Abstract
Botulinum neurotoxins (BoNTs) inhibit cholinergic synaptic transmission by specifically cleaving proteins that are crucial for neurotransmitter exocytosis. Due to the lethality of these toxins, there are elevated concerns regarding their possible use as bioterrorism agents. Moreover, their widespread use for cosmetic purposes, and as medical treatments, has increased the potential risk of accidental overdosing and environmental exposure. Hence, there is an urgent need to develop novel modalities to counter BoNT intoxication. Mammalian motoneurons are the main target of BoNTs, however, due to the difficulty and poor efficiency of the procedures required to isolate the cells, they are not suitable for high-throughput drug screening assays. Here, we explored the suitability of embryonic stem (ES) cell-derived motoneurons as a renewable, reproducible, and physiologically relevant system for BoNT studies. We found that the sensitivity of ES-derived motoneurons to BoNT/A intoxication is comparable to that of primary mouse spinal motoneurons. Additionally, we demonstrated that several BoNT/A inhibitors protected SNAP-25, the BoNT/A substrate, in the ES-derived motoneuron system. Furthermore, this system is compatible with immunofluorescence-based high-throughput studies. These data suggest that ES-derived motoneurons provide a highly sensitive system that is amenable to large-scale screenings to rapidly identify and evaluate the biological efficacies of novel therapeutics.
Keywords: Embryonic stem cells, botulinum neurotoxin, motoneurons, high-throughput, drug discovery
Introduction
Botulinum neurotoxins (BoNTs), composed of seven biochemically distinct serotypes (BoNT/A–G) secreted by anaerobic bacteria Clostridium botulinum, are extremely potent inhibitors of neurotransmitter exocytosis at neuromuscular junctions [1–3]. Furthermore, BoNTs have been weaponized [4], and there is a significant concern that one or more of these toxins could easily be used during an act of bioterror [5]. Indeed, BoNTs, as the most poisonous of known bacteria toxins [6], are listed as category A bio-threat agents by the Centers for Disease Control and Prevention [7].
On the other hand, and in spite of their unmatched toxicities, pharmaceutical grade BoNT serotypes A and B are widely used, in quantitatively minute, localized doses: (i) to treat various movement and hyperactivity disorders and (ii) cosmetically for wrinkle reduction [8–10]. In fact, Botox™ (BoNT/A) treatment is FDA approved, and according to statistics provided by the American Society for Aesthetic Plastic Surgery [11], is one of the top non-surgical cosmetic procedures performed in the United States. Furthermore, to extend BoNT potency for clinical neurology applications, chimeric and modified BoNTs have been generated using protein engineering methods to expedite the toxin’s cellular entry, increase its substrate specificity, and extend its duration of activity in neuronal and possibly non-neuronal cells [9, 12–14].
However, as BoNTs are increasingly being used for medical and cosmetic purposes, the possibility of accidental overdosing in the clinic, in addition to unintentional environmental exposure through contaminated food or liquids, is also increasing. Moreover, as indicated above, BoNTs represent a major concern with regard to counter-bioterrorism efforts. And yet, there is still no effective therapeutic means for countering these toxins post-neuronal internalization [3]. To date, there are only two FDA approved anti-BoNT antibodies: babyBIG (human serum-derived anti-BoNT immunoglobulins) and bivalent (BoNT A/B) equine toxin [15, 16]. However, the antibody treatments must be administered to intoxicated individuals within a very narrow timeframe to prevent the progression of neuronal paralysis – since the antibodies cannot inhibit the toxins post-neuronal penetration. As a result, the antibodies are only marginally beneficial for recovery, since patients still require respiratory aid for weeks. Hence, there is an urgent need to develop novel and more effective medical treatments to limit/counter BoNT intoxication [17].
Mechanistically, BoNTs inhibit neurotransmitter exocytosis by cleaving specific soluble N-ethylmaleimide-sensitive fusion protein attachment (SNARE) proteins. The cleavage of SNARE proteins results in impaired muscle function, respiratory arrest, and if mechanical respiration is not available, death [1, 18, 19]. Structurally, BoNTs are composed of a heavy chain (HC) (100kDA), which is mainly responsible for neuronal internalization, and a light chain (LC) metalloprotease (50kDa) [20, 21]. The HC and the LC are linked by a disulfide bridge [3]. The C-terminal region of the BoNT HC interacts with SV2 (Synaptic Vesicle Protein 2) receptors, and mediates toxin endocytosis [22, 23]. Following cellular entry, the LC dissociates from the HC and cleaves SNARE proteins in the neuronal cytosol [2]. Importantly, it is the LC’s proteolytic activity that is responsible for the extreme potencies of these toxins; LC at very low concentrations is sufficient to block the activity of a motoneuron. For example, BoNT/A, specifically removes 9 amino acids from the C-terminus of SNARE component synaptosomal-associated protein of 25 kDA (SNAP-25), and this simple cleavage is sufficient to inhibit neurotransmitter release [24–28]. Moreover, while BoNT/A mainly targets motoneurons, it is stable in cells for weeks [29–31], and its active form can travel in the nervous system and reach distant synapses through retrograde transportation [32, 33]
There is a significant need to develop novel therapeutics to counter BoNT poisoning – especially post-neuronal internalization [34]. However, there are currently no reliable, large-scale cell-based drug screening assays to facilitate such research. This is mainly due to a lack of relevant and well-characterized experimental model systems. Specifically, while cell culture-based assays employing mammalian neuroblastoma cells and primary spinal cord cells from chickens or rodents have been used previously to study the effects of small molecules on BoNT intoxication [34–39], these models possess limitations. For example, immortalized cells can be produced in large quantities, but lack sensitivity [34]. Moreover, such cell lines carry tumorigenic properties, and therefore do not mimic physiologically normal/relevant conditions. In contrast, primary embryonic spinal cord cells from rodents are physiologically relevant and very sensitive to BoNT intoxication [40]; however, these cells are very difficult to obtain in large quantities because primary embryonic spinal motoneurons are post-mitotic. Moreover, spinal cord dissections are technically challenging and inconsistent, as they can yield different ratios of motoneurons (versus other cells) from culture to culture. Therefore, these strategies are not optimal for developing high-throughput, neuron-based screening assays.
Recently, it was shown that mammalian motoneurons can be differentiated from embryonic stem (ES) cells [41–44]. Therefore, we tested the hypothesis that ES-derived motoneurons can be used as a renewable cellular source for large-scale drug screening assays to identify BoNT therapeutics, as well as examine the general biological effects of intoxication on the host’s cellular pathways. The results presented herein demonstrate that ES-derived motoneurons do provide a highly sensitive, easily accessible, and renewable cell model system that is compatible with high-throughput screening and experimentation. Furthermore, the presented cellular model is also a unique tool for investigating host-based genes and proteins that are responsive to BoNT intoxication. Significantly, while this study focused on BoNT/A intoxication, the established model is also applicable for studying all other BoNT serotypes.
Results
Differentiation and characterization of mouse ES-derived motoneurons
It is established the mouse ES cells can be differentiated into functional motoneurons via the application of Retinoic Acid (RA) and Sonic Hedgehog (Shh) protein [42–44]. For these experiments, we employed an HBG3 ES cell line carrying the motoneuron specific transcription factor Hb9 promotor, which drives the expression of the enhanced green fluorescence gene (eGFP). This allows for the identification of differentiated motoneurons based on eGFP expression [42]. In addition, such specific eGFP expression permits motoneuron isolation and/or enrichment using a fluorescence activated cell sorter (FACS).
Pluripotent ES cells were co-cultured with primary mouse embryonic fibroblasts, and at 70–80% confluency, the ES cells were separated from the fibroblasts (see Materials and Methods), aggregated as embryoid bodies (EBs), and induced in suspension culture with RA, Shh, and Purmorphamine. After 7 days in suspension, the EBs were dissociated gently and plated onto matrigel-coated cell culture dishes/slides for an additional 3 days prior to characterization.
Western blot analyses of differentiated ES cell lysates were performed at various time points to demonstrate changes in the protein expression profile of markers that are characteristic of stem cells, neuronal cells, and motoneurons. First, we found that eGFP expression increased in response to motoneuron inducing factors, suggesting activation of the motoneuron specific HB9 promotor (Fig. 1A). Furthermore, expression of the neural markers Tau (Tau5) and β-III tubulin specific Tuj1 were transiently up-regulated. Similarly, after day 5, Choline Acetyl Transferase (ChAT), an enzyme that is required for the synthesis of the neurotransmitter acethylcholine, was also up-regulated (Fig. 1A). By contrast, pluripotency markers Oct4 and Sox2 that are expressed in ES cells were down-regulated during differentiation. Taken together, these data suggest that the protocol used is very effective for differentiating ES cells into the neuronal lineage.
Figure 1. Characterization of ES-derived motoneurons.
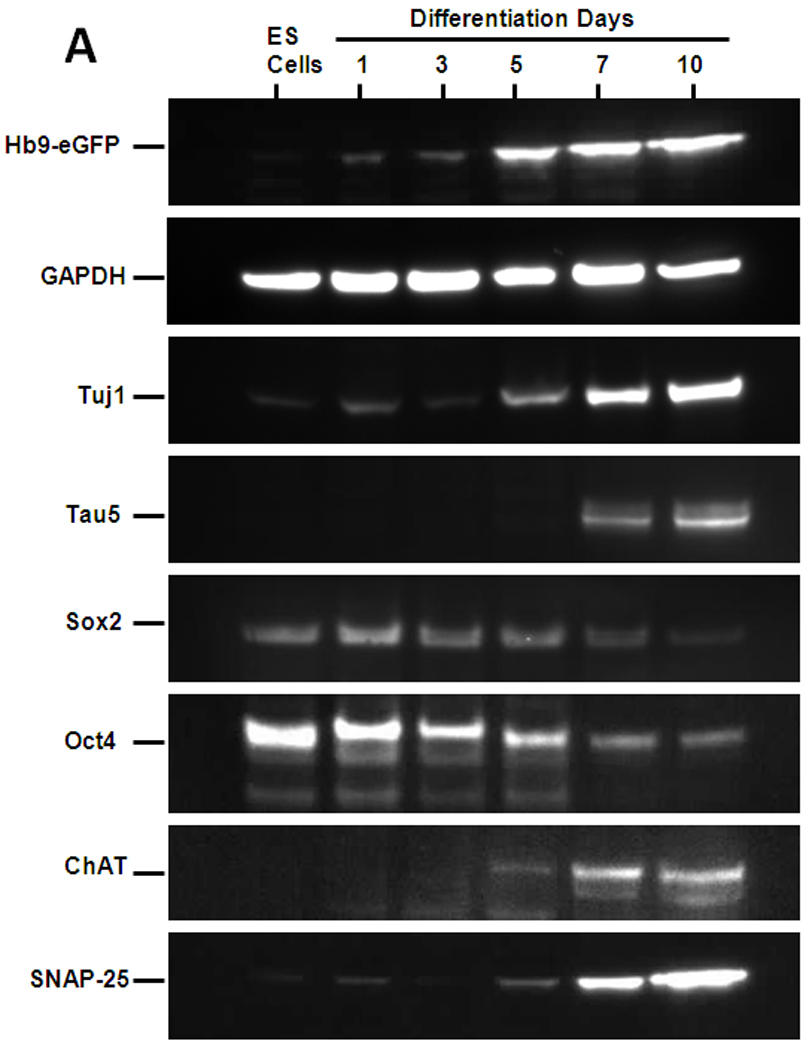
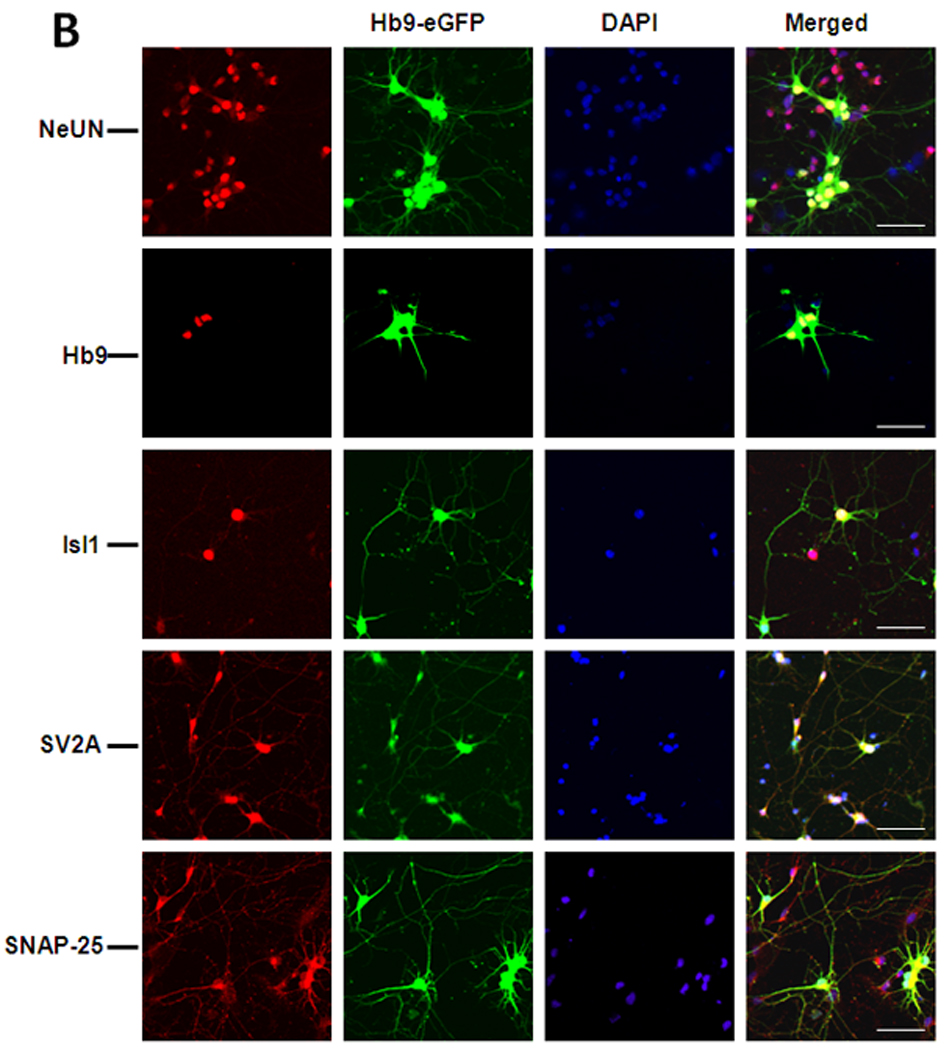
(A) Western blot analyses of cellular markers expressed at different stages of the differentiation process. Markers included eGFP driven by Hb9 motoneuron promotor (Hb9-eGFP) genes expressed by pluripotent ES cells, Oct4 and Sox2; neuronal cells, Tuj1 and Tau; and post-mitotic motoneurons, ChAT protein. Similarly, SNAP-25 protein expression, the only-known intracellular target of BoNT/A, was also evaluated. Total protein loaded per lane was 10–15 µg and GAPDH was used as loading control. (B) Immunostaining for neural markers in the differentiated cultures. Cells from embryoid bodies dissociated at day 7 and cultured on matrigel-coated culture slides for an additional 3 days were immunostained with antibodies against neuronal marker, NeUN; post-mitotic motoneuron markers Hb9, and Isl1; SNARE protein, SNAP-25, and SV2A, the receptor required for BoNT/A entry into neurons. Shown are also the Hb9-eGFP and DAPI nuclear staining. The scale bar is 50 µm.
To further characterize (at the cellular level) the identity of the differentiated cells, immunocytochemistry was used to investigate the expression profiles of specific motoneuron markers (Fig. 1B and Sup. Fig1). Importantly, we confirmed that the Hb9::GFP expression overlaps accurately with the endogenous Hb9 protein. The specificity of the differentiation procedure was further demonstrated by the expression of motoneuron markers Isl1 (Fig. 1B) and LIM3 (Sup. Fig1). Moreover, confocal analyses indicated that ChAT was expressed by all GFP+ cells, thereby indicating the mature phenotype of the motoneurons. Occasionally, some GFP-negative cells also expressed ChAT with other neuronal markers (such as NeUN (Fig. 1B), Tau, and Tuj1 (Sup. Fig1)). These data suggested that the majority of the differentiated cells at day 10 were motoneurons, but that other neural cells could also form.
FACS analysis quantification of GFP+ cells at day 7 indicated a motoneuron differentiation rate of 30–40%, which is consistent with the efficiency observed in previous studies employing similar protocols (data not shown; [44]).
SNAP-25 protein and the SV2A receptor are present in ES-derived motoneurons
SNAP-25, the intracellular target of the BoNT/A LC, is required for acetylcholine exocytosis and is abundantly expressed in primary spinal motoneurons [3, 45]. Therefore, we investigated its expression in ES-derived motoneurons to establish the relevance of these cells with respect to BoNT/A intoxication experiments. Western blot analyses revealed increased SNAP-25 protein expression during differentiation up to day 10 (Fig. 1A). Immunocytochemical analyses further confirmed the presence of SNAP-25 in the ES-derived motoneurons. Similarly, the SV2A receptor, which is crucial for BoNT/A entry into the neuron cytosol, was also expressed by GFP+ motoneurons (Fig. 1B). Thus, the presence of BoNT/A receptor SV2A, and the toxin’s intracellular target (i.e., SNAP-25) in the differentiated motoneurons strongly suggested that this system should be sensitive to BoNT/A intoxication.
ES-derived motoneurons are acutely sensitive to BoNT/A intoxication in a dose-dependent manner
Since ES-derived motoneurons express both SV2A and SNAP-25, we evaluated their sensitivity to various concentrations of BoNT/A (0–1000 picomolar) after 3 hours (Fig. 2A). Via Western blot analysis, we visualized the BoNT/A-mediated cleavage of SNAP-25 by examining its shift in molecular weight from ~25kDa to ~24kDa. As shown in Fig. 2A, we observed a direct, dose-dependent correlation between cleaved SNAP-25 and BoNT/A concentration. ES-derived motoneurons were very sensitive to BoNT/A, since concentrations as low as 10 pM caused SNAP-25 cleavage (Fig. 2A). Next, we compared the BoNT/A intoxication sensitivity of ES-motoneurons with the BoNT/A intoxication sensitivities of primary mouse spinal cord cells and DRG neurons (Fig. 2B). As previously reported, primary spinal cord cells exhibit BoNT/A sensitivity at concentrations that are comparable to ES motoneurons (although starting at 25 pM, [40], Fig. 2B). DRG neurons were found to be less sensitive to BoNT/A intoxication than either ES-derived motoneurons or primary spinal cord cells, since the BoNT/A effect became apparent at 100pM (Fig. 2C). Additionally, immortalized mouse spinal cord cells derived from the NSC-34 cell line demonstrated a complete lack of sensitivity to BoNT/A within the toxin concentration range that was examined in this study (Fig. 2D). However, this result was not surprising, as immortal neural cell lines have been shown to usually require either higher BoNT concentrations or longer exposure times (up to 48 hours) to display SNAP-25 cleavage [34, 37]. Taken together, these data suggest that ES-derived motoneurons are at least as sensitive to BoNT/A intoxication as primary spinal cord neurons (as revealed by the SNAP-25 cleavage profiles shown in Fig. 2). Moreover, these experiments also indicated that, although other cells can arise during the ES motoneuron differentiation protocol, no further purification or enrichment of the ES-derived motoneurons is required to obtain a BoNT/A-mediated SNAP-25 proteolysis level comparable to that observed for primary cells.
Figure 2. Concentration-dependent effects of BoNT/A on SNAP-25 protein cleavage in mouse ES-derived motoneurons, primary mouse spinal neurons and DRG neurons, and the motoneuron like NSC-34 cell line.
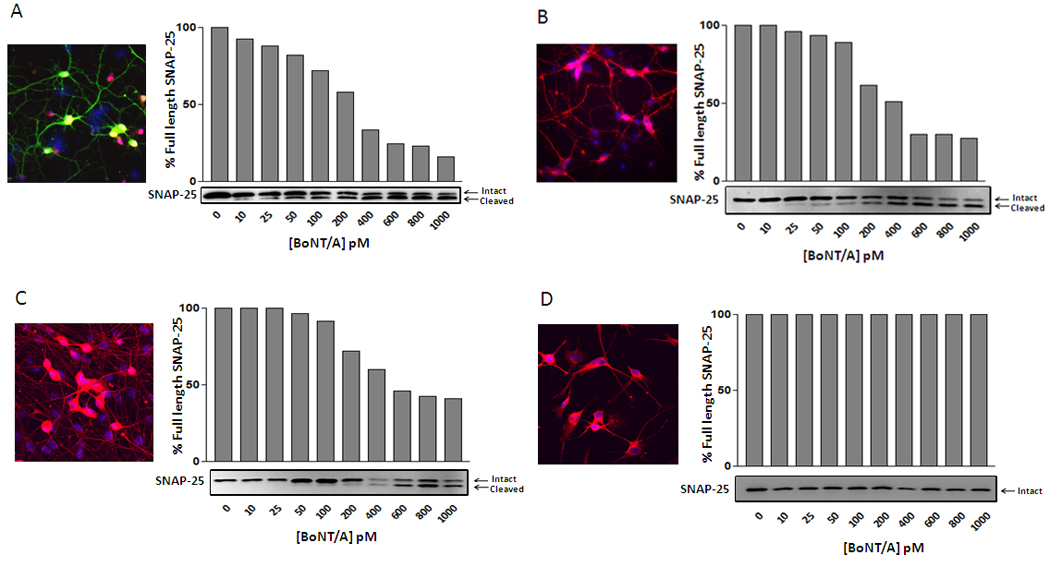
A–D; Graphic representation of the percentage of uncleaved SNAP-25 protein following cell treatment with various concentrations of BoNT/A (0–1000 pM). The graphs represent the quantification of each blot shown at the bottom of the x-axis. The top band is uncleaved SNAP-; the bottom band is the BoNT/A mediated cleavage fragment. Inset in each panel are representative image of : (A) ES-derived motoneuron cultures stained with the neuronal marker NeUN (red), Hb9-eGFP (green) and DAPI (blue); (B) primary mouse spinal cord cells and (C) DRG neurons stained with cytoplasmic neuronal markers Tuj1 (Red) and DAPI (Blue); (D) immortalized NSC-34 cells stained with neuronal marker Tau5 (Red) for total Tau protein and DAPI imaging. The scale bar is 50 µm.
Evaluation of the time dependency of BoNT/A action on SNAP-25 proteolysis
To further evaluate the effect of internalized BoNT/A over time, ES-derived motoneurons were treated with 1 nM BoNT/A for 1 hr, washed with media to remove extracellular toxin, and lysed at different time points (Fig. 3). Western blot analyses of the cell lysates indicated that after only 1 hr exposure, 1nM BoNT/A cleaved 41% of the SNAP-25 in our ES motoneurons (Fig. 3). After 1.5 hrs, 70% of the SNAP-25 was cleaved (Fig. 3). These data suggest that the BoNT/A mediated cleavage of SNAP-25 is rapid and effective, as observed by the fact that cleavage of this protein was very close to a plateau point after only 1.5 hrs post-intoxication. Therefore, 1.5 hrs-3hrs incubation time frame is reasonable for assays to screen inhibitors to counter BoNT/A poisoning in already intoxicated cells.
Figure 3. Time dependent effects of BoNT/A on SNAP-25 cleavage.

Western blot analysis and graphical representation of BoNT/A mediated cleavage of SNAP-25 over time. Cultures were exposed to 1 nM of BoNT/A for 1 hr, after which time the toxin was removed and cells were lysed at the indicated time points. The top band in the Western blot is the uncleaved SNAP-25; the bottom band is the BoNT/A mediated cleavage fragment.
BoNT/A inhibitors impede SNAP-25 proteolysis in ES cell-derived motoneurons
To examine if the observed SNAP-25 cleavage in ES-derived motoneurons was BoNT/A specific, we examined the effects of three known inhibitors on the toxin’s efficacy. We first used the plant protein TVL, an extracellular BoNT antagonist that binds to cell surfaces and interferes with BoNT/A entry into neurons [36, 46]. Following differentiation, motoneurons were treated with TVL for 1 hr and incubated with BoNT/A for 3 hrs. The media was then removed, and the cells were rinsed with fresh media three times (to remove any extracellular toxin). As shown in Figure 4, TVL pre-treatment blocked BoNT/A mediated SNAP-25 proteolysis, providing an effect similar to that observed in primary isolated motoneurons.
Figure 4. Inhibition of BoNT/A-mediated SNAP-25 cleavage with BoNT/A neutralizing antibodies, Triticum Vulgaris Lectin (TVL) and Bafilomycin A1.
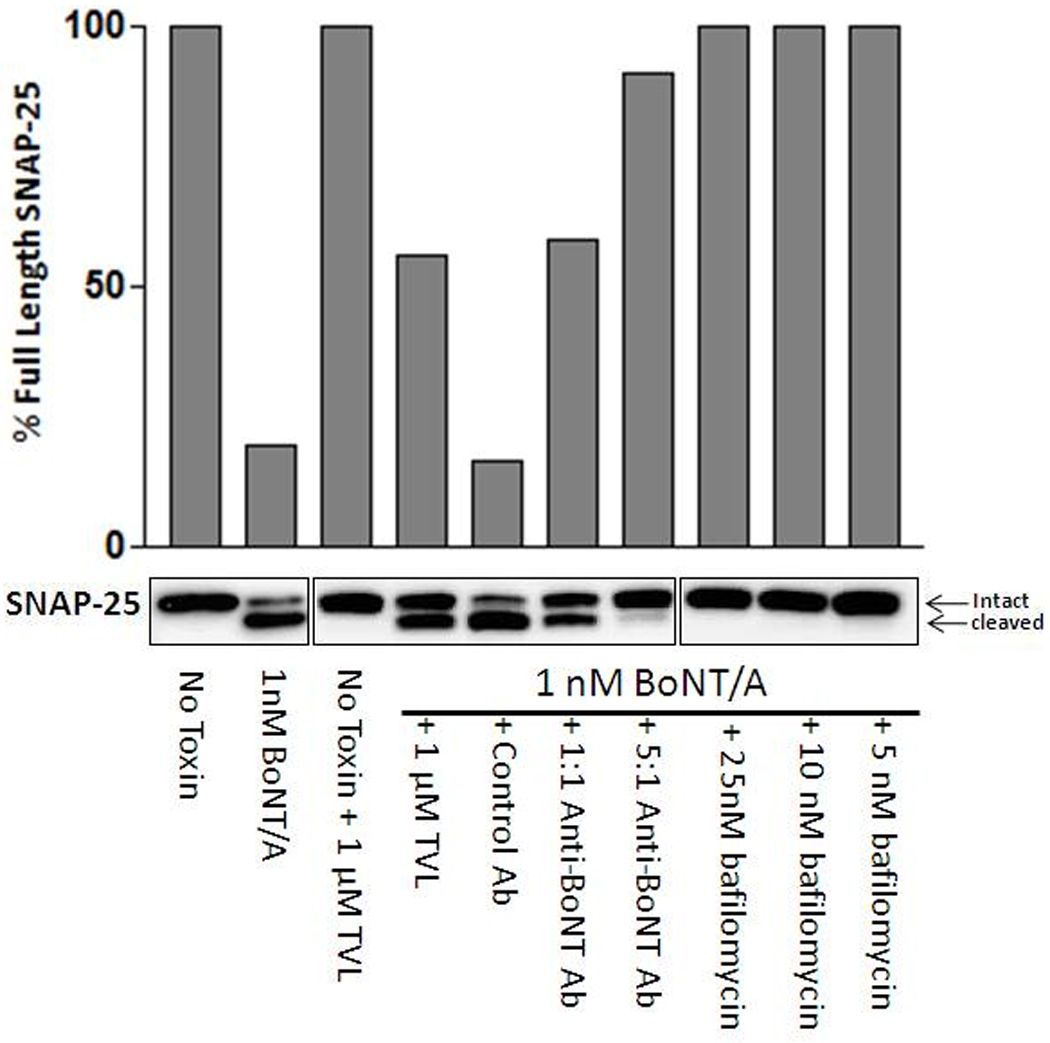
Western blot analysis and graphical representation of BoNT/A mediated cleavage of SNAP-25 in ES cell-derived motoneurons incubated with 1nM BoNT/A in the presence of the indicated inhibitors for 3 hours. Note that while 1 µM TVL only partially blocked SNAP-25 cleavage, BafilomycinA1 completely inhibited BoNT/A mediated SNAP-25 cleavage at any concentration, while the efficacy of the 4A2-4 antibody tightly correlates with the antibody to toxin ratio.
Similar SNAP-25 protection was also obtained employing the neutralizing antibody 4A2-4, which inhibited BoNT/A mediated SNAP-25 cleavage in a dose dependent manner. The same level of protection has also been observed using a chick primary motoneuron assay (Fig.4) [37, 47]. Finally, we examined the effect of Bafilomycin A1, an ATPase inhibitor that blocks endosome acidification, which is a process required for receptor mediated BoNT entry into the neuronal cytoplasm (for all BoNT serotypes) [40, 48, 49]. ES-derived motoneurons were incubated with BoNT/A and various concentrations of Bafilomycin A1 for 3 hrs, and then washed 3 times with fresh media to remove extracellular toxin. As shown in Fig. 4, BafilomycinA1 completely inhibited SNAP-25 proteolysis at all concentrations. Overall, these data strongly suggest that the ES-derived motoneuron cell system can be used to effectively evaluate inhibitor mediated SNAP-25 protection in the presence of intracellular BoNT/A.
ES-derived motoneurons are applicable for high throughput assays measuring BoNT/A activity
Immunofluorescence-based high-throughput studies to screen compounds at high speed (to measure their abilities to inhibit BoNT/A mediated proteolysis) would require (i) specific antibodies to quantify protein cleavage, and (ii) sensitive cell culture systems that are amenable to large scale studies. We previously developed BoNT/A cleavage sensitive (BACS) antibodies, which are highly specific to full-length SNAP-25, but not to truncated fragments resulting from BoNT/A cleavage [47]. These antibodies, when used in conjunction with commercially available non-cleavage sensitive SNAP-25 antibodies, are unique biological tools to quantify SNAP-25 cleavage in high-throughput studies. Herein, with the knowledge that ES-derived motoneurons are highly sensitive to BoNT/A, we sought to determine whether this system is compatible with immunofluorescence-based high-throughput studies; i.e., high content imaging [50] and Li-Cor imaging assays [47].
As a prelude, we first verified the efficacy of BACS antibodies in this system using high content imaging. Mouse ES cell-derived motoneurons were cultured in 96-well plates and immunolabeled with total SNAP-25 (N-terminal specific antibody staining) (green) and full length SNAP-25 antibodies (BACS antibody staining) (red) in the control and BoNT/A intoxicated samples. As shown in Fig. 5A, a 3hr BoNT/A (1 nM) treatment diminished immunostaining resulting from the BACS antibodies (red), whereas immunostaining with the N-terminal-specific antibody was not affected by BoNT/A exposure (lower panels). Using a high content imaging assay, we next measured the effects of BoNT/A at varying concentrations (0–1000 pM) on SNAP-25 cleavage with BACS antibodies (following 3 hrs intoxication) (Fig. 5B). The ratio of the integrated fluorescence intensities in both channels was used to measure the change in SNAP-25 cleavage as a function of BoNT/A concentration (Fig. 5B). We further tested utility of a simple scanning fluorescence assay, Li-Cor imaging, for measuring intracellular BoNT/A activity under similar conditions in 96-well plates (Fig. 5C). Cells were treated with increasing doses of BoNT/A (0–1000 pM), incubated for 3 hrs, and fixed and stained with the antibody combinations described above. Plates were then imaged and analyzed using a Li-Cor Odyssey infrared imaging system [47]. The measured dose response in both high content imaging and Li-Cor imaging assays showed a BoNT/A concentration dependent increase in SNAP-25 proteolysis. Immunoblotting experiments utilizing N-terminal-specific SNAP-25 antibodies (Fig. 2) exhibited a similar dose-dependent change in SNAP-25 (Fig. 5B and C). Taken together, these data suggest that ES-derived motoneurons can serve as a renewable cell source for immunofluorescence based high-throughput assays to measure BoNT/A activity by detecting SNAP-25 proteolysis with BACS antibodies.
Figure 5. Measuring BoNT/A activity in cell based assays using BoNT/A cleavage-sensitive (BACS) antibodies.
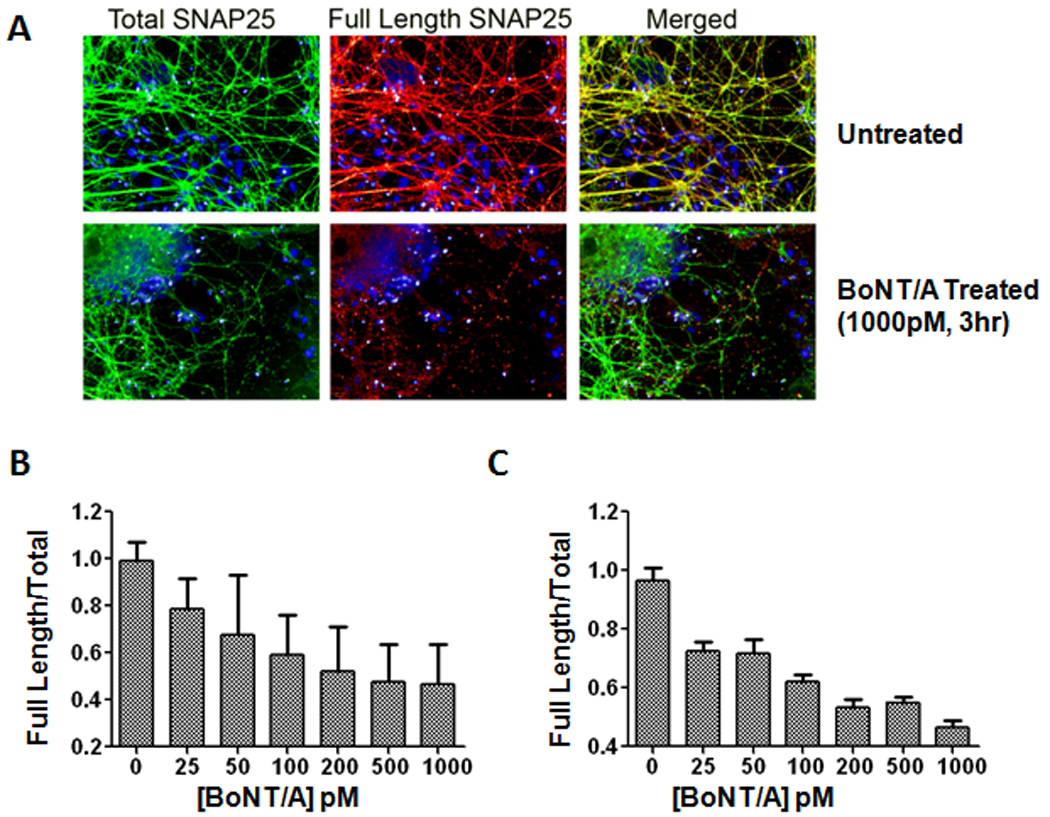
(A) ES cell-derived motoneurons were fixed and immunolabeled with antibodies to detect total SNAP-25 (N-terminal specific antibody labeling) (green) and full length SNAP-25 (BACS antibody labeling) (red). The nuclei in all images were labeled with Hoechst dye (blue). After a 3 hr BoNT/A intoxication, truncated SNAP-25 was recognized by total SNAP-25 antibodies, but not by BACS antibodies, which resulted in a reduction in red fluorescence. (B) High content imaging assay for measuring BoNT/A activity. ES cell-derived motoneurons were treated with a range of BoNT/A concentrations (0–1000 pM) and following 3 hr incubation were fixed and stained with total SNAP-25 and BACS antibodies as described in part A. (C) Licor imaging assay for measuring intracellular BoNT/A activity. Cells were treated with BoNT/A (0–1000 pM), incubated for 3 hrs and fixed and stained with the antibody combinations described above. The measured dose response in both high content imaging and Li-Cor imaging assays correlated well with BoNT/A concentrations. For each condition, images from 6 fields/well were acquired with the script calculating the integrated fluorescence intensities in whole cell regions. Error bars represent standard errors of the averages.
Discussion
Efficient cell-based, high-throughput assays should employ cell culture systems that are: (i) physiologically relevant, (ii) sensitive, (iii) consistent, (iv) well-characterized, and (v) renewable. The primary goal of this study was to establish a physiologically relevant motoneuron cell culture model suitable for the identification of BoNT inhibitors using a high-throughput approach. Based on our results, we have demonstrated that ES-cell derived primary neurons can provide such a system, as they can be produce in relatively large quantities, are very sensitive to BoNT intoxication, and are suitable for the biochemical analyses of cellular pathways that are affected during BoNT intoxication.
Although primary mouse embryonic spinal motoneurons are ideal for BoNT studies, the difficulty in obtaining and culturing these cells makes them unattractive for high-throughput screening purposes. Specifically, these cells are difficult to obtain in large quantities, as only 5,000–15,000 cells per embryo can be obtained [51]. Moreover, it is difficult to obtain ‘pure’ motoneurons because other cells are present in the dissected spinal cord. Consequently, to attempt to address these issues, several methods have been established to isolate or enrich primary motoneurons from dissected spinal cords. These include either simple density-based techniques, or more advanced antibody-based isolations [51, 52]. However, these methods have provided only varying degrees of success, require expensive reagents, are time consuming, and yield limited quantities of motoneurons. Therefore, we tested a previously established ES cell-to-motoneuron differentiation methodology to obtain large quantities of mammalian motoneurons.
As for the characterization of this system, our results are in line with other studies utilizing the HBG3 cell line, thus indicating that differentiated motoneurons exhibit the morphological and physiological properties of their in vivo counterparts, including the formation of cell processes and the expression of motoneuron specific markers [41, 42, 53] (Fig. 1, Fig. 2, and Supplementary Fig. 1). It is also noteworthy to report the observation that, upon transplantation, these motoneurons successfully integrate into the chick spinal cord [42]. Furthermore, in line with the literature [41, 54], we have found that differentiated motoneurons can innervate, and lead to postsynaptic acetylcholine receptor clustering, which is detectable by alpha-bungarotoxin labeling when co-cultured with C2C12 skeletal muscle cells, thereby suggesting neuro-muscular junction formation (Supplementary Fig. 2). Additionally, we have also found that ES-derived motoneurons exhibit the same neurotrophin receptor expression profiling that is observed for embryonic spinal motoneurons (data not shown) [55–57].
Previous research employing mammalian and chick primary cells has demonstrated that spinal motoneurons are extremely sensitive to BoNT/A when compared to poorly sensitive DRGs. Our analysis is consistent with those findings, indicating that ES cell-derived motoneurons, which are equivalent to primary spinal motoneurons, are more sensitive to BoNT/A than DRG neurons (Fig. 2). Moreover, we confirmed that the NSC-34 cell line also fails to respond effectively to BoNT intoxication under the conditions that we employed (Fig. 2), further suggesting that, contrary to our system, these cells are not adequate for BoNT toxicity studies.
Although the described ES-derived motoneuron model leads to the generation of fully functional motoneurons, other cells also arise when applying the differentiation protocol. However, it should be noted that primary motoneurons suffer from a similar limitation, with other cells also being isolated during the plating of the dissected spinal cords. Irrespective, ES-derived motoneurons, without further isolation or enrichment, are very sensitive to BoNT/A, thereby providing a robust read-out of its toxicity (Fig. 2). Moreover, since immunofluorescence analysis (Fig. 1) showed that non-neuronal cells express relatively low levels of SNAP-25 compared to GFP+ motoneurons, and the receptor required for BoNT entry into the cells, SV2A, is mainly expressed in GFP+ cells, it is unlikely that non-neuronal cells present in our cultures significantly contributed to the pool of truncated SNAP-25 after 3 hours of BoNT/A intoxication. If needed, eGFP expression, which is under the control of the motoneuron specific Hb9 promotor, would allow for the isolation of pure or enriched motoneuron populations by FACS. Indeed, we have performed such experiment and found that FACS sorted motoneurons exhibit elevated sensitivity to BoNT/A intoxication (data not shown). However, sorted cells do not survive well unless they are cultured on skeletal muscle cells, which are believed to secrete neurotrophic factors required to regulate the growth and maturation of motoneurons. Regardless, although other cells also arise during directed motoneuron differentiation, the non-FACS sorted motoneurons provide a robust and sensitive system in which BoNT/A mediated SNAP-25 proteolysis can be quantified (Fig. 2).
A large body of biochemical and cellular biology data supports the conclusion that BoNT mediated SNAP-25 cleavage inhibits cholinergic exocytosis, leading to the paralysis associated with the disease state botulism [24–28]. This is a strong indicator that SNAP-25 cleavage is a critical read-out to assess the effects of potential BoNT inhibitors. Although functional assays such as neurotransmitter release assay could be also utilized, SNAP-25 cleavage assay allows us to directly monitor BoNT/A activity by detecting cleavage of its endogenous substrate and serves as the most time-efficient and sensitive assay for large scale BoNT/A inhibitor screenings. Herein, we demonstrated that: (i) ES-derived motoneurons are very sensitive to BoNT/A mediated SNAP-25 cleavage (Fig. 2 and 3), (ii) the efficacies of BoNT/A inhibitors can be determined in these motoneurons by quantifying SNAP-25 protection during intoxication (Fig. 4), and (iii) immunofluorescence-based high-throughput studies, taking advantage of both renewable motoneurons and BACS antibodies, can be successfully utilized to screen BoNT inhibitors at high speed (Fig. 5) [47].
Conclusions
Directed differentiation of ES-cell motoneurons, without the need for further purification or enrichment, can provide a very efficient, sensitive, and reliable cell system that may be used to facilitate both basic research, geared toward understanding the biological effects of BoNTs on motoneurons, as well as high-throughput drug discovery efforts to identify novel inhibitors/antagonists of BoNTs.
Materials and methods
Directed differentiation of mouse ES cells into motoneurons
Mouse ES cells (HBG3 cell line), in which GFP expression is driven by mouse motoneuron specific Hb9 promotor, were a kind gift from Dr. Thomas M. Jessell and Dr. Hynek Wichterle (Columbia University, New York, NY). The directed differentiation of HBG3 cells into spinal motoneurons was achieved by Retinoic Acid/Sonic Hedgehog/Purmorphamine based-induction methods, as described previously [41–44, 58], but with slight modifications. Briefly, pluripotent ES cells were maintained on mitomycin-inactivated primary mouse embryonic fibroblast (MEF) cells in HBG3 medium. The medium consisted of DMEM for ES cells (Chemicon, Billerica, MA) supplemented with 15% ES-cell tested fetal bovine serum (Hyclone, Waltham, MA), 1% Nucleosides (Chemicon), 1000 Unit/ml Leukemia Inhibitory Factor (LIF) (Millipore, Billerica, MA), β-mercaptoethanol (final 0.1mM), 1% Penicilin/Streptomycin, 1% Glutamax, and 1% Non-essential amino acids. Unless otherwise stated, all reagents were obtained from Invitrogen (Carlsbad, CA). For motoneuron induction, the ES cells were trypsinized and re-suspended in differentiation medium including 1:1 Advanced DMEM/F12 plus Neurobasal medium supplemented with 1% Penicilin/Streptomycin, 1% Glutamax, β-mercaptoethanol (final 0.1mM), and 15 % Knockout serum replacer. To remove MEFs by selective adherence, the single cell mixtures were then plated onto tissue culture treated plates for 30 minutes. MEFs adhered to the plates more tightly than ES cells. ES cells in the supernatant were collected, counted using a Cellometer AutoT4 (Nexcelom Biosciences, Lawrence, MA), and seeded onto AggreWell 400 (Stem Cell Technologies, Vancouver, Canada) plates, at a density of 500 cells per microwell, to form embryoid bodies (EBs). EBs were harvested the next day (Day1), and maintained in suspension culture (in fresh differentiation medium) for an additional 24 hours. Following 2 days of differentiation, EBs were treated with Retinoic Acid (RA) (1µM, Sigma, St. Louis, MO) for 24 hrs to induce neuralization and caudalization, and then Sonic Hedgehog (Shh) (100ng/ml, R&D Systems, Minneapolis, MN) protein was added to the same medium on day 3 to induce motoneuron specification. Purmorphamine (Calbiochem, Gibbstown, NJ) was added to the cultures at a concentration of 1 µM (24 hours after Shh was added (Day4)). On day 5, EBs were transferred to dishes including fresh motoneuron medium consisting of 1:1 Advanced DMEM/F12 plus Neurobasal medium, which was supplemented with 1% Penicilin/Streptomycin, 1% Glutamax, and 2% B27 serum-free supplement (Invitrogen), brain-derived neurotrophic factor (10ng/ml, Chemicon), Glial cell-derived neurotrophic factor (100ng/ml, R&D Systems), Cliary-derived neurotrophic facors (10 ng/ml, Chemicon), and Neurotrophin3 (10 ng/ml, Chemicon). Finally, on day 7, EBs were dissociated using a papain dissociation system (Worthington, Lakewood, NJ), and plated onto matrigel (BD Biosciences, San Jose, CA) coated dishes for an additional 3 days to allow for the degree of neurite elongation required for experimental purposes. The efficiency of motoneuron differentiation indicated by GFP expression was quantified using a fluorescence activated cell sorter (FACSCalibur Instrument, BD Biosciences).
Isolation and culture of primary mouse spinal cord cells and Dorsal Root Ganglion (DRG) neurons
Embryonic spinal motoneurons and DRG cells were dissected and cultured from wild-type E13.5-old mouse (C57BL/6) embryos as described previously [51], but with modifications. Briefly, dissected embryonic spinal cord and DRG cells were dissociated using a papain dissociation system for 30 min at 37°C, according to the manufacturer’s protocol. Dissociated cells were then pelleted and triturated until evenly suspended in medium containing papain inhibitor. Following, intact cells were separated from tissue fragments with a discontinuous density gradient provided in the papain dissociation kit. The cell population was enriched with motoneurons by selective adherence. The single cell mixtures were plated onto tissue culture treated dishes for 30 minutes. Non-neuronal cells adhered to the dishes more tightly than neuronal cells. The neuronal cells that remained in the supernatant were collected, counted, and plated onto matrigel-coated 6-well culture plates in motoneuron media including neurotrophic factors (as described above) for 5 days prior to the addition of toxin.
Culture and differentiation of NSC-34 cells
NSC-34 cells were purchased from CELLutions Biosystems Inc. (Ontario, Canada), and cultured and differentiated as described previously [59]. Briefly, undifferentiated cells were maintained in media consisting of high glucose DMEM supplemented with 10% fetal bovine serum (Hyclone), 1% Penicillin/Streptomycin, and 1% Glutamax. For the experiments, cells were differentiated in medium containing 1:1 DMEM/Ham’s F12 supplemented with 1% fetal bovine serum, 1% Penicillin/Streptomycin, and 1% Non-essential amino acids for 7 days.
Western blot analysis for the characterization of ES-derived motoneurons
The ES cells and differentiated cells were harvested at different stages, washed with PBS, and lysed in NP-40 cell lysis buffer. The lysates were then centrifuged at 10,000 rpm for 10 min at 4°C. The total protein concentration of the supernatant was determined using a BCA protein assay kit (Thermo Scientific). Equal amounts of total protein were run on 4–12% or 14% polyacrylamide gels (Invitrogen), transferred to nitrocellulose membranes, blocked in 5% non-fat milk for 1hr, and incubated with primary antibodies against eGFP (Invitrogen), GAPDH (Millipore), Tuj1 (R&D Systems), Tau5 (Thermo Scientific), Sox2 (Millipore), Oct4 (Sigma), Choline Acethyltransferase (ChAT) (Millipore), and SNAP-25 (BD Biosciences), in TBST buffer containing 5% milk overnight at 4°C. Horse radish peroxidase conjugated secondary antibodies used in these experiments were obtained from Millipore. The membrane was visualized using a Pierce ECL Western detection kit (Thermo Scientific). The immunoblotting results were scanned with a Syngene (Frederick, MD) gel documentation and analysis system.
Immunocytochemistry
Day 7 differentiated embryoid bodies were dissociated with papain and plated onto matrigel (BD Biosciences) coated 4- or 8-well BD BioCoat culture slides for 3 days. Following, cells were fixed in 4% paraformaldehyde for 10 minutes, and permeabilized with 0.1% Triton X-100. After being blocked with 1% appropriate serum, the cells were stained with antibodies against NeUN (Millipore), Hb9 (Developmental Studies Hybridoma Bank (DSHB, Iowa City, IO)), Isl1 (DSHB), SV2A (Millipore), and SNAP-25 (BD Biosciences) (as shown in Fig1B); as well as for Tau5 (Thermo Scientific), Tuj1 (R&D Systems), ChAT (Millipore), and LIM3 (Millipore) (as shown in Supplementary Fig1). Primary antibodies were incubated overnight in PBS with 10% serum (or 1% BSA). Appropriate secondary antibodies, conjugated with Alexa488 and Alexa 594, were incubated the following day for 2 hours at room temperature. Cells were washed in PBS and mounted in ProLong Gold antifade reagent with DAPI (Invitrogen) for nuclear staining. Images were collected employing Zeiss ApoTome (ImagerZ1) and Zeiss confocal microscopes (Zeiss, Thornwood, NY).
BoNT intoxication and immunoblotting analyses to quantify SNAP-25 cleavage
Neuron cultures (day10) were intoxicated with various concentrations of BoNT/A (MetaBiologics, Madison, WI) and incubated at 37°C for 3 hr. Following intoxication, toxin containing media was removed and cells were rinsed with fresh motoneuron media three times. SNAP-25 protein cleavage was quantified using standard immunoblotting procedures employing SNAP-25 antibodies - as described previously [47]. For the Western blot analysis shown in Fig. 3, ES-derived motoneurons (Day10) were intoxicated for 1 hr, and then toxin remaining in the medium was cleared with washing. Following, cells were incubated at 37 °C for additional time frames - as indicated in the Fig. 3. This protocol allowed us to observe the activity of internalized BoNT/A over time. For inhibitor studies (Fig. 4), Triticium vulgaris Lectin was used at 1µM, and BafilomycinA1 was used at titrated concentrations. Both reagents were obtained from Sigma-Aldrich. BoNT/A neutralizing antibody (4A2-4) (produced at the US Army Medical Research Institute of Infectious Diseases) and the control antibody (Anti staphylococcal enterotoxin B) (Toxin Technology, Sarasota, FL) were simultaneously added with BoNT/A at the time of intoxication. The indicated neutralizing antibody to BoNT/A ratio was based on approximate molar concentrations.
Measuring BoNT/A activity in cell-based assays using BoNT/A cleavage sensitive antibodies
For high content imaging, automated image acquisition and analysis were performed as described previously [50]. Briefly, ES-derived motoneurons were cultured on matrigel-coated 96-well plates (BD Falcon Imaging Microplates) and intoxicated with various concentrations of BoNT/A (0–1000pM) for 3hr. SNAP-25 protein cleavage was detected using standard immunofluorescence procedures employing total SNAP-25 antibody (N-terminal specific antibody, SMI-81, Abcam, Cambridge, MA) and full length SNAP-25 antibodies (BoNT/A cleavage sensitive (BACS) F2070 antibodies) [47]. The images were acquired using an Opera confocal high content imager (Opera model 3842-Quadruple Excitation High Sensitivity (QEHS), PerkinElmer, Waltham, MA) through 2 exposures with 20X water objectives. In the first exposure, the excitations of GFP and fluorophores binding to total SNAP-25 were directed to sample using 488 nm and 640 nm lasers. In the second exposure, 568nm and UV 350nm were used to excite fluorophores binding to full length SNAP-25 and nuclei. Images were analyzed within the Opera environment using Acapella scripts. The algorithm was used to identify objects such as nuclei based on Hoechst dye and cytoplasm based on expression of GFP inside the ES-derived motoneurons. The intensity and cellular localization of total SNAP-25 and full length SNAP-25 were determined by Alexa 568 or Alexa 633 fluorescence. For each condition, images from 6 fields/well were acquired with the script calculating the integrated fluorescence intensities in whole cell regions. The Li-Cor imaging assay was performed using a Li-Cor odyssey infrared imaging system (Li-Cor, Lincoln, NE), as described previously [47].
Supplementary Material
Cells such as those in Fig. 1B were fixed and immunostained for the neuronal markers Tau (Tau5), β-III Tubulin specific Tuj1, ChAT and LIM3. Shown are also Hb9-eGFP and DAPI nuclear staining. The scale bar is 50 µm.
ES cell-derived motoneurons (Hb9-eGFP, green) were co-cultured as partially dissociated embryoid bodies with differentiated C2C12 mouse myoblasts (American Type Culture Collection) and postsynaptic acetylcholine receptor clustering was detected by alpha-bungarotoxin (red) labeling, as previously described[41, 54]. The nuclei in all images were labeled with DAPI (blue). A–C, obtained using fluorescent microscopy at different magnifications. The scale bars: A, 240 µm; B, 60 µm; 40 µm.
Acknowledgements
We are indebted to Dr. Thomas M. Jessell and Dr. Hynek Wichterle (Columbia University, New York, NY) for the generous gift of HBG3 mouse ES cell line. Also, we are grateful to Eileen Southon for kindly providing mouse embryonic fibroblast cells and helping with stem cell culture, Jodi Becker for providing assistance with microscopy and mouse spinal cord dissections, and Lyal Tressler for providing assistance with Li-Cor studies. Additionally, we thank the members of the Tessarollo, Gussio and Bavari labs for their insightful discussion and generosity. This work was supported by Defense Threat Reduction Agency (CBS.MEDBIO_01_10_RD_014). Furthermore, for JCB, this project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E.
The abbreviations used are
- ES
embryonic stem
- RA
retinoic acid
- Shh
sonic hedgehog
- EBs
embryoid bodies
- WT
wild type
- FACS
fluorescence activated cell sorter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The views, findings, interpretations, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Department of Health and Human Services or the U.S. Army.
Disclosure statement
The authors indicate no potential conflicts of interest.
References
- 1.Neale EA, Bowers LM, Jia M, Bateman KE, Williamson LC. Botulinum neurotoxin A blocks synaptic vesicle exocytosis but not endocytosis at the nerve terminal. The Journal of cell biology. 1999;147:1249–1260. doi: 10.1083/jcb.147.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossetto O, Morbiato L, Caccin P, Rigoni M, Montecucco C. Presynaptic enzymatic neurotoxins. Journal of neurochemistry. 2006;97:1534–1545. doi: 10.1111/j.1471-4159.2006.03965.x. [DOI] [PubMed] [Google Scholar]
- 3.Montecucco C, Molgo J. Botulinal neurotoxins: revival of an old killer. Current opinion in pharmacology. 2005;5:274–279. doi: 10.1016/j.coph.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K. Botulinum toxin as a biological weapon: medical and public health management. Jama. 2001;285:1059–1070. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 5.Wein LM, Liu Y. Analyzing a bioterror attack on the food supply: the case of botulinum toxin in milk. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9984–9989. doi: 10.1073/pnas.0408526102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamanna C. The most poisonous poison. Science (New York, N.Y. 1959;130:763–772. doi: 10.1126/science.130.3378.763. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. [Accessed November 02, 2010];Specific Bioterrorism Agents. Available at http://www.bt.cdc.gov/agent/agentlist-category.asp.
- 8.Jankovic J. Botulinum toxin in clinical practice. Journal of neurology, neurosurgery, and psychiatry. 2004;75:951–957. doi: 10.1136/jnnp.2003.034702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolly JO, Lawrence GW, Meng J, Wang J, Ovsepian SV. Neuro-exocytosis: botulinum toxins as inhibitory probes and versatile therapeutics. Current opinion in pharmacology. 2009;9:326–335. doi: 10.1016/j.coph.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Brin MF. Development of future indications for BOTOX. Toxicon. 2009;54:668–674. doi: 10.1016/j.toxicon.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 11.American Society for Aesthetic Plastic Surgery. [Accessed November 02, 2010];Cosmetic Surgery National Data Bank Statistics. doi: 10.1093/asj/sjz164. Available at http://www.surgery.org/sites/default/files/2008stats.pdf. [DOI] [PubMed]
- 12.Chen S, Barbieri JT. Engineering botulinum neurotoxin to extend therapeutic intervention. Proceedings of the National Academy of Sciences of the United States of America. 2009 doi: 10.1073/pnas.0903111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Meng J, Lawrence GW, Zurawski TH, Sasse A, Bodeker MO, Gilmore MA, Fernandez-Salas E, Francis J, Steward LE, Aoki KR, Dolly JO. Novel chimeras of botulinum neurotoxins A and E unveil contributions from the binding, translocation, and protease domains to their functional characteristics. The Journal of biological chemistry. 2008;283:16993–17002. doi: 10.1074/jbc.M710442200. [DOI] [PubMed] [Google Scholar]
- 14.Foster KA, Adams EJ, Durose L, Cruttwell CJ, Marks E, Shone CC, Chaddock JA, Cox CL, Heaton C, Sutton JM, Wayne J, Alexander FC, Rogers DF. Re-engineering the target specificity of Clostridial neurotoxins - a route to novel therapeutics. Neurotoxicity research. 2006;9:101–107. doi: 10.1007/BF03354881. [DOI] [PubMed] [Google Scholar]
- 15.Infant Botulism Treatment and Prevention Program. [Accessed November 02, 2010];Division of Communicable Disease Control, California Department of Public Health. What is BabyBIG? Available at http://www.infantbotulism.org/.
- 16.Larsen JC. U.S. Army Botulinum Neurotoxin (BoNT) Medical Therapeutics Research Program: Past Accomplishments and Future Directions, Drug Development Research. 2009:266–278. [Google Scholar]
- 17.Burnett JC, Henchal EA, Schmaljohn AL, Bavari S. The evolving field of biodefence: therapeutic developments and diagnostics. Nature reviews. 2005;4:281–297. doi: 10.1038/nrd1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldwin MR, Barbieri JT. Association of botulinum neurotoxins with synaptic vesicle protein complexes. Toxicon. 2009;54:570–574. doi: 10.1016/j.toxicon.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossetto O, Schiavo G, Montecucco C, Poulain B, Deloye F, Lozzi L, Shone CC. SNARE motif and neurotoxins. Nature. 1994;372:415–416. doi: 10.1038/372415a0. [DOI] [PubMed] [Google Scholar]
- 20.Grumelli C, Verderio C, Pozzi D, Rossetto O, Montecucco C, Matteoli M. Internalization and mechanism of action of clostridial toxins in neurons. Neurotoxicology. 2005;26:761–767. doi: 10.1016/j.neuro.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Simpson LL. Identification of the major steps in botulinum toxin action. Annual review of pharmacology and toxicology. 2004;44:167–193. doi: 10.1146/annurev.pharmtox.44.101802.121554. [DOI] [PubMed] [Google Scholar]
- 22.Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER. SV2 is the protein receptor for botulinum neurotoxin A. Science (New York, N.Y. 2006;312:592–596. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- 23.Mahrhold S, Rummel A, Bigalke H, Davletov B, Binz T. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS letters. 2006;580:2011–2014. doi: 10.1016/j.febslet.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 24.Kalandakanond S, Coffield JA. Cleavage of SNAP-25 by botulinum toxin type A requires receptor-mediated endocytosis, pH-dependent translocation, and zinc. The Journal of pharmacology and experimental therapeutics. 2001;296:980–986. [PubMed] [Google Scholar]
- 25.Apland JP, Adler M, Oyler GA. Inhibition of neurotransmitter release by peptides that mimic the N-terminal domain of SNAP-25. J Protein Chem. 2003;22:147–153. doi: 10.1023/a:1023423013741. [DOI] [PubMed] [Google Scholar]
- 26.Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Sudhof TC, Niemann H, Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365:160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- 27.Apland JP, Biser JA, Adler M, Ferrer-Montiel AV, Montal M, Canaves JM, Filbert MG. Peptides that mimic the carboxy-terminal domain of SNAP-25 block acetylcholine release at an Aplysia synapse. J Appl Toxicol. 1999;19 Suppl 1:S23–S26. doi: 10.1002/(sici)1099-1263(199912)19:1+<s23::aid-jat609>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 28.Bajohrs M, Rickman C, Binz T, Davletov B. A molecular basis underlying differences in the toxicity of botulinum serotypes A and E. EMBO reports. 2004;5:1090–1095. doi: 10.1038/sj.embor.7400278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adler M, Keller JE, Sheridan RE, Deshpande SS. Persistence of botulinum neurotoxin A demonstrated by sequential administration of serotypes A and E in rat EDL muscle. Toxicon. 2001;39:233–243. doi: 10.1016/s0041-0101(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 30.Keller JE, Neale EA, Oyler G, Adler M. Persistence of botulinum neurotoxin action in cultured spinal cord cells. FEBS letters. 1999;456:137–142. doi: 10.1016/s0014-5793(99)00948-5. [DOI] [PubMed] [Google Scholar]
- 31.Fagan RP, McLaughlin JB, Middaugh JP. Persistence of botulinum toxin in patients' serum: Alaska, 1959–2007. J Infect Dis. 2009;199:1029–1031. doi: 10.1086/597310. [DOI] [PubMed] [Google Scholar]
- 32.Caleo M, Antonucci F, Restani L, Mazzocchio R. A reappraisal of the central effects of botulinum neurotoxin type A: by what mechanism? Journal of neurochemistry. 2009;109:15–24. doi: 10.1111/j.1471-4159.2009.05887.x. [DOI] [PubMed] [Google Scholar]
- 33.Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28:3689–3696. doi: 10.1523/JNEUROSCI.0375-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hakami RM, Ruthel G, Stahl AM, Bavari S. Gaining ground: assays for therapeutics against botulinum neurotoxin. Trends in microbiology. 2010;18:164–172. doi: 10.1016/j.tim.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Sheridan RE, Smith TJ, Adler M. Primary cell culture for evaluation of botulinum neurotoxin antagonists. Toxicon. 2005;45:377–382. doi: 10.1016/j.toxicon.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Coffield JA, Yan X. Neuritogenic Actions of Botulinum Neurotoxin A on Cultured Motor Neurons. The Journal of pharmacology and experimental therapeutics. 2009 doi: 10.1124/jpet.108.147744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stahl AM, Ruthel G, Torres-Melendez E, Kenny TA, Panchal RG, Bavari S. Primary cultures of embryonic chicken neurons for sensitive cell-based assay of botulinum neurotoxin: implications for therapeutic discovery. J Biomol Screen. 2007;12:370–377. doi: 10.1177/1087057106299163. [DOI] [PubMed] [Google Scholar]
- 38.Dong M, Tepp WH, Johnson EA, Chapman ER. Using fluorescent sensors to detect botulinum neurotoxin activity in vitro and in living cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14701–14706. doi: 10.1073/pnas.0404107101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellett S, Tepp WH, Clancy CM, Borodic GE, Johnson EA. A neuronal cell-based botulinum neurotoxin assay for highly sensitive and specific detection of neutralizing serum antibodies. FEBS letters. 2007;581:4803–4808. doi: 10.1016/j.febslet.2007.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keller JE, Cai F, Neale EA. Uptake of botulinum neurotoxin into cultured neurons. Biochemistry. 2004;43:526–532. doi: 10.1021/bi0356698. [DOI] [PubMed] [Google Scholar]
- 41.Miles GB, Yohn DC, Wichterle H, Jessell TM, Rafuse VF, Brownstone RM. Functional properties of motoneurons derived from mouse embryonic stem cells. J Neurosci. 2004;24:7848–7858. doi: 10.1523/JNEUROSCI.1972-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 43.Wichterle H, Peljto M, Nedelec S. Xenotransplantation of embryonic stem cell-derived motor neurons into the developing chick spinal cord. Methods in molecular biology (Clifton, N.J. 2009;482:171–183. doi: 10.1007/978-1-59745-060-7_11. [DOI] [PubMed] [Google Scholar]
- 44.Wichterle H, Peljto M. Differentiation of mouse embryonic stem cells to spinal motor neurons, Current protocols in stem cell biology, Chapter 1. 2008 doi: 10.1002/9780470151808.sc01h01s5. Unit 1H 1 1-1H 1 9. [DOI] [PubMed] [Google Scholar]
- 45.Keller JE, Neale EA. The role of the synaptic protein snap-25 in the potency of botulinum neurotoxin type A. The Journal of biological chemistry. 2001;276:13476–13482. doi: 10.1074/jbc.M010992200. [DOI] [PubMed] [Google Scholar]
- 46.Bakry N, Kamata Y, Simpson LL. Lectins from Triticum vulgaris and Limax flavus are universal antagonists of botulinum neurotoxin and tetanus toxin. The Journal of pharmacology and experimental therapeutics. 1991;258:830–836. [PubMed] [Google Scholar]
- 47.Nuss JE, Ruthel G, Tressler LE, Wanner LM, Torres-Melendez E, Hale ML, Bavari S. Development of cell-based assays to measure botulinum neurotoxin serotype A activity using cleavage-sensitive antibodies. J Biomol Screen. 2010;15:42–51. doi: 10.1177/1087057109354779. [DOI] [PubMed] [Google Scholar]
- 48.Simpson LL, Coffield JA, Bakry N. Inhibition of vacuolar adenosine triphosphatase antagonizes the effects of clostridial neurotoxins but not phospholipase A2 neurotoxins. The Journal of pharmacology and experimental therapeutics. 1994;269:256–262. [PubMed] [Google Scholar]
- 49.Savino PJ, Maus M. Botulinum toxin therapy. Neurologic clinics. 1991;9:205–224. [PubMed] [Google Scholar]
- 50.Panchal RG, Kota KP, Spurgers KB, Ruthel G, Tran JP, Boltz RC, Bavari S. Development of high-content imaging assays for lethal viral pathogens. J Biomol Screen. 2010;15:755–765. doi: 10.1177/1087057110374357. [DOI] [PubMed] [Google Scholar]
- 51.Wiese S, Herrmann T, Drepper C, Jablonka S, Funk N, Klausmeyer A, Rogers ML, Rush R, Sendtner M. Isolation and enrichment of embryonic mouse motoneurons from the lumbar spinal cord of individual mouse embryos. Nature protocols. 2009;5:31–38. doi: 10.1038/nprot.2009.193. [DOI] [PubMed] [Google Scholar]
- 52.Camu W, Henderson CE. Purification of embryonic rat motoneurons by panning on a monoclonal antibody to the low-affinity NGF receptor. Journal of neuroscience methods. 1992;44:59–70. doi: 10.1016/0165-0270(92)90114-s. [DOI] [PubMed] [Google Scholar]
- 53.Soundararajan P, Miles GB, Rubin LL, Brownstone RM, Rafuse VF. Motoneurons derived from embryonic stem cells express transcription factors and develop phenotypes characteristic of medial motor column neurons. J Neurosci. 2006;26:3256–3268. doi: 10.1523/JNEUROSCI.5537-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harper JM, Krishnan C, Darman JS, Deshpande DM, Peck S, Shats I, Backovic S, Rothstein JD, Kerr DA. Axonal growth of embryonic stem cell-derived motoneurons in vitro and in motoneuron-injured adult rats. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7123–7128. doi: 10.1073/pnas.0401103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moscatelli I, Pierantozzi E, Camaioni A, Siracusa G, Campagnolo L. p75 neurotrophin receptor is involved in proliferation of undifferentiated mouse embryonic stem cells. Experimental cell research. 2009;315:3220–3232. doi: 10.1016/j.yexcr.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 56.Domeniconi M, Hempstead BL, Chao MV. Pro-NGF secreted by astrocytes promotes motor neuron cell death. Molecular and cellular neurosciences. 2007;34:271–279. doi: 10.1016/j.mcn.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pitts EV, Potluri S, Hess DM, Balice-Gordon RJ. Neurotrophin and Trk-mediated signaling in the neuromuscular system. International anesthesiology clinics. 2006;44:21–76. doi: 10.1097/00004311-200604420-00004. [DOI] [PubMed] [Google Scholar]
- 58.Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW, Zhang SC. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem cells (Dayton, Ohio) 2008;26:886–893. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, Dahrouge S, Antel JP. Neuroblastoma × spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn. 1992;194:209–221. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cells such as those in Fig. 1B were fixed and immunostained for the neuronal markers Tau (Tau5), β-III Tubulin specific Tuj1, ChAT and LIM3. Shown are also Hb9-eGFP and DAPI nuclear staining. The scale bar is 50 µm.
ES cell-derived motoneurons (Hb9-eGFP, green) were co-cultured as partially dissociated embryoid bodies with differentiated C2C12 mouse myoblasts (American Type Culture Collection) and postsynaptic acetylcholine receptor clustering was detected by alpha-bungarotoxin (red) labeling, as previously described[41, 54]. The nuclei in all images were labeled with DAPI (blue). A–C, obtained using fluorescent microscopy at different magnifications. The scale bars: A, 240 µm; B, 60 µm; 40 µm.


