Abstract
Background
Among the patients with bladder cancer, a group is still at risk of disease recurrence, progression, and death from their cancer after curative treatment. Angiogenesis is a crucial pathogenic mechanism for this type of urothelial carcinoma and is a potential therapeutic target.
Objectives
To quantify tumor angiogenesis in bladder cancer and determine whether it correlates with tumor stage and grade.
Patients and methods
A series of 42 archival samples from carcinomas of the urinary bladder were graded, staged, and analyzed for microvessel density (MVD) by a double immunohistochemical technique using Factor VIII (FVIII) and CD31 antibodies. The correlation between MVD and histopathological grade and tumor stage was evaluated.
Results
FVIII and CD31 immunoreactivity was observed in 100% of cases and more intensely with CD31. Significantly higher MVD was determined in invasive tumors than in superficial tumors (p<0.05). MVD increased with tumor grade and stage (p<0.05); MVD was not affected by age or sex of the patients.
Conclusion
These data demonstrate that MVD in bladder carcinoma correlates with the tumor grade and stage. Quantification of tumor angiogenesis may allow selection of the type of treatment for bladder cancer patients.
Keywords: angiogenesis, bladder carcinoma, Factor VIII, CD31, microvessel density
Angiogenesis is the growth of new blood vessels, which is an important natural process at various anatomic sites. Angiogenesis occurs as an essential part of normal healing of wounds as well as in restoring blood flow to the tissues after injury. In many disease states, however, the body loses its normal control of angiogenesis. In the case of cancer, excessive angiogenesis occurs when cancer cells produce abnormal amounts of angiogenic growth factors, overwhelming the effects of natural angiogenesis inhibitors. These new vessels allow tumors to grow and cells to escape into the circulation and lodge in other organs (1). Taking this into account, potential anti-angiogenic therapies may have an important role in the treatment procedure (2, 3).
Bladder cancer is the 5th most common malignant disease and the 10th most common cause of cancer deaths, with more than 67,000 new cases diagnosed annually in the United States (4). About 382,660 cases are estimated worldwide in 2008 accounting for 5.3% of all cancers in both sexes (5). Like all solid tumors, it requires an active angiogenesis to support its growth and progression (6). Bladder tumor-associated angiogenesis is emerging as an important prognostic factor and represents a promising potential therapeutic target for cancer treatment (7).
CD31 is a 130 kDa integral membrane protein, also known as PECAM-1 (Platelet Endothelial Cell Adhesion Molecule-1), a member of the immunoglobulin superfamily, which mediates cell-to-cell adhesion. CD31 is expressed constitutively on the surface of adult and embryonic endothelial cells and is weakly expressed on many peripheral leukocytes and platelets. It has also been detected on bone marrow-derived hematopoietic stem cells and embryonic stem cells. Another important glycoprotein present in human endothelial cells is FVIII-related antigen, also present in megakaryocytes and platelets. Immunohistochemical staining for FVIII-related antigen could be used to determine if the benign and malignant neoplastic lesions are of endothelial cell origin (8).
Many researchers aimed for qualitative and quantitative assessment of angiogenesis in urinary bladder carcinoma (9), while others used the morphometric characteristics of the tumor microvasculature as predictor for prognosis (10). The present study was conducted to quantify the microvessel density (MVD), identifying the vessels by their immunohistochemical expression of CD31 and FVIII antigens. MVD was correlated with a variety of clinicopathological variables to get insight in their potential prognostic and predictive value in bladder cancer.
Materials and methods
Patients
A series of 42 Libyan patients (6 females, 36 males) with invasive urinary bladder carcinoma were retrospectively studied. Age of the patients ranged from 43 to 81 years (mean 63.9 years). All carcinomas were selected from the archives of the Department of Pathology, derived from the period from 2007 to 2010, based on availability of representative paraffin blocks. An experienced pathologist confirmed all histological diagnoses. All tumors were classified using the histopathological criteria of the World Health Organization (WHO) classification, and staging was made according to the American Joint Committee on Cancer system. Four normal bladder samples were used as control group. Clinical data of the patients are presented in Table 1.
Table 1.
Key characteristics of the patients and their tumors
| Variable | Number or value | Percentage%* |
|---|---|---|
| Patients | ||
| Male | 36 | 85.7 |
| Female | 6 | 14.3 |
| Age (years) | ||
| Range | (43–81) | –– |
| Mean | 63.95 | –– |
| Median | 65.00 | –– |
| Standard deviation | 9.80 | –– |
| Histological type | ||
| TCC | 39 | 92.9 |
| AC | 1 | 2.4 |
| SCC | 1 | 2.4 |
| TCC & SCC | 1 | 2.4 |
| Grade | ||
| Low grade | 27 | 64.3 |
| High grade | 15 | 35.7 |
| Stage | ||
| Stage 1 | 30 | 71.4 |
| Stage 2 | 11 | 26.2 |
| Stage 3 | 1 | 2.4 |
When applicable; TCC: transitional cell carcinoma; AC: adenocarcinoma; SCC: squamous cell carcinoma.
Immunohistochemical method
Paraffin-embedded blocks of the primary tumors were cut serially at 5 µm for immunohistochemical (IHC) staining. The IHC analysis was done using the automatic system (Bench-Mark XT, Ventana Medical System, Tucson, Arizona). This fully automated processing for bar code labeled slides included baking of the slides, solvent free deparaffinization, antigen retrieval in a cell conditioning buffer CC1 (mild: 36 min conditioning, and standard: 60 min conditioning), incubation with polyclonal FVIII antibody (clone: rabbit polyclonal, Ventana Medical Systems, Inc. Tucson, Arizona, USA) at a dilution 1:100/(60 min at room temperature). The same method was followed for monoclonal CD31 antibody (clone: AC-1A1, Ventana Medical Systems, Inc. Tucson, Arizona, USA) at a dilution 1:50/(60 min at room temperature). The IHC staining was visualized using Ultra ViewTM universal DAB, containing the following reagents: ultra view universal HRP, ultra view universal DAB inhibitor, ultra view universal DAB chromogen, ultra view universal DAB H2O2, and ultra view universal DAB copper. This was followed by counterstaining with hematoxylin 2021 for 4 min, and post-counterstaining with bluing reagent (2037) for 4 min. After staining, the sections were dehydrated in ethanol, cleared in xylene, and covered with Mountex and cover slips. Tonsil tissue was used as an external positive control and four normal urothelial samples were used as a normal reference.
Evaluation of IHC staining
The CD31 and FVIII positivity was indicated by the presence of cytoplasmic or membranous brown staining. Microvessel counting was performed in areas with maximal neovascularization within the tumors, outside any areas of artifact, necrosis, or inflammation, and without prior knowledge of the patient outcome. Low-power light microscopy (at ×40) magnification was used to scan the heterogeneous tumor sections for these areas. At ×100 magnification, counts were made of all distinct brown staining endothelial cells over three fields in each slide. The microvessel density (MVD) was defined as the average value of the three readings.
Statistical analysis
SPSS for Windows (18.0.2 SPSS, Inc. Chicago, Illinois, USA) was used for statistical analysis. Frequency tables were analyzed using the Chi-square test, with Fisher's exact test (where appropriate), or likelihood ratio (LR) statistics to assess the significance between categorical variables. Differences in the means of continuous variables between the groups were analyzed using ANOVA (analysis of variance) or non-parametric tests (Mann–Whitney, Kruskal–Wallis) tests. Reported p-values are from two-sided tests, and in all analysis p=0.05 was regarded as the limit of statistical significance.
Results
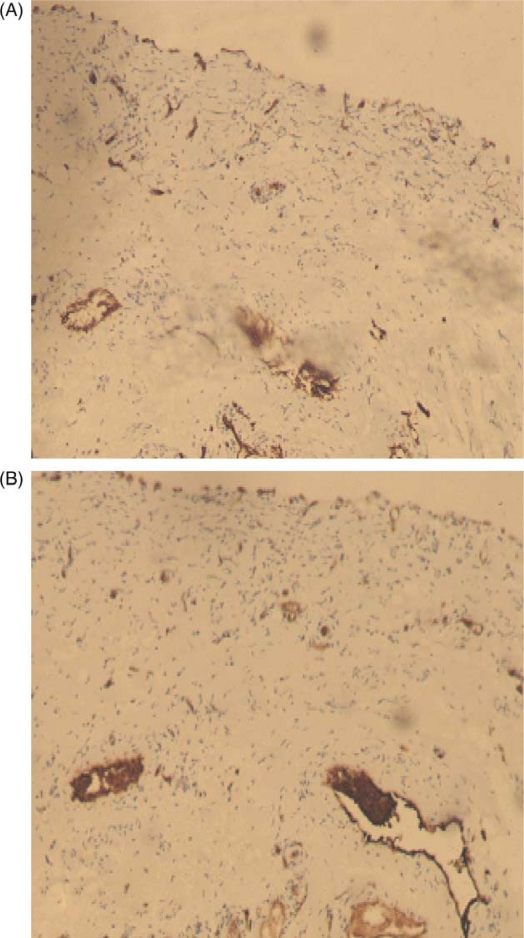
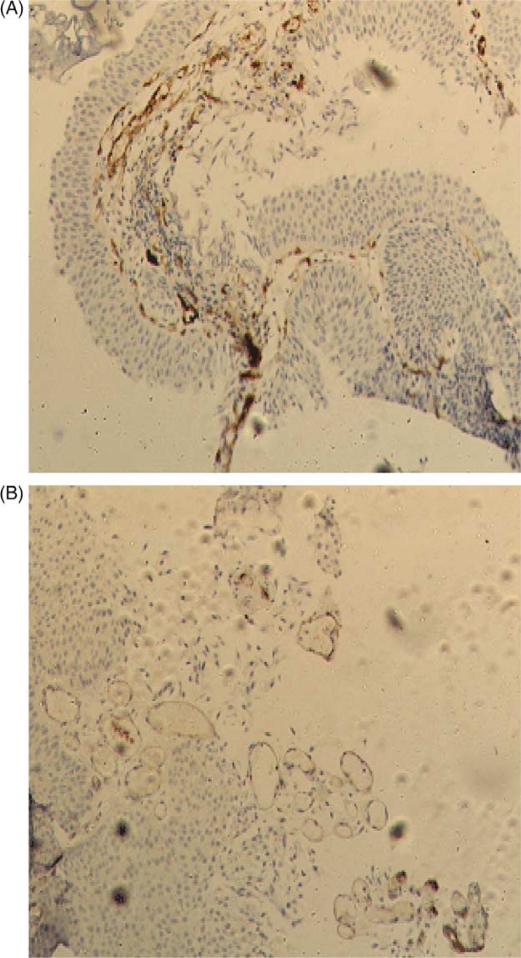
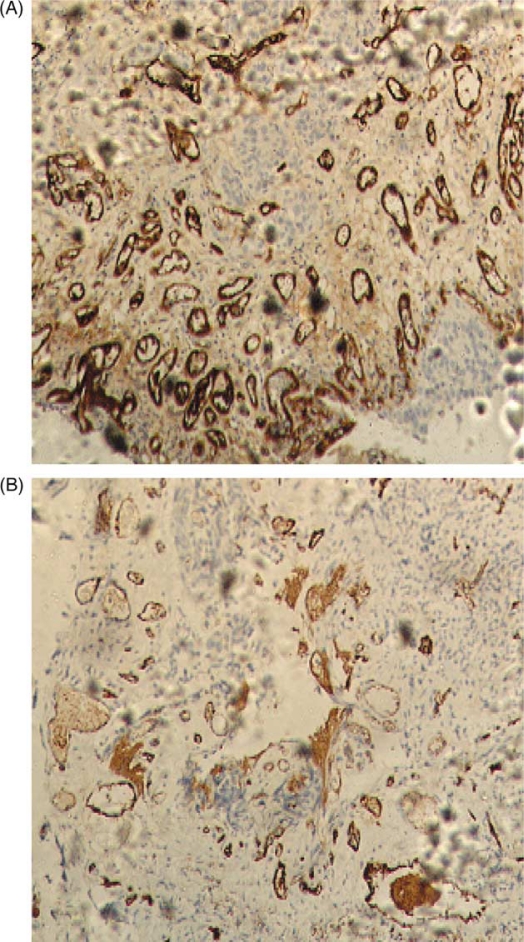
The FVIII and CD31 expression patterns in normal bladder, low grade urothelial carcinoma, and high grade urothelial carcinoma are illustrated in Figs. 1, 2, and 3, respectively. The IHC staining of FVIII and CD31 was successful in all 42 cases. The expression of these two markers was much higher in urinary bladder carcinoma in comparison to normal urinary bladder. The expression pattern of FVIII was cytoplasmic and membranous, whereas the expression of CD31 was predominantly membranous. The expression of CD31 was slightly stronger than that of FVIII, with mean MVD count of 80.29 and 78.53, respectively.
Fig. 1.
(A) Factor VIII expression in normal bladder (×100). (B) CD31 expression in normal bladder (×100).
Fig. 2.
(A) Factor VIII expression in low-grade urothelial carcinoma (×100). (B) CD31 expression in low-grade urothelial carcinoma (×100).
Fig. 3.
(A) Factor VIII expression in high-grade urothelial carcinoma (×100). (B) CD31 expression in high-grade urothelial carcinoma (×100).
The expression of both FVIII and CD31 was related to all available clinical variables including age, sex, histological type, grade, and stage (Table 2). Interestingly, the expression of both markers increased with tumor stage and grade, with significant correlation to tumor stage: p =0.001 and p =.002, respectively, and a highly significant correlation with tumor grade (p =0.0001 for both) (Table 2). Despite the small number of squamous cell carcinomas (SCC) and adenocarcinomas (AC) included in the present series, the MVD counts were higher in these two histological types than in classical transitional cell carcinoma. Both SCC and AC also presented with higher grades and stages. The MVD was not related to age and sex of the patients.
Table 2.
MVD as defined by Factor VIII and CD31 expression
| Result | Factor VIII | CD31 |
|---|---|---|
| Mode of expression | Cytoplasmic and membranous | Membranous only |
| Mean MVD count | ||
| Normal bladder | 28.75 | 31.46 |
| Low grade carcinoma | 67.34 | 65.83 |
| High grade carcinoma | 98.68 | 106.32 |
| Mean MVD count | ||
| Stage 1 | 70.72 | 72.11 |
| Stage 2 | 99.69 | 104.75 |
| Stage 3 | 80.00 | 86.60 |
| MVD as related to: | p-Value | p-Value |
| Age | 0.67 | 0.78 |
| Sex | 0.80 | 0.72 |
| Grade | 0.0001 | 0.0001 |
| Stage | 0.001 | 0.002 |
MVD: microvessel density.
Discussion
Over the past two decades, assessment of angiogenesis has emerged as a potentially useful biological prognostic and predictive factor in all solid human tumors. With the development of highly specific endothelial markers that can be assessed in archival specimens, several quantitative studies have been performed on urinary bladder carcinomas (11, 12). The majority of published studies have shown a positive correlation between intra-tumoral MVD (a measure of tumor angiogenesis) and prognosis in bladder tumors (13, 14). A minority of studies have failed to demonstrate such an association (9, 15, 16). These seemingly contradictory observations may be attributed to some crucial differences in the techniques used in sample selection in IHC staining, antibodies, vessel counting, and statistical analysis, although a number of biological differences may account for the discrepancy.
Papillary bladder cancer is a pathological entity with a well-developed branching fibrovascular core. In fact, it has been speculated that angiogenesis is the initial pathogenic mechanism for this type of urothelial carcinoma (6). This architectural pattern in papillary urothelial carcinoma increases the difficulty of MVD assessment; therefore, the discrimination between prior microvessels (fibrovascular core) and new ones (neovascularization) is a hard task. Based on our observation, anti-CD31 antibody recognizes small caliber vessels that are associated with angiogenesis in bladder cancer more efficiently than anti-FVIII antibody. Similar results were observed by Bochner and others (17).
In the present series, FVIII expression was both membranous and cytoplasmic, whereas CD31 expression was only membranous. These observations are in agreement with the staining protocol of these endothelial cells markers and with the results observed by Santos et al. (18). Interestingly, despite the small number of cases, angiogenesis was higher in AC and SCC samples, which also substantiates the recently published data (15). This corroborates with the known fact that AC and SCC of the urinary bladder have a higher potential for progression, and they usually present with a more advanced stage than transitional cell carcinomas.
We observed no significant relationship between tumor angiogenesis and the gender of the patients, in contrast to Emad and others (19), who in their study of 154 patients, reported that men had tumors with a higher vascularity than did women (p =0.0015). These discrepant observations might be due to differences in sample selection; they studied Schistosoma-associated SCC only. In addition, the number of patients may also influence the results.
Significant correlations between MVD and tumor stage and grade were observed in the current study, angiogenesis increasing in parallel with increasing tumor stage and grade (Table 2). This is fully consonant with the observations by Ozer et al. (20), who found that Grade III Stage pT1 bladder tumors have higher angiogenic activity and worse prognosis. Another study by Canoglu and coworkers (21) also confirmed that MVD in bladder carcinoma correlates with the tumor grade, stage, and its malignant potential thus substantiating the previous observations.
In distinct contrast to our results and those of other previous studies (13, 20, 21), however, Herrmann et al. (15) reported that MVD count did not show any relationship to disease-free or overall survival, and these authors even suggested that MVD could be considered as a favorable prognostic sign. Chabannes et al. (3) also found that all patients with lower MVD had a shorter survival time and, thus, confirm the consideration of MVD as a good prognostic factor. The most likely explanation to this discrepancy is the different markers used by these authors because different antibodies can give different staining patterns. Other possible reasons might include differences in the vessels counting, and the use of qualitative techniques for microvasculature assessment that significantly affects the results. Finally, it must be emphasized that MVD evaluation under the microscope is somewhat subjective and inter-observer variation can be substantial.
Conclusion
Taken together, even if based on a limited number of cases, the present data implicate that angiogenesis in urinary bladder carcinoma increases with the tumor grade and stage and also varies between the histological types of the tumor. Additional studies with larger cohorts and extended follow-up are necessary to fully elucidate the prognostic value of IHC-based MVD counting in bladder cancer and its potential predictive role in selecting the treatment options on more individual basis.
Acknowledgements
The skilful technical assistance of Ms. Huda El-Labbar in preparing sections for IHC is gratefully acknowledged.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Folkman J. Angiogenesis and angiogenesis inhibition: an overview. EXS. 1997;79:1–8. doi: 10.1007/978-3-0348-9006-9_1. [DOI] [PubMed] [Google Scholar]
- 2.Inoue K, Slaton JW, Karashima T. The prognostic value of angiogenesis factor expression for predicting recurrence and metastasis of bladder cancer after neoadjuvant chemotherapy and radical cystectomy. Clin Cancer Res. 2000;6:4866–73. [PubMed] [Google Scholar]
- 3.Chabannes E, Bernardini S, Wallerand H, Bittard H. Angiogenesis in bladder: prognosis indicator and therapeutic target. Prog Urol. 2001;11:417–27. [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E. Cancer statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Lyon, France: International Agency for Research on Cancer; 2010. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 10 [Internet] Available from: http://globocan.iarc.fr. [Google Scholar]
- 6.Chaudhary R, Bromley M, Clarke NW, Betts CD, Barnard RJ, Ryder WD, et al. Prognostic relevance of micro-vessel density in cancer of the urinary bladder. Anticancer Res. 1999;19:3479–84. [PubMed] [Google Scholar]
- 7.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 8.Delisser HM. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–77. [PMC free article] [PubMed] [Google Scholar]
- 9.Bartoletti R, Cai T, Nesi G, Sardi I, Rizzo M. Qualitative and quantitative analysis of angiogenetic factors in transitional cell bladder carcinoma: relationship with clinical course at 10 years follow-up. Oncol Rep. 2005;14:251–55. [PubMed] [Google Scholar]
- 10.Korkolopoulou P, Konstantinidou AE, Kavantzas N, Patsouris E, Pavlopoulos PM, Christodoulou P, et al. Morphometric microvascular characteristics predict prognosis in superficial and invasive bladder cancer. Virchows Arch. 2001;438:603–11. doi: 10.1007/s004280100400. [DOI] [PubMed] [Google Scholar]
- 11.Sagol O, Yorukoglu K, Sis B, Tuna B, Ozer E, Guray M, et al. Does angiogenesis predict recurrence in superficial transitional cell carcinoma of the bladder? Urology. 2001;57:895–99. doi: 10.1016/s0090-4295(01)00905-0. [DOI] [PubMed] [Google Scholar]
- 12.Chikazawa M, Inoue K, Fukata S, Karashima T, Shuin T. Expression of angiogenesis-related genes regulates different steps in the process of tumor growth and metastasis in human urothelial cell carcinoma of the urinary bladder. Pathobiology. 2008;75:335–45. doi: 10.1159/000164218. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki K, Morita T, Tokue A. Vascular endothelial growth factor-C (VEGF-C) expression predicts lymph node metastasis of transitional cell carcinoma of the bladder. Int J Urol. 2005;12:152–58. doi: 10.1111/j.1442-2042.2005.01010.x. [DOI] [PubMed] [Google Scholar]
- 14.Beecken WD, Engl T, Jonas D, Blaheta RA. Expression of angiogenesis inhibitors in human bladder cancer may explain rapid metastatic progression after radical cystectomy. Int J Mol Med. 2009;23:261–66. [PubMed] [Google Scholar]
- 15.Herrmann E, Bogemann M, Bierer S, Eltze E, Toma MI, Kopke T, et al. The role of the endothelin axis and microvessel density in bladder cancer – correlation with tumor angiogenesis and clinical prognosis. Oncol Rep. 2007;18:133–38. [PubMed] [Google Scholar]
- 16.Stavropoulos NE, Bouropoulos C, Ioachim IE, Michael M, Hastazeris K, Tsimaris I, et al. Prognostic significance of angiogenesis in superficial bladder cancer. Int Urol Nephrol. 2004;36:163–67. doi: 10.1023/b:urol.0000034676.50636.e0. [DOI] [PubMed] [Google Scholar]
- 17.Bochner B, Esrig D, Groshen S, Dickinsin M, Weidner N, Nichols PW. Relationship of tumor angiogenesis and nuclear p53 accumulation in invasive bladder cancer. Clin Cancer Res. 1997;3:1615–22. [PubMed] [Google Scholar]
- 18.Santos L, Koch M, Costa C, Pereira S, Amaro T, Cardoso F, et al. Neovascularisation is a prognostic factor of early recurrence in T1/G2 urothelial bladder tumors. Ann Oncol. 2003;14:1419–24. doi: 10.1093/annonc/mdg377. [DOI] [PubMed] [Google Scholar]
- 19.Emad E, El-Baz M, Gomha M, Abol-Enein H, Shaaban AA. Prognostic value of angiogenesis in schistosoma-associated squamous cell carcinoma of the urinary bladder. Urology. 2002;60:69–73. doi: 10.1016/s0090-4295(02)01669-2. [DOI] [PubMed] [Google Scholar]
- 20.Ozer E, Mungan MU, Tuna B, Kazimoglu H, Yorukoglu K, Kirkali Z. Prognostic significance of angiogenesis and immunoreactivity of cathepsin D and type IV collagen in high-grade stage T1 primary bladder cancer. Urology. 1999;54:50–55. doi: 10.1016/s0090-4295(99)00026-6. [DOI] [PubMed] [Google Scholar]
- 21.Canoglu A, Gogus C, Beduk Y, Orhan D, Tulunay O, Baltaci S. Microvessel density as a prognostic marker in bladder carcinoma: correlation with tumor grade, stage and prognosis. Int Urol Nephrol. 2004;36:401–5. doi: 10.1007/s11255-004-8869-9. [DOI] [PubMed] [Google Scholar]