Abstract
Although novel buprenorphine induction strategies are emerging, they have been inadequately studied. To examine our newly developed patient-centered home-based inductions, we conducted a subgroup analysis of 79 opioid-dependent individuals who had buprenorphine inductions at an urban community health center. Participants chose their induction strategy; standard-of-care office-based inductions were physician-driven, with multiple assessments, and observed, and patient-centered home-based inductions emphasized patient self-management and included a “kit” for induction at home. We conducted interviews and extracted medical records. Using mixed non-linear models, we examined associations between induction strategy and opioid use and any drug use. Compared to those with standard-of-care office-based inductions, participants with patient-centered home-based inductions had no significant differences in opioid use (AOR=0.63, 95%CI=0.13–2.97), but greater reductions in any drug use (AOR=0.05, 95%CI=0.01–0.37). Taking into account the limitations of our observational cohort study design, we conclude that participants with patient-centered home-based inductions had similar reductions in opioid use and greater reductions in any drug use than those with standard-of-care office-based inductions. It is essential that new induction strategies be based on existing models or theories and well-studied.
1. Introduction
Despite increasing rates of opioid dependence in the U.S., opioid dependence remains severely undertreated (Cicero, T. J., Inciardi, J. A., & Munoz, A., 2005; Sung, H. E., Richter, L., Vaughan, R., Johnson, P. B., & Thom, B., 2005a; Substance Abuse and Mental Health Services Administration [SAMHSA], 2008a; SAMHSA, 2008b; SAMHSA, 2009). To address this, federal legislation was enacted which allows for opioid addiction treatment with buprenorphine to occur outside of drug treatment programs. Buprenorphine treatment is associated with positive health outcomes, including reduction in opioid use and HIV risk behaviors (Marsch, L. A. et al., 2005; Sullivan, L. E. et al., 2008; Carrieri, M. P. et al., 2003; Lott, D. C., Strain, E. C., Brooner, R. K., Bigelow, G. E., & Johnson, R. E., 2006; Fudala, P. J. et al., 2003; Johnson, R. E. et al., 1995; Johnson, R. E., Jaffe, J. H., & Fudala, P. J., 1992; Ling, W., Wesson, D. R., Charuvastra, C., & Klett, C. J., 1996; Pani, P. P., Maremmani, I., Pirastu, R., Tagliamonte, A., & Gessa, G. L., 2000; Johnson, R. E. et al., 2000; Petitjean, S. et al., 2001; Schottenfeld, R. S., Pakes, J. R., Oliveto, A., Ziedonis, D., & Kosten, T. R., 1997; Strain, E. C., Stitzer, M. L., Liebson, I. A., & Bigelow, G. E., 1996). Despite these benefits, buprenorphine treatment is not widespread in the U.S. (Fiellin, D. A., 2007). One reason for limited buprenorphine treatment is the challenge patients and providers face with buprenorphine induction (Cunningham, C. O., Kunins, H. V., Roose, R. J., Elam, R. T., & Sohler, N. L., 2007; Walley, A. Y. et al., 2008).
Of the three phases of buprenorphine treatment (induction, stabilization, maintenance), induction, the initiation of buprenorphine treatment, is key to treatment success. In fact, a substantial proportion of buprenorphine treatment failures occur during the first seven days of treatment (e.g., during the induction) (Lee, J. D., Grossman, E., DiRocco, D., & Gourevitch, M. N., 2009; O'Connor, P. G. et al., 1998; Whitley, S. D. et al., 2009). U.S. National guidelines recommend that the induction process includes multiple assessments to evaluate opioid withdrawal and titrate buprenorphine dose in medical settings (Center for Substance Abuse Treatment., 2007). Because this process is difficult to carry out, physicians have begun developing new induction strategies. In a study of buprenorphine-prescribing physicians in Massachusetts, 42% offered unobserved home-based inductions (Walley, A. Y. et al., 2008). Despite this emerging change in clinical practice, unobserved home-based induction protocols have not been thoroughly described, and essential protocol components remain unknown. Additionally, few studies have evaluated these new induction strategies (Lee, J. D., Grossman, E., DiRocco, D., & Gourevitch, M. N., 2009; Alford, D. P. et al., 2007; Sohler, N. L. et al., 2010).
To evaluate this emerging change in clinical practice, we conducted a subgroup analysis of our prospective cohort study to compare our newly developed patient-centered home-based inductions to standard-of-care office-based inductions, examining drug use outcomes.
2. Methods
We conducted a subgroup analysis of a longitudinal cohort study of opioid-dependent individuals who initiated buprenorphine treatment at an urban community health center. Original study aims were to identify factors predicting positive treatment outcomes among participants receiving buprenorphine treatment integrated into primary care. Participants were followed for six months, and data collection included interviews and medical record extraction. The study was approved by the medical center’s institutional review board.
2.1 Setting
The study was conducted in a Bronx community health center from November 2004 to December 2009, immediately following the establishment of a buprenorphine treatment program (Cunningham, C. et al., 2008). Briefly, six general internists work closely with a clinical pharmacologist to provide buprenorphine treatment in the context of general primary care medicine. The health center, which provides comprehensive services such as adult and pediatric medicine, obstetrics/gynecology, dentistry, radiology, pharmacy and other specialty and ancillary services, is located in an urban area that has been disproportionately affected by drug use (Olson EC, Van Wye G, Kerker B, Thorpe L, & Frieden TR, 2006).
2.2 Participants
Individuals who inquired about buprenorphine treatment at the health center, were clinically appropriate, and gave written informed consent were enrolled.
Study eligibility criteria included: 1) undergoing buprenorphine induction, 2) HIV infection, and 3) English fluency. After securing additional funding, the last two criteria were expanded in January 2007 to include participants with and without HIV infection, and participants fluent in English or Spanish. To receive buprenorphine treatment at the health center, participants had to be at least18 years old, dependent on opioids (per DSMIV criteria (1994)), and insured by a health plan accepted at the health center or willing to pay for treatment on a sliding scale fee. Consistent with national guidelines (Center for Substance Abuse Treatment., 2007), participants were excluded from receiving buprenorphine treatment if they were: 1) hypersensitive to buprenorphine or naloxone, 2) pregnant, 3) alcohol dependent (per DSM-IV criteria (1994)), 4) benzodiazepine dependent (per DSM-IV criteria (1994)), 5) with transaminase levels greater than five times normal, 6) diagnosed with severe, untreated psychiatric illness, and 7) taking more than 60 mg of methadone daily in the past month.
2.3 Inductions
During the initial portion of the study, when buprenorphine treatment was newly implemented at the health center, all participants received standard-of-care observed office-based inductions. After recognizing the barriers associated with standard-of-care inductions, we developed a patient-centered unobserved home-based induction strategy and introduced it into clinical practice. Thus, during the latter portion of the study, participants chose either standard-of-care office-based inductions or patient-centered home-based inductions. After participants were assessed and determined to be appropriate for buprenorphine treatment, providers briefly discussed both induction treatment strategies and ascertained participants’ preferences. Below we describe these induction strategies.
2.3.1 Standard-of-care office-based inductions
Standard-of-care office-based inductions followed recommendations of national guidelines (Center for Substance Abuse Treatment., 2007). Participants attended a preparatory office visit to obtain information about buprenorphine treatment, were scheduled for the induction visit, and instructed to present to the visit in opioid withdrawal. At that first induction visit, participants were assessed with a standardized scale to ensure they were in adequate withdrawal (Wesson, D. R. & Ling, W., 2003), and then given 1–2 tablets of 2/0.5mg of buprenorphine/naloxone. (All participants were given buprenorphine/naloxone rather than buprenorphine monotherapy, and all medication was obtained from the pharmacy located with the community health center and billed to participants’ insurance plans.) After this 20-minute visit, participants were re-assessed 60 minutes later to determine their response to buprenorphine/naloxone. Depending on opioid withdrawal symptoms, participants were subsequently given 1–2 tablets of 2/0.5 mg of buprenorphine/naloxone. This process was repeated until participants’ opioid withdrawal was substantially diminished or until a maximum of 16/4 mg of buprenorphine/naloxone was taken. Physicians also prescribed ancillary medications (e.g., ibuprofen, clonidine, and loperamide hydrochloride) to help manage participants’ withdrawal symptoms accordingly. All together, with repeated assessments, the initial induction visit lasted 2–4 hours.
Participants had their second induction visit within 1–2 days, during which time they were assessed for opioid withdrawal and buprenorphine/naloxone doses were titrated accordingly. This second induction visit typically lasted 15–20 minutes. Participants were encouraged to call their provider with questions or concerns that arose between visits. After participants were stabilized, they usually had monthly visits for buprenorphine treatment. The duration of maintenance buprenorphine treatment was determined on a case-by-case basis and typically continued for an indefinite period of time.
2.3.2 Patient-centered home-based inductions
Patient-centered home-based inductions were developed in response to challenges experienced with standard-of-care office-based inductions. For example, with standard-of-care office-based inductions, employed patients had difficulties missing work, patients felt uncomfortable experiencing opioid withdrawal symptoms in a busy waiting room, and physicians struggled with time demands. Our new patient-centered home-based induction strategy was designed to be more patient-centered and to make opioid addiction management consistent with other chronic disease management in primary care. Thus, patient-centered home-based inductions were guided by the Chronic Care Model (CCM) (Wagner, E. H. et al., 2001; Wagner, E. H., 1998; Watkins, K., Pincus, H. A., Tanielian, T. L., & Lloyd, J., 2003). One specific aspect of the CCM that was emphasized was greater patient self-management of addiction. After developing, pilot testing, and refining this new induction strategy, we introduced it into clinical practice.
For patient-centered home-based inductions, participants attended a preparatory office visit to obtain information about buprenorphine treatment and plan their home-based inductions. During this 30-minute visit, self-management of opioid addiction was promoted by: 1) educating participants about opioid addiction and buprenorphine treatment; 2) using motivational techniques to encourage participants to take the steps necessary to succeed in managing their opioid addiction; 3) emphasizing empowerment by involving participants as the primary drivers of their health; and 4) providing resources to assist participants to self-manage their opioid addiction. Resources consisted of a home induction kit, which included an instruction sheet, ten 2/0.5 and four 8/2 buprenorphine/naloxone pills, and six pills each of ibuprofen, clonidine, and loperamide hydrochloride (see Table 1). Because a total of 52/13 mg of buprenorphine/naloxone pills were included in the kit, the maximum average dose of buprenorphine/naloxone over three days of the induction was 16/4-18/4.5 mg of buprenorphine/naloxone, which was similar to the maximum dose received with standard-of-care office-based inductions. The instruction sheet had six sections that explained the contents of the kit, when to start taking buprenorphine/naloxone, things not to do, how to take buprenorphine/naloxone, plans to guide treatment and facilitate follow-up, and a log to track medications taken. Participants were encouraged to call their provider if questions or concerns arose during the induction.
Table 1.
Contents of the tool kit for patient-centered home-based inductions
| Instruction sheet | |||
|---|---|---|---|
| Section | What the section addresses | ||
| What’s in the tool kit? | Guides when/how to use medications in the kit | ||
| When to start Suboxone | Guides the timing of treatment initiation | ||
| Things not to do | Warns against common mistakes or misunderstandings | ||
| How to take Suboxone | Facilitates correct dosing method | ||
| Plan | Guides treatment, provides support, and facilitates follow-up | ||
| What was taken | Facilitates keeping track of dosing | ||
| Medications | |||
| # Pills | Medication | Dose (mg) | Rationale |
| 10 | Buprenorphine/naloxone | 2/0.5 | Initiate buprenorphine treatment (day 1) |
| 4 | Buprenorphine/naloxone | 8/2 | Buprenorphine treatment (days 2–3) |
| 6 | Ibuprofen | 200 | ↓ Withdrawal symptoms (pain) |
| 6 | Clonidine | 0.1 | ↓ Withdrawal symptoms (anxiety) |
| 6 | Loperamide hydrochloride | 2.0 | ↓ Withdrawal symptoms (diarrhea) |
Participants were given a follow-up appointment within three days, at which time long term maintenance was discussed. Similar to the treatment received by those with standard-of-care office-based inductions, the duration of maintenance treatment for those with patient-centered home-based inductions was determined on a case-by-case basis, typically continued for an indefinite period of time, and usually consisted of monthly visits (after participants were stabilized).
2.4 Data Collection
2.4.1 Interviews
Participants were interviewed at baseline (prior to induction), and 1, 3, and 6 months after induction by a research assistant. Interviews lasted 45–60 minutes and occurred in a private room at the health center. Interviews were conducted using audio computer-assisted self-interview (ACASI) technology in which questions were displayed on a computer while an audio recording of the question was played. Participants entered responses directly on the computer, which may result in more accurate reporting of sensitive behavior than other survey methods (Turner, C. F. et al., 1998). Participants received $15 travel reimbursement for each interview.
Interview data included: age (continuous); gender (male, female); race/ethnicity (Hispanic, non-Hispanic black, non-Hispanic other); education (less than a high school diploma or GED, at least a high school diploma or GED); employment (employed, unemployed); housing status (stably housed defined as reporting living in an apartment or home; unstably housed defined as living in any other situation); ever incarcerated at least three days (yes, no); drug use in the 30 days prior to baseline (heroin; methadone; opioid analgesics; cocaine; sedatives, hypnotics, or tranquillizers; amphetamines; barbiturates; hallucinogens; inhalants); and ever injection drug use (yes, no). Demographic questions were from a buprenorphine/HIV multi-site study funded by the Health Resources and Services Administration, and drug use questions were from the Addiction Severity Index (McLellan, A. T. et al., 1992).
2.4.2 Medical record extraction
At the 6-month follow-up period, data were extracted from electronic medical records. To confirm initiation of buprenorphine treatment, only participants with new buprenorphine/naloxone prescriptions after the baseline interview were included in this analysis. In addition, type of induction strategy (patient-centered home-based, standard-of-care office-based) was extracted from standardized progress notes. In cases where participants had more than one induction during their course of buprenorphine treatment (e.g. they were induced, stopped treatment, and then were re-induced), they were categorized according to their initial induction strategy.
2.5 Outcomes
The primary outcome was self-report of opioid use during the 6-month follow-up period. Opioid use was defined as using heroin, methadone, or opioid analgesics in the 30 days prior to each interview. Because treatment guidelines recommend that buprenorphine not be used in combination with other opioids (Center for Substance Abuse Treatment., 2007), we considered any opioid use (prescribed or non-prescribed, illicit or licit) as buprenorphine treatment failure. The secondary outcome was self-report of any drug use during the 6-month follow-up period. Any drug use was defined as using any of the following drugs (prescribed or non-prescribed, illicit or licit) in the 30 days prior to each interview: heroin; methadone; opioid analgesics; cocaine; sedatives, hypnotics, or tranquilizers; amphetamines; barbiturates; hallucinogens; or inhalants. Although urine toxicology tests were available in medical records, they were conducted for clinical care rather than research. Therefore, they were not routinely collected in a standardized manner, and are not used in either outcome measure.
2.6 Data Analysis
We first explored how time was associated with opioid use for the entire cohort and by induction strategy. We conducted frequencies to describe the proportion of people who used opioids during each follow-up period in the entire cohort, the standard-of-care office-based induction group, and the patient-centered home-based induction group. Next, we constructed a mixed effects non-linear model for the entire cohort using time (baseline, 1 month, 3 months, and 6 months) as our independent variable and opioid use as our dependent variable. We then repeated this entire process by examining any drug use.
To test whether opioid use over the 6-month follow-up period was different by induction strategy, we first examined how our main independent variable, induction strategy, was associated with our main dependent variable, opioid use, while adjusting for baseline opioid use using mixed effects non-linear models. To adjust for potential confounding, we then repeated this analysis and included baseline opioid use, age, gender, and ethnicity in the model. Finally, we repeated this entire process by examining any drug use.
Of the 114 screened and eligible participants, 108 (94.7%) enrolled in the study. Of these, 3 withdrew from the study, 14 never initiated buprenorphine treatment, and 4 had no follow-up interviews. Of the remaining 87 participants, 8 had inductions elsewhere prior to receiving buprenorphine treatment at the health center (e.g., they had inductions in drug detoxification programs or hospitals, and then transferred treatment to the health center). Thus, 79 participants are included in this analysis. From these 79 participants, 70 (88.6%) interviews were conducted at 1 month, 72 (91.1%) at 3 months, and 66 (83.5%) at 6 months.
3. Results
3.1 Participant characteristics
Participants’ mean age was 43 years, and most were men (73.4%), Hispanic (70.9%), unemployed (68.4%), unstably housed (63.3%), with histories of incarceration (69.6%) (see Table 2). Regarding baseline drug use, 70.9% reported using heroin, 50.6% methadone, 25.3% opioid analgesics, and 39.2% cocaine. Half (50.6%) reported a history of injection drug use.
Table 2.
Characteristics associated with buprenorphine induction strategy
| Participants’ characteristics | Total N (%) | Induction strategy |
|
|---|---|---|---|
| Standard-of-care office-based inductions (n=13) N (%) | Patient-centered home-based inductions (n=66) N (%) | ||
| Age (mean years ± SD) | 43.1 ± 9.1 | 47.5 ± 4.6 | 42.2 ± 9.6 |
| Male | 58 (73.4) | 9 (69.2) | 49 (74.2) |
| Race/ethnicity | |||
| Hispanic | 56 (70.9) | 9 (69.2) | 47 (71.2) |
| Non-Hispanic black | 16 (20.3) | 4 (30.8) | 12 (18.2) |
| Non-Hispanic other | 3 (3.8) | 0 (0) | 3 (4.6) |
| English fluency | 48 (60.8) | 10 (76.9) | 38 (57.6) |
| High school diploma or GED | 53 (67.1) | 8 (61.5) | 45 (68.2) |
| Employed | 25 (31.7) | 2 (15.4) | 23 (34.9) |
| Stably housed | 29 (36.7) | 5 (38.5) | 24 (36.4) |
| Married | 21 (26.6) | 2 (15.4) | 19 (28.8) |
| Ever incarcerated | 55 (69.6) | 10 (76.9) | 45 (68.2) |
| HIV-positive | 18 (23.1) | 10 (76.9) | 8 (12.3)* |
| Baseline substance use | |||
| Any drug use** | 72 (91.1) | 12 (92.3) | 60 (90.9) |
| Opioid use | 70 (88.6) | 12 (92.3) | 58 (87.9) |
| Heroin | 56 (70.9) | 9 (69.2) | 47 (71.2) |
| Methadone | 40 (50.6) | 7 (53.9) | 33 (50.0) |
| Opioid analgesics | 20 (25.3) | 7 (53.9) | 13 (19.7) |
| Ever injected drugs | 40 (50.6) | 8 (61.5) | 32 (48.5) |
Note: percentages represent column percentages
p<0.05
Any drug use includes heroin, methadone, opioid analgesics, crack/cocaine, sedatives, amphetamines, barbiturates, hallucinogens, or inhalants (prescribed or non-prescribed, illicit or licit).
Thirteen (16.5%) participants had standard-of-care office-based inductions, while 66 (83.5%) had patient-centered home-based inductions. In the latter half of the study, when given the choice of induction strategy, nearly all (94.5%) chose patient-centered home-based inductions. There were no significant differences in sociodemographic characteristics or baseline drug use by induction strategy; however, reflecting changes in our initial eligibility criteria, those with standard-of-care office-based inductions were more likely to report HIV infection than those with patient-centered home-based inductions (76.9% vs. 12.3%, p<0.05) (see Table 2).
3.2 Opioid use and any drug use over time
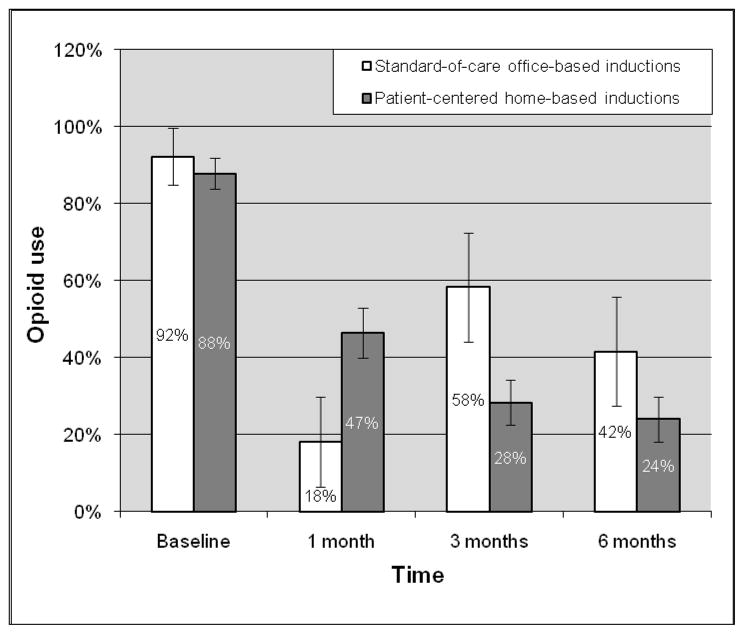
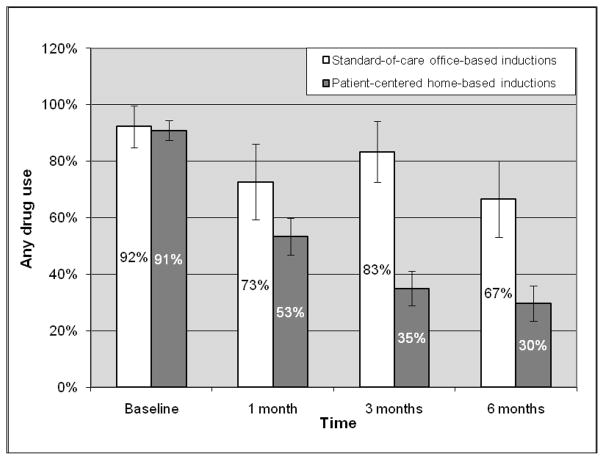
Among all participants, opioid use declined from 88.6% at baseline to 42.0% at 1 month, 33.3% at 3 months, and 27.3% at 6 months. Significant reductions in opioid use occurred between baseline and 1 month (OR=0.09, 95%CI=0.04–0.22), baseline and 3 months (OR=0.06, 95% CI=0.03–0.15), and baseline and 6 months (OR=0.05, 95%CI=0.02–0.12). There was no significant reduction between 1 and 3 months, or 3 and 6 months. Any drug use also declined from 91.1% at baseline to 56.5% at 1 month, 43.1% at 3 months, and 36.4% at 6 months. Significant reductions in any drug use occurred between baseline and 1 month (OR=0.13 95%CI=0.05–0.31), baseline and 3 months (OR=0.07, 95%CI=0.03–0.18), and baseline and 6 months (OR=0.06, 95%CI=0.02–0.14). There was no significant reduction between 1 and 3 months, or 3 and 6 months. Figures 1 and 2 demonstrate reductions in opioid use and any drug use over time by induction strategy. While opioid use and any drug use consistently declined at each time period in participants with patient-centered home-based inductions, this pattern was not observed in those with standard-of-care office-based inductions.
Figure 1.
Opioid use over time by induction strategy
Figure 2.
Any drug use over time by induction strategy
3.3 Opioid use and any drug use by induction strategy
Adjusting only for baseline opioid use, participants with standard-of-care office-based inductions and patient-centered home-based inductions had similar reductions in opioid use (AOR=0.74, 95%CI=0.16–3.50). Adjusting for baseline opioid use, age, gender, and ethnicity, this finding remained (AOR=0.63, 95%CI=0.13–2.97). When examining any drug use and adjusted only for any drug use at baseline, participants with patient-centered home-based inductions had significantly greater reductions in any drug use than those with standard-of-care office-based inductions (AOR=0.07, 95%CI=0.01–0.47). Adjusting for any drug use at baseline, age, gender, and ethnicity, this finding remained (AOR=0.05, 95%CI=0.01–0.37).
4. Discussion
Our study found that patient-centered home-based inductions were feasible and preferred by the vast majority of participants. In addition, taking into account the limitations of our observational cohort study design, patient-centered home-based inductions were equally successful in reducing opioid use and more successful in reducing any drug use than standard-of-care office-based inductions. The greatest reduction in opioid use and any drug use occurred between baseline and 1 month of follow-up, and these reductions endured through 6 months.
To our knowledge, our study is the first to demonstrate better buprenorphine treatment outcomes among patients receiving unobserved home-based inductions, when compared to those receiving observed office-based inductions. We are aware of three other studies that have examined the emerging clinical practice of unobserved inductions occurring outside of medical offices (Lee, J. D., Grossman, E., DiRocco, D., & Gourevitch, M. N., 2009; Alford, D. P. et al., 2007). However, one study by Lee and colleagues examined treatment outcomes of a single group of patients with home-based inductions and had no comparison group (Lee, J. D., Grossman, E., DiRocco, D., & Gourevitch, M. N., 2009). Another study by Alford and colleagues had a substantially different comparison group, as that study’s main goal was to compare buprenorphine treatment outcomes between housed to homeless patients, in which housed patients received phone-guided home-based inductions, and homeless patients received observed office-based inductions (Alford, D. P. et al., 2007). In that study, treatment outcomes were similar between the two groups. The final study, in which we retrospectively compared patients who had home-based versus office-based inductions, we found similar treatment retention rates (Sohler, N. L. et al., 2010). When examining drug use among patients who received unobserved home-based inductions, these studies are consistent with our study’s findings. At 3–6 months, opioid use was reported in approximately 10–30% of patients in these studies (Lee, J. D., Grossman, E., DiRocco, D., & Gourevitch, M. N., 2009; Alford, D. P. et al., 2007), while in our study, at 6 months, opioid use was reported in 24% of participants. Thus, to date, all published studies examining unobserved home-based inductions consistently demonstrate positive buprenorphine treatment outcomes with this induction strategy. Our study advances existing knowledge by further demonstrating that our patient-centered home-based induction strategy had better treatment outcomes than a standard-of-care office-based induction strategy.
Because drug addiction is a chronic medical condition (O'Brien, C. P. & McLellan, A. T., 1996; McLellan, A. T., Lewis, D. C., O'Brien, C. P., & Kleber, H. D., 2000), we used an established model of chronic disease management—the Chronic Care Model (CCM)—to guide our patient-centered home-based buprenorphine induction strategy. The CCM emphasizes the central role of patients, challenges the traditional notion that physicians are the sole people directing care, and allows patients to use their expertise with addiction (Wagner, E. H. et al., 2001; Wagner, E. H., 1998). We developed patient-centered home-based inductions to focus on one component of the CCM—improving self-management support. We believe that activating patients to engage in self-management of their addiction during buprenorphine induction set the stage for improved long-term outcomes.
Although we did not specifically explore why participants with patient-centered home-based inductions (versus standard-of-care office-based inductions) had significantly greater reductions in any drug use and not in opioid use, we believe that patients’ self-management of their opioid addiction affected their self-management of other drug use as well. This is supported by the Figures, which demonstrate that all participants had significant reductions in opioid use and any drug use within the first month after induction. However, when all participants received less intensive treatment during the maintenance phase (e.g. 3 months after induction), and when self-management strategies are needed and important, further reductions in opioid use and any drug use occurred only in those with patient-centered home-based inductions, in which self-management was emphasized. For example, during the maintenance phase, if participants experienced drug cravings, those who practiced self-management skills during patient-centered home-based inductions may have been better equipped to manage their cravings than others who did not practice self management skills and had standard-of-care office-based inductions. Because buprenorphine effectively treats opioid addiction, it is possible that with our small sample size, we could not detect significant differences in opioid use between induction strategies. However, because buprenorphine is not effective in reducing drugs other than opioids, it is likely that self-management, which was promoted in and practiced by those who had patient-centered home-based inductions, was key in reducing other drug use. Further exploration of innovative buprenorphine treatment strategies based on established models of chronic care disease management are needed.
The induction phase is challenging and important. Many treatment failures occur during induction, and those who remain in treatment past induction are likely to remain in treatment long-term (Lee, J. D., Grossman, E., DiRocco, D., & Gourevitch, M. N., 2009; Stein, M. D., Cioe, P., & Friedmann, P. D., 2005; Whitley, S. D. et al., 2009). Our data are consistent with prior studies, demonstrating that significant reduction in opioid use occurs in the first month of treatment and is sustained through six months. Our data reinforce the importance of the induction, which is key in the early reduction of opioid use. Because national guidelines recommend an office-based induction process that requires precise timing, observed dosing, and multiple assessments, orchestrating inductions can be difficult (Center for Substance Abuse Treatment., 2007). Given the importance and challenges associated with buprenorphine inductions, physicians are beginning to offer new buprenorphine induction strategies that are unobserved and occur outside of medical settings (Walley, A. Y. et al., 2008). In addition, when given the choice of induction strategies, nearly all our participants chose patient-centered home-based inductions. Although our current study did not examine adverse effects associated with each induction strategy, in our previous studies we reported similar rates of complicated inductions (e.g., inductions with precipitated or protracted opioid withdrawal) in patients who received patient-centered home-based inductions and standard-of-care office-based inductions (Whitley, S. D. et al., 2010; Sohler, N. L. et al., 2010). Because physicians and patients are interested in home-based inductions, and thoroughly described theory-based home-based induction protocols are lacking, development of evidence-based induction strategies that allow for unobserved inductions to occur outside of medical offices is urgently needed.
4.1 Study Limitations
Our study has several limitations. Participants were not assigned an induction strategy, rather, they chose one; thus, selection bias was possible. Due to changes in our study inclusion criteria, those with standard-of-care office-based inductions were more likely to be HIV-positive than those with patient-centered home-based inductions. To explore the association between HIV status and buprenorphine treatment outcomes, we conducted additional exploratory analyses among participants with patient-centered home-based inductions. We found no significant difference in opioid use (AOR=1.00) or any drug use (AOR=1.04) between participants who were HIV-positive versus -negative. In addition to this difference in HIV status between the two induction groups, unmeasured factors could also have biased our finding. Because patient-centered home-based inductions were available during the latter half of our study, it is possible that temporal factors such as physician or participant experience with buprenorphine may have affected outcomes. However, both induction strategies used standardized clinical protocols, thereby reducing the potential effect that either patient or provider experience may have had on outcomes
Additional limitations included our small sample size, which limited our ability to detect potential differences between the two groups. Because urine toxicology tests were collected for clinical care, and not research, our study relied on self-reported drug use, which may not accurately portray ongoing drug use and is subject to recall bias. Finally, because our study was limited to one clinical site, our findings may not be generalizable to other populations.
4.2 Conclusions
Our study, which compared two different buprenorphine induction strategies, found that patient-centered home-based inductions were feasible and preferred by the vast majority of participants. In addition, taking into account the limitations of our observational cohort study design, participants with patient-centered home-based inductions (versus standard-of-care office-based inductions) had similar reductions in opioid use and greater reductions in any drug use over six months. As innovative treatment strategies related to buprenorphine inductions are emerging, it is essential that they be based on established theories or models and well-studied to optimize treatment outcomes for opioid addiction.
Acknowledgments
This study was supported by the Health Resources and Services Administration, HIV/AIDS Bureau, Special Projects of National Significance, grant 6H97HA00247; the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (NIH AI-51519); NIH R25DA023021; and the Robert Wood Johnson Foundation’s Harold Amos Medical Faculty Development Program. A portion of this work was presented at the Society of General Internal Medicine Annual Conference on April 2010 in Minneapolis, MN. We thank Mia Brisbane and Johanna Rivera for their help conducting this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Diagnostic and Statistical Manual of Mental Disorders. 4. Washington D.C: American Psychiatric Association; 1994. [Google Scholar]
- Alford DP, LaBelle CT, Richardson JM, O'Connell JJ, Hohl CA, Cheng DM, Samet JH. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. Journal of General Internal Medicine. 2007;22:171–176. doi: 10.1007/s11606-006-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri MP, Rey D, Loundou A, Lepeu G, Sobel A, Obadia Y. Evaluation of buprenorphine maintenance treatment in a French cohort of HIV-infected injecting drug users. Drug and Alcohol Dependence. 2003;72:13–21. doi: 10.1016/s0376-8716(03)00189-3. [DOI] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2007. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. 2004. [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA, Munoz A. Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002–2004. The Journal of Pain. 2005;6:662–672. doi: 10.1016/j.jpain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Giovanniello A, Sacajiu G, Whitley S, Mund P, Beil R, Sohler N. Buprenorphine treatment in an urban community health center: what to expect. Family Medicine. 2008;40:500–506. [PMC free article] [PubMed] [Google Scholar]
- Cunningham CO, Kunins HV, Roose RJ, Elam RT, Sohler NL. Barriers to obtaining waivers to prescribe buprenorphine for opioid addiction treatment among HIV physicians. Journal of General Internal Medicine. 2007;22:1325–1329. doi: 10.1007/s11606-007-0264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA. The first three years of buprenorphine in the United States: Experience to date and future directions. Journal of Addiction Medicine. 2007;1:62–67. doi: 10.1097/ADM.0b013e3180473c11. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, Collins J, Raisch D, Casadonte P, Goldsmith RJ, Ling W, Malkerneker U, McNicholas L, Renner J, Stine S, Tusel D. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. The New England Journal of Medicine. 2003;349:949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. The New England Journal of Medicine. 2000;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug and Alcohol Dependence. 1995;40:17–25. doi: 10.1016/0376-8716(95)01186-2. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. The Journal of American Medical Association. 1992;267:2750–2755. [PubMed] [Google Scholar]
- Lee JD, Grossman E, DiRocco D, Gourevitch MN. Home buprenorphine/naloxone induction in primary care. Journal of General Internal Medicine. 2009;24:226–232. doi: 10.1007/s11606-008-0866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Wesson DR, Charuvastra C, Klett CJ. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Archives of General Psychiatry. 1996;53:401–407. doi: 10.1001/archpsyc.1996.01830050035005. [DOI] [PubMed] [Google Scholar]
- Lott DC, Strain EC, Brooner RK, Bigelow GE, Johnson RE. HIV risk behaviors during pharmacologic treatment for opioid dependence: a comparison of levomethadyl acetate [corrected] buprenorphine, and methadone. Journal of Substance Abuse Treatment. 2006;31:187–194. doi: 10.1016/j.jsat.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Stothart ME, Quesnel KJ, Stanger C, Brooklyn J. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Archives of General Psychiatry. 2005;62:1157–1164. doi: 10.1001/archpsyc.62.10.1157. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. The Journal of American Medical Association. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, McLellan AT. Myths about the treatment of addiction. The Lancet. 1996;347:237–240. doi: 10.1016/s0140-6736(96)90409-2. [DOI] [PubMed] [Google Scholar]
- O'Connor PG, Oliveto AH, Shi JM, Triffleman EG, Carroll KM, Kosten TR, Rounsaville BJ, Pakes JA, Schottenfeld RS. A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinic. The American Journal of Medicine. 1998;105:100–105. doi: 10.1016/s0002-9343(98)00194-6. [DOI] [PubMed] [Google Scholar]
- Olson EC, Van Wye G, Kerker B, Thorpe L, Frieden TR. Take care Highbridge and Morrisania. NYC Community Health Profiles. (2) 2006 (Rep. No. 6 (42)). Available at http://www.nyc.gov/html/doh/downloads/pdf/data/2006chp-106.pdf.
- Pani PP, Maremmani I, Pirastu R, Tagliamonte A, Gessa GL. Buprenorphine: a controlled clinical trial in the treatment of opioid dependence. Drug and Alcohol Dependence. 2000;60:39–50. doi: 10.1016/s0376-8716(99)00140-4. [DOI] [PubMed] [Google Scholar]
- Petitjean S, Stohler R, Deglon JJ, Livoti S, Waldvogel D, Uehlinger C, Ladewig D. Double-blind randomized trial of buprenorphine and methadone in opiate dependence. Drug and Alcohol Dependence. 2001;62:97–104. doi: 10.1016/s0376-8716(00)00163-0. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Archives of General Psychiatry. 1997;54:713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- Sohler NL, Li X, Kunins HV, Sacajiu G, Giovanniello A, Whitley S, Cunningham CO. Home- versus office-based buprenorphine inductions for opioid-dependent patients. Journal of Substance Abuse Treatment. 2010;38:153–159. doi: 10.1016/j.jsat.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Cioe P, Friedmann PD. Buprenorphine retention in primary care. Journal of General Internal Medicine. 2005;20:1038–1041. doi: 10.1111/j.1525-1497.2005.0228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Buprenorphine versus methadone in the treatment of opioid dependence: self-reports, urinalysis, and addiction severity index. Journal of Clinical Psychopharmacology. 1996;16:58–67. doi: 10.1097/00004714-199602000-00010. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2008a. NSDUH Series H-34, DHHS Publication No. (SMA) 08–4343. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Drug Abuse Warning Network, 2006: National Estimates of Drug-Related Emergency Department Visits. Rockville, MD: 2008b. DAWN Series D-30, DHHS Publication No. (SMA) 08–4339. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. National Admissions to Substance Abuse Treatment Services. Rockville, MD: 2009. Treatment Episode Data Set (TEDS). Highlights - 2007. DASIS Series: S-45, DHHS Publication No. (SMA) 09-4360. [Google Scholar]
- Sullivan LE, Moore BA, Chawarski MC, Pantalon MV, Barry D, O'Connor PG, Schottenfeld RS, Fiellin DA. Buprenorphine/naloxone treatment in primary care is associated with decreased human immunodeficiency virus risk behaviors. Journal of Substance Abuse Treatment. 2008;35:87–92. doi: 10.1016/j.jsat.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung HE, Richter L, Vaughan R, Johnson PB, Thom B. Nonmedical use of prescription opioids among teenagers in the United States: trends and correlates. Journal of Adolescent Health. 2005;37:44–51. doi: 10.1016/j.jadohealth.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Turner CF, Ku L, Rogers SM, Lindberg LD, Pleck JH, Sonenstein FL. Adolescent sexual behavior, drug use, and violence: increased reporting with computer survey technology. Science. 1998;280:867–873. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Effective Clinical Practice. 1998;1:2–4. [PubMed] [Google Scholar]
- Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Affairs (Millwood) 2001;20:64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]