Abstract
Context
Most environmental risk factors for psychiatric disorders cannot be studied experimentally, making causal attributions difficult. Can we address this question by using together 2 major methods for causal inference: natural experiments and specialized statistical methods?
Objective
To determine the causal relationship between dependent stressful life events (dSLEs) and prior depressive episodes (PDEs) and major depression (MD).
Design
Assessment of risk factors and episodes of MD at interview. Statistical analyses used the co-twin control and propensity score–matching methods.
Setting
General community.
Participants
Four thousand nine hundred ten male and female twins from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders.
Main Outcome Measure
Episodes of MD.
Results
We found that dSLEs were strongly associated with risk for MD in female (odds ratio [OR], 5.85) and male (4.55) twins in the entire sample and, at considerably lower levels, in female (2.29) and male (2.19) monozygotic twins discordant for dSLE exposure. A case-control sample matched on propensity score showed a moderate association in female (OR, 1.79) and male (1.53) twins. A PDE strongly predicted risk for MD in female (OR, 3.68) and male (5.20) twins in the entire sample. In monozygotic pairs discordant for exposure, the association was weaker in male (OR, 1.41) and absent in female (1.00) twins. A case-control sample matched on propensity score showed a moderate association between PDE and depressive episodes in male (OR, 1.58) and female twins (1.66).
Conclusions
Although dSLEs have a modest causal effect on the risk for MD, a large proportion of the observed association is noncausal. The same pattern is seen for PDEs, although the causal impact is somewhat more tenuous. For environmental exposures in psychiatry that cannot be studied experimentally, co-twin control and propensity scoring methods—which have complementary strengths and weaknesses—can provide similar results, suggesting their joint use can help with the critical question of causal inference.
Elucidating causal pathways to psychiatric illness is a critical research goal. However, with many risk factors for psychopathology, causal inference is problematic because randomized controlled trials—the gold standard for clarifying causation—are, for ethical or practical reasons, impossible. Two approaches remain to evaluate causal processes: natural experiments and statistical methods.1–3
In this report, we examine whether dependent stressful life events (dSLEs4–6; events likely influenced by the individual’s behavior) and prior depressive episodes (PDEs) increase risk for major depression (MD). Increased rates of MD follow dSLEs7–9 and PDEs.10–12 However, the causal nature of these associations is unclear.
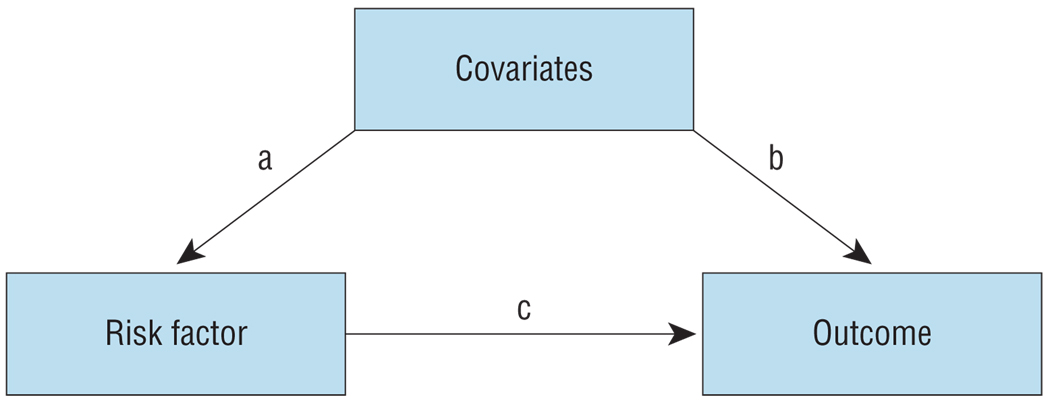
The critical question is whether the association between these risk factors and MD is causal (path c in Figure 1) or arises from covariates influencing both risk factors (path a) and disease (path b). As articulated by the counterfactual/interventionist theories of causation,13–15 if the association is causal, reducing risk factor exposure will reduce rates of disease. If the association is noncausal, altering risk factor exposure will not affect illness rates.
Figure 1.
The interrelationship among a risk factor, a potential covariate, and an outcome. The causal interrelationship between the risk factor and the outcome is depicted by path c, whereas the noncausal pathway is captured by paths a and b.
Many factors predispose to both dSLEs and MD, including neuroticism, PDEs, and genetic risk for MD.16–21 Thus, the association between dSLEs and MD might result from these shared covariates. Because numerous risk factors for MD—including genes and childhood adversities—have long-lasting effects,11,12,22,23 the tendency for MD to recur could arise without one depressive episode having a causal effect on the next.
In this report, we address these questions using a natural experiment (twins) and a specialized statistical method (propensity analysis). The co-twin control method examines outcomes in twin pairs discordant for risk factor exposure. Twin pairs raised together are matched for their rearing environment and their genes (partly for dizygotic [DZ] and completely for monozygotic [MZ] pairs) although not for environmental experiences unique to each twin.
Propensity analysis simulates a case-control study in a cohort assessed for the risk factor, outcome, and covariates predicting risk factor exposure.24–26 For each case (an individual exposed to the risk factor), an unexposed control is selected matched on the probability of being a case as predicted by the covariates (ie, the propensity to risk factor exposure). The co-twin control and propensity analyses attempt to isolate the impact of single risk factors and discriminate their effect from the background context in which these risk factors arise. We applied these 2 methods separately in male-male and female-female twin samples for the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders.27
METHODS
SAMPLE
Participants derive from 2 related studies in white same-sex twin pairs from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders.27 Participants were ascertained from the birth-certificate–based Virginia Twin Registry. Female-female twin pairs, born 1934 through 1974, were eligible if both members responded to a mailed questionnaire in 1987 through 1988. We use data from all 4 waves (FF1, FF2, FF3, and FF4), which included 85% to 92% of eligible twins.27 Mean interwave intervals were 17.3 months for FF1 to FF2, 45.0 months for FF2 to FF3, and 36.1 months for FF3 to FF4.27 Male-male pairs, born 1940 through 1974, were ascertained from registry records by a telephone interview (MM1), with a 72% response rate, and followed up with a face-to-face interview (MM2), with an 83% response rate. The mean interwave interval was 19.0 months. Zygosity was determined by discriminate function analyses using “twin questions” validated against DNA genotyping.28 The mean (SD) age and education of the twins were 36.3 (8.2) and 14.3 (2.2) years, respectively, at the FF4 interview and 37.0 (9.1) and 13.6 (2.6) years, respectively, at the MM2 interview.
This project was approved by the human subject committees at Virginia Commonwealth University. Written informed consent was obtained before the MM2 interviews, and verbal consent was obtained before the MM1 interviews.
MEASUREMENTS
We assessed the occurrence, to the nearest month during the year before the MM2 interview, of 11 classes of personal and 4 classes of network events.29 Interrater reliability (κ) values for the occurrence and dating of our SLE categories were 0.93 and 0.82, respectively.30
Dependence of SLEs, conceptualized as the probability that a respondent’s own behavior contributed to the SLE, was interviewer rated on a 4-point scale as clearly independent, probably independent, probably dependent, and clearly dependent. Interrater reliability was assessed from tape recordings of 92 randomly selected SLEs. Test-retest reliability was obtained by blindly reinterviewing 191 respondents at a mean interval of 4 weeks. Using the Spearman correlation (rs) and weighted kappa (κ),31 we determined the test-retest and interrater reliabilities to be rs=0.77/κ=0.63 and rs=0.89/κ=0.79, respectively. We defined a dSLE as probably or clearly dependent. Diagnoses of MD in the past year were made according to DSM-IV criteria.32 For each reported episode, the respondents described its duration and date of onset and offset. Of the MD episodes examined in our dSLE and PDE analyses, 36.4% and 40.4%, respectively, denied prior episodes (ie, were potentially first onsets).
We explored the relationship between dSLEs and risk for depressive episode onset in the month of the dSLE and 2 subsequent months in the FF4 and MM2 interviews.29 For our propensity analysis, we chose 18 covariates as predictors of dSLE exposure. Beginning with an extensive list of variables used in 2 prior comprehensive analyses of the etiology of MD in this sample,11,12 we selected variables that were assessed before the FF4 and MM2 interviews or were collected to reflect events occurring before that interview. These variables (and, where available, the original scales and publications showing their association with MD) were (1) birth year, (2) mean parental warmth measured by the Parental Bonding Instrument,33,34 (3) childhood sexual abuse,35,36 (4) parental loss due to death or divorce in childhood,37 (5) neuroticism measured by the short version of the Eysenck Personality Questionnaire,38,39 (6) introversion (extraversion reversed) measured by the short version of the Eysenck Personality Questionnaire,38,39 (7) self-esteem measured by the Rosenberg scale,11,12,40,41 (8) early-onset anxiety disorder,11,12 (9) conduct disorder,11,12,32 (10) lifetime traumas,11,12 (11) social support,11,12 (12) lifetime diagnosis of alcohol abuse or dependence,32 (13) lifetime diagnosis of nicotine dependence,42,43 (14) lifetime diagnosis of illicit drug abuse or dependence,32 (15) history of divorce,11,12 (16) history of MD more than 1 year before the FF3 or MM1 interview,11,12 (17) marital quality (high risk is defined as being married in the bottom 20% of quality44; medium risk, unmarried; and low risk, married in the top 80% of quality),11,12 and (18) difficulties, described as the number of life events in the past year outside the 3-month window before the depressive episode onset for cases (or chosen at random for controls).11,12
To evaluate the relationship between PDE and risk for a subsequent depressive episode, MD episodes reported at the MM1 and FF1 interviews were used as risk factors, and those reported at the MM2 and FF2 interviews were used as the outcomes. We began with the same possible list of covariates, but herein, to avoid possible bias, our final list was shorter because it had to reflect events or risk factors present before the MM1 or FF1 interview. We selected the following 10 covariates as potential predictors of PDE: birth year, years of education, childhood sexual abuse, neuroticism, history of MD before the past year, introversion, self-esteem, social support, difficulties, and parental warmth. Two additional covariates used were marital quality (ordinal variable of increasing risk of depression onset based on marital status and quality, where 0 indicates married with adequate quality; 1, never married; 2, married with low quality; 3, divorced; and 4, separated or widowed) and SLEs in the 3-month interval before the depressive episode in the MM1 or FF1 interviews or a random 3-month interval if no episodes were reported.
STATISTICAL ANALYSIS
For our co-twin control analyses, we obtained twin pairs discordant for the risk factor exposure, separately by zygosity group. Only same-sex pairs were used. Male-male and female-female samples were analyzed separately.
We calculated odds ratios (ORs) using standard logistic regression as operationalized in the SAS procedure.45 Conditional logistic regression is more appropriate for our matched samples (discordant DZ and MZ pairs and propensity matching) but could not be applied in the entire sample. For comparability, therefore, we used logistic regression. We repeated all analyses in our matched samples using conditional logistic regression and found very similar results.
We wished to compare propensity matching to the co-twin control approach. To understand the potential success of our approach, we examined covariate imbalance, that is, the degree to which our groups (general population, discordant twins, and our propensity score–constructed case-control sample) were matched on our determined covariates. Covariate imbalance was assessed by standardizing the sample around the grand mean for each covariate and then calculating means for each group. Consistent with previous approaches,25,46,47 we defined covariate imbalance as a difference of 0.25 SD or more in the mean of the standardized covariate in the 2 groups.
In our propensity score analyses, we used a nearest neighbor or a greedy matching algorithm implemented in an SAS macro48 to create matched pairs discordant for exposure. This algorithm examines cases sequentially and finds the closest match among the controls on the basis of propensity score (the probability of being a case from multivariate logistic regression). On the first iteration, a match is retained if propensity scores are within 0.00001. The matched pair is then removed from the data set and not considered further. Additional iterations allow matches within 0.0001, 0.001, 0.01, and 0.1. If a match has not been found at that point, the case is excluded. So that we could include individuals with missing values for some variables, we used a multiple imputation approach. The method used was that of Raghunathan et al,49 as implemented in IVEware software.50 We created 10 imputed data sets for analysis, and the results were combined with estimates and standard errors calculated as described by Rubin51 and Li et al.52 The mean results from these 4 regression analyses predicting dSLEs and PDEs in male and female twins are given in eTable 1 and eTable 2 (http://www.archgenpsychiatry.com).
We report the C statistic from the co-twin control and propensity analyses; this reflects the degree to which exposed and unexposed individuals in the initial population differ on their propensity score. A C value of 0.5 indicates no predictive power (ie, a correct prediction half the time). A C value of 1.0 means perfect prediction, in which cases and controls reflect entirely separate distributions. For optimal propensity score matching, a moderate overlap of the 2 distributions (eg, C values of approximately 0.7) is ideal. At high C values, it becomes increasingly difficult to match cases and controls because many cases have covariate values outside the range observed in controls.
Confidence intervals and P values are based on profile likelihoods. Because of clear a priori expectations that dSLEs and PDEs should increase the risk for MD, we present 1-tailed P values and 90% confidence intervals (CIs).
RESULTS
dSLEs AS A CAUSAL RISK FACTOR FOR AN MD EPISODE
Male-Male Pairs
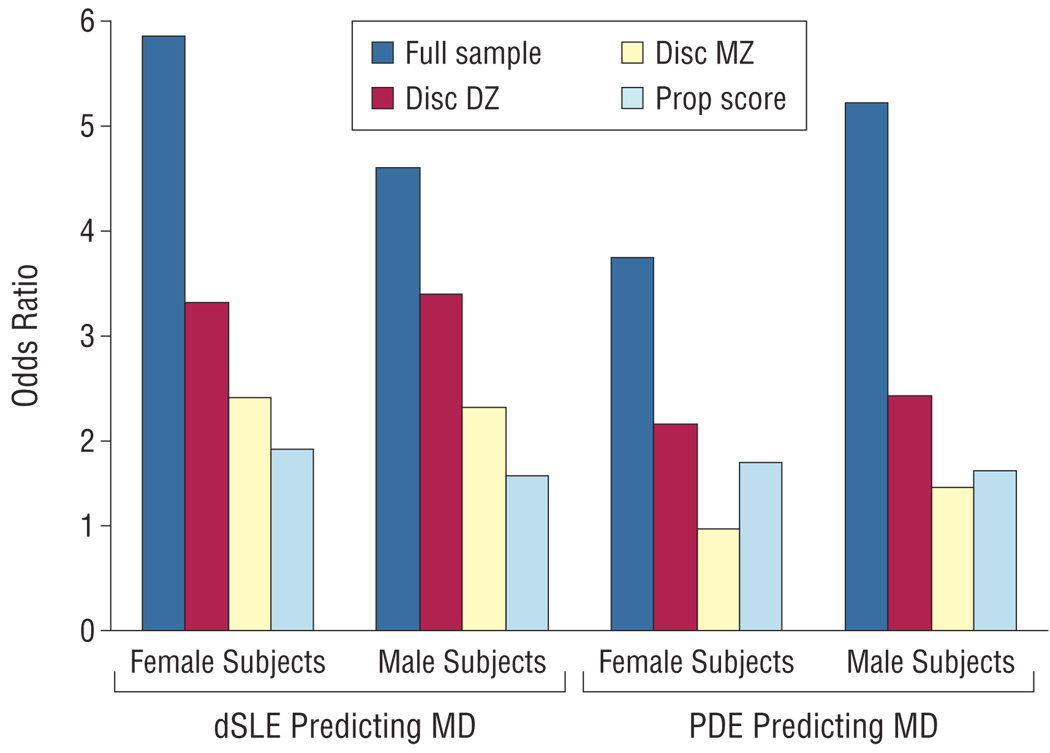
We began with a sample of 2394 members of male-male twin pairs, including both members of 706 MZ and 491 DZ pairs. Of these, 496 were exposed to a dSLE in the year before the MM1 interview. Exposure to a dSLE was strongly associated with an onset of MD within 3 months (OR, 4.55; 90% CI, 3.49–5.90; P<.001) (Figure 2). Participants in this sample who were vs were not exposed to the dSLE were poorly matched, differing substantially on a number of the covariates selected to reflect risk factors for dSLEs (Table 1). The 2 groups displayed covariate imbalance on the following 10 variables: parental warmth, neuroticism, conduct disorder, lifetime traumas, alcohol abuse/dependence, illicit drug abuse/dependence, history of MD before the last year, marital quality, difficulties, and birth year.
Figure 2.
The odds ratio for the prediction of an episode of major depression (MD) as predicted by the occurrence of a dependent stressful life event (dSLE) or a prior depressive episode (PDE) in the full twin sample, in monozygotic twins discordant for the risk factor exposure (Disc MZ), in dizygotic twins discordant for the risk factor exposure (Disc DZ), and in simulated case-control pairs matched on propensity scores (Prop score). These results are presented separately in male and female twins.
Table 1.
Standardized Differences in the Means of Covariates in Male Individuals Exposed and Unexposed to a Dependent Stressful Life Eventa
| Scores | ||||
|---|---|---|---|---|
| Covariate | Total Sample |
Discordant DZ Twins |
Discordant MZ Twins |
Propensity Score–Matched Sample |
| Years of education | 0.045 | 0.116 | 0.046 | 0.027 |
| Mean parental warmth | 0.253 | 0.314 | 0.044 | 0.031 |
| Childhood sexual abuse | 0.167 | 0.038 | 0.078 | 0.040 |
| Childhood parental loss | 0.194 | 0.036 | 0.012 | 0.021 |
| Neuroticism | 0.326 | 0.332 | 0.072 | 0.026 |
| Introversion | 0.016 | 0.214 | 0.068 | 0.034 |
| Self-esteem | 0.117 | 0.338 | 0.040 | 0.033 |
| Early-onset anxiety disorder | 0.211 | 0.146 | 0.066 | 0.044 |
| Conduct disorder | 0.250 | 0.092 | 0.026 | 0.015 |
| Traumas | 0.259 | 0.170 | 0.078 | 0.028 |
| Social support | 0.211 | 0.242 | 0.112 | 0.027 |
| Alcohol abuse/dependence | 0.256 | 0.014 | 0.066 | 0.025 |
| Nicotine dependence | 0.128 | 0.016 | 0.078 | 0.023 |
| Illicit drug abuse/dependence | 0.368 | 0.224 | 0.038 | 0.040 |
| Ever divorced | 0.098 | 0.288 | 0.000 | 0.032 |
| History of MD >1 y before the FF3 or MM1 interview | 0.356 | 0.334 | 0.134 | 0.027 |
| Marital quality | 0.395 | 0.408 | 0.324 | 0.024 |
| Difficulties | 0.355 | 0.136 | 0.030 | 0.025 |
| Birth year | 0.359 | 0.000 | 0.000 | 0.013 |
Abbreviations: DZ, dizygotic; FF3, third wave; MD, major depression; MM1, telephone contact; MZ, monozygotic.
Scores with a value of at least 0.25, which meet the definition of a covariate imbalance (a difference of ≥0.25 SD in the mean of the standardized covariate in the 2 groups) are given in boldface type. The propensity score reflects the mean of 10 iterations.
This sample contained 312 twin pairs discordant for dSLE exposure, of whom 133 were DZ and 179 were MZ. Compared with observations in the entire sample, the association between dSLE exposure and depressive episode onset was still statistically significant but considerably lower in magnitude in discordant DZ pairs (OR, 3.31; 90% CI, 1.54–7.84; P=.004) and lower still in discordant MZ pairs (2.19; 1.25–3.93; P=.01). As seen in Table 1, the DZ pairs discordant for dSLE exposure were better matched than the entire sample but still had covariate imbalance on the following 6 variables: parental warmth, neuroticism, self-esteem, history of divorce, history of MD before the past year, and marital quality. The MZ pairs discordant for dSLE exposure were much better matched than the 2 previous groups, having a covariate imbalance on only the variable of marital quality.
In our propensity analysis, we obtained a mean C statistic across the 10 imputations of 0.711, which indicates a good degree of overlap in propensity scores between the exposed and unexposed groups. As expected, we did well with our matching, obtaining a mean of 491.9 controls for our 496 cases. The case-control sample created by our propensity analysis had no covariate imbalance (Table 1). That is, the samples were well matched on all the selected risk factors. The OR between dSLE exposure and a depressive outcome in this analysis was 1.53 (90% CI, 1.24–1.88; P<.001).
Female-Female Pairs
We began with a sample of 1938 members of female-female twin pairs, which included both members of 503 MZ and 326 DZ twins as well as 3 twin pairs of unknown zygosity and 274 twins, without their co-twin. Of this sample, 155 had been exposed to a dSLE in the year before the FF4 interview. In this entire sample, exposure to a dSLE was strongly associated with an onset of MD within 3 months (OR, 5.85; 90% CI, 4.22–8.06; P<.001). Exposed and unexposed participants were again poorly matched, demonstrating covariate imbalance on the following 8 variables: parental loss, conduct disorder, lifetime traumas, alcohol abuse/dependence, illicit drug abuse/dependence, marital quality, difficulties, and birth year.
This sample contained 108 twin pairs discordant for dSLE exposure, of whom 46 were DZ and 62 were MZ. Compared with that seen in the entire sample, the association between dSLE exposure and depressive episode onset was considerably lower but remained significant in discordant DZ pairs (OR, 3.23; 90% CI, 1.30–8.85; P=.02) and lower still in discordant MZ pairs (2.29; 1.02–5.44; P=.05). The DZ pairs discordant for dSLE exposure had covariate imbalance on the following 3 variables: self-esteem, illicit drug abuse/dependence, and marital quality. The MZ pairs discordant for dSLE exposure were even better matched, with covariate imbalance only on marital quality.
Our propensity analysis achieved a mean C statistic of 0.713, indicating a substantial degree of overlap in propensity scores between cases and controls. As a result, we were able to obtain matched controls for a mean of 153.5 of 155 cases. The selected case-control sample demonstrated no covariate imbalance. The OR between dSLE exposure and a depressive outcome in this sample was 1.79 (90% CI, 1.33–2.41; P=.001).
PDEs AS CAUSAL RISK FACTORS FOR SUBSEQUENT DEPRESSIVE EPISODES
Male-Male Pairs
We began with a sample of 2908 members of male-male twin pairs personally assessed at our MM2 interview, 162 of whom reported 1 or more PDEs in the year before our MM1 interview. In this sample, exposure to a PDE in the year before the MM1 interview was strongly associated with the occurrence of 1 or more depressive episodes in the year before the MM2 interview (OR, 5.20; 90% CI, 3.67–7.27; P<.001). As seen in Table 2, exposed and unexposed twins in this sample were poorly matched, having covariate imbalance on the following 10 variables: a history of MD, neuroticism, childhood sexual abuse, social support, self-esteem, marital quality, SLEs, difficulties, parental warmth, and birth year.
Table 2.
Standardized Differences in the Means of Covariates in Male Individuals Exposed and Unexposed to a Prior Depressive Episodea
| Scores | ||||
|---|---|---|---|---|
| Covariate | Total Sample |
Discordant DZ Twins |
Discordant MZ Twins |
Propensity Score–Matched Sample |
| Years of education | 0.172 | 0.028 | 0.072 | 0.053 |
| Mean parental warmth | 0.358 | 0.232 | 0.090 | 0.058 |
| Childhood sexual abuse | 0.328 | 0.166 | 0.249 | 0.066 |
| Neuroticism | 1.078 | 0.704 | 0.728 | 0.051 |
| Introversion | 0.083 | 0.110 | 0.038 | 0.068 |
| Self-esteem | 0.669 | 0.698 | 0.498 | 0.057 |
| Social support | 0.289 | 0.180 | 0.166 | 0.076 |
| Marital quality | 0.483 | 0.408 | 0.464 | 0.039 |
| SLEs | 0.849 | 0.490 | 0.438 | 0.055 |
| History of MD before the past year | 0.553 | 0.244 | 0.324 | 0.031 |
| Difficulties | 0.629 | 0.414 | 0.064 | 0.059 |
| Birth year | 0.311 | 0.000 | 0.000 | 0.069 |
Abbreviations: DZ, dizygotic; MD, major depression; MZ, monozygotic; SLEs, stressful life events.
Scores with a value of at least 0.25, which meet the definition of a covariate imbalance (a difference of ≥0.25 SD in the mean of the standardized covariate in the 2 groups) are given in boldface type. The propensity score reflects the mean of 10 iterations.
This sample contained 119 twin pairs discordant for exposure to a PDE, of whom 51 were DZ and 68 were MZ. Compared with that observed in the entire sample, the association between PDEs and depressive episode onset was considerably lower in discordant DZ pairs (OR, 2.31; 90% CI, 0.96–5.91; P=.06) and lower still in discordant MZ pairs (1.41; 0.64–3.14; P=.24). The DZ and MZ pairs discordant for a PDE had covariate imbalance on 5 variables (Table 2). For DZ pairs, these variables were neuroticism, self-esteem, marital quality, SLEs, and difficulties. For MZ pairs, these were a history of MD, neuroticism, self-esteem, marital quality, and SLEs.
Our propensity score analysis produced a mean C statistic of 0.840, which is higher than ideal. However, we were successful in obtaining matched controls for a mean of 160.3 of 162 cases. No covariate imbalance was found in these cases and controls (Table 2). The OR between PDE exposure and a depressive outcome in this analysis was 1.58 (90% CI, 0.87–2.88; P=.10).
Female-Female Pairs
We began with a sample of 2002 members of female-female twin pairs personally assessed at our FF2 interview, 184 of whom reported 1 or more PDEs in the year before our FF1 interview. In that sample, exposure to a PDE was strongly associated with the occurrence of 1 or more PDE onsets in the year before the FF4 interview (OR, 3.68; 90% CI, 2.66–5.04; P<.001). Exposed and unexposed twins were poorly matched, with covariate imbalance on the following 8 variables: a history of MD, neuroticism, social support, self-esteem, marital quality, SLEs, difficulties, and parental warmth.
This sample contained 132 twin pairs discordant for exposure to a PDE, of whom 63 were DZ and 69 were MZ. Compared with the results in the entire sample, the association between a PDE and a subsequent depressive episode was considerably lower in discordant DZ pairs (OR, 2.04; 90% CI, 0.97–4.47; P=.06) and disappeared entirely in discordant MZ pairs (1.00; 0.45–2.23; P=.99). The DZ and MZ pairs discordant for a PDE in the year before our FF1 interview had covariate imbalance on 4 variables: a history of MD, neuroticism, self-esteem, and SLEs for DZ pairs and neuroticism, self-esteem, marital quality, and SLEs for MZ pairs.
Our propensity analysis produced a mean C statistic of 0.857, again indicating potential problems due to the limited overlap of the propensity score distribution of the exposed and unexposed twins. Indeed, we obtained, on average across our 10 iterations, matched controls for 170.2 of the 184 cases, with no covariate imbalance between the 2 groups. The OR between exposure to a PDE and a subsequent depressive episode in this analysis was 1.66 (90% CI, 0.95–2.89; P=.07).
COMMENT
The goal of this report was to use 2 different and complementary approaches—co-twin control and propensity analysis—to determine in a single longitudinally assessed community sample the causal relationship between exposure to SLEs or PDEs and risk for MD. A double-blind study to clarify the causal relationship of either of these exposures with MD would not be feasible or ethical. We examine these results in turn and then review what we have learned about the problems of causal inference in such situations.
dSLEs AS A CAUSAL RISK FACTOR FOR AN MD EPISODE
We specifically studied dSLEs because their causal relationship with psychiatric illness is inherently unclear and their association with MD is typically stronger than that observed for independent SLEs.11,12 Indeed, the occurrence of dSLEs was strongly related to the risk for MD episodes in our entire sample in both male and female twins. In our detailed set of covariates, those exposed to dSLEs (cases) frequently differed from those who were unexposed (controls), immediately raising concern that the association between dSLEs and subsequent depressive episodes may not be entirely causal and may instead be at least partly mediated via these covariates along paths a and b in Figure 1.
When we examined DZ and MZ twin pairs discordant for dSLE exposure, the association with MD fell substantially but remained statistically significant in both groups. As expected, the OR was lower in the MZ than in the DZ pairs given that the former controls entirely for genetic risk factors. In participants matched for family background and partially (DZ pairs) or completely (MZ pairs) for genes, dSLE remained predictive of depressive episodes. Of interest, MZ twin pairs discordant for dSLE exposure were very similar on our covariates, differing in male and female twins only in marital quality.
Our propensity method worked as expected in obtaining controls well matched to our cases. The OR observed in the propensity score analysis was similar and statistically significant in both male and female twins but also substantially lower than that observed in the general population.
Although the OR produced by the propensity score method was lower in both male and female twins than that obtained using the co-twin control method in MZ pairs, these 2 estimates of causal effect had widely overlapping CIs in both sexes. For male and female twins, the CIs for our 2 best estimates of causal effect overlapped in the range of 1.2 to 1.9 and 1.3 to 2.4, respectively. In both sexes, these 2 methods have produced broadly similar results showing a moderate causal impact of dSLEs on risk for MD.
PDEs AS CAUSAL RISK FACTORS FOR SUBSEQUENT DEPRESSIVE EPISODES
The PDEs recorded at our FF1 interviews strongly predicted risk for depressive episodes assessed at our FF2 interviews. In our set of covariates, those exposed to PDEs differed even more greatly than was seen with the dSLEs. Given these results and the fact that the risk factor and outcome variables were the same (ie, both are episodes of MD) and should share most predictors, the a priori probability that the observed association might be substantially noncausal is particularly high.
Indeed, when we examined DZ and MZ twin pairs discordant for PDE exposure, the association with MD fell sharply from that seen in the general population. None of the 4 resulting analyses (2 twin types in male and female twins) achieved statistical significance, although 2 of them produced trends suggesting a causal relationship. In participants matched completely for family background and partially (DZ pairs) or completely (MZ pairs) for genes, the evidence that PDEs are causally related to future MD episodes is modest and statistically inconclusive. However, MZ twins discordant for exposure to PDEs differed from each other on our covariates much more than those pairs discordant for dSLE exposure.
Our propensity method worked well in obtaining matched controls for our exposed cases and produced ORs that were modest and similar in male and female twins (1.6–1.7). Both results approached statistical significance (P values of .07 and .10). Given that the effect of risk for MD observed in our propensity analysis of PDEs was very similar to that seen for dSLEs, our less definitive results for PDEs may stem from the lower frequency of this risk factor and the concomitant reduction in statistical power.
Overall, the results of the propensity analysis of the PDE-MD relationship were somewhat stronger than those obtained from the co-twin control analyses. From both analyses, it is clear that a large proportion of the substantial association between PDEs and subsequent depressive episodes is not causal. However, a direct causal effect probably exists. This interpretation is consistent with previous analyses in this11,12 and other10 samples in which, in the presence of many covariates, PDEs modestly predict risk for future depressive episodes.
STRENGTHS AND LIMITATIONS OF THE CO-TWIN CONTROL AND PROPENSITY MATCHING METHODS FOR CAUSAL INFERENCE
Strengths
Does our discordant twin or our propensity analysis provide a more accurate picture of causal processes? Critically, their strengths and limitations differ. The co-twin control method provides excellent matching for all shared environmental exposures and, for MZ twins, all genes. All risk factors falling into these 2 categories, including those we do not know about, are well controlled. However, environmental experiences unique to 1 member of the pair are not controlled for by this method. If such experiences influence the risk factors and the outcome, the co-twin control method could overestimate causal effects.
If we knew of and measured all the relevant factors that influence risk exposure, the propensity method would provide accurate and unbiased estimates of causal effects. Although this is not realistic for any behavioral outcome, our longitudinal twin cohort has a particularly rich set of depression-related risk factors. If covariates that influence risk factor exposure and outcome are left out of propensity matching, this could also result in an overestimation of causal effects. However, propensity matching can also overcontrol for variables and thereby downwardly bias causal effects. Covariates have to be causes and not consequences of risk factor exposure. If assessment of a covariate is influenced by risk factor exposure, this could produce an underestimation of the causal effects of the risk factor. For example, our propensity analysis of dSLEs used neuroticism that was measured before dSLE exposure. If we had measured it after dSLE exposure, it could reflect the reaction to the event. Inclusion as a covariate would then downwardly bias our estimates of the causal effect of dSLEs on MD.
We have somewhat more confidence in the results of our analyses of dSLE than of PDEs. Dependent SLEs are, at least in part, exogenous risk factors that proved relatively easy to control for, as demonstrated by the very low rate of covariate imbalance in our discordant MZ twins. By contrast, PDEs are a more endogenous variable, reflecting internal vulnerabilities to depressive illness. As might be expected, this proved harder to control for, as evidenced by the larger number of covariate imbalances seen in our MZ twins discordant for PDEs.
Our confidence in scientific results should increase when similar answers are obtained by different methods, especially when these 2 methods have different strengths and weaknesses, for example, in the congruent evidence of genetic influences on schizophrenia from twin and adoption studies.53 The present report is in this situation. The co-twin control and propensity analyses each suggest that dSLEs have a moderate true causal effect on the risk for MD. Both methods also agree broadly with the more tenuous evidence of a modest causal effect on risk for MD of PDEs. The congruence of these findings substantially increases the probability of their veracity.
Limitations
These results should be interpreted in the context of 2 potentially important methodological limitations. First, despite our large sample size, power was limited, especially for the discordant twin analyses and with the rarer risk factor of PDEs. Low power may contribute to the lack of more definitive findings.
Second, the co-twin control and propensity analyses assess the impact of a risk factor given the background exposures of the study population. If a risk factor is causally potent only in the presence of an uncommon background factor, it will typically produce a weak overall effect.
CONCLUSIONS
Clarifying causal processes is problematic in psychiatric epidemiology, in which controlled trials are often impossible. We examined 2 typical problems (the relationships between dSLEs and PDEs and MD) in which strong associations are present but causal processes are much harder to elucidate. Applying examples of the 2 major available approaches (twins as a natural experiment and propensity scoring as a statistical method), we obtained reassuringly similar answers. Although the observed associations were largely noncausal, a moderate causal effect of dSLEs on MD was convincingly demonstrated, whereas the causal impact of PDEs was somewhat weaker and more tentative. Natural experiments and statistical methods, especially when used together in carefully collected samples, can provide substantial help in clarifying the nature of the causal pathways to psychiatric illness.
Acknowledgments
Funding/Support: This study was supported in part by grants MH-068643, DA-011287, and MH-49492 from the National Institutes of Health (NIH). The Mid-Atlantic Twin Registry (MATR), of which the Virginia Twin Registry is now part, has received support from the NIH, the Carman Trust, and the W. M. Keck, John Templeton, and Robert Wood Johnson foundations.
Role of the Sponsor: The NIH played no direct role in the design or conduct of the study or in the collection, management, analysis, and interpretation of the data and did not review or approve this manuscript.
Footnotes
Author Contributions: Dr Kendler had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
Online-Only Material: The eTables are available at http://www.archgenpsychiatry.com.
Additional Contributions: Linda Corey, PhD, provided assistance with the ascertainment of twins from the Virginia Twin Registry. Judy Silberg, PhD, directs the MATR. Carol Prescott, PhD, contributed to the design and implementation of this study. Kate Lapane, PhD, provided helpful advice on the propensity score analyses.
REFERENCES
- 1.Rutter M. Proceeding from observed correlation to causal inference: the use of natural experiments. Perspect Psychol Sci. 2007;2:377–395. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 2.Rutter M. Identifying the Environmental Causes of Disease: How Should We Decide What to Believe and When to Take Action? London, England: Academy of Medical Sciences; 2007. [Google Scholar]
- 3.Rutter M. Epidemiological methods to tackle causal questions. Int J Epidemiol. 2009;38(1):3–6. doi: 10.1093/ije/dyn253. [DOI] [PubMed] [Google Scholar]
- 4.Brown GW, Sklair F, Harris TO, Birley JL. Life-events and psychiatric disorders, I: some methodological issues. Psychol Med. 1973;3(1):74–87. doi: 10.1017/s0033291700046365. [DOI] [PubMed] [Google Scholar]
- 5.Paykel ES. Methodology of life events research. Adv Psychosom Med. 1987;17:13–29. doi: 10.1159/000414004. [DOI] [PubMed] [Google Scholar]
- 6.Kendler KS, Karkowski LM, Prescott CA. The assessment of dependence in the study of stressful life events: validation using a twin design. Psychol Med. 1999;29(6):1455–1460. doi: 10.1017/s0033291798008198. [DOI] [PubMed] [Google Scholar]
- 7.Williamson DE, Birmaher B, Anderson BP, al-Shabbout M, Ryan ND. Stressful life events in depressed adolescents: the role of dependent events during the depressive episode. J Am Acad Child Adolesc Psychiatry. 1995;34(5):591–598. doi: 10.1097/00004583-199505000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 9.Surtees PG, Miller PM, Ingham JG, Kreitman NB, Rennie D, Sashidharan SP. Life events and the onset of affective disorder: a longitudinal general population study. J Affect Disord. 1986;10(1):37–50. doi: 10.1016/0165-0327(86)90047-9. [DOI] [PubMed] [Google Scholar]
- 10.Sjöholm L, Lavebratt C, Forsell Y. A multifactorial developmental model for the etiology of major depression in a population-based sample. J Affect Disord. 2009;113(1–2):66–76. doi: 10.1016/j.jad.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in women. Am J Psychiatry. 2002;159(7):1133–1145. doi: 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- 12.Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in men. Am J Psychiatry. 2006;163(1):115–124. doi: 10.1176/appi.ajp.163.1.115. [DOI] [PubMed] [Google Scholar]
- 13.Kendler KS, Campbell J. Interventionist causal models in psychiatry: repositioning the mind-body problem. Psychol Med. 2009;39(6):881–887. doi: 10.1017/S0033291708004467. [DOI] [PubMed] [Google Scholar]
- 14.Woodward J. Making Things Happen. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 15.Pearl J. Causality Models, Reasoning, and Inference. Cambridge, England: Cambridge University Press; 2000. [Google Scholar]
- 16.Hammen C. Generation of stress in the course of unipolar depression. J Abnorm Psychol. 1991;100(4):555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- 17.Kendler KS, Gardner CO, Prescott CA. Personality and the experience of environmental adversity. Psychol Med. 2003;33(7):1193–1202. doi: 10.1017/s0033291703008298. [DOI] [PubMed] [Google Scholar]
- 18.Kendler KS, Karkowski-Shuman L. Stressful life events and genetic liability to major depression: genetic control of exposure to the environment? Psychol Med. 1997;27(3):539–547. doi: 10.1017/s0033291797004716. [DOI] [PubMed] [Google Scholar]
- 19.Kercher AJ, Rapee RM, Schniering CA. Neuroticism, life events and negative thoughts in the development of depression in adolescent girls. J Abnorm Child Psychol. 2009;37(7):903–915. doi: 10.1007/s10802-009-9325-1. [DOI] [PubMed] [Google Scholar]
- 20.Hammen C. Stress generation in depression: reflections on origins, research, and future directions. J Clin Psychol. 2006;62(9):1065–1082. doi: 10.1002/jclp.20293. [DOI] [PubMed] [Google Scholar]
- 21.Harkness KL, Stewart JG. Symptom specificity and the prospective generation of life events in adolescence. J Abnorm Psychol. 2009;118(2):278–287. doi: 10.1037/a0015749. [DOI] [PubMed] [Google Scholar]
- 22.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of 1-year prevalence of major depression in women. Arch Gen Psychiatry. 1993;50(11):843–852. doi: 10.1001/archpsyc.1993.01820230009001. [DOI] [PubMed] [Google Scholar]
- 23.Fergusson DM, Mullen PE. Childhood Sexual Abuse: An Evidence Based Perspective. Thousand Oaks, CA: Sage Publications Inc; 1999. [Google Scholar]
- 24.Harder VS, Stuart EA, Anthony JC. Adolescent cannabis problems and young adult depression: male-female stratified propensity score analyses. Am J Epidemiol. 2008;168(6):592–601. doi: 10.1093/aje/kwn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27(12):2037–2049. doi: 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 26.Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf. 2004;13(12):841–853. doi: 10.1002/pds.969. [DOI] [PubMed] [Google Scholar]
- 27.Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. New York, NY: Guilford Press; 2006. [Google Scholar]
- 28.Kendler KS, Prescott CA. A population-based twin study of lifetime major depression in men and women [published correction appears in Arch Gen Psychiatry. 2000;57(1):94–95] Arch Gen Psychiatry. 1999;56(1):39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- 29.Kendler KS, Karkowski LM, Prescott CA. Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. J Nerv Ment Dis. 1998;186(11):661–669. doi: 10.1097/00005053-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, Eaves LJ. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry. 1995;152(6):833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- 31.Fleiss JS, Cohen J, Everett BS. Large sample standard errors of kappa and weighted kappa. Psychol Bull. 1969;72:323–327. [Google Scholar]
- 32.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 33.Parker G, Tupling H, Brown L. A parental bonding instrument. Br J Med Psychol. 1979;52:1–10. [Google Scholar]
- 34.Kendler KS, Myers J, Prescott CA. Parenting and adult mood, anxiety and substance use disorders in female twins: an epidemiological, multi-informant, retrospective study. Psychol Med. 2000;30(2):281–294. doi: 10.1017/s0033291799001889. [DOI] [PubMed] [Google Scholar]
- 35.Martin J, Anderson J, Romans S, Mullen P, O’Shea M. Asking about child sexual abuse: methodological implications of a two stage survey. Child Abuse Negl. 1993;17(3):383–392. doi: 10.1016/0145-2134(93)90061-9. [DOI] [PubMed] [Google Scholar]
- 36.Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- 37.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Childhood parental loss and adult psychopathology in women: a twin study perspective. Arch Gen Psychiatry. 1992;49(2):109–116. doi: 10.1001/archpsyc.1992.01820020029004. [DOI] [PubMed] [Google Scholar]
- 38.Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the Psychoticism Scale. Pers Individ Dif. 1985;6:21–29. [Google Scholar]
- 39.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of personality and major depression in women. Arch Gen Psychiatry. 1993;50(11):853–862. doi: 10.1001/archpsyc.1993.01820230023002. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- 41.Roberts SB, Kendler KS. Neuroticism and self-esteem as indices of the vulnerability to major depression in women. Psychol Med. 1999;29(5):1101–1109. doi: 10.1017/s0033291799008739. [DOI] [PubMed] [Google Scholar]
- 42.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 43.Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29(2):299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- 44.Spotts EL, Prescott CA, Kendler KS. Examining the origins of gender differences in marital quality: a behavior genetic analysis. J Fam Psychol. 2006;20(4):605–613. doi: 10.1037/0893-3200.20.4.605. [DOI] [PubMed] [Google Scholar]
- 45.SAS Institute Inc. SAS 9.2 documentation. SAS Institute Inc Web site. [Accessed September 15, 2010];2008 http://support.sas.com/documentation/cdl_main/index.html.
- 46.Imai K, King G, Stuart EA. Misunderstandings between experimentalists and observationalists about causal inference. J R Stat Soc Ser A Stat Soc. 2008;171(2):481–502. [Google Scholar]
- 47.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 48.Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques; Proceedings of the Twenty-sixth Annual SAS Users Group International Conference; Cary, NC: SAS Institute Inc; 2001. pp. 214–226. [Google Scholar]
- 49.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27(1):85–95. [Google Scholar]
- 50.Raghunathan TE, Solenberger P, Van Hoewyk J. IVEware: Imputation and Variance Estimation Software User Guide. Ann Arbor: University of Michigan; 2002. [Google Scholar]
- 51.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 52.Li KH, Raghunathan TE, Rubin DB. Large-sample significance levels from multiply imputed data using moment-based statistics and an F-reference distribution. J Am Stat Assoc. 1991;86(416):1065–1073. [Google Scholar]
- 53.Kendler KS, Diehl SR. The genetics of schizophrenia: a current, genetic-epidemiologic perspective. Schizophr Bull. 1993;19(2):261–285. doi: 10.1093/schbul/19.2.261. [DOI] [PubMed] [Google Scholar]