Abstract
Evidence regarding the health benefits of carotenoids is controversial. Effects of serum carotenoids and their interactions on mortality have not been examined in a representative sample of US adults. The objective was to examine whether serum carotenoid concentrations predict mortality among US adults. The study consisted of adults aged ≥20 years enrolled in the National Health and Nutrition Examination Survey (NHANES) III, 1988–1994, with measured serum carotenoids and mortality follow-up through 2006 (N=13,293). Outcomes were all-cause, cardiovascular disease (CVD), and cancer mortality. In adjusted Cox proportional hazards models, participants in the lowest total carotenoid quartile (<1.01µmol/L) had significantly higher all-cause mortality (mortality rate ratio=1.38; 95% confidence interval:1.15—1.65; P=0.005) than those in the highest total carotenoid quartile (>1.75µmol/L). For alpha-carotene, the highest quartile (>0.11µmol/L) had the lowest all-cause mortality rates (P<0.001). For lycopene, the middle two quartiles (0.29–0.58µmol/L) had the lowest all-cause mortality rates (P=0.047). Analyses with continuous carotenoids confirmed associations of serum total carotenoids, alpha-carotene, and lycopene with all-cause mortality (P<0.001). In a random survival forest analysis, very low lycopene was the carotenoid most strongly predictive of all-cause mortality, followed by very low total carotenoids. Alpha-carotene/beta-cryptoxanthin, alpha-carotene/lutein+zeaxanthin and lycopene/lutein+zeaxanthin interactions were significantly related to all-cause mortality (P<0.05). Low alpha-carotene was the only carotenoid associated with CVD mortality (P=0.002). No carotenoids were significantly associated with cancer mortality. Very low serum total carotenoid, alpha-carotene, and lycopene concentrations may be risk factors for mortality, but carotenoids show interaction effects on mortality. Interventions of balanced carotenoid combinations are needed for confirmation.
Keywords: alpha-carotene, beta-carotene, beta-cryptoxanthin, carotenoids, human subjects, lycopene, lutein, mortality, NHANES, zeaxanthin
1. INTRODUCTION
Diets rich in fruits and vegetables are associated with lower morbidity from chronic diseases and greater longevity[1–5]. Serum carotenoids, the best biomarker for fruit and vegetable consumption[6], may play a role in the health benefits of a plant-rich diet[3]. Carotenoids are best known for antioxidant activities including quenching free radicals, reducing damage from reactive oxidant species, and inhibiting lipid peroxidation. Carotenoids also facilitate cell-to-cell communication which regulates cell growth, differentiation, and apoptosis; and some carotenoids convert to vitamin A[3].
The most abundant carotenoids in human serum are alpha-carotene, beta-carotene, beta-cryptoxanthin, lycopene, lutein, and zeaxanthin. Alpha-carotene and beta-carotene are found in green leafy vegetables and in orange and yellow fruits and vegetables (e.g., carrots, pumpkin, collard greens). The main source of beta-cryptoxanthin is orange and red fruits and vegetables (e.g., pumpkin, papayas, red bell pepper). Lutein and zeaxanthin are also found in green leafy vegetables (e.g., spinach, kale, turnip greens) as well as egg yolk. Tomato products are the primary source of lycopene in the US [3].
Studies examining carotenoid health effects have produced inconsistent findings. Observational studies have shown that individuals with higher carotenoid intake or serum concentrations have lower risk of mortality[7–9], lung cancer[10], prostate cancer[11], and coronary heart disease[12]. In contrast, many interventions of carotenoid supplementation, specifically with beta-carotene, have produced harmful effects (notably among smokers) or no effects[13–16].
Possible reasons for inconsistencies between observational and intervention studies include differences in study design, differences in participant populations, differences in carotenoid concentrations, and carotenoid interactions. Results from observational studies may be confounded, whereas results from randomized intervention studies may be correct. Participants in observational studies with high carotenoid concentrations may have healthy lifestyle factors that have not been fully taken into account, and participants in some intervention studies may not resemble the general population due to increased prevalence of risk factors (e.g., smoking)[3, 17]. Also, interventions have often focused on high-dose supplements of a single carotenoid rather than carotenoid consumption similar to healthy individuals in observational studies. Specifically, interventions have tested beta-carotene supplementation at levels of ≥20 mg/day, much higher than the reported average beta-carotene intakes of 2.5 and 2.9 mg/day for US women and men, respectively[18]. At high concentrations, carotenoids may produce prooxidative breakdown products, which may explain the harmful effects of beta-carotene interventions previously found in smokers[13, 15, 16]. Further, excessive concentrations of one carotenoid can interfere with absorption or bioavailability of others, creating a carotenoid imbalance, such as that shown in basic science experiments between beta-carotene and lycopene[3, 17, 19, 20]. Finally, the effectiveness of individual carotenoids may depend on concentrations of other carotenoids. Carotenoid mixtures appear to have greater antioxidant activity than the sum of individual carotenoids, suggesting that carotenoids may interact synergistically[3, 17] and supplementation with a single carotenoid may be ineffective.
Whether carotenoid concentrations and their interactions relate to mortality has not been examined in the general US adult population. An understanding of the health effects of carotenoid combinations can help inform nutritional interventions and policies. Therefore, this study aims to characterize how serum carotenoid concentrations relate to all-cause and cause-specific mortality among US adults using data from the Third National Health and Nutrition Examination Survey (NHANES III), a nationally representative sample. Our primary hypothesis is that lower concentrations of total carotenoids (sum of alpha-carotene, beta-carotene, beta-cryptoxanthin, lycopene, lutein, and zeaxanthin) are associated with higher mortality risk. Our secondary and tertiary hypotheses are, respectively, that lower concentrations of individual carotenoids have higher mortality risk and that carotenoids interact synergistically. For all hypotheses, all-cause mortality is the primary endpoint; cardiovascular disease (CVD) and cancer mortality are secondary endpoints.
2. METHODS AND MATERIALS
2.1 Study participants
NHANES III is a multi-stage probability survey conducted during 1988–1994 by the National Center for Health Statistics[21]. It was designed to represent non-institutionalized American civilians, and it oversampled Non-Hispanic blacks, Mexican Americans, and older adults[22]. Analyses here only included adults aged ≥20 years eligible for vital status follow-up. Out of 33,994 NHANES III participants, 15,166 were aged<20 years, 28 lacked data for determining vital status, 2,847 had missing carotenoid data, and 2,660 others had incomplete covariates, leaving 13,293 participants included in analyses. The University of Maryland School of Medicine Institutional Review Board determined the secondary analysis of this de-identified dataset to be exempt.
2.2 Study variables
NHANES III survey and data collection procedures are described elsewhere[21]. Briefly, NHANES was an observational study that included an interview, physical examination, and blood draw (regardless of fasting status). Isocratic high-performance liquid chromatography (HPLC)-based methods measured serum alpha-carotene, beta-carotene, beta-cryptoxanthin, lycopene, and lutein+zeaxanthin (Waters HPLC System; Millford, Massachusetts). These methods do not discriminate lutein from zeaxanthin, thus we used the combined concentration, lutein+zeaxanthin, in analyses. Median interassay coefficients of variation (CVs) were 9.4% for alpha-carotene, 7.0% for beta-carotene, 8.7% for beta-cryptoxanthin, 7.7% for lycopene, and 11.0% for lutein+zeaxanthin[23]. Latex-enhanced nephelometry (Behring Nephelometer Analyzer system; Behring Diagnostics, Westwood, Massachusetts) measured serum C-reactive protein (CRP) with median CV 6.3%[24]. Most participants had undetectable CRP concentrations (<0.22 mg/dL), thus CRP was dichotomized at this concentration in statistical analyses. Serum total and high-density lipoprotein (HDL) cholesterol were measured enzymatically (Hitachi 704 Analyzer; Boehringer Mannheim Diagnostics, Indianapolis, Indiana). Cotinine, a nicotine metabolite, was measured using an enzyme immunoassay screen (STC, Inc., Bethlehem, Pennsylvania) with liquid chromatography-tandem mass spectrometry confirmation (SCIEX, PerkinElmer, Wellesley, Massachusetts).
Body mass index (BMI, kg/m2) was calculated using measured height and weight. Diastolic (DBP) and systolic blood pressures (SBP) were averages of up to three measurements obtained at the household interview or mobile examination center. We categorized physical activity level as low (≤3.5 metabolic equivalent tasks [METs]), moderate (3.6–14.9 METs), or high (≥15 METs) according to self-reported participation in activities in the previous month[25]. Alcohol consumption was measured using self-reported number of alcoholic drinks over the previous 30 days. We classified responses of <30, 30–60, and >60 drinks, respectively, as <1, 1–2, and >2 drinks/day. Participants self-reported age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), marital status (married/not married), education (high-school graduate/ non-high school graduate), smoking status (current smoker/non-current smoker), physician diagnosis of comorbidities (congestive heart failure, cancer, diabetes, emphysema, stroke), use of multivitamin/multimineral supplements within the last month and antihypertensive and cholesterol-lowering medications.
2.3 Causes of death
This analysis used the 2010 public-release NHANES III mortality file. Mortality was obtained through December 31, 2006 by probabilistic matching to National Death Index records using 12 identifying variables. Matching was validated via manual review of a subsample of death certificates. Cause of death was classified using the International Classification of Diseases, Ninth Revision, Clinical Modification [26] for deaths during 1988–1998, and using the International Statistical Classification of Diseases, 10th Revision (ICD-10)[27] for deaths during 1999–2006. Deaths during 1988–1998 were recoded based on ICD-10 causes[26]. We categorized deaths caused by CVD and cancer. CVD mortality included deaths attributed to hypertensive disease (codes I10–I13), ischemic heart disease (codes I20–I25), pericardial disease and acute myocarditis (codes I30–31, I40), heart failure (code I50), other heart disease (codes I26–I28, I34–I38, I42–I49, I51), cerebrovascular disease (codes I60–I69), or atherosclerosis/other arterial disease (codes I70–I78). Cancer mortality included deaths attributed to malignant neoplasms (codes C00–C95) including malignant neoplasms of digestive organs (codes C15–C26), respiratory and intrathoracic organs (codes C30–39), and genital organs (codes C50–C63).
2.4 Statistical analyses
To accommodate the complex survey design, we used the survey package of R software version 2.10.0[28]. We calculated standard errors using linearization to account for stratification and clustering. Participant characteristics were examined by total carotenoid quartiles.
Primary analyses used weighted Cox proportional hazards models to assess associations of serum carotenoid concentrations with mortality[29, 30]. Cause-specific mortality rate (hazard) ratios were calculated by censoring deaths attributed to other causes[31]. We first performed analyses for total and individual carotenoids using carotenoid quartiles. P-values resulted from testing the null hypothesis that all four carotenoid quartiles have equal mortality rates. Additional analyses modeled carotenoids as continuous variables with all-cause mortality using restricted cubic splines to address potential nonlinearity. We chose number of degrees of freedom for each carotenoid to minimize Akaike’s information criterion. Three models assessed associations of serum total and individual carotenoids with mortality. First, we fit a model adjusted for age and sex, followed by a partially-adjusted model that additionally included demographics (race/ethnicity, marital status, and education), lifestyle behaviors (alcohol consumption, smoking, multivitamin/multimineral use, and physical activity), and BMI. Finally, we fit a fully-adjusted model that additionally included biomarkers (DBP, SBP, total and HDL cholesterol, and CRP), antihypertensive and cholesterol-lowering medications, and comorbidities (congestive heart failure, cancer, diabetes, emphysema, and stroke). We excluded comorbidities and biomarkers from partially adjusted models because they may be in the causal pathway between carotenoids and mortality. Fully- and partially-adjusted models for individual carotenoids also included other individual carotenoids. We chose the specific demographic and lifestyle variables because they relate to carotenoid concentrations, and the specific biomarkers because they relate to comorbidities associated with low carotenoids and low fruit and vegetable intake[32]. To examine carotenoid interactions, we refit the fully-adjusted all-cause mortality model by including two-way interaction terms between quartile indicators for carotenoid pairs. All p-values are two-sided, and results are unadjusted for multiple comparisons. P<0.05 was considered statistically significant.
Next, we performed a secondary analysis using random survival forests (RSF) to validate our findings and quantify the relative importance of total and individual carotenoids in predicting all-cause mortality[33, 34]. The RSF was an ensemble of 500 survival trees, where each tree was grown via recursive partitioning to identify the optimal node splits for predicting mortality (defined as splits that maximize the logrank statistic comparing survival between two prospective nodes). Candidate predictors for each tree included continuous individual and total carotenoids and all covariates from fully-adjusted models. Random aspects of RSF were 1) randomly drawing separate bootstrap samples to grow each tree and 2) randomly selecting a subset of 5 predictors as candidates to split the nodes. This process ensures independence between trees[33, 34]. We used relative importance to compare predictive strength between variables, which was quantified as the relative increase in ensemble prediction error from excluding competing variables to predict mortality. Ensemble prediction error equaled 1 – concordance (C) index[33, 34], which was calculated using averaged predicted mortality for each participant using trees for which that participant was not selected into the bootstrap sample. This machine-learning approach has the advantages of 1) head-to-head comparisons of predictive importance between individual and total carotenoids, 2) adjustment for potential multi-way interactions in estimating variable importance and mortality, and 3) virtually no modeling assumptions[33, 34]. The randomSurvivalForest package in R was used for RSF analysis[34].
Lastly, sensitivity analyses examined robustness of results from primary analyses. To address missing covariate data, we modeled weighted estimating equations (WEE) using survey weights multiplied by the inverse probability of being observed[30, 35]. We estimated the probability using weighted logistic regression of the indicator for having complete data on age, sex, and race/ethnicity among eligible participants. Survey weights addressed total study refusal, but WEE addressed potential selection bias from item nonresponse. Additional models excluded participants taking beta-carotene, retinol, or lutein supplements (3,073 participants) and included log serum cotinine to improve adjustment for smoke exposure. An additional 236 participants were excluded due to missing cotinine, leaving 9,984 participants for sensitivity analyses.
3. RESULTS
3.1 Participant characteristics and carotenoid concentrations
Table 1 shows baseline participant characteristics, where those with higher total carotenoid concentrations were more often older, female, married, high-school graduates, physically active, and multivitamin/multimineral users (P<0.001). Participants with lower total carotenoid concentrations were more likely to be current smokers, consume more than two drinks per day, and have detectable CRP, higher BMI, and lower total and HDL cholesterol concentrations (P<0.001).
Table 1.
Baseline Characteristics of Participants by Quartiles of Total Carotenoids,1 Third National Health and Nutrition Examination Survey, 1988–1994
| Total Carotenoids2 Quartile (µmol/L) | |||||
|---|---|---|---|---|---|
| Characteristic | Quartile 1 <1.01 (N=3,567) |
Quartile 2 1.01–1.32 (N=3,110) |
Quartile 3 1.33–1.75 (N=3,300) |
Quartile 4 >1.75 (N=3,316) |
P3 |
| Age (Years), mean | 42.1 (0.5) | 40.2 (0.5) | 42.7 (0.5) | 47.0 (0.7) | <0.001 |
| Female, % | 48.7 (1.5) | 48.3 (1.2) | 52.1 (1.5) | 56.1 (1.1) | <0.001 |
| Race/Ethnicity, % | |||||
| Non-Hispanic White | 77.4 (1.8) | 78.4 (1.3) | 76.2 (1.7) | 74.2 (1.8) | |
| Non-Hispanic Black | 11.3 (1.0) | 10.2 (0.7) | 9.6 (0.7) | 10.0 (0.7) | 0.01 |
| Mexican American | 8.7 (1.2) | 8.6 (0.8) | 10.5 (1.1) | 10.3 (1.2) | |
| Other | 2.6 (0.5) | 2.8 (0.7) | 3.6 (0.8) | 5.5 (1.0) | |
| Married, % | 56.9 (1.8) | 61.3 (1.5) | 66.4 (1.4) | 65.0 (1.8) | <0.001 |
| High School Graduate, % | 69.0 (1.3) | 77.5 (1.2) | 80.8 (1.3) | 81.6 (1.2) | <0.001 |
| Alcohol Consumption, % | |||||
| <1 Drinks/Day | 86.4 (1.1) | 89.6 (0.9) | 91.3 (0.7) | 90.1 (1.1) | |
| 1–2 Drinks/Day | 10.2 (1.0) | 8.0 (0.8) | 7.3 (0.6) | 8.8 (1.1) | <0.001 |
| >2 Drinks/Day | 3.5 (0.7) | 2.3 (0.4) | 1.4 (0.3) | 1.0 (0.3) | |
| Current Smoker, % | 43.2 (1.7) | 34.9 (1.3) | 24.2 (1.5) | 12.9 (1.1) | <0.001 |
| Multivitamin/Multimineral Use, % | 23.0 (1.4) | 30.9 (1.1) | 32.5 (1.4) | 41.6 (1.6) | <0.001 |
| Physical Activity Level, % | |||||
| High | 15.6 (1.2) | 21.7 (1.4) | 24.8 (1.4) | 27.5 (1.8) | |
| Moderate | 52.7 (1.7) | 54.5 (1.4) | 53.8 (1.3) | 51.3 (1.7) | <0.001 |
| Low | 31.7 (1.5) | 23.8 (1.5) | 21.4 (1.3) | 21.1 (1.4) | |
| Body Mass Index (kg/m2), mean | 27.7 (0.2) | 26.3 (0.1) | 25.9 (0.1) | 25.4 (0.1) | <0.001 |
| Diastolic Blood Pressure (mmHg), mean | 74.5 (0.3) | 74.3 (0.3) | 73.6 (0.3) | 73.9 (0.2) | 0.17 |
| Systolic Blood Pressure (mmHg), mean | 122.7 (0.5) | 120.4 (0.5) | 119.9 (0.5) | 121.8 (0.6) | <0.001 |
| Total Cholesterol (mg/dL), mean | 186.7 (1.5) | 195.7 (1.3) | 206.4 (1.1) | 221.0 (1.4) | <0.001 |
| HDL Cholesterol (mg/dL), mean | 47.8 (0.4) | 49.4 (0.4) | 51.0 (0.5) | 55.1 (0.6) | <0.001 |
| C-Reactive Protein ≥ 0.22 mg/dL, % | 36.7 (1.8) | 26.4 (1.5) | 23.2 (1.2) | 21.9 (1.2) | <0.001 |
| Antihypertensive Medications Use, % | 11.1 (0.9) | 8.7 (0.8) | 8.9 (0.7) | 12.3 (0.9) | 0.001 |
| Cholesterol-Lowering Medications Use, % | 2.3 (0.4) | 1.6 (0.4) | 2.8 (0.4) | 2.8 (0.4) | 0.15 |
| Congestive Heart Failure, % | 1.8 (0.3) | 1.4 (0.3) | 1.5 (0.3) | 1.5 (0.2) | 0.85 |
| Cancer, % | 5.5 (0.6) | 7.2 (0.8) | 6.4 (0.5) | 8.4 (0.6) | 0.005 |
| Diabetes, % | 5.1 (0.5) | 3.6 (0.4) | 4.5 (0.5) | 3.7 (0.4) | 0.03 |
| Emphysema, % | 2.6 (0.4) | 1.9 (0.4) | 1.0 (0.3) | 1.4 (0.3) | 0.04 |
| Stroke, % | 1.8 (0.3) | 1.4 (0.3) | 1.3 (0.2) | 1.6 (0.3) | 0.49 |
Data are given as mean (standard error) or % (standard error)
Total carotenoids: sum of alpha-carotene, beta-carotene, beta-cryptoxanthin, lycopene, lutein+zeaxanthin
P-values calculated using survey-weighted analysis of variance for continuous variables and Wald tests for categorical variables [30]
Older participants had higher concentrations of all individual carotenoids except lycopene, which was higher among younger adults (P<0.001) (results not shown). Provitamin A carotenoids (alpha-carotene, beta-carotene, beta-cryptoxanthin) were higher among women whereas lycopene was higher among men (P<0.001). All carotenoids were lowest among current smokers (P<0.001) except lycopene, where there was no association (P=0.97). Lycopene was lower among participants with chronic comorbidities (P<0.01). Participants with BMIs <18.5 kg/m2 had the lowest lycopene concentrations, whereas participants with BMIs ≥30.0 kg/m2 had the lowest concentrations of other carotenoids (P<0.001). Except for lutein+zeaxanthin, higher physical activity was associated with higher carotenoid concentrations (P<0.05). Participants with higher total (P<0.001) and HDL cholesterol (P<0.05) and undetectable CRP (P<0.01) had higher carotenoid concentrations.
3.2 Total carotenoid quartiles and mortality
There were 2,933 deaths over a median follow-up time of 14.3 years (interquartile range 12.7–16.1 years). CVD caused 1264 (43%) deaths, and cancer caused 645 (22%) deaths.
After adjusting for age and sex, the mortality rate ratio (MRR) for the lowest total carotenoid group was 1.83 times higher (95% confidence interval [CI]=1.54—2.19; P<0.001) than that for the highest total carotenoid group; the MRR after full covariate adjustment was attenuated to 1.38 (95% CI=1.15—1.65; P=0.005) (Table 2). Age- and sex-adjusted CVD mortality rates differed by total carotenoids (MRR=1.43; 95% CI=0.89—1.50; P=0.03), but fully-adjusted CVD mortality rates did not (P=0.53). Age- and sex-adjusted cancer mortality rates were 1.82 times higher in the lowest versus highest carotenoid group (95% CI=1.35—2.46; P<0.001), but were not significant after full covariate adjustment (MRR=1.32; 95% CI=0.97—1.80; P=0.13).
Table 2.
Rate Ratios of All-Cause and Cause-Specific Mortality by Total Carotenoid Quartiles, Third National Health and Nutrition Examination Survey, 1988–1994
| Total Carotenoids1 Quartile | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 (<1.01 µmol/L) |
Quartile 2 (1.01–1.32 µmol/L) |
Quartile 3 (1.33–1.75 µmol/L) |
Quartile 4 (>1.75 µmol/L) |
P3 | |||||
| All-cause Mortality | MRR2 | 95% CI2 | MRR | 95% CI | MRR | 95% CI | MRR | 95% CI | |
| Age- and Sex-Adjusted | 1.83 | 1.54—2.19 | 1.26 | 1.11—1.42 | 1.10 | 0.96—1.26 | 1.00 | Reference | <0.001 |
| Partially Adjusted4 | 1.43 | 1.21—1.70 | 1.09 | 0.96—1.24 | 1.03 | 0.90—1.17 | 1.00 | Reference | <0.001 |
| Fully Adjusted5 | 1.38 | 1.15—1.65 | 1.07 | 0.93—1.23 | 1.04 | 0.91—1.18 | 1.00 | Reference | 0.005 |
| CVD Mortality | |||||||||
| Age- and Sex-Adjusted | 1.43 | 1.11—1.85 | 1.13 | 0.92—1.39 | 1.02 | 0.85—1.22 | 1.00 | Reference | 0.03 |
| Partially Adjusted4 | 1.08 | 0.85—1.39 | 0.96 | 0.77—1.20 | 0.93 | 0.77—1.12 | 1.00 | Reference | 0.42 |
| Fully Adjusted5 | 1.16 | 0.89—1.50 | 1.01 | 0.82—1.25 | 1.00 | 0.82—1.22 | 1.00 | Reference | 0.53 |
| Cancer Mortality | |||||||||
| Age- and Sex-Adjusted | 1.82 | 1.35—2.46 | 1.27 | 0.85—1.89 | 1.06 | 0.74—1.51 | 1.00 | Reference | <0.001 |
| Partially Adjusted4 | 1.43 | 1.05—1.96 | 1.09 | 0.72—1.63 | 0.97 | 0.68—1.40 | 1.00 | Reference | 0.04 |
| Fully Adjusted5 | 1.32 | 0.97—1.80 | 1.02 | 0.67—1.55 | 0.94 | 0.66—1.33 | 1.00 | Reference | 0.13 |
Total carotenoids: sum of alpha-carotene, beta-carotene, beta-cryptoxanthin, lycopene, lutein+zeaxanthin
MRR, mortality rate ratio; 95% CI, 95% confidence interval
Based on testing the null hypothesis that all four quartiles have equal mortality rates using survey-weighted Cox models [31].
Adjusted for demographics (age, sex, race/ethnicity, marital status, education), lifestyle behaviors (alcohol consumption, smoking status, multivitamin/multimineral use, physical activity) and body mass index
Additionally adjusted for biomarkers (diastolic blood pressure, systolic blood pressure, total cholesterol, HDL cholesterol, C-reactive protein), use of blood pressure medication, use of cholesterol-lowering medication, and comorbidities (congestive heart failure, cancer, diabetes, emphysema, stroke)
3.3 Individual carotenoid quartiles and mortality
After full covariate adjustment, all-cause mortality rates for alpha-carotene were 1.46 (95% CI=1.22—1.75), 1.15 (95% CI=0.96—1.39), and 1.10 (95%CI=0.94—1.29) times higher for participants in the first (lowest), second, and third quartiles, respectively, relative to the fourth (highest) quartile (P<0.001) (Table 3). In contrast, there were no associations between all-cause mortality and fully-adjusted beta-carotene (P=0.58), beta-cryptoxanthin (P=0.44), or lutein+zeaxanthin (P=0.79). Age- and sex-adjusted MRRs for beta-carotene and beta-cryptoxanthin differed from their respective fully-adjusted MRRs, but the partially adjusted models produced MRRs similar to fully-adjusted models. While the lowest lycopene quartile had all-cause mortality rate similar to the highest quartile, the middle two quartiles had the lowest rates, which were, respectively, 18% (95% CI=−2%—33%) and 16% (95% CI=−7%—34%) lower than the highest lycopene quartile (P=0.047).
Table 3.
Rate Ratios of All-Cause and Cause-Specific Mortality by Individual Carotenoid Quartiles, Third National Health and Nutrition Examination Survey, 1988–19941
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Alpha-Carotene | (<0.05 µmol/L)3 | (0.05–0.07 µmol/L) | (0.08–0.11 µmol/L) | (>0.11 µmol/L) | |||||
| All-Cause Mortality | |||||||||
| Age- and Sex- Adjusted | 2.20 | 1.90—2.56 | 1.46 | 1.24—1.72 | 1.19 | 1.02—1.38 | 1.00 | Reference | <0.001 |
| Partially Adjusted4 | 1.49 | 1.25—1.78 | 1.21 | 1.01—1.45 | 1.12 | 0.96—1.31 | 1.00 | Reference | <0.001 |
| Fully Adjusted5 | 1.46 | 1.22—1.75 | 1.15 | 0.96—1.39 | 1.10 | 0.94—1.29 | 1.00 | Reference | <0.001 |
| CVD Mortality | |||||||||
| Age- and Sex- Adjusted | 2.08 | 1.65—2.60 | 1.38 | 1.12—1.71 | 1.20 | 0.91—1.56 | 1.00 | Reference | <0.001 |
| Partially Adjusted4 | 1.76 | 1.33—2.34 | 1.31 | 1.03—1.67 | 1.21 | 0.90—1.61 | 1.00 | Reference | 0.002 |
| Fully Adjusted5 | 1.77 | 1.31—2.38 | 1.30 | 1.01—1.69 | 1.22 | 0.90—1.64 | 1.00 | Reference | 0.002 |
| Cancer Mortality | |||||||||
| Age- and Sex- Adjusted | 2.27 | 1.70—3.02 | 1.48 | 1.04—2.11 | 1.09 | 0.84—1.42 | 1.00 | Reference | <0.001 |
| Partially Adjusted4 | 1.57 | 1.05—2.34 | 1.16 | 0.79—1.71 | 0.96 | 0.72—1.28 | 1.00 | Reference | 0.10 |
| Fully Adjusted5 | 1.51 | 0.96—2.36 | 1.11 | 0.73—1.70 | 0.92 | 0.66—1.29 | 1.00 | Reference | 0.10 |
| Beta-Carotene | (<0.18 µmol/L)3 | (0.18–0.28 µmol/L) | (0.29–0.45 µmol/L) | (>0.45 µmol/L) | |||||
| All-Cause Mortality | |||||||||
| Age- and Sex- Adjusted | 1.81 | 1.56—2.10 | 1.29 | 1.08—1.54 | 1.11 | 0.97—1.27 | 1.00 | Reference | <0.001 |
| Partially Adjusted4 | 0.95 | 0.77—1.16 | 0.88 | 0.73—1.07 | 0.89 | 0.77—1.03 | 1.00 | Reference | 0.43 |
| Fully Adjusted5 | 0.90 | 0.74—1.11 | 0.90 | 0.75—1.08 | 0.91 | 0.79—1.06 | 1.00 | Reference | 0.58 |
| CVD Mortality | |||||||||
| Age- and Sex- Adjusted | 1.48 | 1.16—1.88 | 1.11 | 0.84—1.46 | 1.00 | 0.84—1.18 | 1.00 | Reference | 0.006 |
| Partially Adjusted4 | 0.70 | 0.49—1.01 | 0.71 | 0.52—0.97 | 0.76 | 0.60—0.95 | 1.00 | Reference | 0.11 |
| Fully Adjusted5 | 0.65 | 0.46—0.91 | 0.72 | 0.54—0.95 | 0.79 | 0.63—0.997 | 1.00 | Reference | 0.07 |
| Cancer Mortality | |||||||||
| Age- and Sex- Adjusted | 1.76 | 1.30—2.38 | 1.44 | 1.001—2.08 | 1.25 | 0.94—1.66 | 1.00 | Reference | 0.005 |
| Partially Adjusted4 | 0.88 | 0.58—1.33 | 0.95 | 0.64—1.40 | 1.00 | 0.74—1.37 | 1.00 | Reference | 0.92 |
| Fully Adjusted5 | 0.86 | 0.56—1.30 | 0.96 | 0.64—1.42 | 0.99 | 0.73—1.34 | 1.00 | Reference | 0.89 |
| Beta-Cryptoxanthin | (<0.10 µmol/L)3 | (0.10–0.13 µmol/L) | (0.14–0.20 µmol/L) | (>0.20 µmol/L) | |||||
| All-Cause Mortality | |||||||||
| Age- and Sex- Adjusted | 1.81 | 1.54—2.13 | 1.43 | 1.23—1.66 | 1.19 | 0.99—1.41 | 1.00 | Reference | <0.001 |
| Partially Adjusted4 | 1.11 | 0.91—1.36 | 1.14 | 0.95—1.38 | 1.06 | 0.89—1.25 | 1.00 | Reference | 0.58 |
| Fully Adjusted5 | 1.15 | 0.95—1.38 | 1.15 | 0.96—1.38 | 1.04 | 0.88—1.23 | 1.00 | Reference | 0.44 |
| CVD Mortality | |||||||||
| Age- and Sex- Adjusted | 1.58 | 1.29—1.93 | 1.41 | 1.15—1.73 | 1.08 | 0.87—1.36 | 1.00 | Reference | <0.001 |
| Partially Adjusted4 | 1.09 | 0.83—1.44 | 1.20 | 0.92—1.55 | 1.01 | 0.82—1.24 | 1.00 | Reference | 0.60 |
| Fully Adjusted5 | 1.15 | 0.87—1.51 | 1.20 | 0.94—1.54 | 0.99 | 0.80—1.22 | 1.00 | Reference | 0.48 |
| Cancer Mortality | |||||||||
| Age- and Sex- Adjusted | 1.97 | 1.45—2.69 | 1.56 | 1.10—2.22 | 1.34 | 0.95—1.89 | 1.00 | Reference | <0.001 |
| Partially Adjusted4 | 1.27 | 0.87—1.84 | 1.25 | 0.84—1.86 | 1.17 | 0.81—1.68 | 1.00 | Reference | 0.63 |
| Fully Adjusted5 | 1.28 | 0.87—1.89 | 1.25 | 0.84—1.86 | 1.17 | 0.81—1.68 | 1.00 | Reference | 0.61 |
| Lycopene | (<0.29 µmol/L)3 | (0.29–0.43 µmol/L) | (0.44–0.58 µmol/L) | (>0.58 µmol/L) | |||||
| All-Cause Mortality | |||||||||
| Age- and Sex- Adjusted | 1.33 | 1.11—1.60 | 0.92 | 0.76—1.12 | 0.88 | 0.71—1.09 | 1.00 | Reference | <0.001 |
| Partially Adjusted4 | 1.05 | 0.87—1.26 | 0.83 | 0.68—1.02 | 0.86 | 0.70—1.07 | 1.00 | Reference | 0.02 |
| Fully Adjusted5 | 1.02 | 0.83—1.25 | 0.82 | 0.67—1.02 | 0.84 | 0.66—1.07 | 1.00 | Reference | 0.047 |
| CVD Mortality | |||||||||
| Age- and Sex- Adjusted | 1.06 | 0.80—1.41 | 0.73 | 0.52—1.04 | 0.90 | 0.65—1.23 | 1.00 | Reference | 0.04 |
| Partially Adjusted4 | 0.92 | 0.68—1.25 | 0.71 | 0.50—1.01 | 0.92 | 0.67—1.27 | 1.00 | Reference | 0.16 |
| Fully Adjusted5 | 1.00 | 0.73—1.36 | 0.74 | 0.52—1.06 | 0.93 | 0.67—1.28 | 1.00 | Reference | 0.20 |
| Cancer Mortality | |||||||||
| Age- and Sex- Adjusted | 1.17 | 0.85—1.62 | 0.98 | 0.72—1.33 | 0.63 | 0.38—1.04 | 1.00 | Reference | 0.04 |
| Partially Adjusted4 | 0.93 | 0.66—1.32 | 0.90 | 0.65—1.23 | 0.61 | 0.37—1.01 | 1.00 | Reference | 0.22 |
| Fully Adjusted5 | 0.88 | 0.61—1.27 | 0.85 | 0.62—1.16 | 0.60 | 0.36—0.997 | 1.00 | Reference | 0.22 |
| Lutein+Zeaxanthin | (<0.26 µmol/L)3 | (0.26–0.33 µmol/L) | (0.34–0.46 µmol/L) | (>0.46 µmol/L) | |||||
| All-Cause Mortality | |||||||||
| Age- and Sex- Adjusted | 1.60 | 1.38—1.86 | 1.29 | 1.11—1.50 | 1.09 | 0.93—1.27 | 1.00 | Reference | <0.001 |
| Partially Adjusted4 | 1.08 | 0.90—1.31 | 1.07 | 0.91—1.25 | 0.99 | 0.84—1.16 | 1.00 | Reference | 0.60 |
| Fully Adjusted5 | 1.07 | 0.89—1.29 | 1.02 | 0.86—1.20 | 0.98 | 0.84—1.15 | 1.00 | Reference | 0.79 |
| CVD Mortality | |||||||||
| Age- and Sex- Adjusted | 1.48 | 1.20—1.82 | 1.08 | 0.86—1.34 | 1.10 | 0.90—1.35 | 1.00 | Reference | 0.004 |
| Partially Adjusted4 | 1.10 | 0.84—1.43 | 0.94 | 0.73—1.21 | 1.02 | 0.82—1.28 | 1.00 | Reference | 0.62 |
| Fully Adjusted5 | 1.14 | 0.89—1.47 | 0.92 | 0.72—1.18 | 1.04 | 0.84—1.28 | 1.00 | Reference | 0.44 |
| Cancer Mortality | |||||||||
| Age- and Sex- Adjusted | 1.48 | 1.13—1.92 | 1.48 | 1.13—1.96 | 1.03 | 0.71—1.48 | 1.00 | Reference | <0.001 |
| Partially Adjusted4 | 0.96 | 0.69—1.32 | 1.19 | 0.87—1.62 | 0.89 | 0.61—1.30 | 1.00 | Reference | 0.09 |
| Fully Adjusted5 | 0.90 | 0.63—1.29 | 1.13 | 0.82—1.56 | 0.89 | 0.60—1.30 | 1.00 | Reference | 0.21 |
Data are given as mortality rate ratio with 95% confidence interval
Based on testing the null hypothesis that all four quartiles have equal mortality rates using survey-weighted Cox models [31]
To convert from SI units (µmol/L) to metric (µg/L) divide SI carotenoid concentrations by the following conversion factors: alpha-carotene, beta-carotene, and lycopene = 0.001863; beta-cryptoxanthin= 0.001809; lutein+zeaxanthin = 0.001758.
Adjusted for demographics (age, sex, race/ethnicity, marital status, education), lifestyle behaviors (alcohol consumption, smoking status, multivitamin/multimineral use, physical activity), body mass index and other individual carotenoids
Additionally adjusted for biomarkers (diastolic blood pressure, systolic blood pressure, total cholesterol, HDL cholesterol, C-reactive protein), use of blood pressure medication, use of cholesterol-lowering medication, and comorbidities (congestive heart failure, cancer, diabetes, emphysema, stroke)
Fully-adjusted CVD mortality rates were significantly higher for participants with lower alpha-carotene (first versus fourth quartile: MRR=1.77, 95% CI=1.31—2.38, P=0.002). The MRR was not quite significantly lower for those with lower beta-carotene (first versus fourth quartile: MRR=0.65, 95% CI= 0.46—0.91, P=0.07). There was no association of CVD mortality with fully-adjusted beta-cryptoxanthin (P=0.48), lycopene (P=0.20), or lutein+zeaxanthin (P=0.44).
Although not statistically significant, participants with lower alpha-carotene concentrations had higher fully-adjusted cancer mortality rates (first versus fourth quartile: MRR=1.51, 95% CI=0.96—2.36, P=0.10). There was no association of cancer mortality with fully-adjusted beta-carotene (P=0.89), beta-cryptoxanthin (P=0.61), lycopene (P=0.22), or lutein+zeaxanthin (P=0.21).
3.4 Continuous carotenoids and all-cause mortality
The relationship between continuous total carotenoids and all-cause mortality (Figure 1) shows a steep reduction in mortality up to approximately 1.00 µmol/L, then little change for total carotenoids >1.00 µmol/L (P<0.001). Relationships between continuous individual carotenoids and adjusted all-cause mortality were generally similar to results using quartiles. Higher alpha-carotene concentrations up to approximately 0.11 µmol/L were associated with lower mortality rates followed by little change for alpha-carotene >0.11 µmol/L (P<0.001). Higher lycopene concentrations until approximately 0.40 µmol/L were associated with lower mortality, then starting at approximately 0.50 µmol/L, higher lycopene concentrations were associated with higher mortality rates (P<0.001). There was no significant association of fully-adjusted all-cause mortality with continuous beta-carotene (P=0.32), beta-cryptoxanthin (P=0.23), and lutein+zeaxanthin (P=0.62).
Figure 1. Continuous Carotenoids and All-Cause Mortality, Third National Health and Nutrition Examination Survey, 1988–1994.
Adjusted for demographics (age, sex, race/ethnicity, marital status, education), lifestyle behaviors (alcohol consumption, smoking status, multivitamin/multimineral use, physical activity), biomarkers (body mass index, diastolic blood pressure, systolic blood pressure, total cholesterol, HDL cholesterol, C-reactive protein; models of individual carotenoids included other individual carotenoids), use of blood pressure medication, use of cholesterol-lowering medication, and comorbidities (congestive heart failure, cancer, diabetes, emphysema, stroke)
To convert from SI units (µmol/L) to metric (µg/L) divide SI carotenoid concentrations by the following conversion factors: alpha-carotene, beta-carotene, and lycopene = 0.001863; beta-cryptoxanthin= 0.001809; lutein+zeaxanthin = 0.001758.
25, 50, 75, and 95 are labels for the carotenoid 25th through 95th percentiles
k1 – k5 are labels for locations of carotenoid 1st through 5th knots for restricted cubic spline models. The number of knots ranged from 3 to 5 for each carotenoid.
CI, confidence interval (shown by dashed lines). P-values from survey-weighted Cox models [31].
3.5 Carotenoid interactions and all-cause mortality
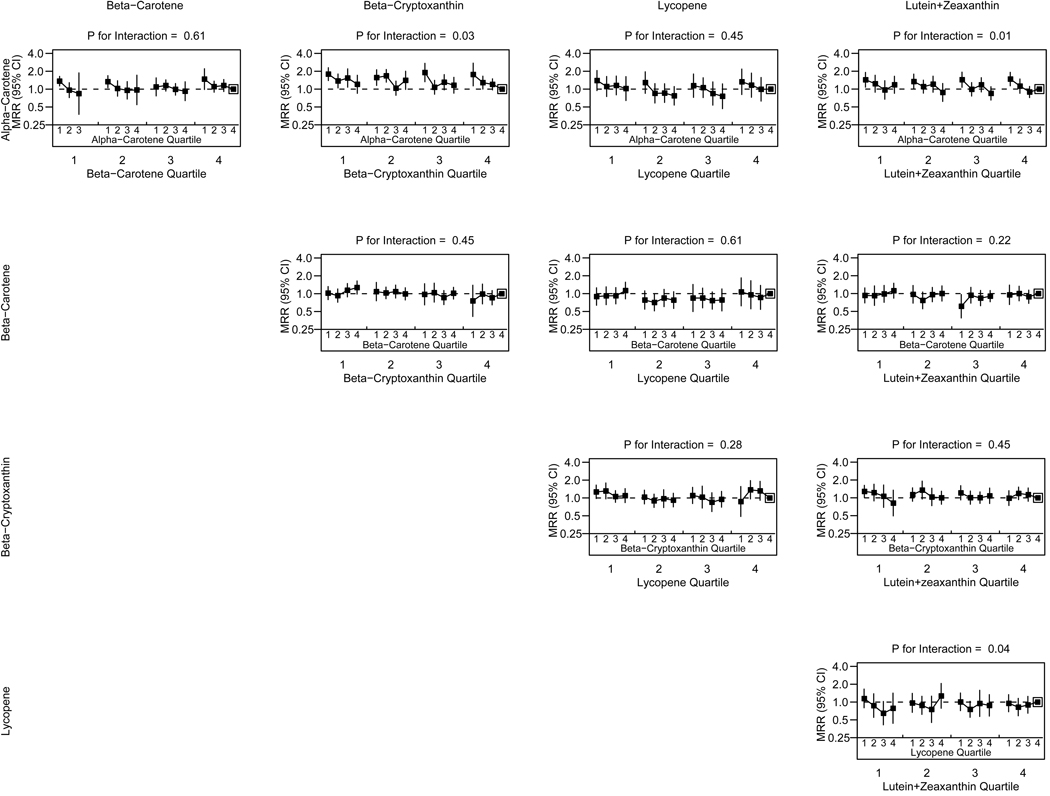
Figure 2 shows joint associations of carotenoid pairs with fully-adjusted all-cause mortality. Alpha-carotene had statistically significant interactions with beta-cryptoxanthin (P=0.03) and lutein+zeaxanthin (P=0.01). Lutein+zeaxanthin also had a statistically significant interaction with lycopene (P=0.04). For participants in the lowest beta-cryptoxanthin quartile, a drop in mortality was only observed for the highest alpha-carotene quartile, but for participants in the highest two beta-cryptoxanthin quartiles, a drop in mortality was observed for the upper three alpha-carotene quartiles. Similarly, participants in the highest two lutein+zeaxanthin quartiles had steeper declines in mortality with higher alpha-carotene than participants in the lowest lutein+zeaxanthin quartile. For participants in the lowest lutein+zeaxanthin quartile, higher lycopene concentrations showed a pronounced reduction then higher in mortality rates, but for those in the highest lutein+zeaxanthin quartile, mortality rates did not differ by lycopene concentration. Interactions between all other pairs of carotenoids were not statistically significant (all P>0.05).
Figure 2. Two-Way Carotenoid Interactions and All-Cause Mortality, Third National Health and Nutrition Examination Survey, 1988–1994.
Adjusted for demographics (age, sex, race/ethnicity, marital status, education), lifestyle behaviors (alcohol consumption, smoking status, multivitamin/multimineral use, physical activity), biomarkers (body mass index, diastolic blood pressure, systolic blood pressure, total cholesterol, HDL cholesterol, C-reactive protein, other individual carotenoids), use of blood pressure medication, use of cholestero-lowering medication, and comorbidities (congestive heart failure, cancer, diabetes, emphysema, stroke)
To convert from SI units (µmol/L) to metric (µg/L) divide SI carotenoid concentrations by the following conversion factors: alpha-carotene, beta-carotene, and lycopene = 0.001863; beta-cryptoxanthin=0.001809; lutein+zeaxanthin = 0.001758.
MRR, mortality rate ratio; CI, confidence interval (shown by error bars). P-values from survey-weighted Cox models [31].
Boxed point indicates the reference category (4th quartile for both carotenoids)
Only 13 participants simultaneously had alpha-carotene in the fourth quartile and beta-carotene in the first quartile, among whom 3 deaths occurred, therefore this combination was removed from analyses. Otherwise, the smallest carotenoid combination had 133 participants, and the smallest number of deaths in a carotenoid combination was 19, which are sufficient for the Cox model.
3.6 Total and Individual Carotenoids and All-Cause Mortality Prediction
The RSF produced a C-index of 87.4% for predicting mortality. The overall shapes of the relationships were similar to those from the splines presented in Figure 1 (results not shown). Lycopene was the carotenoid most strongly predictive of mortality after accounting for other predictors and potential multi-way interactions. Total carotenoids and lutein+zeaxanthin were 59% and 25% as predictive as lycopene, respectively. The individual provitamin A carotenoids; beta-cryptoxanthin, alpha-carotene, and beta-carotene; were, respectively, 13%, 12%, and 10% as predictive of all-cause mortality as lycopene.
3.7 Sensitivity analyses
Overall, neither WEE nor removing participants taking beta-carotene, retinol, or lutein supplements and adding cotinine into models substantially changed results and interpretations. However, the modeling changes resulted in higher beta-carotene becoming significantly associated with higher CVD mortality (P=0.007).
4. DISCUSSION
Using a large, representative sample of US adults, we found that carotenoids relate to mortality, but relationships differed by carotenoid type, were often nonlinear with threshold effects, and were complicated by interactions. Total carotenoids, alpha-carotene, and lycopene were significantly associated with all-cause mortality regardless of analysis method. Total carotenoids had a threshold effect in which concentrations greater than 1.00 µmol/L showed diminishing incremental benefits. Alpha-carotene was the lowest-concentration carotenoid and the only carotenoid that had a monotonic dose-response relationship with mortality, with higher concentrations associated with gradually lower all-cause mortality. The dose-response effect for alpha-carotene may be due to its low concentrations in the study sample. Interestingly, RSF found alpha-carotene to be the second-least predictive carotenoid of all-cause mortality. Lycopene was the highest-concentration carotenoid and had a U-shaped relationship with mortality, where concentrations of approximately 0.40 µmol/L were associated with the lowest all-cause mortality. The combined results using quartiles and continuous lycopene detected a steep drop in mortality with higher lycopene within its lowest quartile. Also, lycopene was the carotenoid most predictive of all-cause mortality. Consistent with many randomized trials[13, 15, 16], there was no evidence that high beta-carotene concentrations related to reduced mortality rates. Low alpha-carotene was the only carotenoid significantly associated with CVD mortality; no carotenoids were significantly associated with cancer mortality.
These overall findings are generally consistent with previous reports. Studies have found that high total carotenoids (and alpha-carotene among nonsmokers) are associated with lower mortality rates[2, 7–9]. A recent report also found a dose-response relationship between alpha-carotene and mortality in NHANES III, but the only other carotenoid for which the authors adjusted was beta-carotene, and the effects of other carotenoids and their interactions on mortality were not examined [36]. Also, previous research found that participants with lower lycopene serum concentrations or consumption have higher mortality rates[2, 9]. Our findings extend previous reports by demonstrating threshold effects of total carotenoids, alpha-carotene, and lycopene; identifying interactions between carotenoids; and determining which carotenoids are most predictive of mortality.
Previous reports have also found associations between carotenoids and disease outcomes and processes that may mediate the association between carotenoids and mortality. Higher total carotenoid concentrations have been shown to be associated with lower cancer and CVD risk. [37–39]. Higher lycopene concentrations are also associated with lower risk of prostate cancer and ischemic stroke [11, 40, 41]. Relationships have also been found between individual and total carotenoids with markers of inflammation and oxidative stress [42, 43].
Alpha-carotene/beta-cryptoxanthin and alpha-carotene/lutein+zeaxanthin interactions were found and appeared synergistic, such that higher concentrations of one carotenoid resulted in stronger effects of the other on mortality. We found a lycopene/lutein+zeaxanthin interaction that appeared to be compensatory, where higher concentrations of one carotenoid resulted in weaker effects of the other. While it is difficult to fully understand the mechanisms and the consequences of these interactions, our findings suggest that carotenoids exert their effects in concert. It is important to note that individual carotenoids have varying antioxidant activity depending on carotenoid structure and polarity of the environment. For example, polar carotenoids (lutein+zeaxanthin and beta-cryptoxanthin) are more effective scavengers of free radicals in polar (e.g., aqueous) environments while non-polar hydrocarbon carotenoids (alpha-carotene, beta-carotene, and lycopene), scavenge more effectively deep within the lipoprotein cell membrane layer[3, 17]. The three pair-wise interactions found in this analysis all involve a polar and non-polar carotenoid, thus it is biologically plausible that alpha-carotene would have a synergistic relationship with beta-cryptoxanthin and lutein+zeaxanthin on mortality. The smaller lycopene effects with higher lutein+zeaxanthin are surprising and may suggest a potential redundancy in their health effects where high concentrations of one carotenoid can compensate for low concentrations of the other.
These findings have important implications for future carotenoid research. First, the differing effects of individual carotenoids on mortality demonstrate the importance of examining individual carotenoids along with total carotenoids. Differences in health effects appear significant and are biologically plausible given the carotenoids’ different molecular properties (polar or non-polar) and activities (provitamin A or non-provitamin A).
Second, carotenoid interactions signify that individual carotenoids should be examined simultaneously, rather than in isolation. The potential beneficial effects of carotenoids may depend on concentrations of other antioxidants in the same milieu[3, 17]. Also, although carotenoids are antioxidants, high concentrations of individual carotenoids may have prooxidant effects in settings of high oxidative stress, especially if not balanced by water-soluble antioxidants like vitamin C; e.g., high beta-carotene among smokers[3].
Lastly, carotenoids remain an important target of intervention despite the negative results of beta-carotene trials. However, the non-monotone effects found in this study suggest that carotenoids may be most beneficial within a certain range; therefore, dietary interventions may be more promising than high-dose supplementation. Carotenoids may not protect against mortality in isolation, but may reflect a dietary pattern associated with better health. For example, high lycopene concentrations suggest a diet rich in tomato products[44], which may also involve high intake of other fruits and vegetables. One such dietary pattern is the Mediterranean diet, which has been associated with decreased oxidative stress[45], and lower risk of metabolic syndrome[46, 47] and mortality from all causes, CVD, and cancer[4, 5].
Some limitations should be noted when interpreting results. First, NHANES III is an observational study; therefore, the potential exists for unmeasured or excluded confounding factors. We attempted to mitigate this possibility by controlling for many known confounders and performing sensitivity analyses. By adjusting for multiple covariates, including other individual carotenoids, we reduce the chance that the effects of each individual carotenoid are merely proxies for an overall healthy diet. Second, this study relied on self-report data for behaviors and comorbidities; however, this limitation was at least partially overcome by including many objectively measured biomarkers. Third, the primary analyses used carotenoid categories (i.e., quartiles), parametric modeling assumptions (proportional hazards, splines), and an inability to directly compare the importance of total and individual carotenoids for predicting mortality while accounting for multi-way interactions; but the non-parametric RSF overcame these limitations and allowed us to examine robustness of study findings. Fourth, carotenoid data were only available at one time point, which may not necessarily reflect long-term dietary exposure; therefore the results are likely to be conservative. Lastly, primary analyses were limited to participants with complete data; however, sensitivity analyses showed robust conclusions. This study also had several strengths. First, no other previous study in the US has examined associations and interactions between as many serum carotenoid measures and mortality over as long a follow-up. Second, the study used data from NHANES III, which is representative of the US population. Lastly, multiple analysis techniques were used to validate findings.
This study supports the beneficial effects of carotenoids[48]. However, for adults with carotenoid concentrations within a certain range, consuming too much of any one carotenoid may not be beneficial. Further work examining the health effects of micronutrient interactions and identifying optimal concentrations of micronutrient combinations is needed to better develop dietary interventions and identify sub-populations who may benefit from these interventions.
ACKNOWLEDGMENT
This research was supported by NIH grants K12 HD043489, K12HD055931, K23 AG019161, R21HD057274, R01 HL094507, R01 AG027012.
Abbreviations
- BMI
body mass index
- C-index
concordance index
- CI
confidence interval
- CRP
C-reactive protein
- CV
coefficient of variation
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- HDL
high-density lipoprotein
- HPLC
High-performance liquid chromatography
- ICD-10
International Classification of Diseases, 10th Revision
- MET
metabolic equivalent task
- MRR
mortality rate ratio
- NHANES
National Health and Nutrition Examination Survey
- RSF
random survival forest
- SBP
systolic blood pressure
- WEE
weighted estimating equations
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report.
REFERENCES
- 1.Bazzano LA, He J, Ogden LG, et al. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr. 2002;76:93–99. doi: 10.1093/ajcn/76.1.93. [DOI] [PubMed] [Google Scholar]
- 2.Agudo A, Cabrera L, Amiano P, et al. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain) Am J Clin Nutr. 2007;85:1634–1642. doi: 10.1093/ajcn/85.6.1634. [DOI] [PubMed] [Google Scholar]
- 3.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 5.Mitrou PN, Kipnis V, Thiebaut AC, et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med. 2007;167:2461–2468. doi: 10.1001/archinte.167.22.2461. [DOI] [PubMed] [Google Scholar]
- 6.US Institute of Medicine. US Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids: Dietary Antioxidants and Related Compounds. Washington, D.C.: National Academy Press; 2000. [Google Scholar]
- 7.Lauretani F, Semba RD, Dayhoff-Brannigan M, et al. Low total plasma carotenoids are independent predictors of mortality among older persons: the InCHIANTI study. Eur J Nutr. 2008;47:335–340. doi: 10.1007/s00394-008-0732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akbaraly TN, Favier A, Berr C. Total plasma carotenoids and mortality in the elderly: results of the Epidemiology of Vascular Ageing (EVA) study. Br J Nutr. 2009;101:86–92. doi: 10.1017/S0007114508998445. [DOI] [PubMed] [Google Scholar]
- 9.Mayne ST, Cartmel B, Lin H, et al. Low plasma lycopene concentration is associated with increased mortality in a cohort of patients with prior oral, pharynx or larynx cancers. J Am Coll Nutr. 2004;23:34–42. doi: 10.1080/07315724.2004.10719340. [DOI] [PubMed] [Google Scholar]
- 10.Holick CN, Michaud DS, Stolzenberg-Solomon R, et al. Dietary carotenoids, serum beta-carotene, and retinol and risk of lung cancer in the alpha-tocopherol, beta-carotene cohort study. Am J Epidemiol. 2002;156:536–547. doi: 10.1093/aje/kwf072. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci E. Tomato products, lycopene, and prostate cancer: a review of the epidemiological literature. J Nutr. 2005;135:2030S–2031S. doi: 10.1093/jn/135.8.2030S. [DOI] [PubMed] [Google Scholar]
- 12.Morris DL, Kritchevsky SB, Davis CE. Serum carotenoids and coronary heart disease. The Lipid Research Clinics Coronary Primary Prevention Trial and Follow-up Study. JAMA. 1994;272:1439–1441. doi: 10.1001/jama.272.18.1439. [DOI] [PubMed] [Google Scholar]
- 13.The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14–23. doi: 10.1093/jnci/djn438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjelakovic G, Nikolova D, Gluud LL, et al. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 16.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 17.El-Agamey A, Lowe GM, McGarvey DJ, et al. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys. 2004;430:37–48. doi: 10.1016/j.abb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Nebeling LC, Forman MR, Graubard BI, et al. Changes in carotenoid intake in the United States: the 1987 and 1992 National Health Interview Surveys. J Am Diet Assoc. 1997;97:991–996. doi: 10.1016/S0002-8223(97)00239-3. [DOI] [PubMed] [Google Scholar]
- 19.Ferrucci L, Perry JR, Matteini A, et al. Common variation in the beta-carotene 15,15'-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009;84:123–133. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.During A, Hussain MM, Morel DW, et al. Carotenoid uptake and secretion by CaCo-2 cells: beta-carotene isomer selectivity and carotenoid interactions. J Lipid Res. 2002;43:1086–1095. doi: 10.1194/jlr.m200068-jlr200. [DOI] [PubMed] [Google Scholar]
- 21.National Center for Health Statistics. Centers for Disease Control and Prevention Web Site. National Health and Nutrition Examination Survey Data; [Accessed April 20, 2010]. http://www.cdc.gov/nchs/nhanes.htm. [Google Scholar]
- 22.Ezzati-Rice TM. Sample design : third National Health and Nutrition Examination Survey. Hyattsville, MD: Centers for Disease Control, National Center for Health Statistics; 1992. [Google Scholar]
- 23.Gunter EW, Lewis BL, Koncikowski SM. Laboratory Methods used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Atlanta, GA: US Department of Health and Human Services; 1996. [Google Scholar]
- 24.Visser M, Bouter LM, McQuillan GM, et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 25.Zhu S, St-Onge MP, Heshka S, et al. Lifestyle behaviors associated with lower risk of having the metabolic syndrome. Metabolism. 2004;53:1503–1511. doi: 10.1016/j.metabol.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. The International classification of diseases, 9th revision, clinical modification : ICD-9-CM. Geneva, Switzerland: World Health Organization; 1991. [Google Scholar]
- 27.World Health Organization. International statistical classification of diseases and related health problems, 10th revision. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 28.R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 29.Binder DA. Fitting Cox's proportional hazards models from survey data. Biometrika. 1992;79:139–147. [Google Scholar]
- 30.Korn EL, Graubard BI. Analysis of health surveys. New York: Wiley; 1999. [Google Scholar]
- 31.Therneau TM, Grambsch PM. Modeling survival data : extending the Cox model. New York: Springer; 2000. [Google Scholar]
- 32.Brady WE, Mares-Perlman JA, Bowen P, et al. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr. 1996;126:129–137. doi: 10.1093/jn/126.1.129. [DOI] [PubMed] [Google Scholar]
- 33.Ishwaran H, Kogalur UB, Blackstone EH, et al. Random survival forests. Ann Appl Statist. 2008;2:841–860. [Google Scholar]
- 34.Ishwaran H, Kogalur UB. Random survival forests for R. R News. 2007;7:25–31. [Google Scholar]
- 35.Robins JM, Rotnitzky A, Zhao LP. Estimation of regression coefficients when some regressors are not always observed. Journal of the American Statistical Association. 1994;89:846–866. [Google Scholar]
- 36.Li C, Ford ES, Zhao G, et al. Serum {alpha}-Carotene Concentrations and Risk of Death Among US Adults: The Third National Health and Nutrition Examination Survey Follow-up Study. Arch Intern Med. 2010 doi: 10.1001/archinternmed.2010.440. in press. [DOI] [PubMed] [Google Scholar]
- 37.Toniolo P, Van Kappel AL, Akhmedkhanov A, et al. Serum carotenoids and breast cancer. Am J Epidemiol. 2001;153:1142–1147. doi: 10.1093/aje/153.12.1142. [DOI] [PubMed] [Google Scholar]
- 38.Hozawa A, Jacobs DR, Jr, Steffes MW, et al. Circulating carotenoid concentrations and incident hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Hypertens. 2009:237–242. doi: 10.1097/HJH.0b013e32832258c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coyne T, Ibiebele TI, Baade PD, et al. Diabetes mellitus and serum carotenoids: findings of a population-based study in Queensland, Australia. Am J Clin Nutr. 2005;82:685–693. doi: 10.1093/ajcn.82.3.685. [DOI] [PubMed] [Google Scholar]
- 40.Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev. 1998;56:35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 41.Hak AE, Ma J, Powell CB, et al. Prospective study of plasma carotenoids and tocopherols in relation to risk of ischemic stroke. Stroke. 2004;35:1584–1588. doi: 10.1161/01.STR.0000132197.67350.bd. [DOI] [PubMed] [Google Scholar]
- 42.Kritchevsky SB, Bush AJ, Pahor M, et al. Serum carotenoids and markers of inflammation in nonsmokers. Am J Epidemiol. 2000;152:1065–1071. doi: 10.1093/aje/152.11.1065. [DOI] [PubMed] [Google Scholar]
- 43.Hozawa A, Jacobs DR, Jr, Steffes MW, et al. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) study. Clin Chem. 2007;53:447–455. doi: 10.1373/clinchem.2006.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganji V, Kafai MR. Population determinants of serum lycopene concentrations in the United States: data from the Third National Health and Nutrition Examination Survey, 1988–1994. J Nutr. 2005;135:567–572. doi: 10.1093/jn/135.3.567. [DOI] [PubMed] [Google Scholar]
- 45.Dai J, Jones DP, Goldberg J, et al. Association between adherence to the Mediterranean diet and oxidative stress. Am J Clin Nutr. 2008;88:1364–1370. doi: 10.3945/ajcn.2008.26528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salas-Salvado J, Fernandez-Ballart J, Ros E, et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one-year results of the PREDIMED randomized trial. Arch Intern Med. 2008;168:2449–2458. doi: 10.1001/archinte.168.22.2449. [DOI] [PubMed] [Google Scholar]
- 47.Esposito K, Marfella R, Ciotola M, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 48.Semba RD, Lauretani F, Ferrucci L. Carotenoids as protection against sarcopenia in older adults. Arch Biochem Biophys. 2007;458:141–145. doi: 10.1016/j.abb.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]