hyperoxia; P14 Control vs. P14 Hyperoxia; and P14 Control vs. P14 Recovery (i.e., 7 d hyperoxia + 7 d recovery). Typically, first strand cDNA was synthesized from 250 ng RNA using the RT2 First Strand Kit (SABiosciences, Frederick, MD USA) according to manufacturer’s protocol with the exception of replacing GE Buffer with water. Due to the lower yield of total RNA in the P14 Hyperoxia group, however, it was necessary to use a smaller quantity of RNA (160 ng) in the cDNA reactions for this group. Since the P14 Control group was compared to both the P14 Hyperoxia and P14 Recovery groups, two separate batches of cDNA were prepared for this group (using 160 ng and 250 ng RNA, respectively). The resulting single-stranded cDNA products were diluted with 14 μl RNase-free water and stored at −20°C until quantitative PCR analysis.
Each gene of interest was assayed on three separate 96-well plates (one plate for each of the three planned comparisons). Individual Control and Hyperoxia cDNA samples were run in triplicate (1 μl cDNA per replicate) for each corresponding gene of interest and for β-actin. Preliminary analysis indicated that β-actin was the most stable reference gene compared to tyrosine hydroxylase, synaptophysin, chromogranin A, and β-III tubulin (geNORM v. 3.5, Ghent University Hospital Center for Medical Genetics, Belgium; data not shown). The following primers were purchased from SABiosciences: β-actin (Actb, cat. #PPR06570B; expected amplicon length: 131 bp), TASK-1 (Kcnk3, cat. #PPR46123A; 126 bp), TASK-3 (Kcnk9, cat. #PPR50306A; 154 bp), and BKCa (Kcnma1, cat. #PPR48434E; 156 bp).
Amplification was performed using RT2 Fast SYBR Green/ROX qPCR Master Mix (SABiosciences) in an M×3000 qPCR System (Stratagene, Cedar Creek, TX, USA) as follows: 95°C × 10 min, 40 cycles of 95°C × 10 s, 60°C × 30 s. Dissociation curve analyses were run to confirm a single gene product in each well, and “no template” and “no reverse transcriptase” controls were included to monitor for genomic DNA and other contamination.
For each cDNA sample, triplicate CT values were averaged to produce one value for the gene of interest and for β-actin. CT values for the gene of interest were then normalized to β-actin CT values using the 2−ΔΔCT method (Livak and Schmittgen, 2001). The averaged CT values from the Control samples were used as the calibrator for that plate, and the data are reported relative to that value (i.e., percent of Control).
2.5. Statistical analysis
Spiking rates and intracellular calcium responses were compared across groups using two-way analysis of variance (ANOVA) with treatment (Control or Hyperoxia) and age as grouping variables. Where significant main effects or treatment × age interactions were detected, pairwise comparisons were made using Tukey post-hoc tests. In order to meet the equal variance assumption for ANOVA, it was necessary to apply a logarithmic (log10) transformation to nerve-responses to 12% O2 and calcium responses to 0% O2. mRNA (2−ΔΔCT values) expression levels were compared between treatment groups (Control vs. Hyperoxia) using independent sample t-tests.
All statistical tests were run using SigmaStat 3.11 (SPSS, Chicago, IL), and P≤0.05 was considered significant. Values are reported as mean±SEM.
3. Results
3.1. Single-unit chemoafferent responses to hypoxia
Single-unit chemoafferent activity was recorded in vitro for neonatal rats chronically exposed to 60% O2 and for age-matched controls (Fig. 1). Baseline (21% O2) discharge rates were low (~0.1 Hz) and did not differ between treatment groups (P=0.78) or across ages (P=0.59). When superfused with hypoxic BSS, however, differences emerged between treatment groups, and these varied with age (treatment × age, P=0.001 and P<0.001 in 12% and 0% O2, respectively) (Fig. 2). Peak discharge rates during hypoxia tended to increase with age in Control rats during the first postnatal week, but a similar trend was only evident for the first few days (i.e., P1-P3) in Hyperoxia rats. Instead, peak activity tended to decrease between P3 and P5 in the Hyperoxia group and remained low through P7. Consequently, peak activity was lower in the Hyperoxia group from P4 through P7 compared to age-matched Controls in both 12% O2 and 0% O2 (all P<0.05, except P6 in 12% O2, P=0.08). By P7, peak single-unit activity in the Hyperoxia group was 83% lower than in Controls at 12% O2 and 76% lower than in Controls at 0% O2 (both P<0.001).
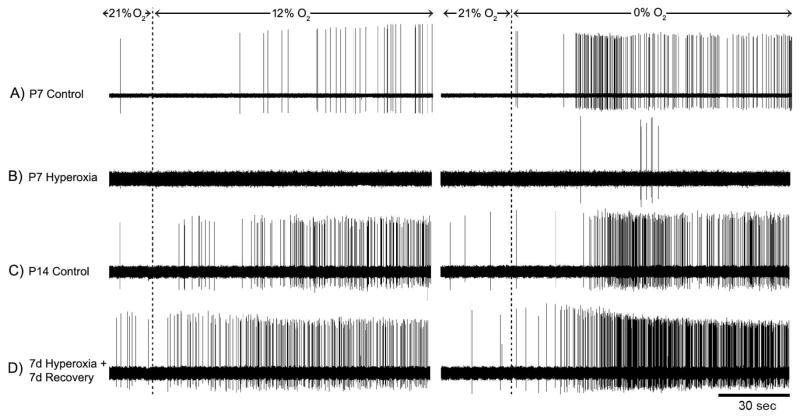
Fig. 1.
Representative recordings of single-unit chemoafferent activity in rats reared in 21% O2 (Control) or 60% O2 (Hyperoxia) through P7 (panels A, B) and after 7 d recovery in room air (i.e., P14; panels C, D). These recordings show raw activity under baseline conditions (21% O2/5% CO2) and during 2-min exposures to mild hypoxia (12% O2/5% CO2) and severe hypoxia (0% O2/5% CO2); there was a 10-min recovery period (21% O2/5% CO2) between hypoxic challenges. Note the robust hypoxic response in Hyperoxia rats after 7 d recovery (panel D) compared to the weak hypoxic response at P7 (panel B).
To determine whether chemosensitivity would spontaneously recover upon return to normoxia, P7 rats were returned to room air and studied 3 d (P10) or 7–8 d (P14-15) later. Although neuronal activity was uniformly low for P7 Hyperoxia rats, even in severe hypoxia (Fig. 1B), hypoxic responses were robust after 7 d recovery in room air (Fig. 1D). Peak hypoxic discharge rates were no longer significantly different from Control after 3 days of recovery (both P>0.08) and were fully recovered within a week (both P>0.11) (Fig. 2). Although we did not study rats maintained in hyperoxia from birth through P14 in the present study, carotid chemoreceptor responses were previously shown to be significantly reduced following this treatment (Donnelly et al., 2005; see also Fig. 2). Therefore, the observed increase in hypoxic responses with age in the Hyperoxia group is caused by the return to room air rather than ageing per se.
3.2. Intracellular calcium responses to KCl and hypoxia in isolated glomus cells
Apparent recovery of chemoafferent nerve responses could reflect compensatory changes in chemoafferent neurons rather than glomus cell O2 sensitivity. To exclude this possibility, intracellular calcium responses were compared among Hyperoxia rats exposed for the first postnatal week (P7), Hyperoxia rats after recovery in room air for 3 d (P10) or 7–8 d (P14-15), and age-matched Controls (e.g., Fig. 3). Responses to high extracellular K+ (20 mM KCl) increased with age independent of the treatment group (P<0.001), with responses at P14-15 being greater than at P7 and P10. However, responses to extracellular K+ did not differ between treatment groups (treatment, P=0.72; treatment × age, P=0.35) (Fig. 4A). Therefore, chronic hyperoxia did not disrupt the ability of glomus cells to respond to a non-specific depolarizing stimulus. In contrast, the intracellular calcium responses to hypoxia varied by treatment and age (treatment × age, P=0.05) (Fig. 4B). At P7, the calcium response to 0% O2 was 55% lower in the Hyperoxia group than in Controls (P<0.001). Although the response to hypoxia tended to increase with age in both groups between P7 and P14-15, this increase was larger in the Hyperoxia group which had been returned to room air. Consequently, the calcium responses were no longer different between Hyperoxia and Control groups after 3 d (P=0.08) or 7–8 d (P=0.30) recovery in room air. Although we did not study rats maintained in hyperoxia from birth through P14 in the present study, glomus cell calcium responses to hypoxia are significantly reduced following this treatment (Kim et al., 2003). Therefore, the observed increase in hypoxic responses with age in the Hyperoxia group is caused by the return to room air rather than ageing per se.
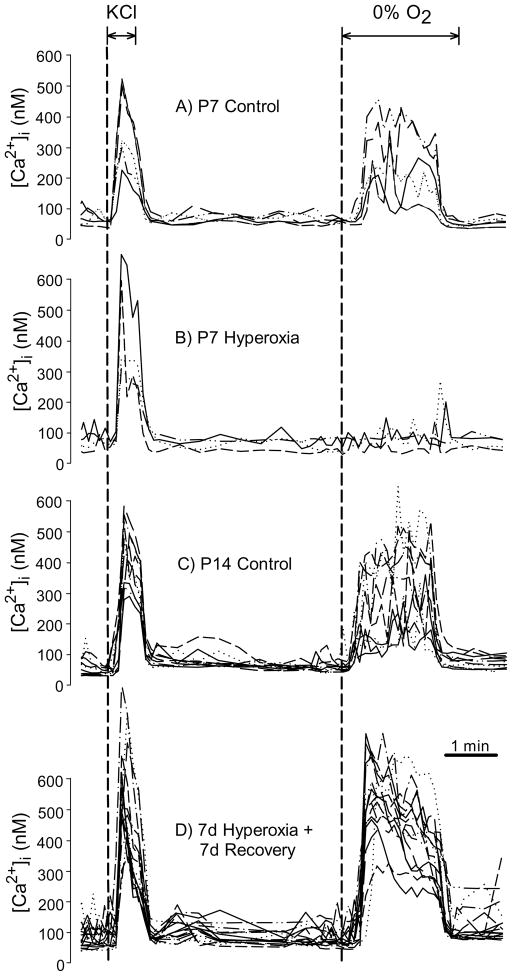
Fig. 3.
Representative glomus cell intracellular calcium ([Ca2+]i) responses to hypoxia for rats reared in 21% O2 (Control) or 60% O2 (Hyperoxia) through P7 (panels A, B) and after 7 d recovery in normoxia (i.e., P14; panels C, D). Each graph panel contains data from several glomus cells plated on a single coverslip, with each line representing serial [Ca2+]i measurements for a single cell during exposure for 30 s to 20mM KCl followed by a 2 min hypoxia challenge (0% O2). Note that glomus cells from P7 Hyperoxia rats remained responsive to KCl (a nonspecific depolarizing stimulus) while the response to hypoxia was markedly reduced.
3.3. Carotid body expression of mRNA for O2-sensitive K+ channels
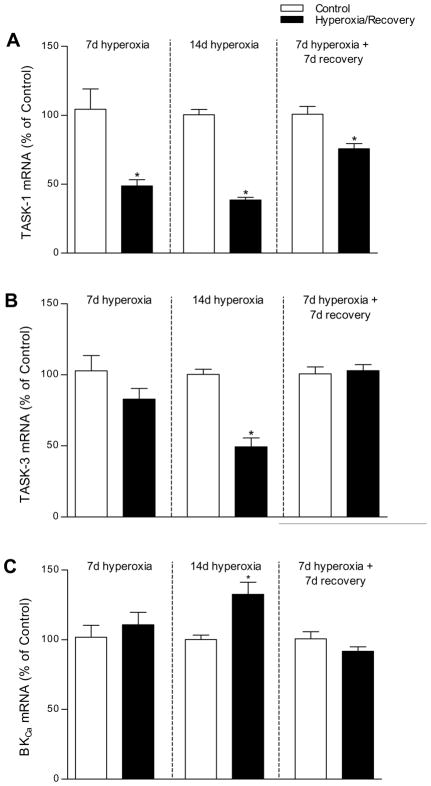
The expression of mRNA for TASK-1, TASK-3, and BKCa potassium channels in the carotid body was influenced by hyperoxia (Fig. 5). TASK-1 transcript levels were significantly reduced at P7 (−51%; P<0.01) and P14 (−61%; P<0.001) in Hyperoxia rat pups exposed to hyperoxia continuously from birth. TASK-3 transcript levels also tended to be reduced in Hyperoxia rats, but this decline was not statistically significant until P14 (−51%; P<0.001). In contrast to TASK mRNA expression, transcript levels for BKCa were significantly increased in P14 Hyperoxia rat pups exposed continuously from birth (+33%; P<0.01). No significant differences in TASK-3 or BKCa mRNA expression were detected between Hyperoxia and Control rats at P7 (P=0.39 and 0.49, respectively).
Fig. 5.
Quantitative RT-PCR analysis of (A) TASK-1, (B) TASK-3, and (C) BKCa mRNA expression (normalized to β-actin) in the carotid bodies of rats exposed to 21% O2 (Control) or 60% O2 (Hyperoxia) from 1 d prior to birth until 7 or 14 d of age, or exposed to hyperoxia for 7 d and allowed to recover in room air for 7 d. Values are mean±SEM; n= 6 for each group, except n=4 for the P14 Hyperoxia group. *P<0.05 vs. Control.
When Hyperoxia rats were returned to room air at P7 and studied after 7 d recovery in room air (i.e., P14 Recovery), TASK-1 mRNA expression tended to return toward the control value (Fig. 5). Although TASK-1 transcript levels were still somewhat reduced compared to P14 Controls (P<0.01), they did not appear to be reduced as much as in P7 Hyperoxia or P14 Hyperoxia rats. Indeed, a one-way ANOVA on normalized mRNA expression for the three groups (i.e., P7 Hyperoxia, P14 Hyperoxia, and P14 Recovery) revealed a significant treatment effect (P<0.001), with a smaller decrease in TASK-1 mRNA expression in the P14 Recovery group (−24%) than in the others (−51% in P7 Hyperoxia and −61% in P14 Hyperoxia; both pairwise comparisons, P<0.05). As was the case for Hyperoxia and Control rats at P7, carotid body expression of TASK-3 and BKCa mRNA was not different between Recovery and Control rats at P14 (P=0.72 and P=0.18, respectively).
4. Discussion
This study revealed that single-unit chemoafferent nerve responses to both moderate and severe hypoxia are diminished after only four days in 60% O2 and remain low during continued hyperoxic exposure. Importantly, hypoxic responses recover rapidly upon return normoxia, with substantial recovery evident after only three days and complete recovery within one week. Based upon intracellular calcium responses to hypoxia, this spontaneous functional recovery of O2 sensitivity occurs at the level of the carotid body glomus cell. The carotid body expression of TASK-1 mRNA tended to vary in parallel with changes in O2 sensitivity, consistent with a potential role for this potassium channel subtype in hyperoxia-induced plasticity. TASK-3 and BKCa mRNA expression also changed as a result of developmental hyperoxia, but these changes occurred over a slower time course (i.e., >7 d).
4.1. Time course for hyperoxia-induced attenuation of chemosensitivity
Young animals often exhibit greater plasticity than their mature counterparts, raising the possibility that hyperoxia might elicit plasticity more easily in the first postnatal week than in the second. We previously studied the time course for changes in carotid body O2 sensitivity in neonatal rats placed into 60% O2 at P7 (Donnelly et al., 2009). In that study, we observed decreased O2 sensitivity after five days of hyperoxia (i.e., P12); no difference was detected after three days of exposure. In the present study, we found that single-unit nerve responses to hypoxia were reduced by P4 in rats placed into hyperoxia approximately one day before birth. Thus, the induction of this plasticity does not occur noticeably faster in the first postnatal week than in the second (approximately 4–5 d in both cases). In the Donnelly et al. (2009) study, however, there was a paradoxical augmentation of the nerve and calcium responses to hypoxia after only one day in 60% O2 (i.e., at P8). No such increase in O2 sensitivity was observed at P1 in the present study, suggesting that the capacity for short periods of hyperoxia to enhance O2 sensitivity increases with postnatal age. This could reflect the relatively immature O2 sensing mechanism in newborn rats (Wasicko et al., 2006) and, consequently, less substrate for enhanced sensitivity at P1 versus P8.
The time course for reduction in carotid chemoreceptor O2 sensitivity during the first postnatal week differs somewhat from changes to the acute hypoxic ventilatory response (HVR) over the same time period. Bavis et al. (2010) measured the HVR of neonatal rats reared in 60% O2. The early, carotid body mediated phase of the HVR (expressed as a percentage increase from baseline) was not statistically different from that of aged-matched controls at P4, while the late phase of the HVR was actually enhanced due to the absence of a secondary ventilatory decline (i.e., no biphasic HVR). To reconcile these observations, it is possible that excitatory plasticity downstream of the chemoafferent neurons compensates for impaired carotid body responses at P4. Hyperoxia has been shown to increase excitability of neurons in the central nervous system (CNS), including those related to the control of breathing (Dean et al., 2004). Continued exposure to hyperoxia ultimately diminishes the HVR by P7 in neonatal rats (Bavis et al., 2010), however, consistent with progressive desensitization of carotid chemoreceptors and/or impairment of chemoafferent neurons.
4.2. Recovery of chemosensitivity following return to normoxia
This is the first study to examine the changes in chemoreceptor function that occur in the period immediately following return to normoxia in hyperoxia-treated rats. Previous studies have assessed chemoreceptor function following a prolonged (2.5–14 mo) return to normoxia. In one study utilizing recordings from the whole CSN, little response to asphyxia or cyanide was observed suggesting a permanent ablation of chemoreceptor function (Fuller et al., 2002). In another, the distribution of chemoreceptor responses appeared bimodal – while some preparations exhibited no response to hypoxia, other preparations had apparently normal hypoxic responses (Prieto-Lloret et al., 2004); however, that study used a mixture of single-unit and pauci-unit recordings, so individual spiking rates were not resolved. Nevertheless, our findings are in general agreement with the latter observation by demonstrating that single-unit chemoafferent activity normalizes following return to normoxia. Moreover, our data reveal that this recovery occurs within the first week of normoxia. Unlike the earlier study (Prieto-Lloret et al., 2004), however, we did not observe a bimodal distribution of chemoafferent responses to hypoxia after recovery in room air: all viable units (as judged by the generation of spontaneous action potentials) responded to hypoxia at P14-15 in our study.
Previous studies have shown that as little as one week exposure to 30–60% O2 is sufficient to attenuate whole-nerve CSN and ventilatory responses to hypoxia into adulthood (Bavis et al., 2002; Bavis et al., 2003; Bisgard et al., 2003). Given the observed recovery of single-unit hypoxic responses, our data suggest that the impaired hypoxic responses observed in adult rats are not caused by decreased sensitivity of individual chemoreceptor cells. Instead, the most parsimonious explanation for long-lasting impairment of the HVR is the permanent reduction in carotid body size and degeneration of chemoafferent neurons in hyperoxia-treated rats (Erickson et al., 1998; Fuller et al., 2002; Wang & Bisgard, 2005).
This finding helps to reconcile two seemingly contradictory observations. In previous studies, phrenic nerve responses to hypoxia were diminished in adult rats exposed to 60% O2 for the first or second postnatal week, but not in adult rats exposed to hyperoxia in the third or fourth postnatal weeks (Bavis et al., 2002) or as adults (Ling et al., 1997b) (i.e., only young rats (≤P14) appeared susceptible to hyperoxia). In contrast, Donnelly et al. (2005) reported that single-unit nerve responses to hypoxia were reduced to similar extents when rats were exposed to 60% O2 at ages P0-P14 or P14-P28; responses were assessed immediately following the hyperoxic exposure. In light of the present study, we propose that chronic hyperoxia reduces carotid body chemoreceptor O2 sensitivity independent of age but that there is a critical period during the first few postnatal weeks in which hyperoxia durably impairs carotid body morphological development. Thus, hyperoxia for 1–2 weeks transiently impairs chemosensitivity regardless of when the exposure is started during the first postnatal month, but carotid body hypoplasia, axonal degeneration, and permanent impairment of HVR only occur when hyperoxia is started in the first or second week of life. Consistent with this model, there is evidence that the first few postnatal weeks comprise a distinct critical period for morphological development of the rat carotid body. For example, carotid chemoafferent neurons initially require trophic support from the carotid body for their postnatal survival, but this dependence is no longer evident after P21 (Hertzberg et al., 1994). Interestingly, carotid body levels of brain-derived neurotrophic factor (BDNF), the molecule implicated in this trophic support, was recently shown to be reduced by chronic hyperoxia (Dmitrieff et al., 2011).
Along with the normalization of single-unit chemoafferent activity levels, there was a correlated return to normal glomus cell calcium response to hypoxia. Previously, it was only determined that the glomus cell calcium response was normal after a 2.5–3.5 month recovery period, and only in a subset of cells (Prieto-Lloret et al., 2004). Thus, for both the onset of hyperoxia-induced attenuation (Donnelly et al., 2009) and normoxia-induced recovery (present study), the nerve response appears to be dependent on the hypoxia-induced increase in glomus cell calcium. However, an understanding of how a calcium increase causes an increase in afferent neuronal activity is elusive and may not be related simply to transmitter release. Although under several experimental conditions there is a good correlation between glomus cell dense-cored granule secretion and nerve activity (as observed by catecholamine release) (Gonzalez et al., 1994), there are clear experimental conditions where the two are dissociated. For instance, carbon monoxide causes both catecholamine release and nerve excitation, but only the nerve excitation is reversed by light (Buerk et al., 1997).
The rapid recovery of glomus cell calcium responses to hypoxia has also been noted upon return to normoxia after chronic postnatal hypoxia. Postnatal hypoxia, like postnatal hyperoxia, diminishes carotid body responses to hypoxia, and this appears to reflect delayed resetting of glomus cell O2 sensitivity following birth (Bavis, 2005). Sterni et al. (1999) showed that glomus cell calcium responses to hypoxia were abolished in chronically hypoxic rat pups at 3, 11 and 18 days of age. However, if the carotid body was harvested from P18 pups that had been returned to normoxia one week earlier (i.e., at P11), hypoxic chemosensitivity had largely returned. Taken together, these data indicate that glomus cells retain their inherent capacity to adapt to prevailing O2 levels despite prolonged hyperoxic or hypoxic challenges. Interestingly, phrenic nerve responses to acute hypoxia can be restored in hyperoxia-treated rats, at least transiently, by exposing them to chronic sustained hypoxia or chronic intermittent hypoxia as adults (Fuller et al., 2001). This induced recovery likely reflects plasticity in the residual carotid body (i.e., increased O2 sensitivity of remaining glomus cells) and CNS (i.e., increased gain for integration of chemoafferent input) identical to that which normally accompanies ventilatory acclimatization to hypoxia in adult mammals (Powell, 2007).
4.3. Potential role of O2-sensitive K+ channels in hyperoxia-induced plasticity
The cellular mechanisms underlying hyperoxia-induced changes in glomus cell O2 sensitivity are not yet known, but it is possible that hyperoxia reversibly alters the expression of genes directly linked to the transduction of hypoxic stimuli. We observed substantial changes in mRNA expression for TASK-1, TASK-3, and BKCa, the putative O2-sensitive K+ channels in the rat carotid body (Gonzalez et al., 2009; Peers et al., 2010). These data must be interpreted cautiously since changes in mRNA expression may not lead to changes in protein expression and since mRNA expression was measured for the whole carotid body (versus only glomus cells). However, these findings are consistent with the hypothesis that hyperoxia influences chemoreceptor O2 sensitivity by altering O2 transduction within the glomus cells. The mRNA expression for all three of these K+ channel subtypes changed after 14 days of hyperoxia, but only TASK-1 was significantly downregulated by seven days of hyperoxia. Since both nerve and calcium responses to hypoxia were reduced by seven days of hyperoxia, this could indicate a relatively greater role for TASK-1 (and/or TASK-1/3 heteromers) in hyperoxia-induced plasticity. This relationship is further supported by the correlated recovery of O2 sensitivity and TASK-1 mRNA expression upon return to room air. While genetic deletion of TASK channels has produced mixed results on mouse carotid body function and HVR (Mulkey et al., 2007; Trapp et al., 2008; Ortega-Sáenz et al., 2010), there is strong evidence that TASK-1/3 heteromers are major determinants of glomus cell O2 sensitivity in neonatal rats (Kim et al., 2009). Decreases in TASK-3 and/or increases in BKCa mRNA expression presumably would only come into play during longer hyperoxic exposures.
4.4. Significance
It is increasingly clear that respiratory control development is sensitive to perinatal oxygenation (Carroll, 2003; Bavis, 2005; Cayetanot et al., 2009; Gauda et al., 2009), and the rapid rise in O2 at birth may be a critical stimulus for normal postnatal maturation. A complete understanding of these processes may be important for infants that experience abnormal O2 profiles as a result of premature birth, cardiorespiratory disease, and/or clinical O2 therapy. The present study confirms that moderate hyperoxia reduces carotid body O2 sensitivity in as little as four days but also reveals that, unlike concurrent changes to carotid body morphology, this intrinsic chemosensitivity recovers upon return to room air. Thus, some effects of hyperoxia may be overlooked in studies focusing on changes to the adult respiratory phenotype. More broadly, the present study highlights the potential use of hyperoxia to reversibly manipulate glomus cell O2 sensitivity. Consequently, developmental hyperoxia may serve as a valuable tool to probe O2 sensing pathways in the carotid body.
Acknowledgments
This study was supported by National Institutes of Health (NIH) grants R15 HL-083972 and P20 RR-016463 (R.W.B.), R01 HL-054621 (J.L.C.), and R01 HL-084520 (D.F.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bavis RW. Developmental plasticity of the hypoxic ventilatory response after perinatal hyperoxia and hypoxia. Respir Physiol Neurobiol. 2005;149:287–299. doi: 10.1016/j.resp.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Olson EB, Jr, Mitchell GS. Critical developmental period for hyperoxia-induced blunting of hypoxic phrenic responses in rats. J Appl Physiol. 2002;92:1013–1018. doi: 10.1152/japplphysiol.00859.2001. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Olson EB, Jr, Vidruk EH, Bisgard GE, Mitchell GS. Level and duration of developmental hyperoxia influence impairment of hypoxic phrenic responses in rats. J Appl Physiol. 2003;95:1550–1559. doi: 10.1152/japplphysiol.01043.2002. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Young KM, Barry KJ, Boller MR, Kim E, Klein PM, Ovrutsky AR, Rampersad DA. Chronic hyperoxia alters the early and late phases of the hypoxic ventilatory response in neonatal rats. J Appl Physiol. 2010;109:796–803. doi: 10.1152/japplphysiol.00510.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgard GE, Olson EB, Jr, Wang ZY, Bavis RW, Fuller DD, Mitchell GS. Adult carotid chemoafferent responses to hypoxia after 1, 2, and 4 wk of postnatal hyperoxia. J Appl Physiol. 2003;95:946–952. doi: 10.1152/japplphysiol.00985.2002. [DOI] [PubMed] [Google Scholar]
- Buerk DG, Chugh DK, Osanai S, Mokashi A, Lahiri S. Dopamine increases in cat carotid body during excitation by carbon monoxide: implications for a chromophore theory of chemoreception. J Auton Nerv Syst. 1997;67:130–136. doi: 10.1016/s0165-1838(97)00098-2. [DOI] [PubMed] [Google Scholar]
- Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol. 2003;94:375–389. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- Cayetanot F, Larnicol N, Peyronnet J. Antenatal environmental stress and maturation of the breathing control, experimental data. Respir Physiol Neurobiol. 2009;168:92–100. doi: 10.1016/j.resp.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Dean JB, Mulkey DK, Henderson RA, III, Potter SJ, Putnam RW. Hyperoxia, reactive oxygen species, and hyperventilation: oxygen sensitivity of brainstem neurons. J Appl Physiol. 2004;96:784–791. doi: 10.1152/japplphysiol.00892.2003. [DOI] [PubMed] [Google Scholar]
- Dmitrieff EF, Wilson JT, Dunmire KB, Bavis RW. Chronic hyperoxia alters the expression of neurotrophic factors in the carotid body of neonatal rats. Respir Physiol Neurobiol. 2011;175:220–227. doi: 10.1016/j.resp.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DF, Kim I, Carle C, Carroll JL. Perinatal hyperoxia for 14 days increases nerve conduction time and the acute unitary response to hypoxia of rat carotid body chemoreceptors. J Appl Physiol. 2005;99:114–119. doi: 10.1152/japplphysiol.01009.2004. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Bavis RW, Kim I, Dbouk HA, Carroll JL. Time course of alterations in pre- and post-synaptic chemoreceptor function during developmental hyperoxia. Respir Physiol Neurobiol. 2009;168:189–197. doi: 10.1016/j.resp.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Mayer C, Jawa A, Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS, Katz DM. Chemoafferent degeneration and carotid body hypoplasia following chronic hyperoxia in newborn rats. J Physiol. 1998;509:519–526. doi: 10.1111/j.1469-7793.1998.519bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Wang ZY, Ling L, Olson EB, Jr, Bisgard GE, Mitchell GS. Induced recovery of hypoxic phrenic responses in adult rats exposed to hyperoxia for the first month of life. J Physiol. 2001;536:917–926. doi: 10.1111/j.1469-7793.2001.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bavis RW, Vidruk EH, Wang ZY, Olson EB, Jr, Bisgard GE, Mitchell GS. Life-long impairment of hypoxic phrenic responses in rats following 1 month of developmental hyperoxia. J Physiol. 2002;538:947–955. doi: 10.1113/jphysiol.2001.012908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauda EB, Carroll JL, Donnelly DF. Developmental maturation of chemosensitivity to hypoxia of peripheral arterial chemoreceptors--invited article. Adv Exp Med Biol. 2009;648:243–255. doi: 10.1007/978-90-481-2259-2_28. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Vaquero LM, López-López JR, Pérez-García MT. Oxygen-sensitive potassium channels in chemoreceptor cell physiology: making a virtue of necessity. Ann NY Acad Sci. 2009;1177:82–88. doi: 10.1111/j.1749-6632.2009.05037.x. [DOI] [PubMed] [Google Scholar]
- Hatton CJ, Carpenter E, Pepper DR, Kumar P, Peers C. Developmental changes in isolated rat type I carotid body cell K+ currents and their modulation by hypoxia. J Physiol. 1997;501:49–58. doi: 10.1111/j.1469-7793.1997.049bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg T, Fan G, Finley JCW, Erickson JT, Katz DM. BDNF supports mammalian chemoafferent neurons in vitro and following peripheral target removal in vivo. Dev Biol. 1994;166:801–811. doi: 10.1006/dbio.1994.1358. [DOI] [PubMed] [Google Scholar]
- Kim D, Cavanaugh EJ, Kim I, Carroll JL. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J Physiol. 2009;587:2963–2975. doi: 10.1113/jphysiol.2009.171181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Donnelly DF, Carroll JL. Modulation of gene expression in subfamilies of TASK K+ channels by chronic hyperoxia exposure in rat carotid body. Adv Exp Med Biol. 2006;580:37–41. doi: 10.1007/0-387-31311-7_6. [DOI] [PubMed] [Google Scholar]
- Kim I, Boyle KM, Carle CM, Donnelly DF, Carroll JL. Perinatal hyperoxia reduces the depolarization and calcium increase in response to an acute hypoxia challenge in rat carotid chemorecepor cells. FASEB J. 2003;17:LB128. (Abstract) [Google Scholar]
- Kumar P. Sensing hypoxia in the carotid body: from stimulus to response. Essays Biochem. 2007;43:43–60. doi: 10.1042/BSE0430043. [DOI] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. J Physiol. 1996;495:561–571. doi: 10.1113/jphysiol.1996.sp021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Developmental plasticity of the hypoxic ventilatory response. Respir Physiol. 1997a;110:261–268. doi: 10.1016/s0034-5687(97)00091-1. [DOI] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Phrenic responses to isocapnic hypoxia in adult rats following perinatal hyperoxia. Respir Physiol. 1997b;109:107–116. doi: 10.1016/s0034-5687(97)00045-5. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- López-Barneo J, Ortega-Sáenz P, Pardal R, Pascual A, Piruat JI. Carotid body oxygen sensing. Eur Respir J. 2008;32:1386–1398. doi: 10.1183/09031936.00056408. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Sáenz P, Levitsky KL, Marcos-Almaraz MT, Bonilla-Henao V, Pascual A, López-Barneo J. Carotid body chemosensory responses in mice deficient of TASK channels. J Gen Physiol. 2010;135:379–392. doi: 10.1085/jgp.200910302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C, Wyatt CN, Evans AM. Mechanisms for acute oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2010;174:292–298. doi: 10.1016/j.resp.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA. 2010;107:10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL. The influence of chronic hypoxia upon chemoreception. Respir Physiol Neurobiol. 2007;157:154–161. doi: 10.1016/j.resp.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Lloret J, Caceres AI, Obeso A, Rocher A, Rigual R, Agapito MT, Bustamante R, Castañeda J, Perez-Garcia MT, López-López JR, González C. Ventilatory responses and carotid body function in adult rats perinatally exposed to hyperoxia. J Physiol. 2004;554:126–144. doi: 10.1113/jphysiol.2003.049445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterni LM, Bamford OS, Wasicko MJ, Carroll JL. Chronic hypoxia abolished the postnatal increase in carotid body type I cell sensitivity to hypoxia. Am J Physiol. 1999;277:L645–L652. doi: 10.1152/ajplung.1999.277.3.L645. [DOI] [PubMed] [Google Scholar]
- Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28:8844–8850. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Bisgard GE. Postnatal growth of the carotid body. Respir Physiol Neurobiol. 2005;149:181–190. doi: 10.1016/j.resp.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Wasicko MJ, Breitwieser GE, Kim I, Carroll JL. Postnatal development of carotid body glomus cell response to hypoxia. Respir Physiol Neurobiol. 2006;154:356–371. doi: 10.1016/j.resp.2006.01.003. [DOI] [PubMed] [Google Scholar]