Abstract
Objective
Obesity increases endometrial cancer risk, yet its impact on disease stage and grade is unclear. We prospectively examined the effects of body mass index (BMI) and waist-to-hip ratio (WHR) on incidence, stage, and grade of endometrial cancer.
Methods
We studied 86,937 postmenopausal women enrolled in the Women’s Health Initiative. Height, weight, and waist and hip circumference were measured at baseline. Endometrial cancer cases were adjudicated by trained physicians and pathology reports were used to determine stage and grade. Cox proportional hazards models generated hazard ratios (HR) for associations between BMI and WHR and risk of endometrial cancer. Logistic regression was used to evaluate associations between BMI and WHR and disease stage and grade.
Results
During a mean 7.8 (standard deviation 1.6) years of follow-up, 806 women were diagnosed with endometrial cancer. Though incidence was higher among Whites, stage and grade were similar between Whites and Blacks. Elevated BMI (HR 1.76, 95% confidence interval [CI] 1.41-2.19) and WHR (HR 1.33, 95% CI 1.04-1.70) increased endometrial cancer risk when comparing women in the highest and lowest categories. No associations were observed between BMI or WHR and disease stage or grade.
Conclusions
Obesity increases endometrial cancer risk independent of other factors, but is not associated with stage or grade of disease. These findings support and validate previous reports. Future research should evaluate the impact of obesity on racial disparities in endometrial cancer survival.
Keywords: Body Mass Index, Waist-Hip Ratio, Endometrial Neoplasms, African Americans, Caucasians
Introduction
Obesity is a well-established risk factor for endometrial cancer [1-3] and may contribute to 40% of the incident cases of this disease [4-6]. However, the impact of obesity on prognostic features of endometrial cancer, such as histologic grade and clinical stage, has not been extensively studied. Some studies have suggested that increased obesity is associated with a lower, and thus more favorable, grade of disease [7-9], though another reported no significant association [10]. Additionally, obesity was associated with earlier stage disease in some [7,8,11], but not all [9,12] previous studies.
Common to these studies, however, is that anthropometric measurements have only been measured at, or close to, the time of diagnosis. It is possible that the disease may have impacted the woman’s body weight at this time, thus confounding any observed associations between obesity and prognostic features. Additionally, these studies have utilized the body mass index (BMI) as their only measure of obesity. Central adiposity, as measured by the waist-to-hip ratio (WHR), may pose a greater health risk, as abdominal fat tends to be more metabolically active than fat in other areas [13]. Data from the Nurses’ Health Study document that WHR and BMI are not strongly correlated with one another [14]. Thus it is important to consider how additional measures of obesity, such as WHR, relate to prognostic features of endometrial cancer.
We utilized data from the Women’s Health Initiative (WHI) to prospectively study associations between measures of obesity and endometrial cancer risk and prognostic features. Specifically, we hypothesized that increased pre-diagnosis BMI and WHR would be positively associated with endometrial cancer incidence and more favorable stage and grade. As an exploratory aim, we evaluated differences between Blacks and Whites.
Methods
Study Population
The WHI study design and methods have been described in detail [15]. Briefly, the WHI is comprised of an observational study (OS) (n=93,676) and three clinical trials (CT) (n=68,132) covering the components of dietary modification, hormone therapy, and supplementation of calcium/vitamin D. The combined WHI cohort consists of 161,808 postmenopausal women from various ethnic and racial backgrounds, including 14,618 (9%) Black participants. Women were recruited at 40 clinical centers nationwide between October 1, 1993 and December 21, 1998. Eligibility criteria for WHI were age 50-79 years, postmenopausal, no plans to move from the area, and an estimated survival ≥ 3 years. We included all WHI participants (Figure 1), except for those reporting cancer (n=14,849) or hysterectomy (n=67,696) prior to baseline or those missing data for either of these factors (n=1,401 cancer; n=86 hysterectomy). Women missing information for baseline BMI (n=799) or WHR (n=421) were excluded from analyses involving those variables. The final sample for analyses involving BMI was 86,228 (n=38,152 from CT and n=48,076 from OS), and the final sample for analyses of WHR was 86,606 (n=38,197 from CT and n=48,409 from OS). All procedures and protocols were approved by the Institutional Review Boards at each participating institution and all participants provided written informed consent.
Figure 1.
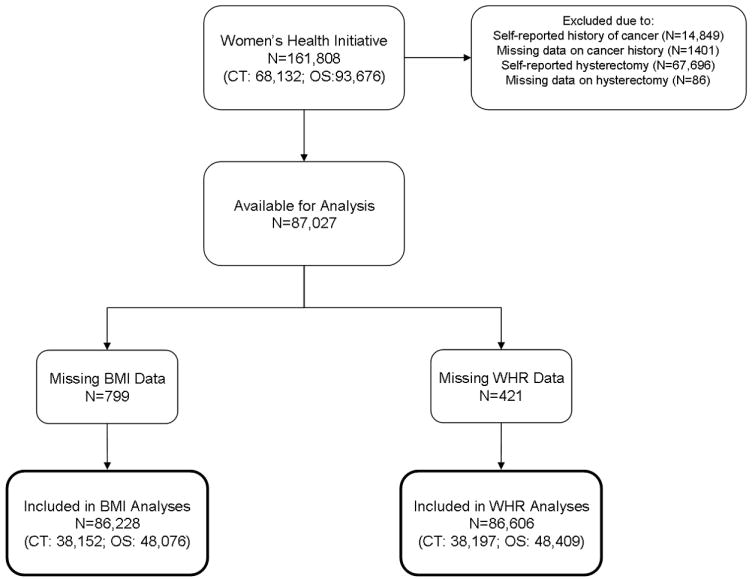
Schematic of participants included in present analysis.
Case Determination
Adjudication and outcome ascertainment for the WHI have been described elsewhere [16]. Briefly, all outcomes were self-reported semi-annually in the CT and annually in the OS. Self-reported outcomes were confirmed by local physician adjudicators who examined the pathology report and any additional information regarding the cancer diagnosis. Trained SEER coders at the Clinical Coordinating Center examined the pathology reports and any additional relevant information on all primary occurrences of endometrial cancer to finalize each cancer outcome. Each case was centrally coded by tumor registry coders to determine grade and stage. Only endometrial cancer cases confirmed by adjudication were included in these analyses (n=806). Due to missing data, 801 cases were included in the analyses with BMI and 802 cases were included in the analyses with WHR. We coded the endometrial cancer cases into histopathological subtypes following World Health Organization and International Society of Gynecological Pathology guidelines [17]. Information on histopathological subtype was missing for 27 cases. The endometrioid subtype was the most frequent (N=639, 82.0%) and cases of other subtypes were too few for analysis: adenosquamous carcinoma, N=1, 0.1%; clear cell adenocarcinoma, N=9, 1.2%; mixed carcinoma, N=28, 3.6%; mucinous adenocarcinoma, N=25, 3.2%; papillary serous adenocarcinoma, N=61, 7.8%; other, N=16, 2.1%. Therefore, we coded histopathological subtype as endometrioid or other for our analyses.
Obesity Variables
Height, weight, and waist and hip circumference were measured according to a standard protocol at clinical center visits. Measurements were obtained by trained and certified clinic staff. Height and weight were measured without shoes or heavy clothing using a wall mounted stadiometer and a calibrated balance beam or digital scale. Waist and hip measurements were taken in light indoor clothing. Baseline values of BMI and WHR were used as measures of obesity in these analyses. BMI was calculated by dividing weight in kilograms by height in meters squared, and WHR was obtained by dividing waist circumference by hip circumference measurements.
Statistical Analysis
Baseline descriptive characteristics were examined separately by BMI category and WHR quartile. BMI was categorized as underweight/normal (<25 kg/m2), overweight (25 - <30 kg/m2), and obese (≥30 kg/m2). Tests of association were conducted using Chi-square tests for categorical variables and two-sample t-tests for continuous variables.
Event rates adjusted to the overall WHI population age distribution were computed for absolute disease rate comparisons. Endometrial cancer rates, separately for BMI category and WHR quartile, were compared using time-to-event methods. Time to event was defined as days from randomization to first diagnosis of endometrial cancer. Follow-up time was censored at a woman’s last known follow-up contact, date of hysterectomy, or date of death. Quantitative comparisons by BMI category and WHR quartile are presented as hazard ratios (HRs) with nominal 95% confidence intervals (CIs) from Cox proportional hazards analyses stratified by WHI study component and adjusted for age, race/ethnicity, income, education, physical activity, smoking, total energy intake, percent energy from fat, fiber intake, fruit and vegetable intake, grain intake, diabetes, history of hypertension, age at menarche, age at menopause, attempted to get pregnant for more than 1 year, age at last term pregnancy, duration of hormone use, duration of oral contraceptive use, NSAID use, and family history of endometrial and ovarian cancers; these covariates were chosen based upon past analyses conducted within the WHI cohort and knowledge of potential associations with obesity and endometrial cancer. We performed separate regression with additional adjustment for BMI or WHR. Dietary variables were categorized into quartiles. These analyses were repeated separately for White and Black racial groups. We also repeated analyses among women with at least two years of follow-up in WHI prior to an endometrial cancer diagnosis in order to assess whether observed associations were influenced by undetected disease at baseline, and using only invasive cancer cases and then only cases of the endometrioid histopathological subtype.
Logistic regression analyses examined differences in grade, stage at diagnosis, and histopathological subtype of endometrial cancer separately by BMI category and WHR category. This analysis included only women who were diagnosed with endometrial cancer during follow-up. Stage of endometrial cancer was categorized as in situ or localized versus regional or distant. Grade of endometrial cancer was categorized as well or moderately differentiated versus poorly differentiated or anaplastic. Analyses were adjusted for age, race/ethnicity, prior hormone use, family history of endometrial cancer, and WHI study component. Odds ratios (ORs) with 95% CIs are reported. All statistical tests performed were two-sided with α≤0.05 considered statistically significant and no adjustment made for multiple comparisons. All analyses were conducted using SAS for Windows, v9.1.3 (Cary, NC).
Results
Women self-reporting a hysterectomy at baseline were excluded from this analysis, as they would not be at-risk for endometrial cancer. Differences in the proportion of potentially eligible WHI subjects excluded for this reason were observed to vary by race (40.4% Whites, 55.6% Blacks, 44.8% Hispanics, and 34.9% Asian/Pacific Islander; p<0.001).
Table 1 describes the baseline characteristics with stratification by BMI category. Statistically significant differences were observed for nearly all characteristics. Noteworthy differences across BMI category include the increased percentage of Blacks in the obese category (12.2%) as compared to the underweight/normal (3.4%) and overweight (6.6%) groups (p<0.0001). Current hormone use was more frequent among underweight/normal (39.4%) compared to the overweight (31.0%) and obese (22.9%) groups. Family history of endometrial cancer was more frequent among the obese (6.3%) compared to the overweight (5.3%) and underweight/normal (4.7%) groups. Overweight (45.8%) and obese (54.2%) women were more likely to be enrolled in the CT as compared to underweight and normal weight women (35.5%; p<0.0001).
Table 1.
Baseline Characteristics of WHI Women by Body Mass Index (kg/m2) Category
| Body Mass Index, kg/m2 |
|||||||
|---|---|---|---|---|---|---|---|
| Underweight/Normal <25 | Overweight 25 - <30 | Obese ≥30 | |||||
| N | % | N | % | N | % | P-value | |
| Age at screening, mean years | 32664 | 62.9 | 29509 | 63.3 | 24055 | 62.5 | <.0001 |
| 50-59 | 11847 | 36.3 | 9761 | 33.1 | 8652 | 36.0 | |
| 60-69 | 13821 | 42.3 | 13283 | 45.0 | 11190 | 46.5 | |
| 70-79 | 6996 | 21.4 | 6465 | 21.9 | 4213 | 17.5 | |
| Race/ethnicity | <.0001 | ||||||
| White | 28590 | 87.5 | 25035 | 84.8 | 19210 | 79.9 | |
| Black | 1097 | 3.4 | 1947 | 6.6 | 2931 | 12.2 | |
| Hispanic | 907 | 2.8 | 1269 | 4.3 | 1164 | 4.8 | |
| American Indian | 85 | 0.3 | 90 | 0.3 | 135 | 0.6 | |
| Asian/Pacific Islander | 1577 | 4.8 | 756 | 2.6 | 253 | 1.1 | |
| Unknown | 408 | 1.2 | 412 | 1.4 | 362 | 1.5 | |
| Physical activity, METs/week | <.0001 | ||||||
| None | 3003 | 9.6 | 3815 | 13.6 | 5255 | 23.0 | |
| <7.2 | 7221 | 23.0 | 8044 | 28.7 | 8181 | 35.8 | |
| 7.2 - <17.2 | 9406 | 29.9 | 8370 | 29.9 | 5568 | 24.3 | |
| ≥17.2 | 11791 | 37.5 | 7779 | 27.8 | 3875 | 16.9 | |
| Smoking | <.0001 | ||||||
| Never | 16656 | 51.6 | 14594 | 50.0 | 11919 | 50.2 | |
| Past | 13042 | 40.4 | 12552 | 43.0 | 10478 | 44.1 | |
| Current | 2597 | 8.0 | 2014 | 6.9 | 1369 | 5.8 | |
| Age at menarche, years | <.0001 | ||||||
| <12 | 5404 | 16.6 | 6031 | 20.5 | 6494 | 27.1 | |
| 12 | 7956 | 24.4 | 7737 | 26.3 | 6404 | 26.7 | |
| 13 | 10287 | 31.6 | 8743 | 29.7 | 6487 | 27.0 | |
| ≥14 | 8938 | 27.4 | 6911 | 23.5 | 4604 | 19.2 | |
| Age at menopause, mean years | 31412 | 50.3 | 28017 | 50.3 | 22608 | 50.3 | 0.40 |
| Tried becoming pregnant >1 year | 5352 | 16.5 | 4698 | 16.0 | 3561 | 14.9 | <.0001 |
| Hormone use | <.0001 | ||||||
| Never | 15358 | 47.1 | 15991 | 54.2 | 15423 | 64.1 | |
| Past | 4435 | 13.6 | 4346 | 14.7 | 3114 | 13.0 | |
| Current | 12848 | 39.4 | 9144 | 31.0 | 5507 | 22.9 | |
| E+P use | <.0001 | ||||||
| Never | 17634 | 54.0 | 18135 | 61.5 | 16953 | 70.5 | |
| Past | 3066 | 9.4 | 2868 | 9.7 | 2024 | 8.4 | |
| Current | 11948 | 36.6 | 8490 | 28.8 | 5071 | 21.1 | |
| Unopposed estrogen use | <.0001 | ||||||
| Never | 28514 | 87.3 | 25989 | 88.1 | 21745 | 90.4 | |
| Past | 3258 | 10.0 | 2880 | 9.8 | 1888 | 7.9 | |
| Current | 879 | 2.7 | 627 | 2.1 | 416 | 1.7 | |
| Non-aspirin NSAID use | 29007 | 88.8 | 25459 | 86.3 | 19552 | 81.3 | <.0001 |
| Family history of endometrial cancer | 1430 | 4.7 | 1472 | 5.3 | 1411 | 6.3 | <.0001 |
| Family history of ovarian cancer | 660 | 2.2 | 616 | 2.2 | 538 | 2.4 | 0.13 |
| Observational Study participant | 21066 | 64.5 | 15996 | 54.2 | 11014 | 45.8 | <.0001 |
| Clinical Trial participant | 11598 | 35.5 | 13513 | 45.8 | 13041 | 54.2 | <.0001 |
Women who developed endometrial cancer were more likely to be of White race/ethnicity (90.5%) than those who remained cancer-free (84.4%; p<0.0001). Total energy intake of ≥1972.1 kcal/day was more common among endometrial cancer cases (31.3%) compared to women without endometrial cancer (24.9%; p<0.0001). Strong associations were observed between exogenous hormone use and endometrial cancer. Women diagnosed with endometrial cancer were more likely to be current hormone therapy users at baseline (41.3%) than those not diagnosed with endometrial cancer (31.8%; p<0.0001). Further, increased duration of hormone therapy use was related to endometrial cancer status, with 22.7% of cases and 10.9% of endometrial cancer-free women reporting hormone use for ≥10 years (p<0.0001).
Average follow-up for this study population was 7.8 years (standard deviation 1.6). Of the 806 cases of endometrial cancer included in this analysis, there were 730 cases among Whites (age-adjusted rate 123.4 cases per 100,000 person-years) and 38 cases among Blacks (age-adjusted rate 82.7 cases per 100,000 person-years, p=0.01). Table 2 reports age-adjusted rates of endometrial cancer among BMI categories and WHR quartiles. Incidence was highest among obese women (187.1 cases per 100,000 person-years) and in the highest WHR quartile (141.8 cases per 100,000 person-years). In adjusted Cox proportional hazards models a positive association was observed between both BMI (p<0.0001) and WHR (p=0.001) and endometrial cancer risk. Obese women had a 76% increased risk of endometrial cancer compared to underweight and normal weight women (HR 1.76, 95% CI 1.41-2.19). Endometrial cancer risk increased 33% among women with a WHR ≥0.8530 versus <0.7554 (HR 1.33, 95% CI 1.04-1.70). Further adjustment for WHR did not appreciably change the associations between BMI and endometrial cancer risk. Associations between WHR and endometrial cancer risk were attenuated and non-significant upon additional adjustment for BMI, however. Results were similar when restricting cases to invasive disease or to the endometrioid subtype and when restricted to follow-up after two years of WHI participation (data not shown).
Table 2.
Multivariate associations of body mass index and waist-to-hip ratio with incidence (age-adjusted rate/100000 PY) of endometrial cancer during WHI
| N | Age-adjusted Rate/100000 PY* | Model 1† HR (95% CI) | P-value‡ | Model 2§ HR (95% CI) | P-value‡ | |
|---|---|---|---|---|---|---|
|
All endometrial cancers (in situ & invasive) | ||||||
| Body Mass Index, kg/m2 | <0.0001 | <0.0001 | ||||
| <25 | 264 | 107.3 | 1.00 | 1.00 | ||
| 25 - <30 | 207 | 92.6 | 0.86 (0.69-1.07) | 0.84 (0.67-1.05) | ||
| ≥30 | 334 | 187.1 | 1.76 (1.41-2.19) | 1.68 (1.33-2.13) | ||
| Waist-to-hip ratio quartiles | 0.001 | 0.54 | ||||
| <0.7554 | 182 | 113.4 | 1.00 | 1.00 | ||
| 0.7554 - <0.8011 | 193 | 117.0 | 0.96 (0.75-1.22) | 0.93 (0.73-1.19) | ||
| 0.8011 - <0.8530 | 198 | 120.3 | 1.12 (0.88-1.43) | 1.04 (0.80-1.33) | ||
| ≥0.8530 | 233 | 141.8 | 1.33 (1.04-1.70) | 1.12 (0.86-1.47) | ||
|
Invasive endometrial cancers | ||||||
| Body Mass Index, kg/m2 | <0.0001 | <0.0001 | ||||
| <25 | 260 | 105.5 | 1.00 | 1.00 | ||
| 25 - <30 | 202 | 90.4 | 0.84 (0.68-1.05) | 0.82 (0.65-1.03) | ||
| ≥30 | 312 | 175.9 | 1.67 (1.33-2.09) | 1.59 (1.25-2.02) | ||
| Waist-to-hip ratio quartiles | 0.001 | 0.41 | ||||
| <0.7554 | 180 | 112.0 | 1.00 | 1.00 | ||
| 0.7554 - <0.8011 | 186 | 113.0 | 0.94 (0.73-1.21) | 0.92 (0.72-1.18) | ||
| 0.8011 - <0.8530 | 186 | 113.1 | 1.06 (0.83-1.36) | 1.00 (0.77-1.29) | ||
| ≥0.8530 | 223 | 137.0 | 1.32 (1.03-1.70) | 1.15 (0.88-1.50) | ||
|
Endometrioid histopathological subtype | ||||||
| Body Mass Index, kg/m2 | <0.0001 | <0.0001 | ||||
| <25 | 212 | 86.0 | 1.00 | 1.00 | ||
| 25 - <30 | 163 | 73.0 | 0.84 (0.65-1.07) | 0.81 (0.63-1.04) | ||
| ≥30 | 262 | 147.1 | 1.69 (1.32-2.17) | 1.60 (1.23-2.09) | ||
| Waist-to-hip ratio quartiles | 0.001 | 0.33 | ||||
| <0.7554 | 148 | 93.2 | 1.00 | 1.00 | ||
| 0.7554 - <0.8011 | 148 | 90.1 | 0.89 (0.67-1.17) | 0.87 (0.66-1.15) | ||
| 0.8011 - <0.8530 | 153 | 92.9 | 1.06 (0.81-1.39) | 0.99 (0.75-1.32) | ||
| ≥0.8530 | 189 | 116.3 | 1.32 (1.01-1.74) | 1.13 (0.85-1.52) | ||
Adjusted to the overall WHI age distribution. PY = person years.
Adjusted for age, race/ethnicity, income, education, physical activity, smoking, total energy intake, percent energy from fat, fiber intake, fruit and vegetable intake, grain intake, diabetes, history of hypertension, age at menarche, age at menopause, tried getting pregnant for >1 year, age at last term pregnancy, duration of hormone use, duration of oral contraceptive use, NSAID use, and family history of endometrial and ovarian cancers and stratified on study component.
Additionally adjusted for BMI or WHR.
Tested using the continuous form of the log-transformed values of body mass index and waist-to-hip ratio.
Hazard ratios were similar when the analyses were restricted to White participants (HR 1.80, 95% CI 1.44-2.27 for BMI ≥30 versus <25; HR 1.33, 95% CI 1.03-1.71 for WHR ≥0.8530 versus <0.7554). Among Blacks the association between both BMI (HR 2.76, 95% CI 0.57-13.51 for BMI ≥30 versus <25) and WHR (HR 4.61, 95% CI 0.50-42.67 for WHR ≥0.8530 versus <0.7554) and endometrial cancer risk appeared to be stronger. There was no significant interaction between race and BMI (p=0.35) or race and WHR (p=0.32).
Among endometrial cancer cases, 13 (1.7%) had in situ, 645 (82.1%) localized, 88 (11.2%) regional, and 40 (5.1%) distant disease. Stage information was missing for 20 (2.5%) cases. Disease grade was well differentiated for 199 (26.6%), moderately differentiated for 309 (41.3%), poorly differentiated for 159 (21.3%), and anaplastic for 81 (10.8%) cases. Grade classification was missing for 58 (7.2%) cases. Stage (p=0.14) and grade were similar between Whites and Blacks (p=0.95; data not shown). BMI and WHR were not associated with higher grade or later stage disease (Table 3). Obese BMI (OR 0.65, 95% CI 0.39-1.08) and WHR ≥0.8011 (OR 0.76, 95% CI 0.50-1.15) were associated with non-significant decreased likelihood of non-endometrioid histopatholgical subtypes. The analyses reported in Table 3 were not repeated with stratification on race/ethnicity due to the small number of Black cases.
Table 3.
Multivariate associations of body mass index and waist-to-hip ratio with grade, stage and histology of invasive disease*
| Well/Moderately Differentiated Grade | Poorly Differentiated/Anaplastic Grade | OR (95% CI) * | |
|---|---|---|---|
| Body Mass Index, kg/m2 | |||
| <25 | 169 | 74 | 1.00 |
| 25 - <30 | 128 | 65 | 1.05 (0.68, 1.62) |
| ≥30 | 205 | 97 | 0.77 (0.50, 1.19) |
| Waist-to-hip ratio quartiles | |||
| <0. 8011 | 246 | 99 | 1.00 |
| ≥0.8011 | 254 | 140 | 1.23 (0.87, 1.74) |
| Localized Stage | Regional/Distant Stage | OR (95% CI)*✽ | |
| Body Mass Index, kg/m2 | |||
| <25 | 223 | 35 | 1.00 |
| 25 - <30 | 164 | 37 | 1.40 (0.82, 2.39) |
| ≥30 | 255 | 54 | 1.05 (0.61, 1.79) |
| Waist-to-hip ratio quartiles | |||
| <0. 8011 | 312 | 51 | 1.00 |
| ≥0.8011 | 329 | 77 | 1.23 (0.80, 1.88) |
| Localized Stage Adjusted for Grade | Regional/Distant Stage Adjusted for Grade | OR (95% CI)*✽ | |
| Body Mass Index, kg/m2 | |||
| <25 | 210 | 32 | 1.00 |
| 25 - <30 | 161 | 32 | 1.32 (0.73, 2.38) |
| ≥30 | 249 | 51 | 1.26 (0.70, 2.25) |
| Waist-to-hip ratio quartiles | |||
| <0. 8011 | 298 | 46 | 1.00 |
| ≥0.8011 | 321 | 71 | 1.20 (0.75, 1.92) |
| Endometrioid Histopathological Subtype | Other Histopathological Subtype | OR (95% CI)*✽ | |
| Body Mass Index, kg/m2 | |||
| <25 | 211 | 48 | 1.00 |
| 25 - <30 | 163 | 39 | 1.04 (0.63, 1.73) |
| ≥30 | 261 | 50 | 0.65 (0.39, 1.08) |
| Waist-to-hip ratio quartiles | |||
| <0. 8011 | 295 | 70 | 1.00 |
| ≥0.8011 | 341 | 67 | 0.76 (0.50, 1.15) |
Odds ratios and 95% confidence intervals are from multinomial logistic regression models adjusted for age, ethnicity, prior hormone use, family history of endometrial cancer, and WHI study component.
Numbers do not sum to 809 due to missing data for BMI, WHR, stage, grade, and/or histopathological subtype
Discussion
We found that elevated pre-diagnosis BMI and WHR were both related to an increased risk of endometrial cancer, yet had no association with prognostic features. Specifically, obese women (BMI ≥30 kg/m2) experienced a 76% increase in endometrial cancer risk, and women with a WHR ≥0.8530 experienced a 33% increase in endometrial cancer risk. BMI and WHR were not associated with disease stage or grade. Results were similar when restricted to invasive cases or to cases of the endometrioid subtype.
Our findings are consistent with previous reports of positive associations of both BMI and WHR with the incidence of endometrial cancer [2]. Friedenreich et al [3] found that women with a BMI of 30 – 40 kg/m2 had a 1.78 times (95% CI 1.41 – 2.26) increased risk of endometrial cancer compared to those of normal BMI, similar to our estimated 76% increased risk. Their estimate for WHR (RR 1.58, 95% CI 1.19-2.10) for highest quartile versus lowest quartile [3] was slightly higher than the 33% increased risk we report, likely due to differences in cut-offs for WHR between the two studies. Interestingly, BMI remained significantly associated with risk upon adjustment for WHR, but WHR was no longer related to disease risk in models adjusted for BMI. Similar observations were noted in other recent studies of adiposity and endometrial cancer [3,18], though another recent study found the WHR was a risk factor for endometrial cancer independent of BMI [19]. As summarized by Friedenreich [3], earlier studies also provide inconsistent evidence regarding whether or not WHR is an independent risk factor for endometrial cancer. Thus it is currently unclear whether general obesity or abdominal obesity, and their respective hormonal and metabolic consequences, is more relevant to endometrial cancer risk.
Current evidence relating body fatness to stage and grade of endometrial cancer is inconsistent [7-9,11,20]. We observed no association between BMI or WHR and stage or grade of disease, contrary to our hypothesis. The close monitoring of WHI participants, especially those in the clinical trials, may have biased our results. While 82.1% of our endometrial cancer cases had localized disease, nationally only 69% of endometrial cancer cases are diagnosed at this stage [21]. It is possible that body composition may be related to stage and/or grade of endometrial cancer in the general population, but this was not the case within the WHI.
In the present study, there was a suggestion that the effect of BMI and WHR on endometrial cancer incidence was greater among Blacks; however, small numbers limited statistical significance. The lack of endometrial cancer cases among Blacks may be related to the increased prevalence of hysterectomy among Blacks (55.6%) in the WHI population. We observed no differences in either stage or grade of disease between racial groups. Other studies have reported that Blacks diagnosed with endometrial cancer often have advanced stage disease and a worse prognosis compared to Whites. This disparity may be due to different genetic etiologies and inequalities in treatment [22]. To the extent that such disparities may be related to differences in socioeconomic status, this effect may not be apparent in our study due to the higher socioeconomic status of Black WHI participants as compared to the general population of U.S. Blacks. Unfortunately, we were unable to examine associations between BMI or WHR and stage or grade separately by race due to the small sample of Black cases. Given that neither BMI nor WHR were associated with stage or grade among our full study population, however, it is unlikely that differences in obesity prevalence explain the racial disparity in endometrial cancer survival. Analyses of SEER data show that poorer endometrial cancer survival among Blacks has persisted over recent decades [23]. Future research, perhaps through pooled data analysis, is needed to determine the reasons for this racial disparity in survival.
The WHI is a well-conducted prospective cohort study of over 160,000 postmenopausal women from various racial and ethnic backgrounds with extensive follow-up. Further strengths include the adjudication and central coding of the endometrial cancer outcomes and the standardized measurement of BMI and WHR. Additionally, BMI and WHR measurements were taken well before participants were diagnosed with endometrial cancer. Previous research studies performed anthropometric assessments at, or close to, the time of diagnosis, thus raising the possibility that such measures were affected by undetected disease. We additionally performed a sensitivity analysis excluding cases diagnosed within two years of enrollment and obtained results similar to those observed among the full cohort. Thus we believe that undetected disease is unlikely to bias our observed associations between BMI and WHR and endometrial cancer incidence.
Unfortunately, our ability to examine racial differences in the association between BMI and endometrial cancer incidence, stage, and grade was limited by the small numbers of cases among racial/ethnic subgroups. Additionally, the external validity of our study may be somewhat limited. The lower prevalence of obesity among both Blacks and Whites in our study population compared to national estimates indicates that our population may have been healthier than the general population. Finally, we measured BMI and WHR at only a single point in time. Women’s BMI or WHR may have changed throughout their WHI follow-up, and it is possible that such changes might be associated (either causally or non-causally) with risk of endometrial cancer.
Our results suggest that obesity may not be important to prognostic features of endometrial cancer, though it clearly has an impact on disease risk. Moreover, our findings suggest areas where prevention and more intense surveillance strategies could be implemented – namely among obese women, and perhaps especially among obese Black women. More research is needed among Black women, however, to more conclusively assess the impact of obesity on the incidence, stage, and grade of endometrial cancer as well as survival from this disease.
Acknowledgments
We acknowledge the contributions of the following WHI investigators:
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Haleh Sangi-Haghpeykar; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence S. Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Erin LeBlanc; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael S. Simon.
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
Footnotes
Conflict of Interest Statment Dr. Cuyun-Carter is currently employed by Eli Lilly and Company. No other authors have conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang SC, Lacey JV, Jr, Brinton LA, Hartge P, Adams K, Mouw T, et al. Lifetime weight history and endometrial cancer risk by type of menopausal hormone use in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. 2007 Apr;16(4):723–730. doi: 10.1158/1055-9965.EPI-06-0675. [DOI] [PubMed] [Google Scholar]
- 2.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008 Feb 16;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 3.Friedenreich C, Cust A, Lahmann PH, Steindorf K, Boutron-Ruault MC, Clavel-Chapelon F, et al. Anthropometric factors and risk of endometrial cancer: the European prospective investigation into cancer and nutrition. Cancer Causes Control. 2007 May;18(4):399–413. doi: 10.1007/s10552-006-0113-8. [DOI] [PubMed] [Google Scholar]
- 4.Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001 Feb 1;91(3):421–430. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1053>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.Bianchini F, Kaaks R, Vainio H. Weight control and physical activity in cancer prevention. Obes Rev. 2002 Feb;3(1):5–8. doi: 10.1046/j.1467-789x.2002.00046.x. [DOI] [PubMed] [Google Scholar]
- 6.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007 Dec 1;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munstedt K, Wagner M, Kullmer U, Hackethal A, Franke FE. Influence of body mass index on prognosis in gynecological malignancies. Cancer Causes Control. 2008 Nov;19(9):909–916. doi: 10.1007/s10552-008-9152-7. [DOI] [PubMed] [Google Scholar]
- 8.Everett E, Tamimi H, Greer B, Swisher E, Paley P, Mandel L, et al. The effect of body mass index on clinical/pathologic features, surgical morbidity, and outcome in patients with endometrial cancer. Gynecol Oncol. 2003 Jul;90(1):150–157. doi: 10.1016/s0090-8258(03)00232-4. [DOI] [PubMed] [Google Scholar]
- 9.Pavelka JC, Ben-Shachar I, Fowler JM, Ramirez NC, Copeland LJ, Eaton LA, et al. Morbid obesity and endometrial cancer: surgical, clinical, and pathologic outcomes in surgically managed patients. Gynecol Oncol. 2004 Dec;95(3):588–592. doi: 10.1016/j.ygyno.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 10.Hill HA, Coates RJ, Austin H, Correa P, Robboy SJ, Chen V, et al. Racial differences in tumor grade among women with endometrial cancer. Gynecol Oncol. 1995 Feb;56(2):154–163. doi: 10.1006/gyno.1995.1024. [DOI] [PubMed] [Google Scholar]
- 11.Anderson B, Connor JP, Andrews JI, Davis CS, Buller RE, Sorosky JI, et al. Obesity and prognosis in endometrial cancer. Am J Obstet Gynecol. 1996 Apr;174(4):1171–8. doi: 10.1016/s0002-9378(96)70659-2. discussion 1178-9. [DOI] [PubMed] [Google Scholar]
- 12.Barrett RJ, 2nd, Harlan LC, Wesley MN, Hill HA, Chen VW, Clayton LA, et al. Endometrial cancer: stage at diagnosis and associated factors in black and white patients. Am J Obstet Gynecol. 1995 Aug;173(2):414–22. doi: 10.1016/0002-9378(95)90261-9. discussion 422-3. [DOI] [PubMed] [Google Scholar]
- 13.Arner P. Regional adipocity in man. J Endocrinol. 1997 Nov;155(2):191–192. doi: 10.1677/joe.0.1550191. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008 Apr 1;117(13):1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 15.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998 Feb;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 16.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003 Oct;13(9 Suppl):S122–8. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 17.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006 Nov;95(Suppl 1):S105–43. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 18.Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med. 2000 Jul 24;160(14):2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 19.Xu WH, Matthews CE, Xiang YB, Zheng W, Ruan ZX, Cheng JR, et al. Effect of adiposity and fat distribution on endometrial cancer risk in Shanghai women. Am J Epidemiol. 2005 May 15;161(10):939–947. doi: 10.1093/aje/kwi127. [DOI] [PubMed] [Google Scholar]
- 20.Douchi T, Ijuin H, Nakamura S, Oki T, Maruta K, Nagata Y. Correlation of body fat distribution with grade of endometrial cancer. Gynecol Oncol. 1997 Apr;65(1):138–142. doi: 10.1006/gyno.1996.4599. [DOI] [PubMed] [Google Scholar]
- 21.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975-2007. National Cancer Institute; Bethesda, MD: 2010. Jun 25, http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site. [Google Scholar]
- 22.Farley J, Risinger JI, Rose GS, Maxwell GL. Racial disparities in blacks with gynecologic cancers. Cancer. 2007 Jul 15;110(2):234–243. doi: 10.1002/cncr.22797. [DOI] [PubMed] [Google Scholar]
- 23.Wright JD, Fiorelli J, Schiff PB, Burke WM, Kansler AL, Cohen CJ, et al. Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time. Cancer. 2009 Feb 9; doi: 10.1002/cncr.24160. [DOI] [PubMed] [Google Scholar]


