Abstract
Nicotinic acetylcholine receptors bind to nicotine and initiate the physiological and pharmacological responses to tobacco smoking. In this report, we studied the association of α5 and α3 subunits with nicotine dependence and with the symptoms of alcohol and cannabis abuse and dependence in two independent epidemiological samples (n = 815 and 1,121, respectively). In this study, seven single nucleotide polymorphisms were genotyped in the CHRNA5 and CHRNA3 genes. In both samples, we found that the same alleles of rs16969968 (P = 0.0068 and 0.0028) and rs1051730 (P = 0.0237 and 0.0039) were significantly associated with the scores of Fagerström test for nicotine dependence (FTND). In the analyses of the symptoms of abuse/dependence of alcohol and cannabis, we found that rs16969968 and rs1051730 were significantly associated with the symptoms of alcohol abuse or dependence (P = 0.0072 and 0.0057) in the combined sample, but the associated alleles were the opposite of that of FTND. No association with cannabis abuse/dependence was found. These results suggested that the α5 and α3 subunits play a significant role in both nicotine dependence and alcohol abuse/dependence. However, the opposite effects with nicotine dependence and alcohol abuse/dependence were puzzling and future studies are necessary to resolve this issue.
Keywords: smoking, alcoholism, cannabis, comorbidity, genetic association
Introduction
Tobacco smoking is one of the most costly health issues facing the world today. According to the World Health Organization, billions of dollars were spent in smoking related health care and in the US alone, 440,000 deaths were attributed to smoking related disorders in 2002 [MMWR, [2004]]. While smoking is a complex behavior in which many environmental factors are involved, genetic-epidemiology studies in the last decade have provided compelling evidence that genetic factors play a significant etiologic role [True et al., [1997]; Lerman et al., [1999]; Sullivan and Kendler, [1999]; Li et al., [2003]; Munafo et al., [2004]]. Genome wide linkage scans [Bergen et al., [1999]; Duggirala et al., [1999]; Straub et al., [1999]; Bierut et al., [2004]; Vink et al., [2004]; Li et al., [2006]; Swan et al., [2006]] and association studies [Ishikawa et al., [1999]; Bierut et al., [2000]; Caporaso et al., [2001]; Sullivan et al., [2001]; Feng et al., [2004]; Ling et al., [2004]; Li et al., [2007]; Nussbaum et al., [2008]] have identified linkage regions and candidate genes for tobacco smoking and nicotine dependence.
Nicotinic acetylcholine receptors (nAChRs) are highly expressed in the central nerve system and their binding to nicotine, the most active pharmacological compound in cigarettes, triggers the physiological and pharmacological responses to tobacco smoking. Animal model studies have shown that these nAChRs are critical to tolerance, reward and the modulation of mesolimbic dopamine function that are essential to the development of nicotine dependence [Mineur and Picciotto, [2008]]. Many genes in the human genome encode for the nicotinic receptors and several genes have been studied in smoking and nicotine dependence in human subjects [Silverman et al., [2000]; Feng et al., [2004]; Li et al., [2005]; Saccone et al., [2007]; Winterer et al., [2007]]. In a recent study, Saccone et al. reported associations in multiple single nucleotide polymorphisms (SNPs) in CHRNA5, CHRNA3, and CHRNB4 gene cluster on 15q25. However, due to the large number of markers tested, the significance of these results did not survive correction for multiple testing. More recently, several other studies provided independent evidence that the CHRNA5, CHRNA3, and CHRNB4 locus is associated with heavy smoking and nicotine dependence [Berrettini et al., [2008]; Bierut et al., [2008]; Thorgeirsson et al., [2008]]. Interestingly, the associations of these genes with other substance abuse/dependence [Grucza et al., [2008]; Wang et al., [2008]] and with lung cancer [Amos et al., [2008]; Hung et al., [2008]; Thorgeirsson et al., [2008]] were also reported.
The comorbidity between nicotine dependence and alcohol abuse/dependence is well documented in the literature [True et al., [1999]; Daeppen et al., [2000]; John et al., [2003]; Falk et al., [2006]]. Nicotine dependent individuals are at increased risk for misusing a variety of other psychoactive substances [Dani and Harris, [2005]; Degenhardt et al., [2007]]. A recent large-scale twin study indicated that approximately two-thirds of the genetic risk factors for nicotine dependence were substance specific in their effect while one-third were non-specific and increased risk for abuse of a range of other psychoactive substances [Kendler et al., [2007]]. We were therefore interested in testing whether these variants in the α5 and α3 nicotinic receptors were associated solely with smoking and nicotine dependence or also with misuse of either another common licit (alcohol) or an illicit psychoactive substance (cannabis).
Materials and Methods
Sample Description
In this study, we selected two independent samples taken from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorder [Kendler et al., [1999]; Prescott and Kendler, [1999]; Kendler and Prescott, [2007]]. These twin studies ascertained a variety of phenotypes, including substance use/abuse/dependence, neuroticism, and other common psychiatric disorders. Tobacco smoking and nicotine dependence were assessed by the Fagerström Tolerance Questionnaire (FTQ) and/or Fagerström Test of Nicotine Dependence (FTND) during the time of heaviest lifetime nicotine use [Fagerström and Schneider, [1989]; Heatherton et al., [1991]]. The VAANX-ND sample was a combined sample of two previously used panels, the Virginia study of nicotine dependence (VAND) panel and the Virginia study of anxiety and neuroticism (VAANX) panel [Chen et al., [2004], [2008]; Hettema et al., [2006]]. The main reason to combine these two panels together was to increase sample size and statistical power. Because the VAND panel was originally designed to study both smoking initiation and nicotine dependence [Sullivan et al., [2001]; Chen et al., [2004], [2007]], never smokers were included. There were two groups of smokers in the VAND panel, one group was with low nicotine dependence (defined as those with an FTQ scores between 0 and 2) and the other group was with high nicotine dependence (defined as those with an FTQ score between 7 and 11). The VAANX panel was initially selected for the study of anxiety and neuroticism [Hettema et al., [2006]]. The inclusion criterion was the top and bottom 25 percentile of a genetic factor score - a composite index representing several internalizing anxiety disorders and the personality trait of neuroticism [Hettema et al., [2006]]. We denoted this composite index as internalizing anxiety and neuroticism genetic factor (IANGF) score. In this study, only regular smokers (defined as those who used at some point in their lives an average of at least seven cigarettes per week for a minimum of 4 weeks) were included (Table I and Supplementary Figure S1).
Table I. Sample Description.
| Sample | FTND | SymAlcAD | SymCanAD |
|---|---|---|---|
| VAANX-ND | A combined sample from two panels: (1) VAANX, regular smokers with high and low IANGF scores; and (2) VAND, regular smokers with high and low FTQ scores. The combined sample (n = 815, 532 males and 283 females) had FTND scores (mean, s.d.) of 5.18, 2.57 and IANGF scores of 0.25, 0.87. See Supplementary Figure S1 for their distribution | DSM IV symptom counts for alcohol abuse and dependence. Only those subjects (n = 1,801) who used five or more drinks in a month were included in the analyses. See Supplementary Figure 2S for symptom count distribution | DSM IV symptom counts for cannabis abuse and dependence. Only those subjects (n = 614) who used cannabis five times or more in a month were included in the analyses. See Supplementary Figure 2S for symptom count distribution |
| VAFTND | Regular smokers (n = 1,128, 727 males, 399 females, and 2 with unknown sex) with FTND scores of 4.60, 2.62 and IANGF scores of 0.08, 0.46. See Supplementary Figure S1 for their distribution |
The VAFTND subjects were selected from the same sources as the VAANX-ND sample. This sample included almost all regular smokers who had FTQ/FTND assessments but were not used in the VAANX-ND sample. We excluded those subjects who themselves or their cotwins were used in previous sample panels to maintain the independence of these samples so they can be used to verify results obtained from previous panels. As in the previous panels, for each twin pair, only one twin was selected. The distributions of the IANGF and FTND scores in the two samples are compared in Supplementary Figure S1. The subjects in both samples were adults, with ages between 18 and 65 at the time of FTND ascertainment and all of them self-reported European ancestry.
In addition to FTQ/FTND measures, the subjects were assessed for abuse and dependence of alcohol and illicit substances using DSM-IV criteria. To evaluate whether the nAChR genes were associated with the abuse/dependence symptoms of these other substances, we queried our twin databases and selected categories with reasonable frequencies (>25%) in our combined VAANX-ND and VAFTND samples. We found that only the symptom counts of alcohol and cannabis abuse/dependence had sufficient frequencies to permit useful analysis. Therefore, we used the symptom counts of alcohol and cannabis abuse/dependence (SymAlcAD and SymCanAD, respectively) as phenotypes instead of a dichotomized affected/unaffected category. In parallel to FTND scores where only regular smokers were assessed, we used a “regular drinker” definition (those who used five or more drinks in a month) as the inclusion criterion for SymAlcAD. Similarly, a “regular cannabis user” definition (those who used cannabis five or more times in a month) was used as inclusion criterion for SymCanAD. The rationale for using these definitions was that we wanted to use only those subjects with sufficient exposures to drinking and cannabis use. In the combined sample, 1,801 and 614 subjects met inclusion criteria for SymAlcAD and SymCanAD, respectively. The frequency distributions of SymAlcAD and SymCanAD are shown in Supplementary Figure S2.
Marker Selection and Genotyping
We selected markers based on the Caucasian dataset of the HapMap project and positive association reports. For the interval covering the CHRNA5, CHRNA3, and CHRNB4 genes, we downloaded the Caucasian dataset from the HapMap website (http://www.hapmap.org) and used the HaploView program (version 4.0) to tag markers with frequency of 5% or greater. Markers with positive reports of association (rs16969968 and rs1051730) were included. Accordingly, we selected 7 SNPs covering the 55 kb genomic distance that encodes CHRNA5 and CHRNA3. No markers located in the CHRNB4 gene were selected, since markers in these three genes were in high LD as shown in HapMap database and previous reports, markers selected in CHRNA5 and CHRNA3 tagged those variants in CHRNB4 effectively.
Genotyping was performed with the TaqMan genotyping method [Livak, [1999]]. Briefly, the PCRs were conducted with 384-well microplates. To ensure the quality of genotyping, negative control samples were included in each plate. The PCRs were performed with 5 ng of genomic DNA, 0.25 μl of TaqMan assay mix (Applied Biosystems, Inc., Foster City, CA) and 2.5 μl of TaqMan universal PCR master mix in a total reaction volume of 5 μl. After activating the polymerase and denaturizing DNA by heating at 95°C for 10 min, 40 cycles of 92°C for 15 sec and 60°C for 1 min were performed. After the reaction, the fluorescence intensities of reporter 1 and 2 (reporter 1: VIC, excitation = 520 ± 10 nm, emission = 550 ± 10 nm; reporter 2: FAM, excitation = 490 ± 10 nm, emission = 510 ± 10 nm) were measured by the Analyst Fluorescence Plate Reader (LJL Biosytems, Sunnyvale, CA). Based on the ratio of fluorescence intensities, genotypes were scored by a Euclidean clustering algorithm developed in our laboratory [van den Oord et al., [2003]]. After genotyping, genotypes were cleaned and checked with the HaploView program [Barrett et al., [2005]] for deviation from Hardy-Weinberg Equilibrium. Details of marker characteristics were shown in Table II. Of the seven markers typed, rs6495038 and rs8192475 had mild deviations in the VAANX-ND and VAFTND samples, respectively (Table II).
Table II. Marker Characteristics.
| Minor allele frequency | HWE P-valuea | |||||||
|---|---|---|---|---|---|---|---|---|
| Marker | Chromosomal position | Polymorphism | Minor allele | Gene/function | VAANX-ND | VAFTND | VAANX-ND | VAFTND |
| rs684513 | 76645705 | C/G | G | CHRNA5/intron | 0.213 | 0.201 | 0.9051 | 0.3543 |
| rs16969968 | 76670230 | A/G | A | CHRNA5/Asp>Asn | 0.314 | 0.356 | 0.5221 | 0.8627 |
| rs578776 | 76675455 | A/G | A | CHRNA3/intron | 0.292 | 0.272 | 0.1220 | 1.0000 |
| rs1051730 | 76681394 | A/G | A | CHRNA3/intron | 0.307 | 0.354 | 0.2008 | 0.9369 |
| rs2869546 | 76694400 | C/T | C | CHRNA3/intron | 0.399 | 0.369 | 0.7343 | 0.5269 |
| rs6495308 | 76694711 | C/T | C | CHRNA3/intron | 0.239 | 0.217 | 0.0087 | 0.3019 |
| rs8192475 | 76698535 | C/T | C | CHRNA3/intron | 0.059 | 0.052 | 0.4116 | 0.0266 |
HWE, Hardy-Weinberg equilibrium.
P-values ≤0.05 were in bold.
Data Analyses
In this study, association analyses with FTND score were conducted with the UNPHASED program (version 3.12) [Dudbridge, [2008]], which uses a log likelihood retrospective regression model. Since the VAANX-ND sample contained individuals selected for high IANGF scores, we thought it prudent to control for its effect by including the IANGF score as a covariate in the regression model. In all association analyses, including those performed for SymAlcAD and SymCanAD, a standard Z-score transformation of raw scores was performed for the phenotypes. For association tests, the SNP spectral decomposition (SNPSpD) method [Nyholt, [2004]] was used to evaluate the effective number of independent tests and P-values reported were uncorrected. Multi-marker haplotype analyses were conducted similarly with the UNPHASED program, and 1% was used as the cutoff for minor haplotypes. Pairwise LD was estimated for all subjects by the HaploView 4.0 software [Barrett et al., [2005]]. Hardy-Weinberg equilibrium tests were also conducted with the HaploView program. To exclude the impact of FTND on the association of SymAlcAD and SymCanAD, FTND scores were included as a covariate when association with SymAlcAD and SymCanAD was analyzed.
Results
LD Structure
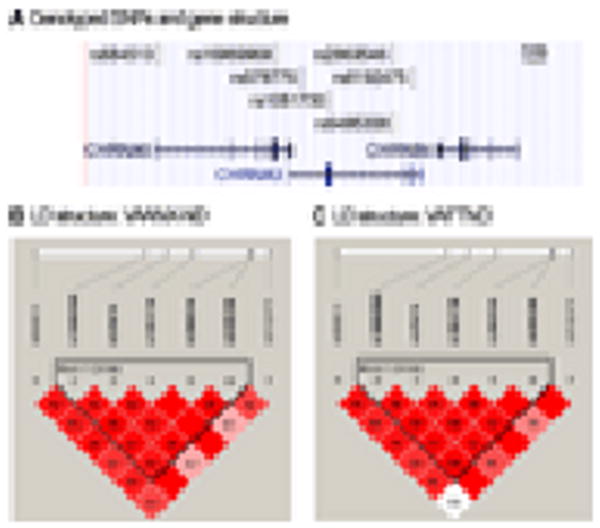
In this study, we genotyped seven SNPs covering the CHRNA5 and CHRNA3 genes in two independent samples selected from our previous twin studies. The first sample, denoted VAANX-ND (n = 815), was a combined sample of two previously used panels, the VAANX panel and the VAND panel [Chen et al., [2004], [2008]; Hettema et al., [2006]]. The second sample, denoted VAFTND (n = 1,121), included subjects who had FTQ/FTND assessments but were not used in the VAANX-ND sample. The characteristics of the markers typed were summarized in Table II. Two markers (rs6495308 and rs8192475) showed mild deviation from Hardy-Weinberg equilibrium. Their location and relative position to the genes were shown in Figure 1A. When linkage disequilibrium (LD) was examined, the two samples showed very similar pairwise LD (D′) among the typed SNPs (Fig. 1B,C). In both samples, rs16969968, rs578776, rs1051730, rs2869546, and rs6495308 were partitioned in a single LD block using default parameters. When haplotype structure was examined, both samples had the same major haplotypes and their frequencies were similar (data not shown). Our data were consistent with those from previous reports and the HapMap database.
Figure 1. Genotyped SNPs and gene structure in the CHRNA5, CHRNA3, and CHRNB4 region (A), and pairwise LD (D′) in the VAANX-ND (B) and VAFTND (C) samples.
Association With FTND
In this study, we used a quantitative design for both VAANX-ND and VAFTND samples. The distribution of FTND scores in the two samples are plotted in Supplementary Figure S1 in the Supplementary Material. The mean FTND score was slightly lower in the VAFTND sample (mean, s.d., 4.27, 2.62) than in the VAANX-ND sample (5.18, 2.57). In the VAANX-ND sample, rs16969968 and rs1051730 were nominally significant (Table III), but only rs16969968 survived correction for multiple testing given the effective number of independent tests estimated at 5 by the SNPSpD method. In VAFTND, four of the seven markers showed nominal significance (Table III), rs16969968 and rs1051730 remained significant after Bonferroni correction. In both samples, the same alleles were over-represented in those subjects with higher FTND scores. For rs16969968, the minor allele A (Asn) was more frequent in those with higher FTND scores; for rs1051730, it was also the minor allele (the A allele) that was over-represented in subjects with higher FTND scores. For both markers, the risk alleles were the same as reported in previous studies [Saccone et al., [2007]; Berrettini et al., [2008]; Thorgeirsson et al., [2008]].
Table III. Single Marker Association With FTND Scoresa.
| VAANX-ND (n = 815) | VAFTND (n = 1,121) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker | Allele | P-value | Cnt | Freq | Effect | 95% CI low bound | 95% CI high bound | P-value | Cnt | Freq | Effect | 95% CI low bound | 95% CI high bound |
| rs684513 | C | 0.9391 | 1,193 | 0.787 | - | - | - | 0.0222 | 1,772 | 0.799 | 0.122 | 0.017 | 0.226 |
| rs16969968 | A | 0.0068 | 470 | 0.314 | 0.166 | 0.045 | 0.287 | 0.0028 | 785 | 0.356 | 0.133 | 0.046 | 0.221 |
| rs578776 | G | 0.6122 | 1,060 | 0.708 | - | - | - | 0.3280 | 1,613 | 0.729 | - | - | - |
| rs1051730 | A | 0.0237 | 464 | 0.307 | 0.139 | 0.018 | 0.260 | 0.0039 | 786 | 0.354 | 0.129 | 0.041 | 0.216 |
| rs2869546 | T | 0.0589 | 891 | 0.601 | 0.043 | -0.002 | 0.088 | 0.0705 | 1,399 | 0.604 | 0.080 | -0.007 | 0.166 |
| rs6495308 | T | 0.3486 | 1,147 | 0.761 | - | - | - | 0.0384 | 1,740 | 0.783 | 0.107 | 0.006 | 0.208 |
| rs8192475 | C | 0.1741 | 89 | 0.059 | - | - | - | 0.5830 | 116 | 0.052 | - | - | - |
Cnt, count; Freq, frequency.
The effect, the low bound, and high bound of 95% confident intervals were shown for only those markers with a P-value less than 0.1. P-values ≤0.05 were in bold.
We conducted haplotype analyses for markers rs16969968, rs1051730, and rs2869546 since these markers showed significant or marginal associations in both VAANX-ND and VAFTND samples. In these analyses, we found that the major haplotypes were the same in the two samples, and that their frequencies were similar. In association analyses, haplotype A-A-T or 2-2-1 was significantly associated FTND scores in both samples (Table IV). These results were consistent with that observed in the single marker analyses.
Table IV. Three-Marker (rs169699698-rs1051730-rs2869546) Haplotype Analysis.
| Global P-value | Haplotype | Count | Frequency | Effect | 95% CI low bound | 95% CI high bound | P-value | |
|---|---|---|---|---|---|---|---|---|
| VAANX-ND | 0.08149 | G-G-T | 421 | 0.290 | 0.000 | 0.000 | 0.000 | 0.5642 |
| G-G-C | 576 | 0.396 | -0.032 | -0.172 | 0.109 | 0.0917 | ||
| A-A-T | 438 | 0.302 | 0.120 | -0.028 | 0.269 | 0.0347 | ||
| VAFTND | 0.00685 | G-G-T | 604 | 0.276 | 0.000 | 0.000 | 0.000 | 0.3450 |
| G-G-C | 802 | 0.366 | -0.021 | -0.127 | 0.085 | 0.0545 | ||
| A-A-T | 766 | 0.350 | 0.130 | 0.022 | 0.237 | 0.0012 |
P-values ≤0.05 were in bold.
Association With SymAlcAD and SymCanAD
To address whether a variant increasing the risk for tobacco smoking and nicotine dependence also increases risks for alcohol and cannabis abuse or dependence, we tested the association with the symptoms of alcohol and cannabis abuse/dependence phenotypes in the combined sample. Table V summarizes the results of these analyses. Two markers (rs16969968 and rs1051730) were significantly associated with SymAlcAD when the effects of FTND scores were taken into consideration by using them as a covariate (P = 0.0072 and 0.0057, respectively) (Table V). Both markers remained significant after correction for multiple testing. However, the risk alleles for high SymAlcAD were the opposite alleles to what we found associated with FTND. Interestingly, the study of Wang et al. [2008] found the same pattern of results. No association was found with SymCanAD in the combined sample.
Table V. Association With SymAlcAD and SymCanAD in the Combined Samplea.
| Marker | Allele | Count | Frequency | Effect | 95% CI low bound | 95% CI high bound | Chi square | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| SymAlcAD (1,801) | rs684513 | G | 737 | 0.207 | - | - | - | 2.68 | 0.1019 |
| rs16969968 | G | 2,350 | 0.662 | 0.224 | 0.060 | 0.387 | 7.23 | 0.0072 | |
| rs578776 | A | 967 | 0.273 | - | - | - | 2.56 | 0.1093 | |
| rs1051730 | G | 2,359 | 0.665 | 0.231 | 0.067 | 0.395 | 7.65 | 0.0057 | |
| rs2869546 | C | 1,356 | 0.387 | - | - | - | 1.92 | 0.1655 | |
| rs6495308 | C | 785 | 0.221 | - | - | - | 1.98 | 0.1594 | |
| rs8192475 | C | 196 | 0.055 | - | - | - | 0.13 | 0.7197 | |
| SymCanAD (614) | rs684513 | G | 268 | 0.220 | - | - | - | 0.02 | 0.8870 |
| rs16969968 | G | 823 | 0.678 | - | - | - | 0.51 | 0.4769 | |
| rs578776 | A | 338 | 0.280 | - | - | - | 0.67 | 0.4122 | |
| rs1051730 | G | 832 | 0.684 | - | - | - | 0.51 | 0.4763 | |
| rs2869546 | C | 472 | 0.393 | - | - | - | 0.01 | 0.9412 | |
| rs6495308 | C | 286 | 0.235 | - | - | - | 0.18 | 0.6732 | |
| rs8192475 | C | 61 | 0.051 | - | - | - | 0.00 | 0.9784 |
The effect sizes were shown for only those markers with a P-value less than 0.1. P-values ≤0.05 were in bold.
Discussion
During preparation of this article, two other reports of association between variants at the CHRNA5, CHRNA3, and CHRNB4 loci at 15q25 and heavy smoking/nicotine dependence were published [Bierut et al., [2008]; Thorgeirsson et al., [2008]]. These and our study replicated the association of CHRNA5 and CHRNA3 with smoking and nicotine dependence. Two previous studies [Berrettini et al., [2008]; Thorgeirsson et al., [2008]] used the number of cigarettes smoked as phenotype. By contrast, ours and other studies [Saccone et al., [2007]; Bierut et al., [2008]] used FTND as phenotype. To compare directly how much difference the phenotypes made, we examined our data using “the number of maximal cigarettes smoked daily” as a phenotype. In our combined sample, the P-value was 0.0010 (effect = 0.111, 95% CI, 0.045-0.178) for rs16969968, which was comparable with the results obtained with FTND scores (see Table III). But for rs2869546, using the number of cigarettes smoked produced a slightly stronger association (P = 0.0060, effect = 0.092, 95% CI, 0.026-0.159). As pointed out in the previous studies [Saccone et al., [2007]; Berrettini et al., [2008]; Thorgeirsson et al., [2008]], rs16969968 and rs1051730 were in high LD (r2 = 0.96 in our combined sample), our results were consistent with this observation. From our study, it seems that a large sample is required to detect the small effect at this locus. For rs16969968, carrying one copy of the minor allele, the A allele (Asn), only increased the mean by 0.13-0.17 standard deviation. Of note, none of the markers typed in this study is located in the CHRNB4 gene. Since the markers used in our study were selected to tag the variants in the CHRNA5/CHRNA3/CHRNB4 genes, where extensive high LD was evident in both our samples and others reported [Saccone et al., [2007]; Berrettini et al., [2008]; Bierut et al., [2008]; Grucza et al., [2008]; Schlaepfer et al., [2008]; Wang et al., [2008]; Weiss et al., [2008]], we believe that the association signal observed at rs16969968 and rs1051730 could extend to CHRNB4 gene. In other words, while we did not observe association directly from CHRNB4 gene, due to its high LD with CHRNA5 and CHRNA3 genes, we cannot exclude it from the association observed at CHRNA5 and CHRNA3 genes.
We also found significant association with symptoms of alcohol abuse and dependence in our combined sample, but the associated alleles were the opposite of those associated with the FTND score. While this finding is similar to those reported recently by others [Grucza et al., [2008]; Wang et al., [2008]], it is not consistent with epidemiological studies where nicotine dependence is consistently found to be positively correlated with alcohol abuse and dependence. In analyses without using FTND as covariate, both rs16969968 and rs1051730 was nominally significant (P = 0.018 and 0.029 respectively, data not shown). However, the association became stronger when accounting for the effect of FTND scores. These analyses further support the hypothesis that the effects of FTND and SymAlcAD at these variants are truly in the opposite direction. It is possible that these findings reflect the different actions of the two substances: nicotine is a stimulant whereas alcohol is a depressant. So, for example, if those with Asn alleles are more predisposed to enjoy the action of stimulants, they may be less likely to enjoy the effects of depressants. Nevertheless, in the light of the strong comorbidity between nicotine and alcohol dependence in the general population, these findings are puzzling and future studies will be necessary to resolve this issue.
To summarize, there were two main findings of this study. First, in two large samples, we verified the association of rs16969968 and rs1051730 with nicotine dependence when FTND scores were used as phenotype. Of these two SNPs, rs16969968 is a non-synonymous polymorphism, changing Asp to Asn at the 398th amino acid position of the CHRNA5 gene. This provides an opportunity to directly test this polymorphism in animal models. Combining these results with those obtained from prior studies [Saccone et al., [2007]; Berrettini et al., [2008]; Bierut et al., [2008]; Thorgeirsson et al., [2008]], we believe that the α5 and α3 genes are established as one of the best replicated genes imposing risks to tobacco smoking and nicotine dependence. Functional studies of these genes in animal and cellular models should follow. Second, we also found significant association with SymAlcAD for the same markers associated with FTND scores. However, the effects were in the opposite direction. While this finding was in agreement with others' reports [Grucza et al., [2008]; Wang et al., [2008]], it is inconsistent with the findings of epidemiological studies.
Supplementary Material
Acknowledgments
This study was supported by grants K01DA019498 to X.C., DA-011287 to K.S.K., and DA-18673 to M.C.N. from the National Institute on Drug Abuse and by funds from the Virginia Tobacco Settlement Foundation through the Virginia Youth Tobacco Project to Virginia Commonwealth University (subcontracted to K.S.K., #5100004ST). The authors thank Dr. Linda Corey for assistance with the ascertainment of twins from the Virginia Twin Registry, now part of the Mid-Atlantic Twin Registry, currently directed by Dr. Judy Silberg. The Mid-Atlantic Twin Registry has received support from the National Institutes of Health, the Carman Trust, and the WM Keck, John Templeton and Robert Wood Johnson Foundations.
Funded by:
▪ National Institute on Drug Abuse; Grant Number: K01DA019498, DA-011287, DA-18673
▪ Virginia Tobacco Settlement Foundation; Grant Number: 5100004ST
Footnotes
How to Cite this Article: Chen X, Chen J, Williamson VS, An S-S, Hettema JM, Aggen SH, Neale MC, Kendler KS. 2009. Variants in nicotinic acetylcholine receptors α5 and α3 increase risks to nicotine dependence. Am J Med Genet Part B.
References
- Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Dong Q, Zhang Q, Gu X, Vijayakrishnan J, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40(5):616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bergen AW, Korczak JF, Weissbecker KA, Goldstein AM. A genome-wide search for loci contributing to smoking and alcoholism. Genet Epidemiol. 1999;17(Suppl 1):S55–S60. doi: 10.1002/gepi.1370170710. [DOI] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13(4):368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Rice JP, Edenberg HJ, Goate A, Foroud T, Cloninger CR, Begleiter H, Conneally PM, Crowe RR, Hesselbrock V, et al. Family-based study of the association of the dopamine D2 receptor gene (DRD2) with habitual smoking. Am J Med Genet. 2000;90(4):299–302. doi: 10.1002/(sici)1096-8628(20000214)90:4<299::aid-ajmg7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Rice JP, Goate A, Hinrichs AL, Saccone NL, Foroud T, Edenberg HJ, Cloninger CR, Begleiter H, Conneally PM, et al. A genomic scan for habitual smoking in families of alcoholics: Common and specific genetic factors in substance dependence. Am J Med Genet Part A. 2004;124A(1):19–27. doi: 10.1002/ajmg.a.20329. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165(9):1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso NE, Lerman C, Audrain J, Boyd NR, Main D, Issaq HJ, Utermahlan B, Falk RT, Shields P. Nicotine metabolism and CYP2D6 phenotype in smokers. Cancer Epidemiol Biomarkers Prev. 2001;10(3):261–263. [PubMed] [Google Scholar]
- Chen X, Wu B, Kendler KS. Association study of the Epac gene and tobacco smoking and nicotine dependence. Am J Med Genet Part B. 2004;129(1):116–119. doi: 10.1002/ajmg.b.30040. [DOI] [PubMed] [Google Scholar]
- Chen X, Che Y, Zhang L, Putman AH, Damaj I, Martin BR, Kendler KS, Miles MF. RhoA, encoding a Rho GTPase, is associated with smoking initiation. Genes Brain Behav. 2007;6(8):689–697. doi: 10.1111/j.1601-183X.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Williamson VS, An SS, Hettema JM, Aggens HS, Neale MC, Kendler KS. The cannabinoid receptor 1 (CNR1) gene association with nicotine dependence. Arch Gen Psychiatry. 2008;65(7):816–824. doi: 10.1001/archpsyc.65.7.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daeppen JB, Smith TL, Danko GP, Gordon L, Landi NA, Nurnberger JI, Jr, Bucholz KK, Raimo E, Schuckit MA. Clinical correlates of cigarette smoking and nicotine dependence in alcohol-dependent men and women. The Collaborative Study Group on the Genetics of Alcoholism. Alcohol Alcohol. 2000;35(2):171–175. doi: 10.1093/alcalc/35.2.171. [DOI] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8(11):1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC. Epidemiological patterns of extra-medical drug use in the United States: Evidence from the National Comorbidity Survey Replication, 2001-2003. Drug Alcohol Depend. 2007;90(2-3):210–223. doi: 10.1016/j.drugalcdep.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudbridge F. Likelihood-based association analysis for nuclear families and unrelated subjects with missing genotype data. Hum Hered. 2008;66(2):87–98. doi: 10.1159/000119108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggirala R, Almasy L, Blangero J. Smoking behavior is under the influence of a major quantitative trait locus on human chromosome 5q. Genet Epidemiol. 1999;17(Suppl 1):S139–S144. doi: 10.1002/gepi.1370170724. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Schneider NG. Measuring nicotine dependence: A review of the Fagerström Tolerance Questionnaire. J Behav Med. 1989;12(2):159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29(3):162–171. [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Niu T, Xing H, Xu X, Chen C, Peng S, Wang L, Laird N, Xu X. A common haplotype of the nicotine acetylcholine receptor alpha 4 subunit gene is associated with vulnerability to nicotine addiction in men. Am J Hum Genet. 2004;75(1):112–121. doi: 10.1086/422194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Wang JC, Stitzel JA, Hinrichs AL, Saccone SF, Saccone NL, Bucholz KK, Cloninger CR, Neuman RJ, Budde JP, et al. A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol Psychiatry. 2008;64(11):922–929. doi: 10.1016/j.biopsych.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hettema JM, An SS, Neale MC, Bukszar J, van den Oord EJ, Kendler KS, Chen X. Association between glutamic acid decarboxylase genes and anxiety disorders, major depression, and neuroticism. Mol Psychiatry. 2006;11(8):752–762. doi: 10.1038/sj.mp.4001845. [DOI] [PubMed] [Google Scholar]
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452(7187):633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Ohtsuki T, Ishiguro H, Yamakawa-Kobayashi K, Endo K, Lin YL, Yanagi H, Tsuchiya S, Kawata K, Hamaguchi H, et al. Association between serotonin transporter gene polymorphism and smoking among Japanese males. Cancer Epidemiol Biomarkers Prev. 1999;8(9):831–833. [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Schumann A, Thyrian JR, Hapke U. Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol Alcohol. 2003;38(6):606–612. doi: 10.1093/alcalc/agg122. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, environment, and psychopathology: Understanding the causes of psychiatric and substance use disorders. New York: Guilford Press; 2007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29(2):299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007;64(11):1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Lerman C, Caporaso NE, Audrain J, Main D, Bowman ED, Lockshin B, Boyd NR, Shields PG. Evidence suggesting the role of specific genetic factors in cigarette smoking. Health Psychol. 1999;18(1):14–20. doi: 10.1037//0278-6133.18.1.14. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98(1):23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Li MD, Beuten J, Ma JZ, Payne TJ, Lou XY, Garcia V, Duenes AS, Crews KM, Elston RC. Ethnic- and gender-specific association of the nicotinic acetylcholine receptor alpha4 subunit gene (CHRNA4) with nicotine dependence. Hum Mol Genet. 2005;14(9):1211–1219. doi: 10.1093/hmg/ddi132. [DOI] [PubMed] [Google Scholar]
- Li MD, Payne TJ, Ma JZ, Lou XY, Zhang D, Dupont RT, Crews KM, Somes G, Williams NJ, Elston RC. A genomewide search finds major susceptibility Loci for nicotine dependence on chromosome 10 in african americans. Am J Hum Genet. 2006;79(4):745–751. doi: 10.1086/508208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Sun D, Lou XY, Beuten J, Payne TJ, Ma JZ. Linkage and association studies in African- and Caucasian-American populations demonstrate that SHC3 is a novel susceptibility locus for nicotine dependence. Mol Psychiatry. 2007;12(5):462–473. doi: 10.1038/sj.mp.4001933. [DOI] [PubMed] [Google Scholar]
- Ling D, Niu T, Feng Y, Xing H, Xu X. Association between polymorphism of the dopamine transporter gene and early smoking onset: An interaction risk on nicotine dependence. J Hum Genet. 2004;49(1):35–39. doi: 10.1007/s10038-003-0104-5. [DOI] [PubMed] [Google Scholar]
- Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14(5-6):143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. Genetics of nicotinic acetylcholine receptors: Relevance to nicotine addiction. Biochem Pharmacol. 2008;75(1):323–333. doi: 10.1016/j.bcp.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MMWR. State-specific prevalence of current cigarette smoking among adults - United States, 2002. MMWR Morb Mortal Wkly Rep. 2004;52(53):1277–1280. [PubMed] [Google Scholar]
- Munafo M, Clark T, Johnstone E, Murphy M, Walton R. The genetic basis for smoking behavior: A systematic review and meta-analysis. Nicotine Tob Res. 2004;6(4):583–597. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- Nussbaum J, Xu Q, Payne TJ, Ma JZ, Huang W, Gelernter J, Li MD. Significant association of the neurexin 1 gene (NRXN1) with nicotine dependence in European and African American smokers. Hum Mol Genet. 2008;17(11):1569–1577. doi: 10.1093/hmg/ddn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74(4):765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156(1):34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16(1):36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, Lessem JM, McQueen MB, Rhee SH, Ehringer MA. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol Psychiatry. 2008;63(11):1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MA, Neale MC, Sullivan PF, Harris-Kerr C, Wormley B, Sadek H, Ma Y, Kendler KS, Straub RE. Haplotypes of four novel single nucleotide polymorphisms in the nicotinic acetylcholine receptor beta2-subunit (CHRNB2) gene show no association with smoking initiation or nicotine dependence. Am J Med Genet. 2000;96(5):646–653. [PubMed] [Google Scholar]
- Straub RE, Sullivan PF, Ma Y, Myakishev MV, Harris-Kerr C, Wormley B, Kadambi B, Sadek H, Silverman MA, Webb BT, et al. Susceptibility genes for nicotine dependence: A genome scan and followup in an independent sample suggest that regions on chromosomes 2, 4, 10, 16, 17 and 18 merit further study. Mol Psychiatry. 1999;4(2):129–144. doi: 10.1038/sj.mp.4000518. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1(Suppl 2):S51–S57. doi: 10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Jiang Y, Neale MC, Kendler KS, Straub RE. Association of the tryptophan hydroxylase gene with smoking initiation but not progression to nicotine dependence. Am J Med Genet. 2001;105(5):479–484. doi: 10.1002/ajmg.1433. [DOI] [PubMed] [Google Scholar]
- Swan GE, Hops H, Wilhelmsen KC, Lessov-Schlaggar CN, Cheng LS, Hudmon KS, Amos CI, Feiler HS, Ring HZ, Andrews JA, et al. A genome-wide screen for nicotine dependence susceptibility loci. Am J Med Genet Part B. 2006;141B(4):354–360. doi: 10.1002/ajmg.b.30315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452(7187):638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True WR, Heath AC, Scherrer JF, Waterman B, Goldberg J, Lin N, Eisen SA, Lyons MJ, Tsuang MT. Genetic and environmental contributions to smoking. Addiction. 1997;92(10):1277–1287. [PubMed] [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56(7):655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- van den Oord EJ, Jiang Y, Riley BP, Kendler KS, Chen X. FP-TDI SNP scoring by manual and statistical procedures: A study of error rates and types. Biotechniques. 2003;34(3):610–620. 622. doi: 10.2144/03343dd04. [DOI] [PubMed] [Google Scholar]
- Vink JM, Beem AL, Posthuma D, Neale MC, Willemsen G, Kendler KS, Slagboom PE, Boomsma DI. Linkage analysis of smoking initiation and quantity in Dutch sibling pairs. Pharmacogenomics J. 2004;4(4):274–282. doi: 10.1038/sj.tpj.6500255. [DOI] [PubMed] [Google Scholar]
- Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, Budde JP, Fox L, Goldstein E, Reyes O, Saccone N, et al. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.42. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, Matsunami N, Singh NA, Baird L, Coon H, McMahon WM, et al. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4(7):e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Musso F, Konrad A, Vucurevic G, Stoeter P, Sander T, Gallinat J. Association of attentional network function with exon 5 variations of the CHRNA4 gene. Hum Mol Genet. 2007;16(18):2165–2174. doi: 10.1093/hmg/ddm168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


