Abstract
Juvenile Fischer 344 rats are known to be less playful than other inbred strains, although the neurobiological substrate(s) responsible for this phenotype is uncertain. In the present study, Fischer 344 rats were compared to the commonly used outbred Sprague-Dawley strain on several behavioral and physiological parameters in order to ascertain whether the lack of play may be related to compromised activity of brain dopamine (DA) systems. As expected, Fischer 344 rats were far less playful than Sprague-Dawley rats, with Fischer 344 rats less likely to initiate playful contacts with a playful partner and less likely to respond playfully to these contacts. We also found that Fischer 344 rats showed less of a startle response and greater pre-pulse inhibition (PPI), especially at higher pre-pulse intensities. The increase in PPI seen in the Fischer 344 rat could be due to reduced DA modulation of sensorimotor gating and neurochemical measures were consistent with Fischer 344 rats releasing less DA than Sprague-Dawley rats. Fast scan cyclic voltammetry (FSCV) revealed Fischer 344 rats had less evoked DA release in dorsal and ventral striatal brain slices and high-performance liquid chromatography revealed Fischer 344 rats to have less DA turnover in the striatum and prefrontal cortex. We also found DA-dependent forms of cortical plasticity were deficient in the striatum and prefrontal cortex of the Fischer 344 rat. Taken together, these data indicate that deficits in play and enhanced PPI of Fischer 344 rats may be due to reduced DA modulation of corticostriatal and mesolimbic/mesocortical circuits critical to the execution of these behaviors.
1. Introduction
The developmental period between weaning and puberty (i.e., early childhood through early adolescence in humans) is a behaviorally rich and dynamic age during which the young of many mammalian species behave in ways that can be quite distinct from what is seen either before weaning or after puberty [1]. One particularly interesting behavior pattern that stands out as relatively unique for this age is play behavior. Play in some form occurs in the young of most mammalian species and has also been observed in other species (e.g., birds, reptiles, invertebrates) as well [2–4]. Although adults will sometimes engage in playful behaviors, play is much less common once puberty has been reached [5, 6] and does not always follow the same “rules” as those observed by younger animals [7–9]. The exact function of play still remains elusive, yet there is general consensus that removing the opportunity to play can have a number of consequences on later behavior and social functioning [10–13].
The presence or absence of play in an otherwise playful species can be a useful indicator for the overall health and psychological well-being of an animal. For example, play can be systematically reduced in the lab by hunger [14–16], fear [17, 18], or other types of stressors [19, 20]. Since play is commonly thought to be an adaptive behavior associated with healthy social and emotional development [2, 4, 21, 22], the relative lack of play in an otherwise playful species could also suggest an underlying pathology. Identifying relevant neural substrates of play behavior could then shed significant light on the etiology and neural correlates of a range of childhood psychiatric disorders in which social play is altered, such as autism and ADHD.
One approach towards identifying neural substrates of play would be to take advantage of known strain differences in playfulness and determine whether these behavioral differences are also reflected by systematic differences in potentially relevant neural systems. For example, brain monoamine systems are thought to be important in modulating levels of playfulness [4, 17, 23–25] and the functioning of monoaminergic systems can be greatly influenced by genetic background [26–29]. The Fischer 344 (Fischer) rat has been shown to play less than either the inbred Buffalo or Lewis strains and a cross-fostering study suggested that these differences are likely due to a heritable component [30, 31]. Fischer rats also differ from other strains on several dimensions of monoaminergic functioning [32–36]. The Fischer rat may then be a particularly useful strain to help identify relevant monoaminergic involvement in play behavior.
Given the fundamental characteristics of play behavior (e.g., energetic, pleasurable, highly motivated), there are many reasons to suppose that brain dopamine (DA) systems may be particularly important for play. However, direct evidence of a specific role for dopamine in the modulation of playfulness is lacking. DA utilization increases during play [37], DA antagonists uniformly reduce play [38–40], and neonatal 6-OHDA lesions impair the sequencing of behavioral elements during a play bout [41]. Increases in play following acute administration of ethanol, nicotine, or compounds that enhance endocannabinoid activity can all be reliably blocked by doses of DA antagonists that have no effect by themselves [42, 43]. These data suggest that at least some aspects of play behavior may be accompanied by increased DAergic activity and that compromising DAergic functioning is incompatible with rough-and-tumble activity. However, this conclusion must be tempered since selective DAergic agonists tend to have mixed effects on play. Low doses of apomorphine increase play [38, 44], although this effect has not been very robust and other investigators have only observed decreases in play with apomorphine and other selective DA agonists [39, 40, 45]. Using a strain of rat such as the Fischer with known differences in dopaminergic physiology and functioning, could help bring clarity to our understanding of how dopamine is involved in play behavior.
A role for mesolimbic dopamine circuitry in motivation, reward, and emotions is well established [46–49] and there is considerable evidence for mesolimbic involvement in modulating social behavior as well. For example, mesolimbic dopamine circuitry is thought to have a pivotal role in pair bonding among prairie voles [50, 51] as well as in social cognition among humans [52]. In rats, mesolimbic dopamine also seems to be critical for the production of 50 kHz ultrasonic vocalizations [53], which are emitted by rats during a variety of affectively positive social behaviors, including play [54, 55]. Dopamine may then influence social behavior in young rats through its action on mesolimbic circuitry.
Dopamine may also influence social behavior through its modulation of cortical synapses and how subcortical circuitry, particularly within the striatum, is influenced through this modulation [51, 52, 56]. Dopamine has been shown to play a key role in modulating the strength of cortical synapses, both acutely during simultaneous activity and chronically through processes such as long-term depression (LTD) and long-term potentiation (LTP) [57–61]. The majority of the research examining dopamine release and its modulation of cortical synapses in the rat has been performed in Sprague-Dawley rats, the outbred strain from which Fischer rats were derived [58, 59, 62–65]. Thus, to gain an understanding about the unique features of the Fischer rat’s dopamine physiology it was important to compare outcomes with the research standards developed in the Sprague-Dawley rat and in this study see how these differences in DA physiology might contribute to play deficits observed through comparisons between Fischer and Sprague-Dawley rats. Indeed, the Sprague-Dawley rat may serve as a better behavioral control as well, as it does not suffer from the impulsive behavior and vulnerability to drug addiction reported to occur in the Lewis rat strain [66]. We observed clear strain differences in play behavior and found that Fischer rats showed a number of associated differences in dopamine physiology which translated into differences in dopamine modulation of cortical synapses.
2. Experiment 1
Fischer rats have been shown to be less playful than either the inbred Buffalo strain [30] or the inbred Lewis strain [31]. Although play in both Buffalo and Lewis strains appears to be comparable to that observed in juveniles of the outbred Sprague-Dawley strain a direct comparison between these two strains has not yet been reported. In this experiment we compared the play behavior of juvenile Fischer rats to that of Sprague-Dawley rats.
In order to compare these two strains on another behavioral measure that may reflect DAergic differences, acoustic startle response and pre-pulse inhibition (PPI) was also assessed in these rats. When a weak auditory stimulus that is 5–15 dB above background noise (pre-pulse) is delivered shortly (100–500 msec) before a loud stimulus (pulse) the startle response to that pulse is attenuated. PPI has been shown to be quite sensitive to disruption by DAergic manipulations [67] and a number of robust strain differences have been reported for baseline PPI and the extent to which DAergic agonists disrupt PPI [68–70]. Indeed, strain differences in PPI may largely reflect differences in DAergic mechanisms [69]. However, few studies have assessed strain differences in PPI among prepubescent rats.
2.1. Methods
2.1.1. Subjects and housing
Male Sprague-Dawley (n = 28) and Fischer-344 (n = 28) rats were obtained from Harlan Sprague-Dawley at approximately 25 days of age. Additional same-age Sprague-Dawley rats were also obtained to serve as target animals for the play experiment. Animals were housed in groups of four in solid bottom cages (48 × 27 × 20 cm) and periodically handled for a few days after arrival in order to acclimate to the laboratory. Food and water were always freely available. The colony room was maintained at 22° C with a 12/12 hr reversed light/dark cycle (lights off at 08:00), with all testing done during the dark phase of the light/dark cycle. All housing and testing was done in compliance with the NIH Guide for Care and Use of Laboratory Animals using protocols approved by the Institutional Animal Care and Use Committees at Gettysburg College.
2.1.2. Play behavior
Play behavior was assessed in a clear Plexiglas chamber (40 × 40 × 50 cm) that was enclosed within a sound-attenuated wooden chamber illuminated by a single 25W red light bulb. The floor of the testing chamber was covered with approximately 3 cm of Aspen pine shavings. Play bouts were recorded as digital video files and scored later using behavioral observation software (Noldus XT: Noldus Information Technology) by an observer unaware of the strain of the animal.
One week after arrival in the laboratory all of the rats were acclimated to the testing chamber by being placed individually in the testing chamber for 5 minutes. On the following day rats were again placed in the testing chamber for 5 minutes but with another rat of the same strain. On the following day all of the rats were isolated for 4 hours before being given a 5 minute opportunity to play with a novel Sprague-Dawley rat that had also been acclimated and isolated in the same manner. Play was quantified by counting the frequency of contacts directed by the test rat towards the nape of the target Sprague-Dawley rat (nape contacts) and the likelihood that a nape contact directed by the target Sprague-Dawley rat to the test rat resulted in a response. A coding scheme similar to that detailed by Pellis and colleagues [71, 72] and used previously in this lab to quantify play between Fischer and Lewis rats [31] was used. If the contacted rat successfully withdrew its nape from the other rat, a response was scored and categorized as one of the following types of responses. A complete rotation was scored if the rat rotated completely to a supine position. A partial rotation was scored if the rat began to rotate along its longitudinal axis but kept the hind paws on the ground. An evasion was scored if the rat rapidly moved away from contact but did not rotate. Nape contacts were quantified by frequency of occurrence, while the different responses were quantified in probabilistic terms by calculating the probability of a particular response occurring in response to a nape contact.
2.1.3. Acoustic startle response and pre-pulse inhibition
One week after testing for play behavior rats were tested for acoustic startle response and pre-pulse inhibition of that response. A commercially available system (SR-Lab; San Diego Instruments) with four chambers was used. Rats were placed individually in a clear acrylic cylindrical chamber (adjusted length = 14 cm; inner diameter = 9 cm) that was situated in a sound-attenuated isolation cabinet. The inside of each cabinet was illuminated during testing and air circulated by a fan. Background white noise was set at 65 dB during testing. A single session of 54 trials was used to assess baseline startle, habituation, and pre-pulse inhibition. Each session began with a 5 minute acclimation period followed by the 54 trials with a variable inter-trial interval having an average of 17 seconds. Pulses consisted of a 40 msec burst of a 110 dB pulse of white noise, with the startle response recorded over a period of 100 msec beginning at the onset of the pulse. Startle response was quantified in arbitrary units that reflected the magnitude of output by an accelerometer attached to the base of the chamber. Three different pre-pulse intensities were used: 5, 10, 15 dB above background. Each pre-pulse was 20 msec in duration and occurred 100 msec before the onset of the 110 dB pulse.
The first 5 trials of the session were pulse-only trials; the first trial was discarded and the next 4 trials, when compared to the last 4 trials of the session, were used to assess habituation across the testing session. An additional 15 pulse-only trials and 10 trials for each of the three pre-pulse intensities were interspersed in a pseudo-random order between the pulse-only trials used to assess habituation.
2.2. Results
2.2.1. Play behavior
As expected, Fischer rats were less playful than Sprague-Dawley rats (Figure 1). Fischer rats directed significantly fewer nape contacts towards the target rats than did Sprague-Dawley rats, t(54) = 3.41, p < .002. Fischer rats were also less likely to respond to nape contacts than were Sprague-Dawley rats, t(54) = 7.32, p < .001. The likelihood of each of the three responses can be seen in Figure 1C. Fischer rats were less likely to respond with a complete rotation, t(54) = 7.36, p < .001, or with an evasion, t(54) = 3.18, p = .002. There was no difference between the 2 strains in responding to a nape contact with a partial rotation.
Figure 1.
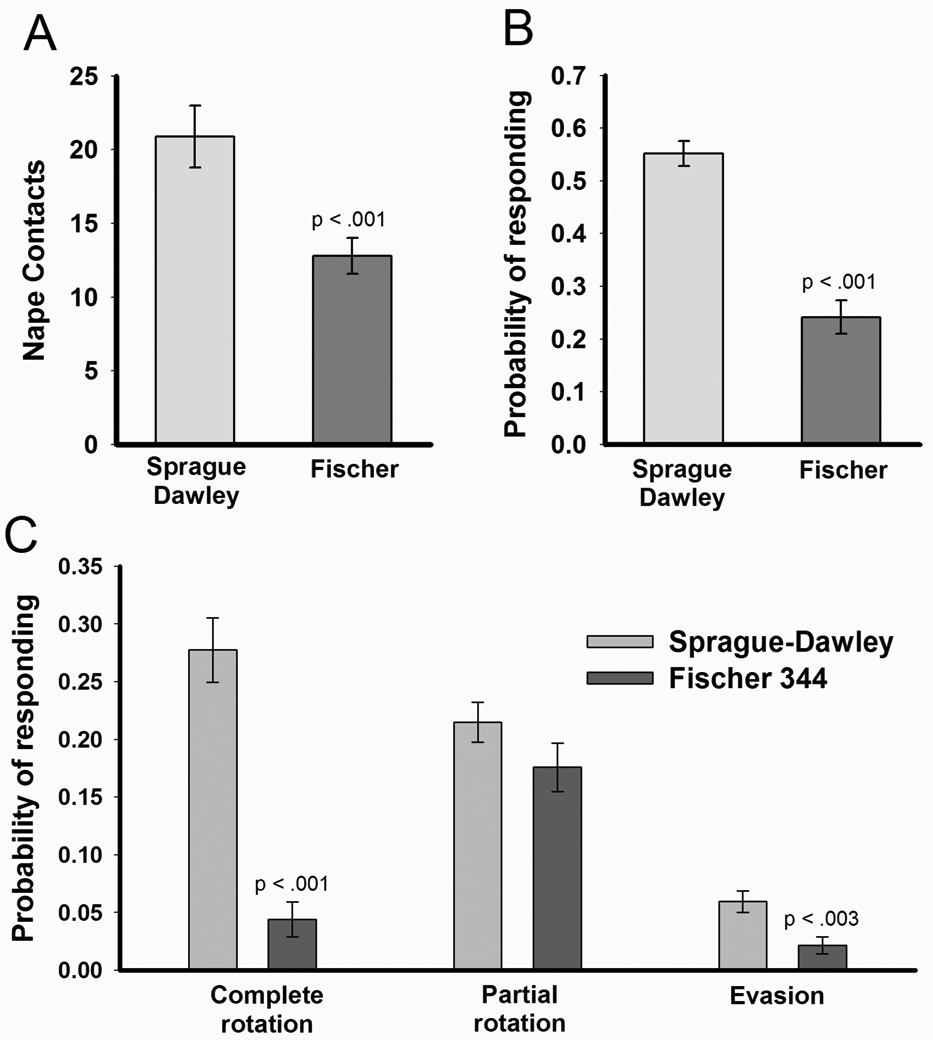
Mean (± SEM) nape contacts (Panel A) and the overall probability of responding to a nape contact (Panel B) in Sprague-Dawley (n=28) and Fischer 344 (n=28) rats. Rats were isolated for 4 hours prior to a 5 minute opportunity to play with a novel and playful Sprague-Dawley partner. Fischer 344 rats directed significantly fewer nape contacts to the partner and were less likely to respond to a nape contact by the playful partner with a complete rotation. Panel C breaks down the probability of responding to a nape contact with either complete rotation, partial rotation, or evasion.
2.2.2. Acoustic startle response and pre-pulse inhibition
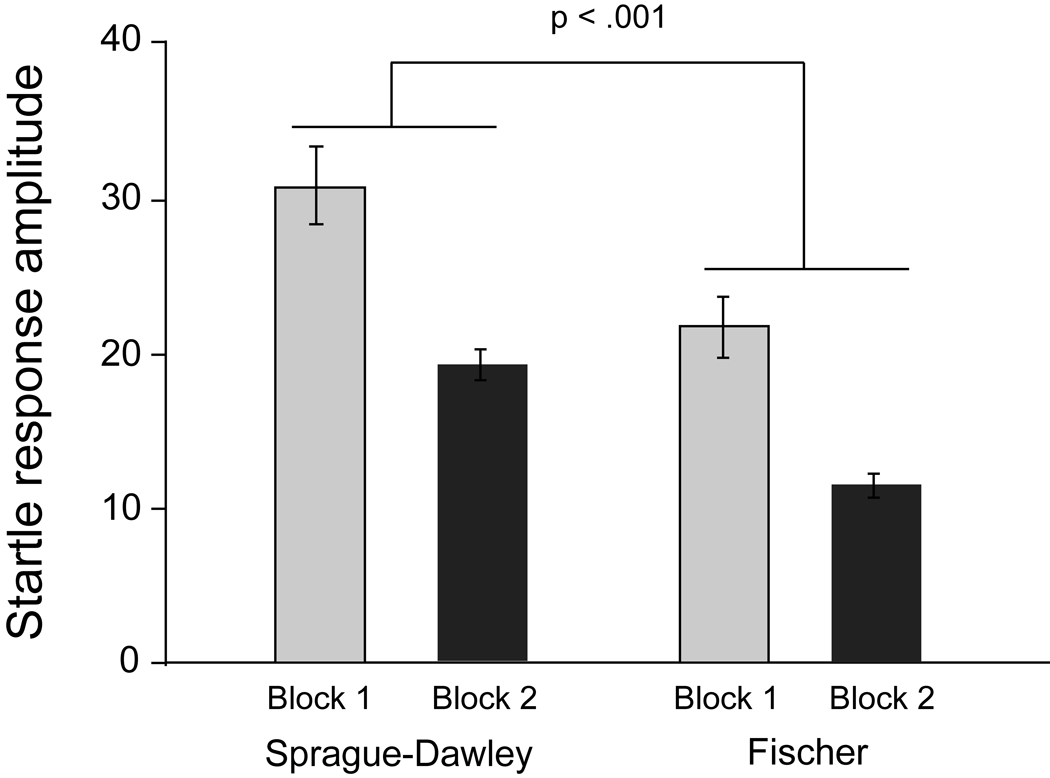
The extent to which habituation of the startle response occurred over the session (Figure 2) was assessed using a 2 × 2 repeated measures Analysis of Variance (ANOVA). There was a significant difference between the strains, F(1,54) = 12.64, p < .002, with Fischer rats having less of a startle response than Sprague-Dawley rats. There was habituation of the startle response as indicated by a significant effect of time, F(1,54) = 20.55, p < .001. The lack of a significant interaction between time and strain indicates that habituation was comparable in the two strains.
Figure 2.
Mean (± SEM) startle response amplitude in Sprague-Dawley and Fischer 344 rats at the beginning (Block 1) and the end (Block 2) of the test session used for assessing PPI in these rats. Habituation between Blocks 1 and 2 occurred in both strains. The startle response in Fischer 344 was significantly less than that of Sprague-Dawley rats and this was consistent across blocks.
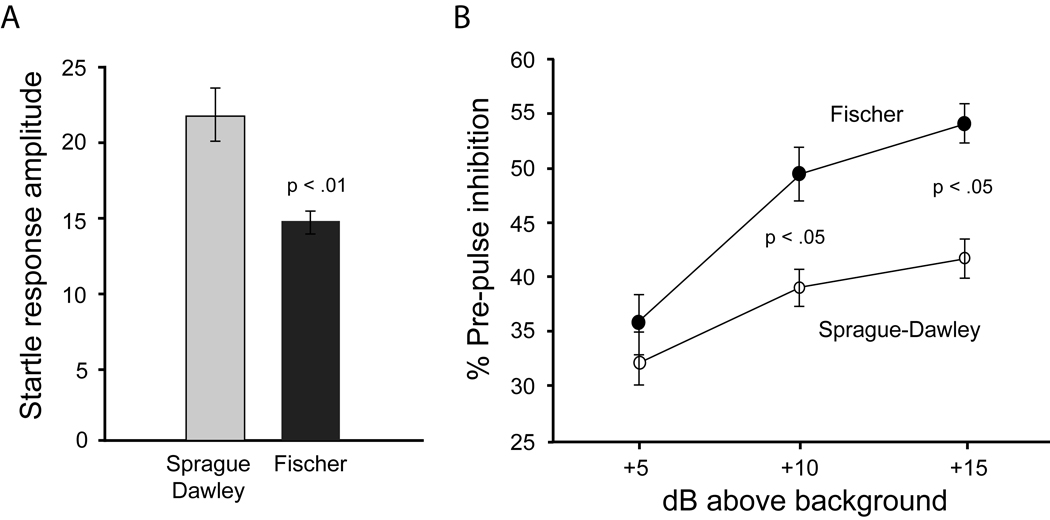
Fischer rats continued to exhibit less of a startle response on those trials used to assess pre-pulse inhibition, t(54) = 3.66, p < .001 (Figure 3). Pre-pulse inhibition (% inhibition) was assessed with a 2 × 3 repeated measures ANOVA. There were significant main effects of both pre-pulse intensity, F(2,108) = 68.43, p < .001, and strain, F(1,54) = 8.62, p < .01. These main effects were tempered by a significant pre-pulse x strain interaction, F(2,108) = 6.87, p < .005. Further analysis of this interaction indicated that while the two strains did not differ at the lowest intensity pre-pulse (5 dB above background), Fischer rats exhibited more inhibition at the two higher pre-pulse intensities (10 and 15 dB above background). In order to determine whether the effect of strain on pre-pulse inhibition was being influenced by baseline startle differences, these data were analyzed using baseline startle as a covariate. When this was done, all of the above-mentioned significant differences remained.
Figure 3.
Mean (± SEM) startle response amplitude (left panel) and pre-pulse inhibition (right panel) in Sprague-Dawley and Fischer 344 rats. Startle response amplitude was significantly lower in Fischer 344 rats. Pre-pulse inhibition was enhanced in Fischer 344 rats but only when the pre-pulse was either 10 or 15 dB above the 65 dB background.
3. Experiment 2
While any exact role for dopamine in play behavior is far from certain at this point, there is ample evidence to suggest that an optimal level of dopamine functioning is required for play to occur [73]. The dorsal and ventral striatum [74] and frontal cortex [75–77] may also be recruited during play behavior and the activity of glutamatergic synapses are modulated by dopamine in these structures [58, 78, 79]. In order to determine whether deficits in dopamine physiology might be associated with the reduced play behavior seen in Fischer rats, we compared Fischer and Sprague-Dawley rats for dopamine content and release as well as dopamine-modulated cortical plasticity.
3.1. Subjects and housing
Male Sprague-Dawley and Fischer rats were obtained from Charles River and housed at either the California State University at San Bernardino (HPLC experiment) or the University of Southern California (voltammetry and electrophysiology experiments) under comparable conditions in colony rooms that were maintained at 22° C with a 12/12 hr light/dark cycle (lights on at 06:00). All housing and testing was done in compliance with the NIH Guide for Care and Use of Laboratory Animals using protocols approved by the Institutional Animal Care and Use Committee at the University of Southern California and California State University at San Bernardino.
3.2. Methods
3.2.1. High performance liquid chromatography (HPLC) analysis of striatal DA and DOPAC
Rats were approximately 38 days old when decapitated and had their striata removed. Following the procedure of Crawford et al. (2000), frozen striatal sections were sonicated in 10 volumes of 0.1 N HClO4 and then centrifuged at 20,000g for 20 min at 4°C. The supernatant was then filtered through a 0.22 mm centrifugation unit for 5 min at 2,000g at 4°C. Twenty microliters of the resulting extracts were assayed for DA content using HPLC (ESA, Chelmsford, MA; 582 pump with a MD-150 column) with electrochemical detection (ESA, Coulochem II EC detector). The mobile phase consisted of 75 mM NaH2PO4, 1.4 mM 1-octane sulfonic acid (OSA), 10 mM EDTA, and 10% acetonitrile at a pH of 3.1 (MD-TM Mobile Phase, ESA) and was pumped at a rate of 0.5 ml/min.
3.2.2. Brain slice preparation
One-month and two-month old rats were anesthetized with halothane and decapitated. Brains were removed and placed in cooled (1–4° C), modified-oxygenated artificial cerebrospinal fluid (aCSF). In the modified aCSF, some of the sodium was replaced with sucrose to reduce tissue excitability during brain slice cutting (sucrose 124 mM, NaCl 62 mM). This solution maintained the osmotic balance found in normal aCSF. Normal aCSF contained (concentrations in mM) NaCl 124, MgSO4 1.3, KCl 3.0, NaH2PO4 1.25, NaHCO3 26, CaCl2 2.4, glucose 10.0, equilibrated with a 95% O2 - 5% CO2 mixture to obtain a pH value of 7.3 – 7.4.
Hemi-coronal striatal slices were cut at a thickness of 400 µm using a Vibratome1000 (Vibratome Co., St. Louis, MO). The slices were immediately placed in an oxygenated aCSF solution and were slowly brought to room temperature (23° C). Single slices were transferred to a recording chamber (Haas ramp style gas interface chamber), and bathed continuously with the oxygenated aCSF solution maintained at a temperature of 32° C.
3.2.3. Fast scan cyclic voltammetry (FSCV) quantification of striatal DA release
Disc carbon fiber electrodes (CFE) were made from 7 mm unsized carbon fibers (Goodfellow Corporation, Devon, PA) by electrophoretic anodic deposition of paint (ALA Scientific Instruments, Inc., Westbury, NY) (Schulte and Chow, 1996). Extracellular DA was monitored at the carbon fiber microelectrode every 100 msec by applying a triangular waveform (−0.4 to +1.0 Volt vs. Ag/AgCl, 300 Volt/second). Currents were recorded with a modified VA-10X Voltammetric and Amperometric Amplifier (NPI Electronic, Tamm, Germany). Data acquisitions were controlled by Clampex 7.0 software (Axon Instruments, Foster City, CA). Electrical stimulation of the brain slice surface across a twisted, bipolar, nichrome electrode was used to evoke DA release. Single constant current pulses of 250 µA and 0.1 msec duration were obtained by using an A360R Constant Current Stimulus Isolator (WPI, Sarasota, FL) and a Master-8 pulse generator (A.M.P.I., Jerusalem, Israel). Stimulus intervals between pulses were not less than 5 min. The CFE’s were inserted 75 to 100 µm into brain slices at a position 100 to 200 µm from the stimulating electrode pair (Miles et al., 2002). Dorsal striatal slices were sampled for DA at 5 sites, which represented medial to lateral and dorsal to ventral dimensions [80, 81]. A separate set of experiments sampled the dorsal striatum, the nucleus accumbens core (Acb core) and the nucleus accumbens shell (Acb shell) in the same brain slice. At least three recordings of evoked DA release were made within each site of the slice. The maximum DA release values were then taken to estimate the evoked DA release per each site. The total DA release per slice was calculated as the average from these sites in each brain slice. Changes in extracellular DA were determined by monitoring the current over a 200 mV window at the peak oxidation potential for DA (for review, see [82]). Subtracting the current obtained before stimulation from the current obtained in the presence of DA created background-subtracted cyclic voltammograms. Electrodes were calibrated with 5 µM DA solutions in aCSF following each experiment to convert the oxidation current to DA concentration. Previous work has demonstrated that this method causes action potential mediated DA release that is TTX sensitive [81–83].
3.2.4. Extracellular stimulation, intracellular recording, and field potential recording
Extracellular stimulation
Bipolar insulated twisted tungsten wire (50 µm diameter) stimulating electrodes were used for delivering single, paired and tetanizing extracellular stimuli to excitatory corticostriatal and prefrontal cortex synapses. In intracellular recordings test stimuli (0.1 ms pulse duration) were delivered as paired stimuli with inter-stimulus intervals of 50 ms at 0.05 Hz . Tetanic stimulation protocols followed those previously published for studies on corticostriatal long-term depression (LTD) [80] and prefrontal cortex long-term potentiation (LTP) [78]. The corticostriatal tetanus consisted of four trains of stimuli separated by 10 sec. Each train lasted 1 sec and was delivered at a frequency of 100 Hz. The tetanus stimulation intensity was set to equal the threshold for orthodromic induction of action potentials. The intensity used to sample excitatory postsynaptic potentials (EPSPs) was then set to half the intensity of the orthodromic threshold. The orthodromic threshold and the threshold for first detection of corticostriatal EPSPs were determined by performing an input-output relationship between stimulation intensity and EPSP amplitude. The prefrontal cortex tetanus consisted of 5 0.5 sec trains of 300-Hz (0.05-ms pulse duration) delivered in 3-min intervals [78].
Striatal neuron intracellular recording
Intracellular recording was used to examine dorsomedial corticostriatal synaptic plasticity, since excitatory corticostriatal synapses are not laminar nor are cellular populations uniform. Intracellular records were obtained with glass microelectrodes pulled by a Flaming-Brown P-87 pipette puller. Electrodes filled with 2 M potassium acetate had resistance values ranging from 100 to 160 MΩ. Intracellular electrodes contained 2% biocytin (SIGMA, St. Louis, MO) in some experiments to verify the cell type based upon morphology. Intracellular signals were amplified with an Axoclamp 2A amplifier, digitized with a Digidata 1200 and stored on disk using pCLAMP software (Molecular Devices, Foster City, CA). Established electrophysiological criteria for striatal medium spiny neurons were used for including cells in this study, which included resting membrane potentials greater than −80 mV, a stable input resistance > 20 MΩ, A-current delayed firing in response to suprathreshold depolarizing injections of current and non-adapting firing of action potentials in response to stronger injections of depolarizing current injections (Akopian et al, 2000; Calabresi et al, 1992). These characteristics were determined for each cell at the beginning of each experiment using 500 msec long injections of current.
Field potential recordings from medial prefrontal cortex (mPFC) pyramidal cell layer
Extracellular recording was obtained by a glass electrode filled with 2 M NaCl placed on layer V of the medial prefrontal cortex, where the basal dendrites and cell bodies of pyramidal neurons are located [78]. Stimuli (0.05 ms pulse duration) were delivered at 0.033 Hz by a bipolar electrode placed on layer II–III of the prefrontal cortex, where the input fibers are located.
Electrophysiological analysis of intracellular and extracellular EPSPs
The peak amplitude of EPSPs were measured with respect to the potential measured just prior to the stimulus artifact off-line using Clampfit 10 analysis software (Molecular Devices, Foster City, CA). Another method of measuring EPSPs is to measure the ascending slope of the response, but previous work from our laboratory and others has shown identical outcomes for measurement of response amplitude and response ascending slope [84, 85].
Synaptic responses recorded with intracellular sharp electrodes were sampled at (0.05 Hz) and the average of 3 samples (1 min) was plotted for each min of the experiment. Each control pretetanus 3-sample time point (see above) was normalized to the average value obtained over the entire 10-min baseline-recording period. Cells were included in the study if they 1) maintained stability in EPSP amplitude over the entire 10-min baseline-recording period (± 5% of the original EPSP amplitude) and 2) they maintained stable responses to current injection, including their pattern of action potential discharge generated by depolarizing injections of current. Posttetanic changes in response amplitude were calculated by expressing the amplitude of each one-min average as a percentage of the average response amplitude generated during the 10 min baseline-sampling period in each cell. In extracellular recordings form mPFC, initial slope (1 ms window) of extracellular field potentials was calculated for each response and values were plotted as percentage of the baseline.
Response descriptive statistics (i.e. mean ± s.e.m) were calculated for short term (3 min posttetanus both for intra and extracellular recordings) and long-term posttetanic samples (average of last 5 min recording of 30 min and 60 min postetanic periods for intra- and extracellular postsynaptic potentials, respectively). Differences in tetanus-induced plasticity were determined through repeated measures analysis of variance (ANOVA) performed across the entire post-tetanus sampling period. Post-hoc comparisons were also performed between each group at 3–4 min posttetanus (posttetanic plasticity) and at 16–20 min posttetanus (LTD, corticostriatal) and 25–30 minutes posttetanus (LTP, prefrontal cortex) using post hoc Student t-tests.
3.3. Results
3.3.1. HPLC analysis of strain differences in Dopamine (DA) and its metabolites (DOPAC & HVA)
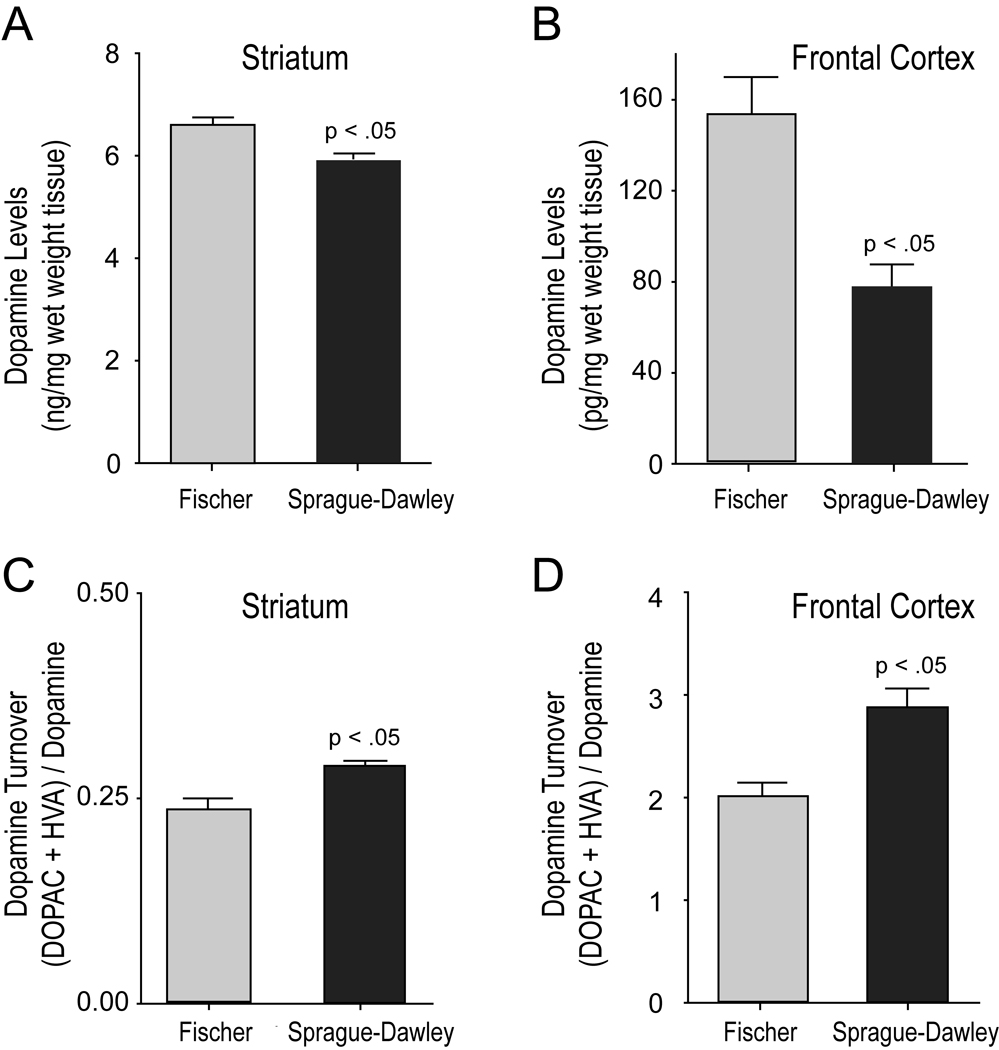
Striata of 1-month-old Fischer rats showed higher dopamine levels (6.566 ± 0.186 ng/mg wet weight tissue; n=7) than Sprague-Dawley rats (5.895 ± 0.170, n=7), t(12)=2.67, p=0.021. No strain differences were seen with dopamine metabolites. DOPAC levels in Fischer rats were 1.355 ± 0.0891 (n=7) and 1.344 ± 0.0518 (n=7) for Sprague-Dawley rats. HVA levels in Fischer rats were 0.318 ± 0.0356 (n=7) and 0.3625 ± 0.0109 (n=7) for Sprague-Dawley rats. However, striatal dopamine turnover as measured by the ratio of metabolites to dopamine levels was greater in Sprague-Dawley rats (0.2896 ± 0.0066; n=7) than in Fischer rats (0.2539 ± 0.0141; n=7), t(12)=−2.297 p=0.04 (Figure 4).
Figure 4.
Fischer 344 rats show elevated DA content and reduced DA turnover in the striatum and prefrontal cortex. Panels A & B show increased DA levels (Mean + SEM) observed in 1 month old male Fischer 344 rats compared to Sprague-Dawley rats for the striatum (A) and the prefrontal cortex (B). Note the difference in DA content scales for striatal versus frontal cortex tissue. Panels C & D show reduced DA turnover levels (Mean + SEM) in 1 month old male Fischer 344 versus Sprague-Dawley rats in the striatum and the frontal cortex. (Fischer 344 n=7; Sprague-Dawley n=7)
Strain-based comparison of dopamine neurochemistry in the frontal cortex revealed similar trends of greater DA content and reduced DA turnover in Fischer rats compared to Sprague-Dawley rats. DA content of Fischer rat frontal cortex was 153.245 ± 16.343 (pg/mg wet weight tissue, n=7) and 77.513 ± 9.694 pg/mg (n=7) in frontal cortex of Sprague-Dawley rats, t(11)=4.128, p=0.002. DA metabolites were also different between strains. DOPAC was 200.439 ± 9.360 pg/mg in Fischer rats and 154.626 ± 15.469 pg/mg in Sprague-Dawley rats (n=7), t(11)=2.534, p=0.034 and HVA was 77.538 ± 5.082 pg/mg in Fischer rats and 57.852 ± 6.917 pg/mg in Sprague-Dawley rats, t(10)=2.120, p=0.060. Dopamine turnover (DOPAC+HVA/DA) was greater in Sprague-Dawley (2.8748 ± .179) than it was in Fischer rats (2.0141 ± .126), t(10) =3.596, p=0.005 (Figure 4).
3.3.2. Fast scan cyclic voltammetry (FSCV) analysis of strain differences in DA release
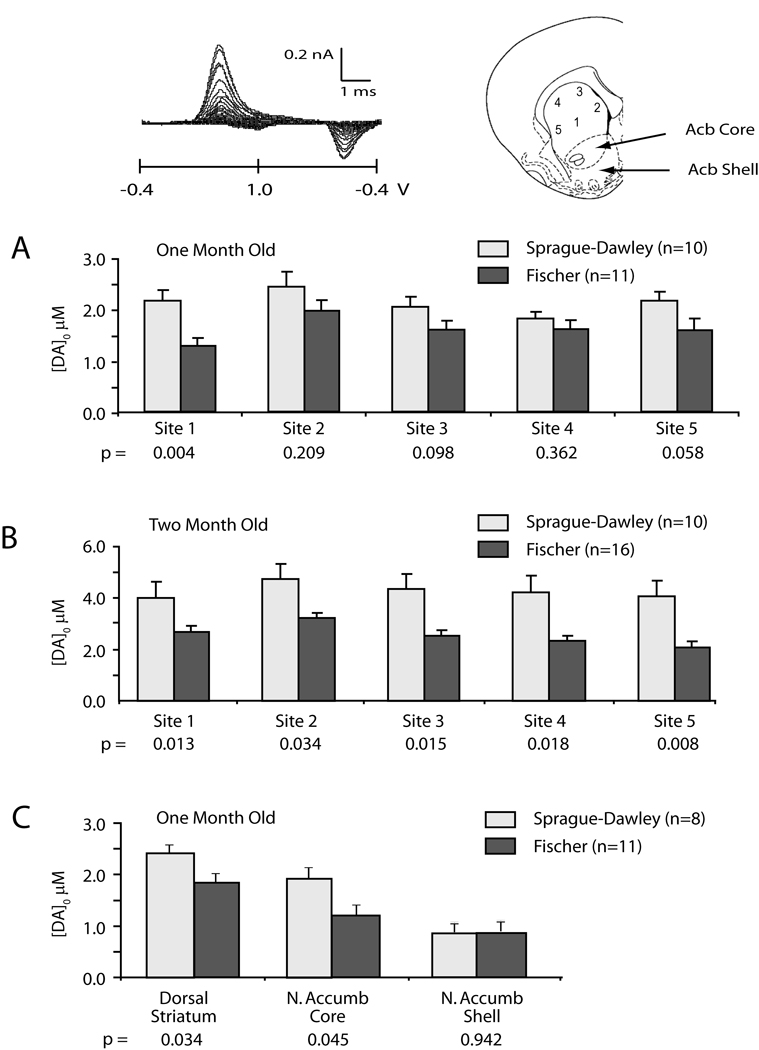
DA release was evaluated across dorsal-ventral and medial-lateral dimensions of the striatum in one set of experiments (Figure 5) and, in a second set of experiments, the dorsal striatum (site 3), the Acb core and the Acb shell were evaluated for strain differences in DA release. FSCV revealed that Sprague-Dawley rats (n=11 at 1 month, n=16 at 2 months) had greater evoked DA release than Fischer rats (n=10 at 1 month and 2 months) across all dimensions of the striatum at both 1 and 2 months postnatal. Comparison between strains across all 5 striatal sampling sites revealed less DA release in Fischer than Sprague-Dawley rats at one month post natal, F(1,19) = 4.434, p < .05, and 2 months postnatal, F(1,24) = 11.45, p < .003. Post-hoc comparison of individual sites in one-month-old rats revealed strain differences at central site 1 (p<0.03) (see inset diagram, Figure 5) and trends seen at dorsal site 3 (p<0.09) and ventrolateral site 5 (p<0.06). Post-hoc comparison of individual sites in two-month-old rats revealed strain differences at all sampling sites (see inset diagram, Figure 5) (site 1 p<0.03; site 2 p<0.01; site 3 p<0.003; site 4 p<0.003; site 5 p<0.002).
Figure 5.
Fischer 344 rats release less striatal DA than Sprague-Dawley rats. A & B: Maximum DA release evoked by a single 0.1 msec, 250 µA intrastriatal stimulus is plotted for 5 striatal sites recorded by FSCV from each coronal brain slice (see inset model of striatum). Strain differences were observed in DA release across all five anatomical sites in 1 and 2 month old rats. C: Maximum DA release evoked by a single 0.1 msec, 250 µA intrastriatal stimulus is plotted for the dorsal striatum (site 3) and the nucleus accumbens core and shell regions (Acb core and Acb shell in inset model). P values under each site reflect outcomes from site-specific post hoc t-tests. Inset shows example voltogram and recording sites in a coronal brain slice.
The second set of experiments examined DA release at dorsal striatal (site 3), Acb core and Acb shell regions and in those slices where release was able to be measured in all 3 regions we found a trend for decreased DA in Fischer slices across all three regions in one-month old rats, F(1,11) = 2.509, p < .15 (Fischer n=6, SD n=8). For those slices in which release could only be measured in dorsal striatum and Acb core, Fischer rats had less DA release than Sprague-Dawley rats, F(1,17) = 7.394, p < .015 (Fischer n=11, SD n= 8). T-tests performed at each site revealed Fischer rats released less DA than Sprague-Dawley rats in the dorsal striatum (p<0.035, Fischer n=11, SD=8), confirming the results in Figure 5a, in the Acb core (p<0.046, Fischer n=11, SD=8), but not in the Acb shell region (p=0.9, Fischer n=6, SD=8).
3.3.3. Dopamine dependent cortical synaptic plasticity
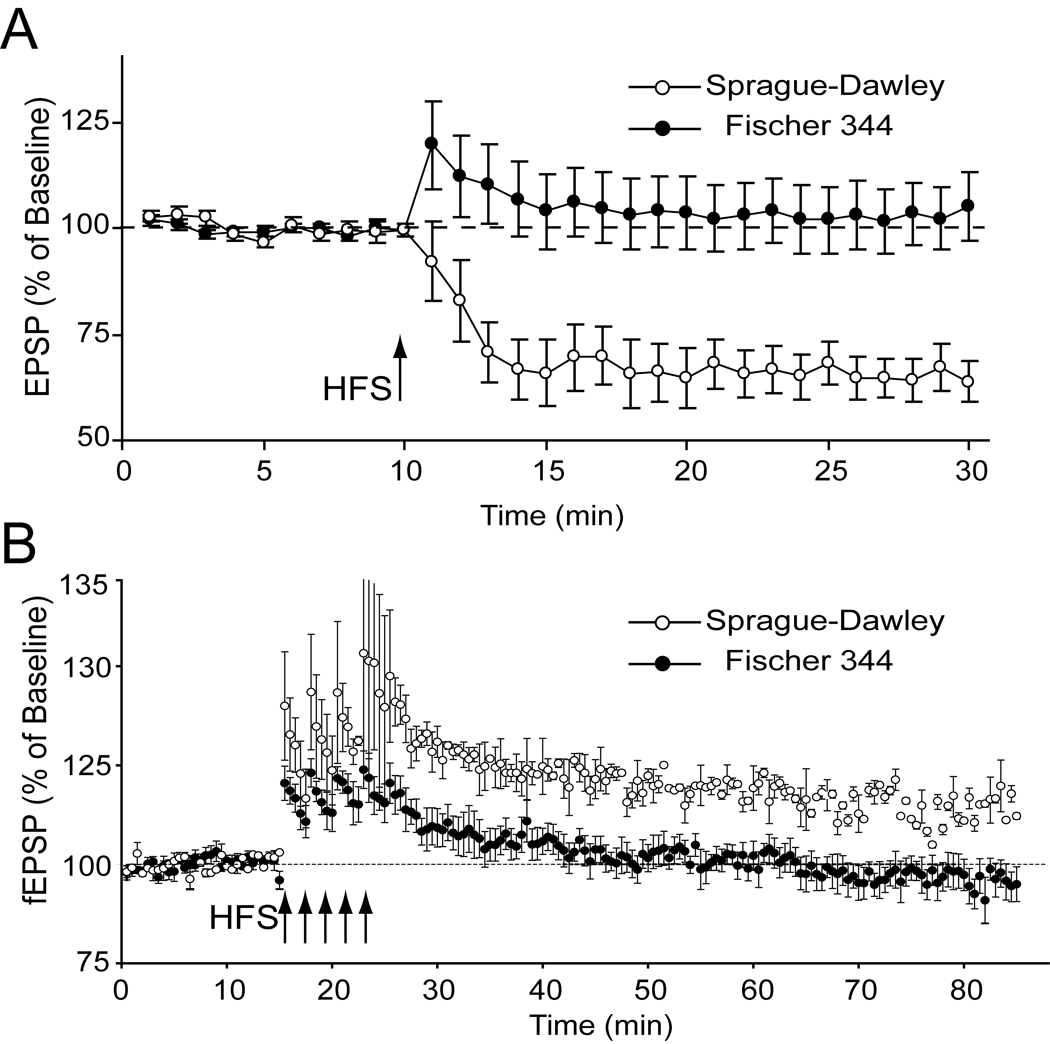
The effect of rat strain was studied for two dopamine-dependent forms of cortical synaptic plasticity; D2 receptor-dependent LTD evoked at corticostriatal synapses [58, 79] and D1 receptor-dependent LTP produced by layer II-III fibers synapsing with layer 5 neurons [78]. Sprague-Dawley showed increased expression of corticostriatal LTD across the entire 20-min post-tetanus sampling period, F(1,27) = 7.88, p<0.01 (Figure 6A). Post-hoc analysis of post-tetanic plasticity (average 0–3 min post-tetanus plasticity) revealed a significant (p = .0168) difference between Sprague-Dawley (81.76 ± 7.98%) and Fischer rats (113.76 ± 9.65%). Long-term plasticity (average 15–20 min post-tetanus) also differed significantly (p = .0003) between Sprague-Dawley (64.80+4.97%) and Fischer rats (102.88+7.60%).
Figure 6.
Juvenile Fischer 344 rats show reduced DA-dependent forms of cortical synaptic plasticity. Panel A shows corticostriatal long-term depression (LTD). Intracellular recordings revealed Fischer 344 rats expressed reduced corticostriatal LTD compared to Sprague-Dawley rats. Strain differences were observed in the tetanus induced change in EPSP amplitude across the entire post-tetatus sampling period. In panel B Fischer 344 rats show reduced prefrontal cortex long-term potentiation (LTP). Field potential recordings taken from layer 5 in the prefrontal cortex revealed Fischer 344 rats expressed reduced intracortical LTP. Strain differences were observed in the tetanus induced change in EPSP ampalitude across the entire post-tetanus sampling period.
Sprague-Dawley also showed increased expression of prefrontal cortex, D1 receptor-dependent LTP across the entire 20-min posttetanus sampling period, F(1,13) = 12.2, p<0.004 (Sprague-Dawley n=5; Fischer n=10) (Figure 6B). Post-hoc analysis of posttetanic plasticity (average 0–3 min posttetanus plasticity) also revealed a difference (Sprague-Dawley 134.92+6.27%, n=5; Fischer 113.14+3.66%, n=10; p=0.0200), as did long-term plasticity (average 15–20 min post-tetanus) (Sprague-Dawley 115.06+3.25%, n=5; Fischer 95.25+3.92%, n=10; p=0.0019).
4. Discussion
Play is so ubiquitous among mammals in general and rodents in particular that identification of a strain of rat that plays substantially less than other strains may provide valuable insight into both the genetics and neurobiological mechanisms behind this complex social behavior. The Fischer rat has been previously shown to be less playful than either inbred Lewis or Buffalo rats [30, 31] and the present data extends these findings by showing that Fischer rats also play less than the outbred Sprague-Dawley strain.
As with our earlier work, both play solicitation and overall responsiveness to playful nape contacts were dampened in the Fischer rats, indicating an overall decrease in playfulness in this strain rather than a deficit in one particular component of play. When responsiveness to nape contacts was analyzed in more detail, Fischer rats were found to be particularly impaired in responding to nape contacts with a complete rotation. Fischer rats were also less likely to respond to nape contacts by evading these contacts but did not differ from Sprague-Dawley rats in the likelihood of responding with a partial rotation. The lack of a strain difference in partial rotations with a robust difference in complete rotations is consistent with what we observed when comparing Fischer rats to Lewis rats [31] and suggests that this pattern of responding is a robust behavioral phenotype of the Fischer rat.
Pellis and his colleagues have compared the play of juveniles to that of young adults and characterized age-related shifts in how male rats respond to playful solicitations as they mature [7, 86]. As juveniles, male rats are most likely to respond to playful solicitations by rotating completely onto their back. As rats mature, they are less likely to respond with complete rotations while more likely to respond with partial rotations. Given the pattern of responsiveness noted in Fischer rats when compared to Sprague-Dawley rats (i.e., fewer complete rotations with no change in partial rotations) combined with less solicitation of play, one possible interpretation of these data is that Fischer rats are more similar to adult rats in how they play and this may reflect an early maturation of this strain. Fischer rats in the present study were tested at approximately 35 days of age and an almost identical pattern of responding was observed in our earlier study when rats were tested at 28 days [31], suggesting that this pattern of responding is robust and stable across development rather than reflecting early maturation. However, a more comprehensive analysis of these strain differences across a wider range of ages is clearly needed.
Fischer rats differ from other strains on several dimensions of monoaminergic functioning, including differences in dopaminergic functioning [32–36]. In order to expand our behavioral comparison between juvenile Fischer and Sprague-Dawley on a measure known to be sensitive to dopaminergic functioning, we also assessed pre-pulse inhibition (PPI) of the acoustic startle response in these two strains. When compared to Sprague-Dawley rats, Fischer rats had less of a startle response and enhanced PPI. The attenuated startle response observed in our hands with young rats is consistent with previous research in adult rats [70, 87–89] and suggests that this phenotypic difference represents a stable trait across development. In direct contrast to the robust strain difference in PPI observed in the present study, only minimal strain differences in PPI have been reported previously [69, 70]. Several possibilities can be suggested to explain the relatively robust differences in this study compared to previous studies. First, pre-pubertal rats were used in this study while earlier studies used adult rats. Since dopaminergic systems are in flux during this age period [1, 90] age-related fluctuations in sensitivity of dopaminergic systems prior to puberty may yield more robust strain differences at this age. Baseline PPI at the lowest pre-pulse intensity (+5 dB) in the present study was also fairly low (approximately 35% inhibition) compared to other studies, perhaps allowing more room for an enhancement to be observed when comparing the two strains.
In Experiment 2 we sought to quantify any differences in DA physiology between young Fischer and Sprague-Dawley rats. The data indicates that Fischer rats may have deficits in handling and delivery of vesicular dopamine, but not in all DA terminal regions. FSCV demonstrated that Fischer rats released significantly less DA than Sprague-Dawley rats across five sampling locations in the dorsal striatum and in the Acb core but not in the Acb shell. The lack of a strain difference in dopamine release in the Acb shell is particularly interesting and suggests that delivery of DA in this region is similar between the two strains. The Acb core has many similarities to dorsal striatum, with both regions involved in orchestrating specific behavior patterns, while the Acb shell seems to be more unique in terms of inputs, outputs, physiology and function [91–93]. Particularly interesting with regards to the current study is that the ACb shell is thought to have direct control of DA release in other parts of the striatum (e.g., dorsal striatum and ACb core) and that this modulation may help invigorate motivationally relevant behaviors [93]. For Fischer rats, an uneven distribution of DA release in the various compartments of the dorsal and ventral striatum may result in an inability of these rats to fully engage in the fluid motor sequences seen in play.
Although DA release among Fischer rats was consistently lower in dorsal striatum and Acb core, dopamine content was higher in Fischer than in Sprague-Dawley rats for both cortical and striatal samples. However, dopamine turn-over was less in Fischer than in Sprague-Dawley rats and this would be consistent with impaired vesicular release. Higher dopamine content associated with reduced dopamine release could be due to a strain-dependent disruption in dopamine transport into vesicles. Cytoplasmic dopamine accumulation is proposed to occur when vesicular dopamine transport is blocked [94] and this would presumably result in less vesicular release of dopamine.
Less vesicular release of dopamine in Fischer rats would be consistent with studies using in vivo microdialysis showing decreased dopamine release in the nucleus accumbens of Fischer rats compared to Lewis rats following nicotine [95] and amphetamine [32]. Since amphetamine is thought to release dopamine into the synapse by both vesicular and non-vesicular means [96] less release of dopamine in Fischer rats could be somewhat problematic from this perspective. For example, if Fischer rats have an accumulation of cytoplasmic dopamine then one might expect amphetamine to yield more dopamine release in Fischer rats. However, Cadoni & di Chiara [32] found that while Fischer rats had less dopamine release in the ACb core at all doses of amphetamine tested, a different pattern emerged with dialysis probes in the ACb shell. A low dose of amphetamine (0.25 mg/kg) resulted in less release of dopamine in Fischer rats while a higher dose (1.0 mg/kg) yielded more release in this strain. An intermediate dose (0.5 mg/kg) yielded no strain difference in release for this brain region. These findings suggest a complex pattern of regional and dose-related differences in how amphetamine can affect dopamine release between strains of rats, a complexity that appears to be reflected in our findings as well (Figure 5).
An important physiological consequence of Fischer rats having less synaptic dopamine would be that cortical synaptic behavior would also be expected to be altered. Cortical synaptic function in the prefrontal cortex and the dorsal striatum is modulated by dopamine and we found evidence of deficiencies in dopamine-dependent forms of synaptic plasticity in both structures in Fischer rats. LTP at excitatory synapses in the prefrontal cortex is enabled by D1 dopamine receptor activation in Sprague-Dawly rats [59, 60, 78]. We found that Sprague-Dawley rats expressed LTP using the paradigm outlined by Huang et al [78], although the same paradigm did not induce LTP in Fischer rats (Figure 6). Corticostriatal synapses in the dorsal striatum express a D2 dopamine-dependent form of corticostriatal LTD in Sprague-Dawley rats [58, 61]. Confirming these studies, we found that young Sprague-Dawley rats expressed DA-dependent LTD. However, Fischer rats were, again, deficient in this DA-dependent form of cortical plasticity (Figure 6). This finding confirms earlier work from our laboratory showing young Fischer rats do not express corticostriatal LTD in vitro [97]. These findings in combination with the deficits in dopamine release we observed in Fischer rats indicates that insufficient dopamine release may underlie abnormalities in long-term plasticity seen with cortical synapses in Fischer rats and may contribute to the behavioral phenotype for this strain as well.
Less release of dopamine in Fischer rats is also consistent with our finding that rats of this strain exhibited more pre-pulse inhibition (PPI) than Sprague-Dawley rats. PPI is a form of sensorimotor gating that is consistently attenuated by indirect and direct dopamine agonists [67, 98–101] and that can also be enhanced by dopamine antagonists [102, 103]. It is especially noteworthy that enhanced PPI among Fischer rats was only apparent when the pre-pulse was at 10 and 15 dB above background, since this is the range suggested to be more indicative of dopamine function than what is seen with 5 dB above background [103]. This suggests that the enhanced PPI observed among Fischer rats in the present study may be a behavioral reflection of impaired dopamine release in this strain.
The deficits in handling and release of dopamine in Fischer rats may also be partly involved in the relative lack of play in this strain. Although a role for dopamine in modulating play behavior is far from clear, there is still abundant evidence for dopaminergic involvement. Dopamine utilization increases during play bouts [37], dopamine antagonists uniformly reduce play [38–40], and neonatal 6-OHDA lesions impair the sequencing of behavioral elements during play bouts [41]. While it has been difficult to obtain consistent increases in play with dopamine agonists [38, 40, 104], dopamine antagonists block the increases in play following alcohol, nicotine, and indirect cannabinoid agonists [105, 106]. Taken together, these data suggest that play is associated with increased release of dopamine and that an optimal level of dopamine functioning is necessary for play to occur [73]. With impairments in the ability to release and utilize synaptic dopamine, Fischer rats may be unable to readily execute the behavioral patterns needed for active engagement in rough-and-tumble activities.
Due to the robust behavioral differences and unique dopamine physiology observed in Fischer rats, this strain may be particularly useful for systematic studies addressing the genetic and neurobiological substrates underlying complex social behaviors, such as play. Considerable insight into the genetic foundations for other neurobehavioral processes have been made through the use of transgenic and knock-out mouse models [107], although the relative lack of play in the mouse limits their use for studying the dynamics of reciprocal social interactions commonly seen during behaviors such as play. While mice exhibit a rudimentary type of social play [108–110] they do not show the same type of reciprocal give-and-take seen during play bouts of rats and any other mammals, including human children. The Fischer rat may then be a useful addition to our genetic arsenal.
The Fischer rat may also be useful for gaining insight into certain aspects of neurodevelopmental disorders that may be marked by impaired playfulness and deficits in DA functioning. For example, attention deficit/hyperactivity disorder (ADHD) is thought to be associated with dampened DA functioning [111–114], although the strength of this association has recently been questioned [115]. Few studies have systematically quantified play in ADHD children and only a handful of those studies have looked at children not on medication at the time of testing [116, 117], although children with ADHD engaged in less social play than non-ADHD peers in both of these studies. ADHD children on medication have also been reported to score lower on the Test of Playfulness [118] and engage in less social play when compared to a non-ADHD comparison group [119]. However, it is not clear in these latter studies if the deficits in play are symptomatic of the disorder in these children or are a consequence of their treatment, especially given the extent to which psychomotor stimulants can reduce play in rats [38, 44, 120]. While it is unlikely that any particular strain of rat, including the Fischer strain, can model all aspects of a multifaceted disorder such as ADHD, this strain may still be quite useful for better understanding aspects of those disorders where normal playful interactions are impaired and in identifying the neurobiological substrate(s) associated with that specific impairment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 2.Burghardt GM. The Genesis of Animal Play: Testing the Limits. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- 3.Fagen R. Animal Play Behavior. New York: Oxford University Press; 1981. [Google Scholar]
- 4.Pellis SM, Pellis VC. The Playful Brain: Venturing to the Limits of Neuroscience. Oxford: Oneworld Publications; 2009. [Google Scholar]
- 5.Meaney MJ, Stewart J. A descriptive study of social development in the rat (Rattus norvegicus) Animal Behaviour. 1981;29:34–45. [Google Scholar]
- 6.Panksepp J. The ontogeny of play in rats. Developmental Psychobiology. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- 7.Pellis SM, Pellis VC. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Developmental Psychobiology. 1990;23:215–231. doi: 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- 8.Smith LK, Fantella S-LN, Pellis SM. Playful defensive responses in adult male rats depend on the status of the unfamiliar opponent. Aggressive Behavior. 1999;25:141–152. [Google Scholar]
- 9.Smith LK, Forgie ML, Pellis SM. Mechanisms underlying the absence of the pubertal shift in the playful defense of female rats. Developmental Psychobiology. 1998;33:147–156. [PubMed] [Google Scholar]
- 10.Burghardt GM, Chiszar D, Murphy JB, Romano J, Walsh T, Manrod J. Behavioral complexity, behavioral development, and play. In: Murphy JB, Ciofi C, de La Panouse C, Walsh T, editors. Komodo Dragons: Biology and Conservation. Washington: Smithsonian Institution Press; 2002. pp. 78–117. [Google Scholar]
- 11.Pellis SM, Pellis VC. Rough-and-tumble play and the development of the social brain. Current Directions in Psychological Science. 2007;16:95–98. [Google Scholar]
- 12.Spinka M, Newberry RC, Bekoff M. Mammalian play: Training for the unexpected. The Quarterly Review of Biology. 2001;76:141–168. doi: 10.1086/393866. [DOI] [PubMed] [Google Scholar]
- 13.Van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Developmental Psychobiology. 1999;34:129–138. [PubMed] [Google Scholar]
- 14.Almeida SS, De Araujo M. Postnatal protein malnutrition affects play behavior and other social interactions in juvenile rats. Physiology and Behavior. 2001;74:45–51. doi: 10.1016/s0031-9384(01)00554-6. [DOI] [PubMed] [Google Scholar]
- 15.Baldwin JD, Baldwin JI. Effects of food ecology on social play: A laboratory simulation. Z Tierpsychol. 1976;40:1–14. doi: 10.1111/j.1439-0310.1976.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 16.Siviy SM, Panksepp J. Energy balance and juvenile play in rats. Physiology and Behavior. 1985;35:435–441. doi: 10.1016/0031-9384(85)90320-8. [DOI] [PubMed] [Google Scholar]
- 17.Panksepp J. Affective Neuroscience: The Foundations of Human and Animal Emotions. New York: Oxford University Press; 1998. [Google Scholar]
- 18.Siviy SM, Harrison KA, McGregor IS. Fear, risk assessment, and playfulness in the juvenile rat. Behavioral Neuroscience. 2006;120:49–59. doi: 10.1037/0735-7044.120.1.49. [DOI] [PubMed] [Google Scholar]
- 19.Romeo RD, Karatsoreos IN, McEwen BS. Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Hormones and Behavior. 2006;50:463–468. doi: 10.1016/j.yhbeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Vanderschuren LJMJ, Niesink RJM, Spruijt BM, Van Ree JM. Influence of environmental factors on social play behavior of juvenile rats. Physiology and Behavior. 1995;58:119–123. doi: 10.1016/0031-9384(94)00385-i. [DOI] [PubMed] [Google Scholar]
- 21.Panksepp J. Attention deficit hyperactivity disorders, psychostimulants, and intolerance of childhood playfulness: A tragedy in the making? Current Directions in Psychological Science. 1998;7:91–98. [Google Scholar]
- 22.Pellegrini AD, Dupuis D, Smith PK. Play in evolution and development. Dev Rev. 2007;27:261–276. [Google Scholar]
- 23.Burghardt GM. Play: Attributes and neural substrates. In: Blass EM, editor. Handbook of Behavioral Neurobiology. New York: Kluwer Academic/ Plenum Publishers; 2001. pp. 317–356. [Google Scholar]
- 24.Siviy SM. Neurobiological substrates of play behavior: glimpses into the structure and function of mammalian playfulness. In: Bekoff M, Byers JA, editors. Animal Play: Evolutionary, Comparative, and Ecological Perspectives. Cambridge: Cambridge University Press; 1998. pp. 221–242. [Google Scholar]
- 25.Vanderschuren LJMJ, Niesink RJM, Van Ree JM. The neurobiology of social play behavior in rats. Neuroscience and Biobehavioral Reviews. 1997;21:3090–3326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 26.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Reif A, Lesch K-P. Toward a molecular architecture of personality. Behavioural Brain Research. 2003;139:1–20. doi: 10.1016/s0166-4328(02)00267-x. [DOI] [PubMed] [Google Scholar]
- 28.Guo S. Linking genes to brain, behavior and neurological diseases: what can we learn from zebrafish? Genes, Brain, And Behavior. 2004;3:63–74. doi: 10.1046/j.1601-183x.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 29.Gainetdinov RR, Caron MG. Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 30.Siviy SM, Baliko CN, Bowers KS. Rough-and-tumble play behavior in Fischer-344 and Buffalo rats: Effects of social isolation. Physiology and Behavior. 1997;61:597–602. doi: 10.1016/s0031-9384(96)00509-4. [DOI] [PubMed] [Google Scholar]
- 31.Siviy SM, Love NJ, DeCicco BM, Giordano SB, Seifert TL. The relative playfulness of juvenile Lewis and Fischer-344 rats. Physiology and Behavior. 2003;80:385–394. doi: 10.1016/j.physbeh.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Cadoni C, di Chiara G. Differences in dopamine responsiveness to drugs of abuse in the nucleus accumbens shell and core of Lewis and Fischer 344 rats. Journal of Neurochemistry. 2007;103:487–499. doi: 10.1111/j.1471-4159.2007.04795.x. [DOI] [PubMed] [Google Scholar]
- 33.Guitart X, Kogan JH, Berhow M, Terwilliger RZ, Aghajanian GK, Nestler EJ. Lewis and Fischer rat strains display differences in biochemical, electrophysiological and behavioral parameters: studies in the nucleus accumbens and locus coeruleus of drug naive and morphine-treated animals. Brain Research. 1993;611:7–17. doi: 10.1016/0006-8993(93)91770-s. [DOI] [PubMed] [Google Scholar]
- 34.Gulley JM, Everett CV, Zahniser NR. Inbred Lewis and Fischer 344 rat strains differ not only in novelty- and amphetamine-induced behaviors, but also in dopamine transporter activity in vivo. Brain Research. 2007;1151:32–45. doi: 10.1016/j.brainres.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris HW, Nestler EJ. Immunohistochemical studies of mesolimbic dopaminergic neurons in Fischer 344 and Lewis rats. Brain Research. 1996;706:1–12. doi: 10.1016/0006-8993(95)01088-2. [DOI] [PubMed] [Google Scholar]
- 36.Helmeste DM, Seeman P, Coscina DV. Relation between brain catecholamine receptors and dopaminergic stereotypy in rat strains. European Journal of Pharmacology. 1981;69:465–470. doi: 10.1016/0014-2999(81)90450-7. [DOI] [PubMed] [Google Scholar]
- 37.Panksepp J. Rough and tumble play: A fundamental brain process. In: MacDonald K, editor. Parent-Child Play. Albany: SUNY Press; 1993. pp. 147–184. [Google Scholar]
- 38.Beatty WW, Costello KB, Berry SL. Suppression of play fighting by amphetamine: Effects of catecholamine antagonists, agonists and synthesis inhibitors. Pharmacology Biochemistry and Behavior. 1984;20:747–755. doi: 10.1016/0091-3057(84)90194-1. [DOI] [PubMed] [Google Scholar]
- 39.Niesink RJM, Van Ree JM. Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology. 1989;28:411–418. doi: 10.1016/0028-3908(89)90038-5. [DOI] [PubMed] [Google Scholar]
- 40.Siviy SM, Fleischhauer AE, Kerrigan LA, Kuhlman SJ. D2 dopamine receptor involvement in the rough-and-tumble play behavior of juvenile rats. Behavioral Neuroscience. 1996;110:1–9. doi: 10.1037//0735-7044.110.5.1168. [DOI] [PubMed] [Google Scholar]
- 41.Pellis SM, Casteneda E, McKenna MM, Tran-Nguyen LT, Whishaw IQ. The role of the striatum in organizing sequences of play fighting in neonatally dopamine-depleted rats. Neuroscience Letters. 1993;158:13–15. doi: 10.1016/0304-3940(93)90600-p. [DOI] [PubMed] [Google Scholar]
- 42.Trezza V, Baarendse PJJ, Vanderschuren LJMJ. Prosocial Effects of Nicotine and Ethanol in Adolescent Rats Through Partially Dissociable Neurobehavioral Mechanisms. Neuropsychopharmacology. 2009;34:2560–2573. doi: 10.1038/npp.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trezza V, Vanderschuren LJMJ. Divergent effects of anandamide transporter inhibitors with different target selectivity on social play behavior in adolescent rats. The Journal of Pharmacology and Experimental Therapeutics. 2009;328:343–350. doi: 10.1124/jpet.108.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanderschuren LJMJ, Trezza V, Griffioen-Roose S, Schiepers OJG, Van Leeuwen N, De Vries TJ, et al. Methylphenidate disrupts social play behavior in adolescent rats. Neuropsychopharmacology. 2008;33:2946–2956. doi: 10.1038/npp.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panksepp J, Normansell L, Cox JF, Crepeau LJ, Sacks DS. Psychopharmacology of social play. In: Olivier B, Mos J, Brain PF, editors. Ethopharmacology of agonistic behaviour in animals and humans. Dordrecht, Holland: Martinus Nijhoff Publishers; 1987. pp. 132–141. [Google Scholar]
- 46.Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: An affective neuroethological perspective. Brain Research Reviews. 2007;56:283–321. doi: 10.1016/j.brainresrev.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 48.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Research Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 49.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 50.McGraw La, Young LJ. The praire vole: an emerging model organism for undertanding the social brain. Trends in Neuroscience. 2009;33:103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young LJ, Young AZM, Hammock EAD. Anatomy and neurochemistry of the pair bond. The Journal of Comparative Neurology. 2005;493:51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
- 52.Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends in Cognitive Sciences. 2009;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: Electrode mapping, lesion, and pharmacology studies. Behavioural Brain Research. 2007;182:274–283. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 54.Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. Journal of Comparative Psychology. 2008;122:357–367. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- 55.Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. Journal of Comparative Psychology. 1998;112:65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- 56.Leblois A, Wendel BJ, Perkel DJ. Striatal Dopamine Modulates Basal Ganglia Output and Regulates Social Context-Dependent Behavioral Variability through D1 Receptors. J Neurosci. 2010;30:5730–5743. doi: 10.1523/JNEUROSCI.5974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine Modulates Release from Corticostriatal Terminals. J Neurosci. 2004;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. The Journal of Neuroscience. 1992;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gurden H, Takita M, Jay TM. Essential Role of D1 But Not D2 Receptors in the NMDA Receptor-Dependent Long-Term Potentiation at Hippocampal-Prefrontal Cortex Synapses In Vivo. J Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. 106RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proceedings of the National Academy of Science (USA) 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang KC, Lowe ML, Grandy DK, Lovinger DM. Dopamine-dependent synaptic plasticity in striatum during development. Proceedings of the National Academy of Science (USA) 2001;98:1255–1260. doi: 10.1073/pnas.031374698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Broderick PA. In vivo voltammetric studies on release mechanisms for cocain with gamma-butyyrolactone. Pharmacology Biochemistry and Behavior. 1991;40:969–975. doi: 10.1016/0091-3057(91)90113-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: An in vivo voltammetric study. The Journal of Neuroscience. 1994;14:442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kolomiets B, Marzo A, Caboche J, Vanhoutte P, Otani S. Background Dopamine Concentration Dependently Facilitates Long-term Potentiation in Rat Prefrontal Cortex through Postsynaptic Activation of Extracellular Signal-Regulated Kinases. Cereb Cortex. 2009;19:2708–2718. doi: 10.1093/cercor/bhp047. [DOI] [PubMed] [Google Scholar]
- 65.Stamford JA, Kruk ZL, Millar J. Measurement of stimulated dopamine release in the rat by in vivo voltammetry: The influence of stimulus duration on drug responses. Neuroscience Letters. 1986;69:70–73. doi: 10.1016/0304-3940(86)90416-7. [DOI] [PubMed] [Google Scholar]
- 66.Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- 67.Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 68.Swerdlow NR, Shoemaker JM, Bongiovanni MJ, Neary AC, Tochen LS, Saint Marie RL. Strain differences in the disruption of prepulse inhibition of startle after systemic and intra-accumbens amphetamine administration. Pharmacology Biochemistry and Behavior. 2007;87:1–10. doi: 10.1016/j.pbb.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swerdlow NR, Shoemaker JM, Crain S, Goins J, Onozuka K, Auerbach PP. Sensitivity to drug effects on prepulse inhibition in inbred and outbred rat strains. Pharmacol Biochem Behav. 2004;77:291–302. doi: 10.1016/j.pbb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 70.Varty GB, Geyer MA. Effects of isolation rearing on startle reactivity, habituation, and prepulse inhibition in male Lewis, Sprague-Dawley, and Fischer-344 rats. Behavioral Neuroscience. 1998;112:1450–1457. doi: 10.1037//0735-7044.112.6.1450. [DOI] [PubMed] [Google Scholar]
- 71.Pellis SM, Pellis VM. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Aggressive Behavior. 1987;13:227–252. [Google Scholar]
- 72.Pellis SM, Pellis VC. Attack and defense during play fighting appear to be motivationally independent behaviors in muroid rodents. The Psychological Record. 1991;41:175–184. [Google Scholar]
- 73.Trezza V, Baarendse PJJ, Vanderschuren LJMJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends in Pharmacological Sciences. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gordon NS, Kollack-Walker S, Akil H, Panksepp J. Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Research Bulletin. 2002;57:651–659. doi: 10.1016/s0361-9230(01)00762-6. [DOI] [PubMed] [Google Scholar]
- 75.Bell HC, McCaffrey DR, Forgie ML, Kolb B, Pellis SM. The role of the medial prefrontal cortex in the play fighting of rats. Behavioral Neuroscience. 2009;123:1158–1168. doi: 10.1037/a0017617. [DOI] [PubMed] [Google Scholar]
- 76.Cheng SY, Taravosh-Lahn K, Delville Y. Neural circuitry of play fighting in golden hamsters. Neuroscience. 2008;156:247–256. doi: 10.1016/j.neuroscience.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 77.Pellis SM, Hastings E, Shimizu T, Kamitakahara H, Komorowska J, Forgie ML, et al. The effects of orbital frontal cortex damage on the modulation of defensive responses by rats in playful and nonplayful social contexts. Behavioral Neuroscience. 2006;120:72–84. doi: 10.1037/0735-7044.120.1.72. [DOI] [PubMed] [Google Scholar]
- 78.Huang YY, Simpson E, Kellendonk C, Kandel ER. Genetic evidence for the bidirectional modulation synaptic plasticity in the prefrontal cortex by D1 receptors. Proceedings of the National Academy of Science (USA) 2004;101:3236–3241. doi: 10.1073/pnas.0308280101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 80.Akopian G, Crawford C, Beal MF, Cappelletti M, Jakowec MW, Petzinger GM, et al. Decreased Striatal Dopamine Release Underlies Increased Expression of Long-Term Synaptic Potentiation at Corticostriatal Synapses 24 h after 3-Nitropropionic-Acid-Induced Chemical Hypoxia. J Neurosci. 2008;28:9585–9597. doi: 10.1523/JNEUROSCI.5698-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, et al. Effects of Treadmill Exercise on Dopaminergic Transmission in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Lesioned Mouse Model of Basal Ganglia Injury. J Neurosci. 2007;27:5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patel J, Rice ME. Monitoring DA release in brain slices. In: Grimes CA, Dickey EC, Pishko MV, editors. Encylopedia of Sensors. Stevenson Ranch, CA: American Scientific Publishers; 2006. pp. 313–334. [Google Scholar]
- 83.Patel J, Mooslehner KA, Chan PM, Emson PC, Stamford JA. Presynaptic control of striatal DA neurotransmission in adult vesicular monoamine transporter 2 (VMAT2) mutant mice. Journal of Neurochemistry. 2003;85:898–910. doi: 10.1046/j.1471-4159.2003.01732.x. [DOI] [PubMed] [Google Scholar]
- 84.Kerr JND, Wickens JR. Dopamine D1/D5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J Neurophysiol. 2001;85:117–124. doi: 10.1152/jn.2001.85.1.117. [DOI] [PubMed] [Google Scholar]
- 85.Villar FDS, Walsh JP. Modulation of long-term synaptic plasticity at excitatory striatal synapses. Neuroscience. 1999;90:1031–1041. doi: 10.1016/s0306-4522(98)00504-1. [DOI] [PubMed] [Google Scholar]
- 86.Pellis SM, Pellis VC, McKenna MM. Some subordinates are more equal than others: Play fighting amongst adult subordinate male rats. Aggressive Behavior. 1993;19:385–393. [Google Scholar]
- 87.Glowa JR, Geyer MA, Gold PW, Sternberg EM. Differential startle amplitude and corticosterone response in rats. Neuroendocrinology. 1992;56:719–723. doi: 10.1159/000126298. [DOI] [PubMed] [Google Scholar]
- 88.Gomez-Serrano MA, Tonelli L, Listwak S, Sternberg EM, Riley AL. Effects of cross fostering on open-field behavior, acoustic startle, lipopolysaccharide-induced corticosterone release, and body weight in Lewis and Fischer rats. Behav Genet. 2001;31:427–436. doi: 10.1023/a:1012742405141. [DOI] [PubMed] [Google Scholar]
- 89.Stohr T, Szuran T, Welzl H, Pliska V, Feldon J, Pryce CR. Lewis/Fischer rat strain differences in endocrine and behavioural responses to environmental challenge. Pharmacology Biochemistry and Behavior. 2000;67:809–819. doi: 10.1016/s0091-3057(00)00426-3. [DOI] [PubMed] [Google Scholar]
- 90.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Basar K, Sesia T, Groenewegen H, Steinbusch HWM, Visser-Vandewalle V, Temel Y. Nucleus accumbens and impulsivity. Progress in Neurobiology. 2010;92:533–557. doi: 10.1016/j.pneurobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 92.Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Progress in Neurobiology. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 93.Voorn P, Vanderschuren LJMJ, Groenewegen HJ, Robbins TW, Pennartz CMA. Putting a spin on the dorsal-ventral divide of the striatum. Trends in Neuroscience. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 94.Fleckenstein AE, Hanson GR. Impact of psychostimulants on vesicular monoamine transporter function. European Journal of Pharmacology. 2004;479:283–289. doi: 10.1016/j.ejphar.2003.08.077. [DOI] [PubMed] [Google Scholar]
- 95.Cadoni C, Muto T, Di Chiara G. Nicotine differentially affects dopamine transmission in the nucleus accumbens shell and core of Lewis and Fischer 344 rats. Neuropharmacology. 2009;57:496–501. doi: 10.1016/j.neuropharm.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 96.Sulzer D, Sonders MS, Poulsen Nw, Galli A. Mechanisms of neurotransmitter release by amphetamines: A review`. Progress in Neurobiology. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 97.Akopian G, Walsh JP. Reduced expression of short- and long-term facilitation at aged corticostriatal synapses Synapse. 2006;60:223–238. [Google Scholar]
- 98.Davis M, Mansbach RS, Swerdlow NR, Campeau S, Braff DL, Geyer MA. Apomorphine disrupts the inhibition of acoustic startle induced by weak prepulses in rats. Psychopharmacology. 1990;102:1–4. doi: 10.1007/BF02245735. [DOI] [PubMed] [Google Scholar]
- 99.Swerdlow NR, Mansbach RS, Geyer MA, Pulvirenti L, Koob GF, Braff DL. Amphetamine disruption of prepulse inhibition of acoustic startle is reversed by depletion of mesolimbic dopamine. Psychopharmacology. 1990;100:413–416. doi: 10.1007/BF02244616. [DOI] [PubMed] [Google Scholar]
- 100.Sills TL. Amphetamine dose dependently disrupts prepulse inhibition of the acoustic startle response in rats within a narrow time window. Brain Research Bulletin. 1999;48:445–448. doi: 10.1016/s0361-9230(99)00036-2. [DOI] [PubMed] [Google Scholar]
- 101.Breier MR, Lewis B, Shoemaker JM, Light GA, Swerdlow NR. Sensory and sensorimotor gating-disruptive effects of apomorphine in Sprague Dawley and Long Evans rats. Behavioural Brain Research. 2010;208:560–565. doi: 10.1016/j.bbr.2009.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Depoortere R, Perrault G, Sanger DJ. Potentiation of prepulse inhibition of the startle reflex in rats: pharmacological evaluation of the procedure as a model for detecting antipsychotic activity. Psychopharmacology. 1997;132:366–374. doi: 10.1007/s002130050357. [DOI] [PubMed] [Google Scholar]
- 103.Feifel D. Individual differences in prepulse inhibition of startle as a measure of individual dopamine function. Behavioral Neuroscience. 1999;113:1020–1029. doi: 10.1037//0735-7044.113.5.1020. [DOI] [PubMed] [Google Scholar]
- 104.Field EF, Pellis SM. Differential effects of amphetamine on the attack and defense components of play fighting in rats. Physiology and Behavior. 1994;56:325–330. doi: 10.1016/0031-9384(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 105.Trezza V, Vanderschuren LJMJ. Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology. 2008;197:217–227. doi: 10.1007/s00213-007-1025-3. [DOI] [PubMed] [Google Scholar]
- 106.Trezza V, Baarendse PJJ, Vanderschuren LJMJ. Prosocial effects of nicotine and ethanol in adolescent rats through partially dissociable neurobehavioral mechanisms. Neuropsychopharmacology. 2009;34:2560–2573. doi: 10.1038/npp.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crawley JN. Behavioral Phenotyping of Transgenic and Knockout Mice. New York: Wiley-Liss; 2000. What's Wrong With My Mouse? [Google Scholar]
- 108.Pellis SM, Pasztor TJ. The developmental onset of a rudimentary form of play fighting in C57 mice. Developmental Psychobiology. 1999;34:175–182. [PubMed] [Google Scholar]
- 109.Terranova ML, Laviola G. Scoring of social interactions and play in mice during adolescence. Current Protocols in Neuroscience. 2005 Supplement 26 doi: 10.1002/0471140856.tx1310s26. 13.0.1-.0.1. [DOI] [PubMed] [Google Scholar]
- 110.Walker C, Byers JA. Heritability of locomotor play in house mice, Mus domesticus. Animal Behavior. 1991;42:891–897. [Google Scholar]
- 111.Pliszka SR. The Neuropsychopharmacology of Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2005;57:1385–1390. doi: 10.1016/j.biopsych.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 112.Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attentin-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences. 2005;28:397–468. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- 113.Tripp G, Wickens JR. Neurobiology of ADHD. Neuropharmacology. 2009;57:579–589. doi: 10.1016/j.neuropharm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 114.Volkow ND, Wang G-J, Newcorn J, Telang F, Solanto MV, Fowler JS, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults With Attention-Deficit/Hyperactivity Disorder. Arch Gen Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- 115.Gonon F. The dopaminergic hypothesis of attention-deficit/hyperactivity disorder needs re-examining. Trends Neurosci. 2009;32:2–8. doi: 10.1016/j.tins.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 116.Alessandri SM. Attention, play, and social behavior in ADHD preschoolers. Journal of Abnormal Child Psychology. 1992;20:289–302. doi: 10.1007/BF00916693. [DOI] [PubMed] [Google Scholar]
- 117.Cordier R, Bundy A, Hocking C, Einfeld S. Comparison of the play of children with attention deficit hyperactivity disorder by subtypes. Australian Occupational Therapy Journal. 2010;57:137–145. doi: 10.1111/j.1440-1630.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 118.Leipold EE, Bundy AC. Playfulness in children with attention deficit hyperactivity disorder. The Occupational Therapy Journal of Research. 2000;20:61–82. [Google Scholar]
- 119.Hubbard JA, Newcomb AF. Initial dyadic peer interaction of attention deficit-hyperactivity disorder and normal boys. Journal of Abnormal Child Psychology. 1991;19:179–195. doi: 10.1007/BF00909977. [DOI] [PubMed] [Google Scholar]
- 120.Beatty WW, Dodge AM, Dodge LJ, White K, Panksepp J. Psychomotor stimulants, social deprivation and play in juvenile rats. Pharmacology Biochemistry and Behavior. 1982;16:417–422. doi: 10.1016/0091-3057(82)90445-2. [DOI] [PubMed] [Google Scholar]