Abstract
Dendritic spines are the predominant sites of excitatory neurotransmission in the adult brain, and brain-derived neurotrophic factor (BDNF) is a well-characterized determinant of dendritic spine number and morphology. The relationship between BDNF expression and dendritic spine number is particularly evident in the hippocampus, where environmental conditions that enhance hippocampal BDNF levels also promote local increases in dendritic spine density. However, the relationship between physiological variability in hippocampal BDNF expression and spine number has yet to be assessed. To determine whether natural variability in BDNF expression is associated with hippocampal dendritic spine number, correlations between BDNF protein levels and dendritic spine density among Golgi-impregnated neurons in the hippocampal dentate gyrus and CA1 subfields were assessed in adult male C57Bl/6J mice. In the dentate gyrus, but not in the apical oblique dendrites of CA1 pyramidal cells, BDNF protein expression was significantly correlated with dendritic spine density. This observation suggests that there may be a subregionally specific relationship between hippocampal BDNF expression and the density of spines.
Keywords: hippocampus, dendritic spine, brain-derived neurotrophic factor
Dendritic spines are a structural substrate for plasticity in the central nervous system. Changes in spine morphology and number influence local calcium dynamics along dendritic segments [1], thereby regulating neuronal excitability. Brain-derived neurotrophic factor (BDNF) is a well-characterized modulator of both spines and synapses [2]. Environmental conditions associated with BDNF upregulation also promote spinogenesis [3], while environmental conditions that suppress BDNF expression induce atrophy among spines [4]. Direct application of BDNF enhances spine number and promotes plasticity [5], while genetic inhibition of BDNF expression has the opposite effect [6]. Both in vitro and in vivo studies have demonstrated a clear relationship between BDNF expression and dendritic spine density.
BDNF heterozygous knockout mice exhibit a fifty percent reduction in BDNF mRNA expression [7], while BDNF overexpressing mice show nearly tenfold upregulation of BDNF protein levels [8]. While these genetic models are informative, they are also extreme, and the extent to which normal fluctuations in BDNF expression modulate dendritic spine density merits further exploration. To this end, the current study evaluates this relationship using a correlational approach. BDNF protein expression was assessed using enzyme-linked immunosorbent assay (ELISA). Dendritic spine density was characterized in two areas that are known to exhibit associative plasticity: the middle molecular layer of the dentate gyrus, where the medial perforant path synapses are located, and the stratum radiatum of area CA1, where the Schaffer collateral synapses are located. BDNF expression was correlated with dendritic spine density in the dentate gyrus, but not in CA1, indicating that dentate gyrus granule cells may be more sensitive to changes in BDNF expression than CA1 pyramidal cells.
Materials and Methods
Male C57Bl/6J mice (n=8) were purchased from Jackson Laboratories at three weeks of age and housed in groups of four. When the mice were two months old, they were euthanized by rapid decapitation under light Isoflurane anesthesia, two to four hours after the onset of the light period. Brains were removed, and the hippocampus was dissected out from one hemisphere and frozen on dry ice. The other hemisphere was kept for Golgi impregnation and analysis. To control for possible lateralization effects, left and right hemispheres were counterbalanced between the BDNF assay and spine density studies. All animal procedures followed National Institutes of Health Guide for the Care and Use of Laboratory Animals.
The BDNF ELISA followed previously published methods [3]. Briefly, hippocampi were homogenized in lysis buffer (137 mM NaCl, 20 mM Tris, 1% NP-40 detergent, 10% glycerol, 1 mM phenylmethylsulfonylflouride, 10 μg/mL aprotinin, 1 μg/mL leupeptin, and 0.5 mM sodium orthovanadate; pH 7.2) at 4°C. Homogenates separated by centrifugation at 14,000 rpm for 15 min (4°C), and supernatants were used for ELISA analysis according to the manufacturer’s instructions (Promega Corp., Madison, WI). Protein content was determined in the supernatant using the Bradford assay and BDNF expression was normalized to total protein.
A commercially available kit (FD Neurotechnologies kit #SS201) was used for Golgi impregnation according to the manufacturer’s instructions. Following two weeks incubation in Golgi-Cox solution, the tissue was sectioned on the transverse plane (100 μm) using a Vibratome. After visualizing impregnated cells, the sections were dehydrated in increasing concentrations of ethanol, cleared in Histoclear, and coverslipped under Permount. Cells were selected for analysis as described [3]. Briefly, cells were required to exhibit a fully impregnated cell body, and dark brown or black dendrites, with sufficient separation from other labeled cells, and no apparent severed dendrites. Dendritic segments selected for analysis of spine density among dentate gyrus granule cells were on second- or third-order dendrites of cells located in the superficial portion of the granule cell layer. Dendritic segments selected for analysis of CA1 pyramidal neurons were apical oblique dendrites in the stratum radiatum.
To acquire images for analysis of dendritic spine density, segments were scanned using a 63× oil-immersion objective on a Zeiss LSM 510 confocal microscope. The configuration used Argon and HeNe lasers, with visible light channeled through the rhodamine path, to acquire images against a white background. Image stacks were acquired at 4.0× optical zoom with a 1.0μm step size, exported as .tiff files, and imported into Reconstruct (freely available at http://synapses.bu.edu). Reconstruct was then used to analyze the density of dendritic spines on five segments per cell, from five cells per mouse. The relationship between the mean spine density and BDNF expression was evaluated using Pearson’s correlation. For all analyses, significance was set at p < 0.05. All statistical analyses were conducted using GraphPad Prism 5.0 software (La Jolla, CA).
Results
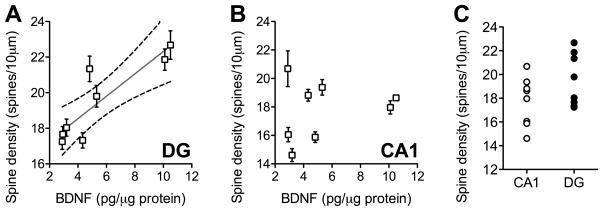
BDNF expression was positively correlated with dendritic spine density in the dentate gyrus (Figure 1A; Pearson’s r=0.87, p=0.004) but not in the CA1 subfield (Figure 1B; Pearson’s r=0.21, p=0.62). Comparison of dendritic spine densities in the dentate gyrus and CA1 subfields revealed that the secondary and tertiary dendrites of dentate granule neurons are not significantly different from CA1 apical oblique dendrites with respect to their mean spine densities (Figure 1C; t14=1.63, p=0.13) or their variability (t14=1.17, p=0.84). Golgi impregnation effectively visualized a large number of CA1 pyramidal neurons (Figure 2A) and dentate gyrus granule cells (Figure 2B). Spines were detectable on the dendritic shafts of apical oblique dendrites on CA1 pyramidal neurons (Figure 2C) and along the secondary and tertiary dendrites of dentate granule neurons (Figure 5D).
Figure 1. Variability in BDNF protein expression correlates with dendritic spine density in the dentate gyrus but not CA1 subfield of the mouse hippocampus.
(A) BDNF protein levels are positively correlated with dendritic spine density along the secondary and tertiary dendrites of Golgi-impregnated granule cells in the hippocampal dentate gyrus. (B), No such correlation was detected in the CA1 subfield. For both graphs, each symbol represents one mouse, and error bars depict the s.e.m. derived from the variability between cells from each mouse. For the dentate gyrus only, the Pearson’s correlation was significant at p < 0.01. (C), Dendritic spine densities are similar along the apical dendrites of CA1 pyramidal neurons and dentate gyrus granule cells.
Figure 2. Representative images of golgi-impregnated cells in the mouse hippocampus.
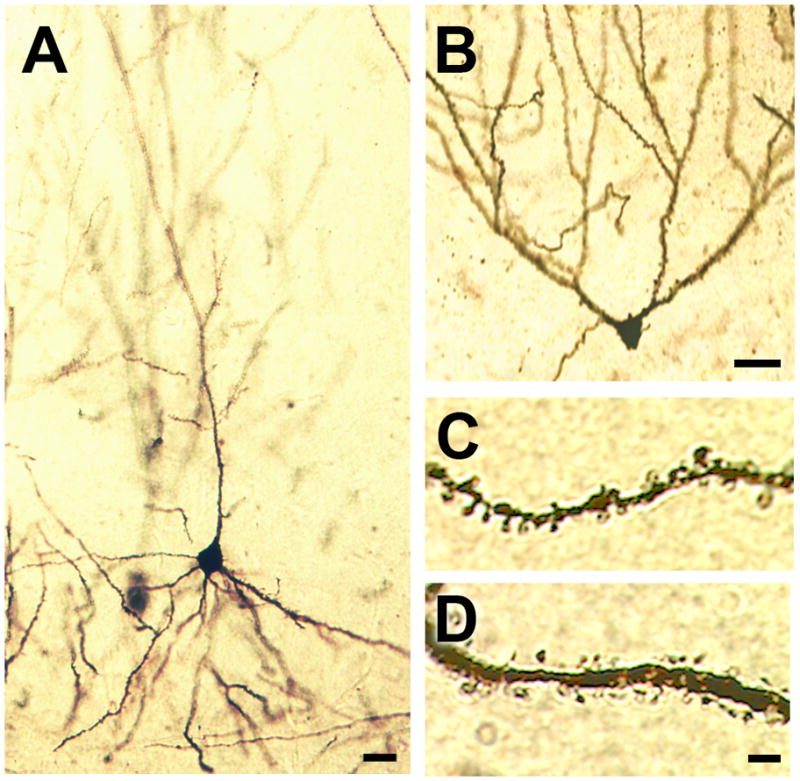
(A), CA1 pyramidal neuron visualized with Golgi impregnation. (B), Dentate gyrus granule cell in the mouse hippocampus. For panels (A–B), scale bar = 20μm. (C), Apical oblique dendritic segment from a CA1 pyramidal neuron in the mouse hippocampus. (D), Secondary dendrite from a Golgi-impregnated dentate gyrus granule cell. For panels (C–D), scale bar = 5.0μm.
Discussion
Genetic and pharmacological manipulations that affect BDNF expression typically do so by elevating or suppressing BDNF levels outside of the physiological range. The experiments in this study have explored whether normal variability in BDNF expression is associated with dendritic spine density in two hippocampal subfields that differ in their endogenous BDNF expression levels. BDNF mRNA is more strongly expressed in the dentate gyrus, relative to the CA1 subfield [9]. Based on this subregional variability in BDNF expression, the structure of dentate gyrus granule cells is likely to be more sensitive to fluctuations in BDNF than CA1 pyramidal neurons. This was upheld in the current experiment, as BDNF protein expression was correlated with dendritic spine density in the dentate gyrus, but not in the CA1 subfield of the hippocampus.
BDNF expression follows a circadian rhythm. While levels of BDNF in the dentate are consistently greater than CA1, the regional disparity is strongest at the onset of the light phase [10]. This is significant because the current experiment examined the association between BDNF and dendritic spine density at the onset of the light phase. It is possible that circadian variability in dentate gyrus BDNF expression might drive changes in spines, while area CA1 might have a more stable synaptic profile over a twenty-four hour period. Future studies will be needed to determine whether the association between BDNF and spine density among dentate gyrus granule neurons varies over the circadian cycle.
The relationship between spine density and BDNF protein in the dentate could be a function of the higher BDNF production in the dentate gyrus, relative to the CA1 subfield, but this correlation could also be attributable to differential cellular coupling between BDNF and its downstream signaling targets across the two regions. Although the dentate gyrus and CA1 subfield express comparable levels of mRNA for the TrkB receptor, the extent to which TrkB receptor activation recruits downstream signaling targets, such as phosphorylation of cyclic-AMP response element binding protein (CREB), could be different in the two hippocampal subfields. These two hypotheses are not mutually exclusive and may simultaneously distinguish the functional role of BDNF as a regulator of structural plasticity in the dentate gyrus and CA1 subfields.
The dentate gyrus and CA1 subfield participate in different aspects of hippocampal function. While activity among CA1 neurons has classically been associated with spatial navigation, the dentate gyrus is involved in disambiguation of environmental contexts [11]. Microinjections of BDNF into the dentate gyrus, but not the CA1 subfield, attenuated learned helplessness behavior and reduced immobility in the forced swim test [12], indicative of a possible role for dentate gyrus BDNF signaling in the antidepressant response. While adult neurogenesis is one feature of the dentate gyrus that responds to BDNF [13], changes also occur among spines and synapses, and these changes may contribute to improved discrimination between contexts that signal stress and contexts that signal safety.
Conclusions
BDNF signaling directly contributes to changes in the number and morphology of synapses. In the current report, we have observed correlations between BDNF expression and dendritic spine density among dentate granule neurons, but not CA1 pyramidal cells. This suggests that, within the physiological range of expression, dendritic spine density is tightly regulated by BDNF in the dentate gyrus.
Acknowledgments
A.M.S. is currently supported by start-up funds from the Physiology Department at Georgia Health Sciences University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–9. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Bramham CR. Local protein synthesis, actin dynamics, and LTP consolidation. Curr Opin Neurobiol. 2008;18:524–31. doi: 10.1016/j.conb.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–61. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabrese F, Molteni R, Racagni G, Riva MA. Neuronal plasticity: a link between stress and mood disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S208–16. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Ji Y, Lu Y, Yang F, Shen W, Tang TT, Feng L, Duan S, Lu B. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat Neurosci. 2010;13:302–9. doi: 10.1038/nn.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23:6856–65. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–60. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci U S A. 2006;103:13208–13. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stranahan AM, Arumugam TV, Mattson MP. Lowering corticosterone levels reinstates hippocampal BDNF and TrkB expression without influencing deficits in hypothalamic BDNF expression in leptin receptor deficient mice. Neuroendocrinology. 2011;93:58–64. doi: 10.1159/000322808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaaf MJ, Duurland R, de Kloet ER, Vreugdenhil E. Circadian variation in BDNF mRNA expression in the rat hippocampus. Brain Res Mol Brain Res. 2000;75:342–4. doi: 10.1016/s0169-328x(99)00314-9. [DOI] [PubMed] [Google Scholar]
- 11.Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behav Neurosci. 2008;122:16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- 12.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–61. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–75. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]