Abstract
Background
The purpose of this study was to evaluate the safety and efficacy of cetuximab (C225), an antibody that inhibits epidermal growth factor receptor (EGFR) activity, with cisplatin and to explore associations between EGFR protein expression with patient demographics or clinical outcome.
Methods
Women with advanced, persistent, or recurrent carcinoma of the cervix were eligible. The women received cisplatin at 30 mg/m2 days 1 and 8 with a loading dose of cetuximab at 400 mg/m2 followed by 250 mg/m2 days 1, 8, and 15 in a 21 day cycle. Adverse events were assessed with CTCAE v 3.0. Primary measure of efficacy was tumor response by RECIST. The study was stratified by prior chemotherapy (CT). EGFR protein expression in pre-treatment tumor was analyzed by immunohistochemistry.
Results
Between September 2004 and March 2008, 76 patients were enrolled. Of these, 69 were eligible and evaluable; 44 (64%) received prior chemotherapy. There were 4 responses in each group, prior chemotherapy and no chemotherapy, 9% and 16%, respectively. Grade 4 toxicities included anemia (1), allergy (1), metabolic (1), and vascular (1). The most common grade 3 toxicities were metabolic (15), dermatologic (8), fatigue (6), and gastrointestinal (6). EGFR protein was expressed in 47/48 (98%) of tumors analyzed with a median cellular expression of 81%. Exploratory analyses revealed a trend between the percentage of cells expressing EGFR protein and PFS (hazard ratio =1.76, 95% confidence interval=0.96–3.21).
Conclusions
The combination of cetuximab with cisplatin was adequately tolerated but did not indicate additional benefit beyond cisplatin therapy.
Keywords: Cisplatin, Cetuximab, Cervical Cancer
INTRODUCTION
A survival benefit has been shown for patients with advanced stage cervical carcinoma from the addition of chemotherapy (CT) to the primary radiation therapy (RT) regimen [1–3]. The Gynecologic Oncology Group (GOG) evaluated the role of chemosensitization in their landmark study comparing cisplatin alone, cisplatin, 5-FU, and hydroxyurea, or hydroxyurea alone in patients with locally advanced cervical cancer [3]. In this study, both groups of patients receiving cisplatin had longer progression-free survival (PFS) than those receiving hydroxyurea alone. Stehman et al. confirmed that addition of cisplatin to radiation therapy (RT) for patients with bulky stage IB cervical cancers significantly increased their survival (relative risk of death = 0.54), compared to radiation therapy alone [4,1]. Despite these exciting results, there remains a subset of patients with a relatively poor prognosis. This includes patients with histologic evidence of metastatic disease to the lymph nodes [5–9].
Epidermal growth factor receptor (EGFR) is a transmembrane protein involved in cell survival signaling pathways. EGFR overexpression has correlated with tumor resistance to cytotoxic agents and radiation therapy [10–13]. EGFR blockade generally increases radiosensitivity in vitro by modulating apoptosis, proliferation, and also increases radioresponse in vivo [11–13]. C225 (cetuximab) is a chimeric monoclonal antibody that binds EGFR and competes with ligand binding and tyrosine kinase activation [10–13]. Cancer cells treated with chemotherapeutic agents and cetuximab sequentially display a supraadditive increase in growth inhibition versus cells treated with chemotherapy alone [14].
In head and neck cancer, the combination of cisplatin, cetuximab, and concurrent radiation produced a 100% overall response rate, 87% complete response, and 13% partial response [15]. The observed 2 year disease free survival was 65%. A majority of cervical cancer patients overexpress EGFR [16–19]. Fifty-four to 71% of patients with early and late FIGO stage cervical cancer patients have moderate to strong expression of EGFR protein [16,19,20]. This overexpression is a poor prognostic factor independent of lymph node status [16,19].
Given the evidence of cetuximab activity in squamous carcinomas of the head and neck, the superior activity of cisplatin in the recurrent setting to date, and the poor prognostic effect of EGFR protein overexpression observed in cervical cancer, this study evaluated the efficacy of concurrent cisplatin and cetuximab chemotherapy in the cervical cancer population.
MATERIALS and METHODS
Study Design
Eligible women had advanced, persistent, or recurrent carcinoma of the cervix, were at least 4 weeks from last radiotherapy, at least 18 years of age, had a GOG performance status of 0, 1, or 2, had disease measurable by physical exam or medical imaging according to GOG Response Evaluation Criteria in Solid Tumors (RECIST), and had adequate hematologic, renal, hepatic, pulmonary, and cardiac function with no active infections. Cervical cancer diagnosis was confirmed by retrospective chart review of pathologic reports. Women were ineligible if they had a concomitant or prior malignancy (other than a non-melanoma skin cancer), within the preceding 5 years, had prior chemotherapy for cervical cancer (except as radiosensitizers), or had any prior anti-EGFR therapy. Patients provided written informed consent consistent with federal, state, and local requirements and gave authorization permitting the release of personal health information.
Assessments
Patients were assessed prior to each cycle of treatment. Radiographic disease measurements were required every other cycle using standard RECIST version 3.0 response criteria [21]. OS was defined as length of time from date of enrollment to death or the date of last contact. PFS was defined as the period from study entry until disease progression, death, or the last date of contact. Progression-free interval (PFI) was defined as length of time from date of diagnosis until the first recurrence before entering this study. Patients who received any study drug were evaluable for efficacy and toxicity.
Treatment
Treatment consisted of cetuximab weekly (400mg/m2 for first dose followed by 250mg/m2 in all subsequent doses) and cisplatin 30mg/m2 given day 1 and 8, every 21 days. Cetuximab was given on days 1, 8, and 15. Three weeks constituted a cycle. A cycle of therapy was not administered unless the absolute neutrophil count was ≥1500 and platelets were ≥100,000ul. Creatinine was required to be ≤1.5X institutional upper limit of normal (ULN). Bilirubin was required to be ≤1.5× ULN (CTCAE v3.0 grade 1). SGOT and alkaline phosphatase were required to be ≤2.5 × ULN (CTCAE v3.0 grade 1). Sensory and motor neuropathy for each patient was required to be < grade 2.
Cisplatin dose adjustments were based on the hematologic toxicity. Treatment delays of up to 14 days were permitted. Only women who experienced recurrent febrile neutropenia or recurrent documented grade 4 neutropenia, persisting >7 days after dose reduction, were allowed to receive growth factor support. Treatment modifications applied equally for day 1 and 8, with day 8 treatment held if the ANC was <1500/mcl cells or platelets were <75,000/mcl. Subjects who failed to recover adequate counts within a 2-week delay were removed from study. Amifostine or other protective agents were not allowed. Women were removed from study therapy for disease progression, unacceptable toxicity, or by request, and generally followed until death.
Tissue Preparation and Histological Evaluation
Representative institutions submitted paraffin-embedded tumor blocks collected at the time of primary diagnosis and before initiation of first-line chemotherapy and/or radiation therapy for forty-eight patients. Analyses of EGFR expression was done blinded to clinical outcomes.
Immunohistochemistry (IHC)
The EGFR pharmDx™ kit and monoclonal mouse anti-human EGFR (clone 2-18C9) IgG1 antibody (DAKO North America, Carpinteria, CA) were used for EGFR detection. Briefly, deparaffinized tissue sections were microwaved in 0.01 M citrate buffer (pH 6.0) for 15 minutes. Sections were incubated overnight at 4°C with primary antibody (1:100). A biotin-streptavidin-peroxidase detection system and 3,3’-diaminobenzidine substrate was used to detect primary antibody (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA).
Background staining, evaluated using the control antibody in place of primary, was negligible (data not shown). Positive controls were colon cancer and stromal cells (internal). Staining was evaluated by visual counting of at least 1000 cells from at least 10 random high-powered fields (100X). Results were expressed as percentage/proportion of tumor cells exhibiting any staining, regardless of intensity. Additionally, intensity was estimated in comparison to the (internal) stromal cell control and scored as: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. H score was calculated by multiplying the proportion and intensity, resulting in scores of 0–3. The reviewer (JF) was blinded to stage, grade, and histology.
Statistical Analysis
The original study was designed to evaluate efficacy through tumor response with a true probability (PR) of 40% considered clinically interesting against a null value of 20% deemed uninteresting. The desired probabilities of type I and II (α and β) errors were 5%. This required approximately 28 patients to the first stage and a cumulative accrual of 62 to the second stage. The study required more than 6/28 responses to proceed to the second stage and more than 17/62 to deem the regimen interesting. This design were close to Simon’s optimum design but was modified for administrative flexibility [22].
After the study opened, Long et al. published results indicating that prior chemotherapy could be prognostic [23]. Based on this finding, along with a negative interim result, data from clinical trials published by Moore et al, Long et al, and (later) Monk et al was examined by GOG administration [23–25]. This internal study indicated a relationship between the probability of response and prior chemotherapy. Based on this analysis, along with the interim results, a decision was made to reopen the study to second stage with a stratified design utilizing the method by London and Chang [26].
London and Chang’s 2-stage conditional method was used where the null PR for patients with and without prior chemotherapy were 10% and 20%, respectively. The interesting levels of PR were 25% and 40%, respectively. The threshold frequency of responders for deeming the regimen interesting depended on the frequency of patients with and without prior chemotherapy. A cumulative accrual of 66 patients was targeted so that α ≈ β ≈ 0.05.
Given its prognostic significance [23–25], an analysis was performed according to the patient’s disease status upon entry: Advanced (ADV), patients with advanced International Federation of Gynecology and Obstetrics (FIGO) stage, ReCurrent Short duration (RCS), patients who had persistent disease or PFI less than the median value, and ReCurrent Long duration (RCL), patients who had a PFI greater than the median.
Secondary and exploratory analyses were conducted to assess associations between patient demographics, clinical outcomes, and biological characteristics. The purpose was to characterize and screen in a hypothesis generating fashion. Tests with p-values less than 5% were deemed suggestive and expected relationships with p-values between 5% and 10% could be noted as a trend. Confidence intervals were presented without correction for multiplicity. Tests used to assess associations included Fisher’s Exact, exact Chi-square, Kendall’s tau-b correlation, and hazard ratios obtained with Cox modeling [22]. Variables examined included prior chemotherapy, prior radiation therapy, prior PFI, age, disease status, performance status, race, ethnicity, EGFR expression, response, PFS at 6 months, overall PFS, and OS. Lack of suggestive associations should not be interpreted as definitive.
Additionally, an exploratory non-randomized comparison of this study to the cisplatin arms of Moore et al. and Long et al. was conducted with Cox modeling to assess the potential cytostatic activity of this regimen [23,24].
RESULTS
Seventy-six women were accrued between September 20, 2004, and March 3, 2008. Seven subjects were excluded: wrong cell type (1), wrong primary (3), abnormal cardiac (1), improper prior therapy (1), and never treated (1). Sixty-nine patients were eligible and evaluable for treatment efficacy and toxicity. The patient characteristics are presented in Table 1. Median age was 51 years (range 24–77). The majority of patients were: white, had GOG performance status of 0, squamous histology, and were well to moderately differentiated. A total of 56 patients had prior radiation therapy. Forty-four (64%) patients had received prior platinum as a sensitizer to radiation therapy, thus London and Chang’s design required at least 15 responders before the regimen would be deemed interesting, which gave α≈β ≈4%. The median PFI was approximately 15 months.
Table 1.
Patient Characteristics
| Characteristic | Category | No. of Cases | % of Cases |
|---|---|---|---|
| Age | 20–29 | 1 | 1.4 |
| 30–39 | 11 | 15.9 | |
| 40–49 | 18 | 26.1 | |
| 50–59 | 26 | 37.7 | |
| 60–69 | 9 | 13.0 | |
| 70–79 | 4 | 5.8 | |
| Race | Asian | 2 | 2.9 |
| African American | 5 | 7.2 | |
| Hispanic | 9 | 13.0 | |
| White | 53 | 76.8 | |
| Performance Status | 0 | 47 | 68.1 |
| 1 or 2 | 22 | 31.9 | |
| Cell Type | Adenocarcinoma, Unsp. | 21 | 30.4 |
| Clear Cell Carcinoma | 1 | 1.4 | |
| Adenosquamous | 5 | 7.2 | |
| Squamous Cell Carcinoma | 41 | 59.4 | |
| Mucinous Adenocarcinoma | 1 | 1.4 | |
| Grade | 1: Well differentiated | 6 | 8.7 |
| 2: Moderately differentiated | 34 | 49.3 | |
| 3: Poorly differentiated | 29 | 42.0 | |
| Prior Platinum | No | 25 | 36.0 |
| Yes | 44 | 64.0 | |
| Prior Radiation | No | 13 | 18.8 |
| Yes | 56 | 81.2 | |
| Prior Surgery | No | 48 | 69.6 |
| Yes | 21 | 30.4 | |
| Disease Status | ADV | 13 | 18.8 |
| RCL | 26 | 37.7 | |
| RCS | 30 | 43.5 | |
| Prior Cis-RT | No | 25 | 36.2 |
| Yes | 44 | 63.8 |
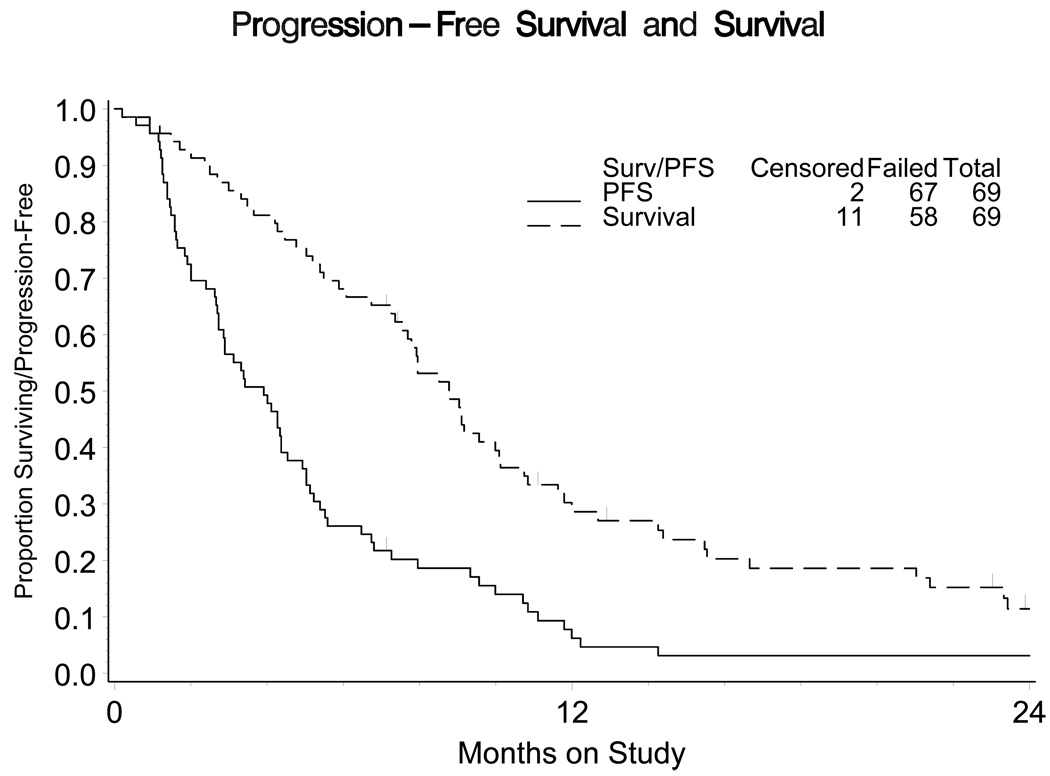
The median number of cycles received in this phase II evaluation was 3. Eight patients responded to therapy (4 in each group; Table 2). The estimated PR was 16% (marginal 90% CI 6% – 33%) and 9% (marginal 90% CI 3% – 20%) in the no prior and prior chemotherapy groups, respectively. The median PFS was 3.91 months (95% CI 2.73 – 4.53) with a median OS of 8.77 months (95% CI 7.56 – 10.09; Figure 1).
Table 2.
Response (n=27)
Response by Prior Cisplatin-Radiation Therapy (CIS-RT)
| Prior CIS-RT | No Prior CIS-RT | ||||
|---|---|---|---|---|---|
| Tumor Response | n | % | n | % | Total |
| Responders | 4 | 9.1 | 4 | 16.0 | 8 |
| Complete | 1 | 2.3 | 0 | 0.0 | 1 |
| Partial | 3 | 6.8 | 4 | 16.0 | 7 |
| Non-Responders | 40 | 90.9 | 21 | 84.0 | 61 |
| Total | 44 | 25 | 69 | ||
Figure 1.
Progression free survival (PFS) and Overall Survival (OS) of study. The median PFS was 3.91 months (95% CI 2.73 – 4.53) with a median OS of 8.77 months (95% CI 7.56 – 10.09).
Pre-treatment EGFR protein expression was analyzed by immunohistochemistry (IHC) in 48 patients. Unfortunately biopsies were obtained at primary diagnosis and not at the time of relapse. EGFR protein was expressed in 98% of tumors analyzed. Many patients (49%) had 3+ staining intensity (Table 3). The percentage of cells staining positive and H-score were dichotomized into higher or lower values based on the median values.
Table 3.
A: Pre-Treatment EGFR IHC Protein Expression Characteristics; B: EGFR IHC Protein Expression and Disease Status; C: Tabulation Between PFS > 6 months and Percent Staining
| IHC Measure | n | % | |
|---|---|---|---|
| Intensity | |||
| 1 | 14 | 30 | |
| 2 | 10 | 21 | |
| 3 | 23 | 49 | |
| P staining | |||
| High | 25 | 52 | |
| Low | 23 | 48 | |
| H-Value | |||
| High | 25 | 52 | |
| Low | 23 | 48 | |
| ADV N(%) |
RCS N(%) |
RCL N(%) |
||
|---|---|---|---|---|
| Intensity | ||||
| 1 | 3 (27) | 5 (25) | 6 (37) | |
| 2 | 1 (9) | 2 (10) | 7 (44) | |
| 3 | 7 (64) | 13 (65) | 3 (19) | |
| P Staining | ||||
| High | 7 (64) | 15 (71) | 3 (19) | |
| Low | 4 (36) | 6 (29) | 13 (81) | |
| H-Value | ||||
| High | 8 (73) | 14 (67) | 3 (19) | |
| Low | 3 (27) | 7 (33) | 13 (81) | |
| PFS> 6 | |||
|---|---|---|---|
| P Staining | Yes | No | |
| N (%) | N (%) | ||
| High | 5 (33) | 20 (60) | |
| Low | 10 (67) | 13 (40) | |
- ADV= advanced; ReCurrent Short (RCS) = Persistent or PFI ≤ median; ReCurrent Long (RCL) = PFI > median
- P Staining = dichotomy of percent staining at the median (0.81). If % staining ≥ 0.81, then P Staining =High.
- H-value = dichotomy of H score (percentage × intensity) at the median (1.44). If H-score ≥ 1.44, then H-Value= High.
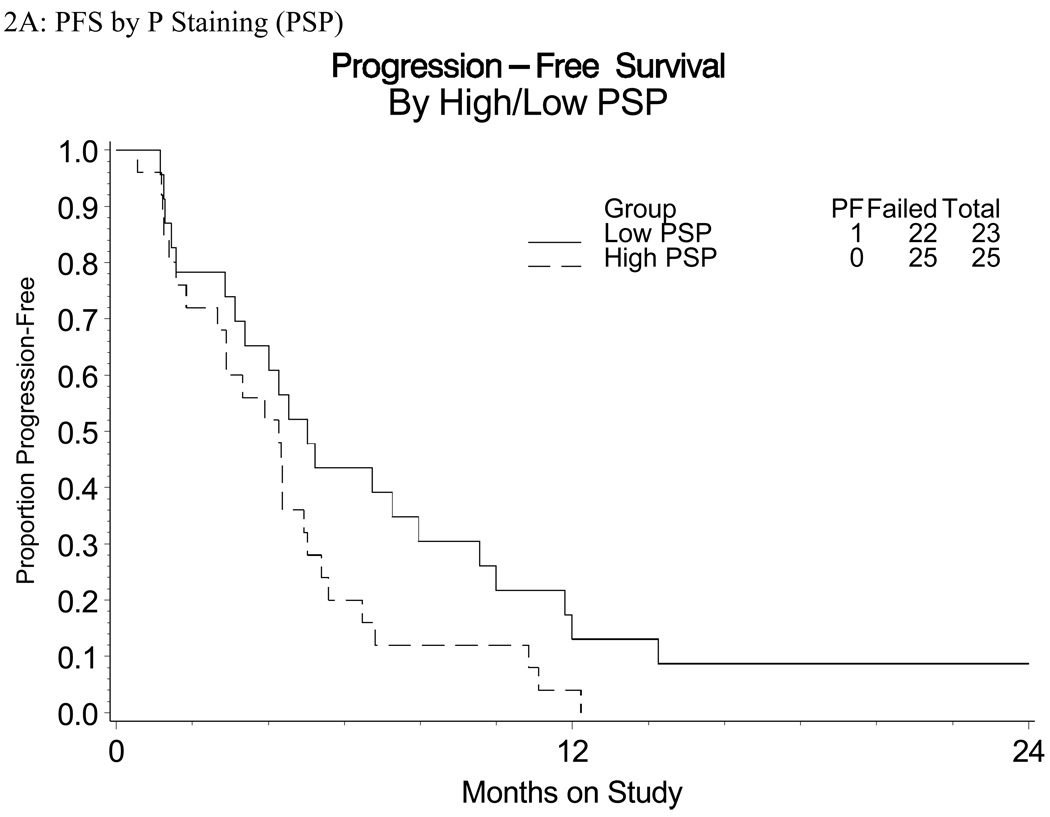
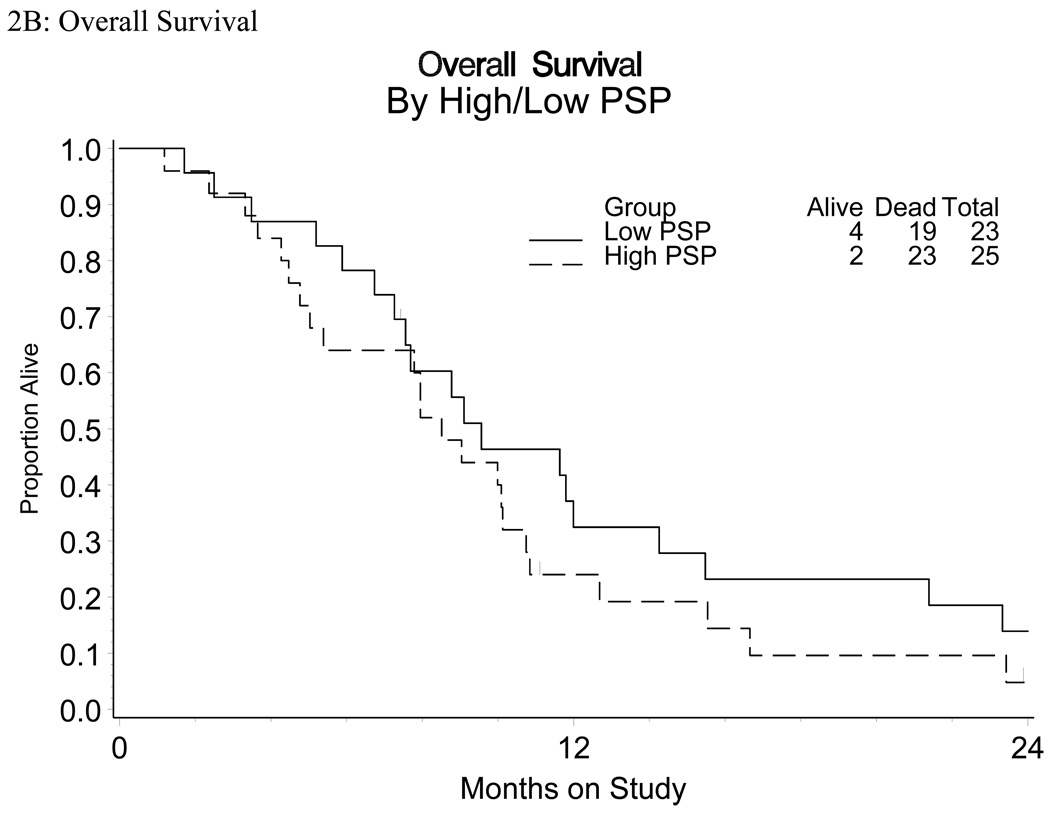
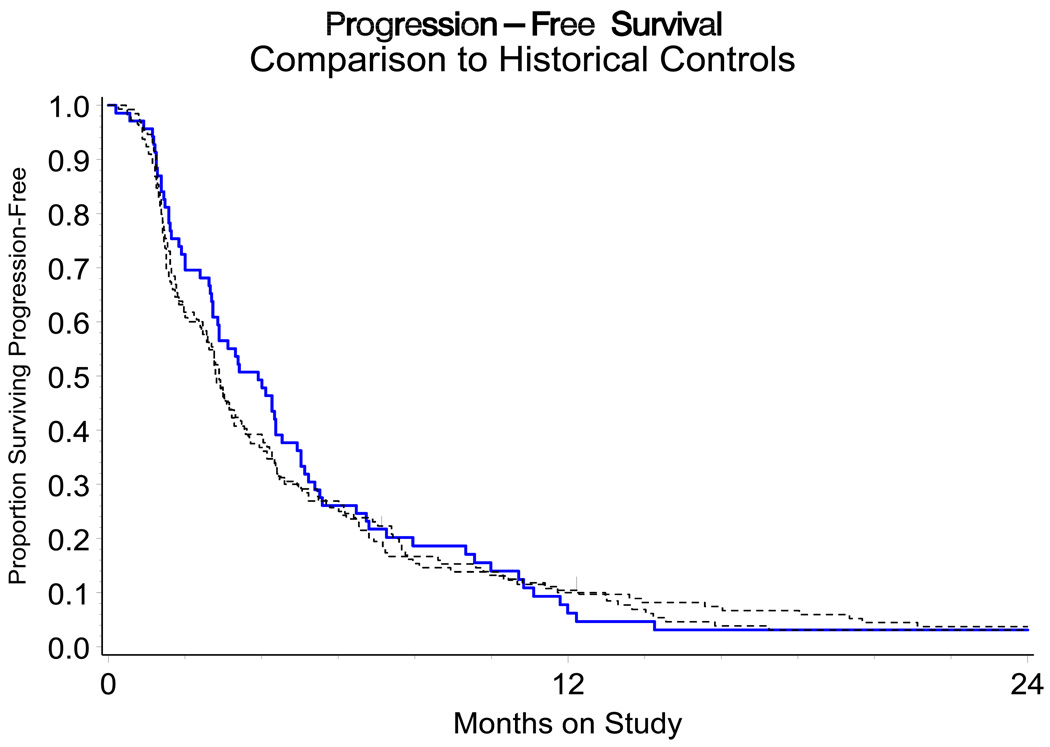
There were suggested associations between disease status and expression of EGFR. For example, a greater proportion of patients in groups ADV and RCS had increased (3+) staining intensity relative to RCL (Table 3B). RCL patients were also more likely to have a lower percentage of cells staining positive (81% patients) and lower H-score (81% patients) compared to their RCS or ADV counterparts (approximately 32% and 30% patients, respectively; Table 3). Those with a higher percentage of cells staining positive had a trend towards shorter PFS (HR=1.76, marginal 95% CI 0.96 – 3.21; Figure 2). There were no other suggested associations or trends observed between EGFR expression (intensity or H score) with PFS or OS. The non-randomized relative hazards for the PFS endpoint for the regimen under study against the cisplatin arms of Moore et al and Long et al were 0.91 (95% CI 0.68 – 1.23) and 0.94 (95% CI 0.70 – 1.26), respectively (Figure 3) [23,24].
Figure 2.
Kaplan-Meyer Survival curves of
A: PFS and Percent (P) Staining (PSP)
B: OS and Percent (P) Staining (PSP)
Figure 3.
A non-randomized comparison of PFS for GOG 0076DD to the cisplatin arms of GOG 169 and GOG 179. The log-rank test statistic is non-significant.
There were no treatment-related deaths (Supplemental Table 1). Observed grade 4 toxicities were anemia (1), allergy (1), metabolic (1), musculoskeletal (1), and vascular (1). Grade 3 toxicities with more than 1 patient were leucopenia (4), neutropenia (3), anemia (5), allergy (3), metabolic (15), dermatologic (8), fatigue (6), gastrointestinal (6), nausea/vomiting (6), genitourinary/renal (3), and infection (5).
DISCUSSION
In the current study, the combination of cetuximab and cisplatin provided a clinical objective response rate of 11%. Additionally, there was little evidence to suggest that cetuximab benefits patients through cytostatic action as indicated by the HRs for PFS obtained for this study against Moore et al and Long et al whose CIs include the value “1” [23,24]. The observed data showed a possible negative impact on one measure of EGFR (high percentage staining positive) and relatively no impact according to the other measures (intensity and H-score). Patients who had longer PFI tended to express EGFR less strongly (by any measure). Disease status has been long known to be a potent prognostic factor with longer PFI associated with better prognosis [23,25].
Previous analysis of EGFR protein expression and prognosis in cervical cancer has yielded conflicting results [19,27–31]. Authors have used a variety of methods to assess EGFR protein expression, determine cut-offs, and percent positive [19,27–31]. Evaluation methods include: IHC, radioligand-binding assay, and enzyme immunoassay [19]. Methods of determining percent positive include: percentage of cells staining positive, intensity of staining and H-score evaluation (calculated by adding or multiplying, depending on the study, the scores for percentage and intensity). In this study we evaluated EGFR protein expression by IHC and determined cut-offs using all 3 of the aforementioned methods: percent positive, intensity, and H-score. Given the robust positive staining for EGFR protein observed, we dichotomized the staining results for percent positive staining and H-score. There was a trend identified between EGFR protein expression (percent staining) and PFS; however, no other associations or trends were observed between EGFR expression (intensity or H-score) with PFS or OS.
The EGFR signaling pathway, which is also frequently activated in colorectal cancer (CRC), has been extensively investigated as a target for CRC therapy [32]. EGFR gene upregulation occurs in approximately 60%–80% of CRC patients with 82% of tumors being EGFR positive by IHC [33]. Expression of EGFR protein, however, has not correlated with survival of CRC patients treated with cetuximab [32]. A randomized trial evaluated cetuximab monotherapy versus cetuximab plus irinotecan in irinotecan refractory EGFR positive tumors. The rate of response in the combination therapy group was significantly higher than that in the monotherapy group (22.9% versus 10.8%, P=0.007). EGFR expression, either as the percentage of EGFR positive tumor cells or as the maximal staining intensity per cell, did not correlate with the clinical response rate (P=0.87 and P=0.64, respectively) [33]. However, data from 16 chemotherapy refractory EGFR negative CRC patients treated with cetuximab confirms cetuximab activity in EGFR deficient patients. In these patients treated either with cetuximab alone (12.5%) or cetuximab plus irinotecan (87.5%), 4 (16%) major responses were seen [34].
The one cancer site in which there is a positive correlation between EGFR expression and response to cetuximab is head and neck cancer [35]. This cancer, like cervical cancer, is thought to be high risk human papillomavirus (HPV) related. High risk HPV expression has been identified in 96%–99% of cervical cancer patients and 20%–22% of head and neck cancer patients [36–38]. Also like cervical cancers, robust expression of EGFR protein is found in the majority of these cancers [35,39]. A randomized controlled phase III trial of cisplatin plus cetuximab or placebo in the treatment of recurrent head and neck cancer found enhancement of response for those patients with EGFR staining in >80% of cells [35]. The median PFS and OS for EGFR high patients randomly assigned to cisplatin with cetuximab were 3.9 and 6.7 months, compared with 3.6 (P = 0.68) and 9.8 months (P = 0.45) for EGFR high patients randomly assigned to cisplatin and placebo. Median PFS and OS for EGFR low patients assigned to cisplatin and cetuximab were 5.0 and 10.0 months, compared with 3.3 (P=0.27) and 7.0 months for patients treated with cisplatin and placebo (P =0.32). Proposed explanations for the lack of clinical response to cetuximab at high levels of EGFR expression included the possible failure of cetuximab at the current dose to saturate the high number of receptors present when EGFR staining is 3+ on > 80% of cells, and stochastic interactions or other ligand-independent mechanisms of EGFR activation [35].
Human papillomavirus (HPV) 16 E6/E7 transcripts have been shown to increase expression of EGFR protein in cervical cancer cell lines [40]. HPV16 E5 transcript has been shown to stimulate EGFR activation by phosphorylation of Akt and ERK1/2 [41]. Given the fact that the Kras mutation rate for cervical cancer is only 1–3%, HPV infection could influence response to cetuximab in these cancers [42,43]. Thus, our results could be interpreted as an enhancement of response to cetuximab in patients with decreased EGFR protein expression in an HPV related malignancy. Alternatively, EGFR expression could be negatively prognostic and the observed data merely reflect a regimen ineffective at countering its affects.
Finally, EGFR gene copy number as determined by fluorescence in situ hybridization (FISH) has been evaluated as a predictor of treatment outcome in cancers to include non-small cell lung cancer (NSCLC) [44]. EGFR gene copy number did not predict survival benefit. However, in NSCLC patients treated with cetuximab; patients with FISH-positive tumors had a median progression-free survival time of 6 months compared with 3 months for FISH-negative patient. Median survival time was 15 months for the FISH-positive group compared with 7 months for patients who were FISH negative [45].
In conclusion, the combination of cetuximab with cisplatin was adequately tolerated but did not indicate additional benefit beyond cisplatin therapy alone in this patient population. There was a suggested trend indicating that patients with more expression of EGFR had shorter PFS. Although the point estimate for the hazard ratio for survival indicated a similar associated risk for death, the evidence was not strong enough to cast doubt on the hypothesis of no relationship between EGFR and OS. It is possible that EGFR protein expression is an important cause of the variability in disease status (and aggressiveness of tumor cells) before study entry and continues to adversely affect the patients’ disease after enrollment. Given the lack of activity observed in this study, these results could be interpreted as a regimen with insufficient disruption of EGFR pathways. A previous study with the EGFR TKI, erlonitinib, in recurrent cervical cancer also showed minimal activity [46]. However, pursuit of anti-EGFR therapy may be a worthwhile endeavor through reformulation of cetuximab or examination of other agents.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Barbara Saczynski for the management of clinical data, the GOG Tissue Bank for their assistance with the banking and distribution of specimens, Beverly Kratzel for the administration of the protocol, and Kim Blaser for publications management. We also wish to thank BMS/ImClone for their industry support.
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical Office (CA 37517). This trial was registered at www.clinicaltrials.gov, NCT 10101192. The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Mississippi Medical Center, Indiana University School of Medicine, Wake Forest University School of Medicine, The Cleveland Clinic Foundation, MD Anderson Cancer Center, Fox Chase Cancer Center, University of Oklahoma, University of Texas – Galveston and Gynecologic Oncology Network.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The views expressed herein are those of the authors and do not reflect the official policy r opinion of the Department of Defense or the United States Army or Navy.
CONFLICT OF INTEREST
Dr. Joan Walker declares financial relationships to Precision Therapeutics, MGI Pharmaceutical, and Intuitive Surgical.
Dr. James Tate Thigpen is a consultant and speaker for Bristol-Myers Squibb and Eli Lilly.
All other co-authors have no conflict of interest to declare.
REFERENCES
- 1.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage 1B cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 2.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic Radiation with Concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 3.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Eng J Med. 1999;341:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 4.Stehman FB, Ali S, Keys HM, Muderspach LI, Chafe WE, Gallup DG, et al. Radiation therapy with or without weekly cisplatin for bulky stage 1B cervical carcinoma: follow-up of A Gynecologic Oncology Group trial. Am J Obstet Gynecol. 2007;197:503.e1–503.e6. doi: 10.1016/j.ajog.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Averette HE, Dudan RC, Ford JH., Jr Exploratory celiotomy for surgical staging of cervical cancer. Am J Obstet Gynecol. 1972;113:1090–1096. doi: 10.1016/0002-9378(72)90741-7. [DOI] [PubMed] [Google Scholar]
- 6.Berman ML, Keys H, Creasman W, DiSaia P, Bundy B, Blessing J. Survival and patterns of recurrence in cervical cancer metastatic to periaortic lymph nodes (A Gynecologic Oncology Group study) Gynecol Oncol. 1984;19:8–16. doi: 10.1016/0090-8258(84)90151-3. [DOI] [PubMed] [Google Scholar]
- 7.Lepanto P, Littman P, Mikuta J, Davis L, Celebre J. Treatment of para-aortic nodes in carcinoma of the cervix. Cancer. 1975;35:1510–1513. doi: 10.1002/1097-0142(197506)35:6<1510::aid-cncr2820350605>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Nelson JH, Jr, Boyce J, Macasaet M, Lu T, Bohorquez JF, Nicastri AD, et al. Incidence, significance and follow-up of para-aortic lymph node metastases in late invasive carcinoma of the cervix. Am J Obstet Gynecol. 1977;128:336–340. doi: 10.1016/0002-9378(77)90633-0. [DOI] [PubMed] [Google Scholar]
- 9.Sudarsanam A, Charyulu K, Belinson J, Averette H, Goldberg M, Hintz B, et al. Influence of exploratory celiotomy on the management of carcinoma of the cervix. A preliminary report. Cancer. 1978;41:1049–1053. doi: 10.1002/1097-0142(197803)41:3<1049::aid-cncr2820410337>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Akimoto T, Hunter NR, Buchmiller L, Mason K, Ang KK, Milas L. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin Cancer Res. 1999;5:2884–2890. [PubMed] [Google Scholar]
- 11.Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000;6:2166–2174. [PubMed] [Google Scholar]
- 12.Milas L, Mason K, Hunter N, Petersen S, Yamakawa M, Ang K, et al. In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res. 2000;6:701–708. [PubMed] [Google Scholar]
- 13.Bianco C, Bianco R, Tortora G, Damiano V, Guerrieri P, Montemaggi P, et al. Antitumor activity of combined treatment of human cancer cells with ionizing radiation and anti-epidermal growth factor receptor monoclonal antibody C225 plus type 1 protein kinase A antisense oligonucleotide. Clin Cancer Res. 2000;6:4343–4350. [PubMed] [Google Scholar]
- 14.Ciardiello F, Bianco R, Damiano V, De Lorenzo S, Pepe S, De Placido S, et al. Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225. Clin Cancer Res. 1999;5:909–916. [PubMed] [Google Scholar]
- 15.Robert F, Ezekiel MP, Spencer SA, Meredith RF, Bonner JA, Khazaeli MB, et al. Phase I study of anti-epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:3234–3243. doi: 10.1200/JCO.2001.19.13.3234. [DOI] [PubMed] [Google Scholar]
- 16.Kersemaekers AM, Fleuren GJ, Kenter GG, Van den Broek LJ, Uljee SM, Hermans J, et al. Oncogene alterations in carcinomas of the uterine cervix: overexpression of the epidermal growth factor receptor is associated with poor prognosis. Clin Cancer Res. 1999;5:577–586. [PubMed] [Google Scholar]
- 17.Lee CM, Shrieve DC, Zempolich KA, Lee RJ, Hammond E, Handrahan DL, et al. Correlation between human epidermal growth factor receptor family (EGFR, HER2, HER3, HER4), phosphorylated Akt (P-Akt), and clinical outcomes after radiation therapy in carcinoma of the cervix. Gynecol Oncol. 2005;99:15–21. doi: 10.1016/j.ygyno.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 18.Gaffney DK, Haslam D, Tsodikov A, Hammond E, Seaman J, Holden J, et al. Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) negatively affect overall survival in carcinoma of the cervix treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:922–928. doi: 10.1016/s0360-3016(03)00209-8. [DOI] [PubMed] [Google Scholar]
- 19.Kim YT, Park Sw, Kim JW. Correlation between expression of EGFR and the prognosis of patients with cervical carcinoma. Gynecol Oncol. 2002;87:84–89. doi: 10.1006/gyno.2002.6803. [DOI] [PubMed] [Google Scholar]
- 20.Cho NJ, Kim YB, Park TK, Kim GE, Park K, Song KJ. P63 and EGFR as prognostic predictors in stage IIB radiation-treated cervical squamous cell carcinoma. Gynecol Oncol. 2003;91:346–353. doi: 10.1016/s0090-8258(03)00504-3. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 23.Long HJ, 3rd, Bundy BN, Grendys EC, Jr, Benda JA, McMeekin DS, Sorosky J, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: A Gynecologic Oncology Group study. J Clin Oncol. 2005;23:4626–4633. doi: 10.1200/JCO.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol. 2004;22:3113–3119. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- 25.Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: A Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.London WB, Chang MN. One- and two-stage designs for stratified phase II clinical trials. Stat Med. 2005;24:2597–2611. doi: 10.1002/sim.2139. [DOI] [PubMed] [Google Scholar]
- 27.Pfeiffer D, Stellwag B, Pfeiffer A, Borlinghaus P, Meier W, Scheidel P. Clinical implications of the epidermal growth factor receptor in the squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 1989;33:146–150. doi: 10.1016/0090-8258(89)90540-4. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi Y, Hachisuga T, Iwasaka T, Fukuda K, Okuma Y, Yokoyama M, et al. Expression of ras oncogene product and EGF receptor in cervical squamous cell carcinomas and its relationship to lymph node involvement. Gynecol Oncol. 1991;40:147–151. doi: 10.1016/0090-8258(91)90107-g. [DOI] [PubMed] [Google Scholar]
- 29.Hale RJ, Buckley CH, Gullick WJ, Fox H, Williams J, Wilcox FL. Prognostic value of epidermal growth factor receptor expression in cervical carcinoma. J Clin Pathol. 1993;46:149–153. doi: 10.1136/jcp.46.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kristensen GB, Holm R, Abeler VM, Tropé CG. Evaluation of the prognostic significance of cathepsin D, epidermal growth factor receptor, and c-erbB-2 in early cervical squamous cell carcinoma. An immunohistochemical study. Cancer. 1996;78:433–440. doi: 10.1002/(SICI)1097-0142(19960801)78:3<433::AID-CNCR9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 31.Scambia G, Ferrandina G, Distefano M, D’Agostino G, Benedetti-Panici P, Mancuso S. Epidermal growth factor receptor (EGFR) is not related to the prognosis of cervical cancer. Cancer Lett. 1998;123:135–139. doi: 10.1016/s0304-3835(97)00421-7. [DOI] [PubMed] [Google Scholar]
- 32.Silvestris N, Tommasi S, Santini D, Russo A, Simone G, Petriella D, et al. KRAS mutations and sensitivity to anti-EGFR monoclonal antibodies in metastatic colorectal carcinoma: an open issue. Expert Opin Biol Ther. 2009;9:565–577. doi: 10.1517/14712590902870394. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 34.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 35.Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Eastern Cooperative Oncology Group. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 36.Slama J, Drazdakova M, Dundr P, Fischerova D, Zikan M, Pinkavova I, et al. High-risk human papillomavirus DNA in the primary tumor, sentinel, and nonsentinel pelvic lymph nodes in patients with early-stage cervical cancer: a correlation with histopathology. Int J Gynecol Cancer. 2009;19:703–707. doi: 10.1111/IGC.0b013e3181a13186. [DOI] [PubMed] [Google Scholar]
- 37.Human papillomavirus 16 and head and neck squamous cell carcinoma. Int J Cancer. 2007;120:2386–2392. doi: 10.1002/ijc.22633. [DOI] [PubMed] [Google Scholar]
- 38.Ringström E, Peters E, Hasegawa M, Posner M, Liu M, Kelsey KT. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res. 2002;8:3187–3192. [PubMed] [Google Scholar]
- 39.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu G, Liu W, Mendelsohn J, Ellis LM, Radinsky R, Andreeff M, et al. Expression of epidermal growth factor receptor and human papillomavirus E6/E7 proteins in cervical carcinoma cells. J Natl Cancer Inst. 1997;89:1271–1276. doi: 10.1093/jnci/89.17.1271. [DOI] [PubMed] [Google Scholar]
- 41.Kim SH, Juhnn YS, Kang S, Park SW, Sung MW, Bang YJ, et al. Human papillomavirus 16 E5 up-regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ERK1,2 and P13K/Akt. Cell Mol Life Sci. 2006;63:930–938. doi: 10.1007/s00018-005-5561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enomoto T, Inoue M, Perantoni AO, Terakawa N, Tanizawa O, Rice JM. K-ras activation in neoplasms of the human female reproductive tract. Cancer Res. 1990;50:6139–6145. [PubMed] [Google Scholar]
- 43.Enomoto T, Inoue M, Perantoni AO, Buzard GS, Miki H, Tanizawa O, et al. K-ras activation in premalignant and malignant epithelial lesions of the human uterus. Cancer Res. 1991;51:5308–5314. [PubMed] [Google Scholar]
- 44.Hirsch FR, Varella-Garcia M, Dziadziuszko R, et al. Fluorescence in situ hybridization subgroup analysis of TRIBUTE, a phase III trial of erlotinib plus carboplatin and paclitaxel in non-small cell lung cancer. Clin Cancer Res. 2008;14:6317–6323. doi: 10.1158/1078-0432.CCR-08-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirsch FR, Herbst RS, Olsen C, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapy. J Clin Oncol. 2008;26:3351–3357. doi: 10.1200/JCO.2007.14.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schilder RJ, Sill MW, Lee YC, Mannel R. A phase II trial of erlotinib in recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Int J Gynecol Cancer. 2009;19:929–933. doi: 10.1111/IGC.0b013e3181a83467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.