1. Introduction
Stressful events or changes in hypothalamic-pituitary-adrenal (HPA) axis function are associated with and precipitate the onset of many psychiatric disorders, including schizophrenia, bipolar disorder, depression and post traumatic stress disorder (PTSD) [1–5]. The HPA axis facilitates adaptation to disruptions in an animal’s internal or external homeostatic environment. The adrenal glucocorticoid (GC) hormones are the principal mediators of the HPA axis. Secretion of GCs is under the stimulatory drive of the neurons in the paraventricular nucleus of the hypothalamus (PVN) that secrete corticotropin releasing hormone (CRH) into the hypophysial circulation and stimulate pituitary release of adrenocorticotrophic hormone (ACTH) leading to the release of GCs from the adrenal gland [6]. Glucocorticoids regulate further CRH and ACTH release via negative feedback loops by binding receptors in the pituitary, PVN, hippocampus (HPC) and prefrontal cortex (PFC) to shut down the stress response in order to return to the homeostatic set point [6]. Corticosterone (CORT), the primary GC in the rat, is the ligand for the glucocorticoid receptor (GR) and mineralocorticoid receptor (MR), steroid hormone receptors that function as transcription factors and regulate neuronal gene transcription and metabolism. GR and MR have different affinities for CORT in the brain. MRs are saturated with circadian levels of CORT and thus mediate daily changes in HPA axis activity, while GRs are saturated with stress levels of CORT and govern stress induced changes in HPA axis function [6,7].
Alterations in GC secretion, as well as GR and MR function have implications not only for stress sensitivity but also for behaviors relevant to neuropsychiatric disorders [8–10]. In fact, shifts in the MR/GR balance, particularly in the HPC, can create a vulnerability to psychiatric disease, especially among genetically predisposed individuals [6,11,12]. The specific susceptibility genes that mediate the interaction with stress in psychiatric populations have not been clearly identified. One candidate may be the neuregulin 1 (NRG1) gene, which has repeatedly been identified by association studies as a susceptibility gene for schizophrenia and bipolar disorder [13–16]. The region of NRG1 that contains many risk haplotype associations with schizophrenia includes the exons that encode Type IV and Type II neuregulin 1 (NRG1) protein, along with additional 5’ non-coding regions [13–15].
In humans, the NRG1 gene is highly complex and can be alternatively spliced into six types (I–VI) of proteins, based on N-terminal structural and functional differences [17,18]. Similar splicing appears to exist in the rat, although there is controversy about the production of some splice variants [19,20]. Types IV and II NRG1 arise from sequences in the 5’ region of the gene, while Types I, III, V and VI are encoded by more downstream 3’ sequences [15,21]. All types of NRG1 signal via an epidermal growth factor (EGF)-like domain that activates ErbB receptor tyrosine kinases, preferentially ErbB4 [22]. NRG1–ErbB4 signaling plays many roles in neural development, including radial neuron migration, axon guidance, myelination, oligodendrocyte development and synapse formation [17,22]. In addition, NRG1 is important for adult neural function, including the regulation of the serotonergic system, NMDA, GABAA and α7 nicotinic receptors, modulation of long-term potentiation, transcriptional regulation and hormonal control of puberty [15,17,23,24]. In the rat, the neurons of the PVN show strong expression of Nrg1 mRNA [25], and Erbb4 mRNA is expressed in neurons [26] and astroglia [27] of the hypothalamus. Additionally, ErbB4 protein is expressed in the medial basal hypothalamus and preoptic area [24]. However, there is virtually no information on the role of NRG1 in HPA axis function, and whether the protein is expressed in the PVN is unknown. Additional evidence to support the need to examine NRG1 in stress regulation comes from reports of gene by environment interactions. In patients with schizophrenia, a single nucleotide polymorphism in the 5’ region of NRG1 interacts with psychosocial stress to affect reactivity to expressed emotion [28]. NRG1 genotype also interacts with job strain to increase risk of heart disease [29]. These studies suggest that NRG1 genotype may create a vulnerability to environmental stimuli.
The present studies utilize a line of Nrg1 mutant rats (Nrg1Tn), generated with the Sleeping Beauty transposon [30]. The transposon inserted into the first intron, which lies between the coding sequences for Type IV and Type II NRG1 protein splice variants. This region corresponds to the 5’ region of the NRG1 gene that is most frequently associated with schizophrenia [13,15,22,31]. Therefore, this novel rat preparation differs from existing mouse models of disrupted Nrg1 expression, which have targeted the 3’ region of the gene. The major goal of the present studies was to establish the utility of Nrg1Tn animals as a model of disrupted Type II NRG1 expression and to test the hypothesis that altering Type II NRG1 expression disrupts stress regulation using neuroendocrine and behavioral measures.
2. Materials and Methods
2.1. Animals
The novel rat preparation utilized by our laboratory was developed and obtained from the PhysGen Program in Genomic Applications (http://pga.mcw.edu/) at the Medical College of Wisconsin. The disruption of the NRG1 gene was created using the Sleeping Beauty transposon system on a Fischer 344 inbred strain background (F344-Nrg1Tn(sb-T2/Bart3)2.183Mcwi) [30]. The transposon randomly inserted into the first intron, which lies between the coding sequences for Type IV and Type II NRG1 protein splice variants (see http://www.knockoutrat.com/ and http://rgd.mcw.edu/tools/strains/strains_view.cgi?id=2290103). All animals were housed at the Maryland Psychiatric Research Center in a light-controlled (lights on 06.00 hr to 20.00 hr) and temperature-controlled facility, with free access to rat chow (Harlan Teklad, Frederick, MD) and water. All procedures conform to guidelines for animal research established by the National Institutes of Health, and were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee.
Genotyping of rats was performed on DNA obtained from tail tip biopsies using polymerase chain reaction (PCR) amplification. Tail biopsies were digested with 100 µg/ml proteinase K in lysis buffer (1.21g Tris Base, 2.5 ml 0.2M EDTA, 4 ml 5M NaCl, 1 ml 100mM CaCl2, 2 ml 10%SDS, distilled H20 to 100 mls) at 56°C overnight. Genomic DNAs were precipitated with isopropanol and washed with 70% ethanol, dried and dissolved in sterile water. Genomic DNA concentrations were measured using the SmartSpec3000 spectrophotometer (Bio-Rad, Hercules, CA). Nrg1 genotype was determined using three primer PCR. Two primers flank the mapped insertion site (Nrg1: 5’- agg aac caa aaa agt aga ctc agt gtg -3’; 3’- gtg agc ttt tct gta agg tgg taa ctt -5’) and a third primer is transposon specific (ctg acc taa gac agg gaa tt). This method was used to determine the presence and zygosity of the transposon insertion. The PCR was performed in a PCR Sprint machine (Thermo Fisher Scientific, Waltham, MA) at 95°C, 10 min for initial PCR activation step, followed by 94°C 30 sec, 60°C 30 sec, 72°C 40 sec, for 25 cycles followed by 72°C 7 min and 15°C holding temperature. Amplified products were separated and visualized by agarose gel electrophoresis. Wild type (WT) rats and rats homozygous for the transposon insertion (Nrg1Tn) were used in the present studies.
2.2. Reverse Transcription PCR
The insertion of the transposon disrupts gene products by interfering with normal splicing patterns. To confirm the disruption of Nrg1 mRNA, reverse transcription PCR (RT-PCR) was utilized. Thirty micrograms of tissue from the cerebral cortex of Nrg1Tn and WT (n=4–5 per group) rats was used to isolate mRNA. Total RNA was extracted with the RNeasy Mini Kit (Qiagen, Valencia, CA). RT-PCR was carried out with Qiagen One-Step RT-PCR kit (Qiagen, Valencia, CA) and contained 75 ng of template RNA, 5× buffer, 10 mM dNTPs, enzyme mix and 0.6 µM primers [Nrg1 forward 5′-aggactcgccaccttcaca -3′, reverse 5′-gctcgttcccattcttgaac -3′, GAPDH forward 5′-ggatttggccgtatcgg-3′, reverse 5′-tcgtggttcacacccatca-3′] for a final volume of 50 µl. The RT-PCR was performed in a PCR Sprint machine (Thermo Fisher Scientific, Waltham, MA) at 50°C, 30 min for reverse transcription, 95°C, 15 min for initial PCR activation step, followed by 94°C 30 sec, 60°C 30 sec, 72°C 1 min, for 30 cycles. Amplified products were separated and visualized by agarose gel electrophoresis.
2.3. Western Blotting
Western blots were performed as previously described [32] with the following modifications. Prefrontal cortex samples from adult WT (n=6) and Nrg1Tn (n=6) rats were used to compare expression levels of Type II NRG1 between genotypes. In a separate group of adult male WT animals, PVN (n=5), HPC (n=5) and PFC (n=5) were extracted to determine localization of Type II NRG1. Pituitary glands (n=11–13 per genotype) were extracted from the skull of adult male WT and Nrg1Tn rats at the basal and peak time points of the acute stress experiment (section 2.5) to examine GR expression. Dorsal HPC (n=5–7 per group) and PVN (n=4 per group) were extracted from brains of adult male WT and Nrg1Tn rats euthanized at the basal time point of the acute stress experiment (section 2.5) to examine GR and MR expression.
Brain regions were identified using the Paxinos and Watson brain atlas [33]. Brain tissue samples from the PFC, HPC and PVN were isolated from 1mm frozen coronal sections using a 1mm rat brain punch (Stoelting, Wood Dale, IL). Bilateral punches were used for each area with a total of 4 punches for the PFC, 6 punches for dorsal HPC, and 2 punches for the PVN. Due to the irregular shape of the PVN, some tissue adjacent to the PVN was likely included in these punches. Tissue samples were homogenized via sonication in lysis buffer with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and protein concentrations were determined using a protein assay kit (Bio-Rad, Hercules, CA). Brain tissue extracts were diluted in Laemmli sample buffer (Bio-Rad, Hercules, CA) to a concentration of 30µg of protein per lane and resolved by electrophoresis on a 10% polyacrylamide gel (Bio-Rad, Hercules, CA). Proteins were electrotransferred from the gel onto PVDF membranes (Bio-Rad, Hercules, CA). Membranes were blocked with 1× Tris-buffered saline with 0.5% Tween-20 (TBS-T) and 3 % bovine serum albumin (BSA) for 1 h and then incubated with the appropriate primary antibody for 24 or 48 h at 4°C: Type II NRG1 (1:1000; AF2015, R&D systems, Minneapolis, MN); GR (BuGR2, 1:1000; ab2768, Abcam, Cambridge, MA); MR (1:1000; sc-11412, Santa Cruz Biotechnology, Santa Cruz, CA). After washing with TBS-T and 3% BSA, membranes were incubated with the appropriate horseradish peroxidase-labeled secondary antibody for 1 h at room temperature: goat anti-rabbit (1:2500; AP307P), goat anti-mouse (1:5000; AP124P) and rabbit anti-goat (1:2500; AP106P) (Millipore, Temecula, CA). Membranes were then washed with TBS-T and the protein detected using Pierce ECL Western blotting substrate (Thermo Fisher Scientific, Waltham, MA). Blots were developed by exposure to chemiluminescent film (Amersham, GE Healthcare, Buckinghamshire, UK). Densitometric measurements of the protein bands were made using AIS 6.0 software (Imaging Research Inc., St. Catharines, ON, Canada). Following image collection, membranes were stripped using Restore plus Western blot stripping buffer (Thermo Fisher Scientific, Waltham, MA) and reprobed for MR (HPC only) and actin (1:10,000; MAB1501, Millipore, Temecula, CA). Protein expression levels were normalized to actin expression. One Nrg1Tn pituitary sample was identified as a statistical outlier using the Grubbs test; therefore this animal was dropped from the study.
2.4. Maternal Behavior
Maternal behavior was measured in WT and Nrg1Tn dams. Arched-back nursing (n=10–15 per group) was measured using a modified version of periodic spot check observation [34]. Observations were recorded over a five day period during the first week of postnatal life. Spot checks occurred three times a day, between 10.00 and 11.00h, 13.00 and 14.00h, and 16.00 and 17.00h. Behavior was recorded randomly during each of the three time intervals. Average percent of observations spent arched-back nursing was calculated and compared between genotypes. To examine pup retrieval, home cages were moved from the housing rack to a cart within the vivarium. Pup retrieval (n=10 per group) was only measured if this mild disturbance elicited the dam to disperse her nursing pups throughout the cage. After the pups detached, latency to retrieve the first pup, resulting in its return to the original or a new nest, was recorded. Latencies were recorded in bins, ≤30s, 31–60s, 61–90s, 91–120s. The upper limit of each bin that the observation fell into was scored for an estimated average latency. This method was utilized to minimize disruption of normal maternal care. Nest building (n=9–14 per group) was measured on the fourth day of observation. Additional bedding was added to the front of each home cage and the time to incorporate the bedding into the nest was scored. A score of 3 was given if the dam began moving the bedding into the nest within 5 minutes, a score of 2 was given if the dam began moving the bedding within 15 minutes and a score of 1 was given if the bedding was moved into the nest after 4 hours. All dams observed moved the bedding to their nest within 4 hours.
2.5. Acute Restraint Stress
Adult (76–79 days of age) and adolescent (43–45 days of age) male WT (n=6–12 per group/per time point) and Nrg1Tn (n=6–11 per group/per time point) rats were moved from the vivarium to a temperature and light controlled behavioral testing room for acclimation. Animals were handled for 2 days for 1–2 minutes. For the next 5 days, animals were handled with light restraint in a towel for 1 minute each day. On the eighth day, beginning at approximately 08.30 h, animals were again lightly restrained in a towel and tail clipping was used for blood collection as outlined by Vahl and colleagues [35]. Animals were then immediately placed in cylindrical plastic restraint tubes for 30 minutes according to our previously published protocol [36]. At the end of the acute restraint, blood was again collected. Animals were then returned to their home cage for 120 minutes, at which point a final blood sample was collected. After the conclusion of blood collection, animals were returned to their home cages. Serum was isolated by centrifugation and stored at −80°C.
Two and five days later, the same animals were again handled for 1 minute. One week after the first acute restraint stress, animals were exposed to a second acute restraint stress. Animals were randomly divided into groups and each group was euthanized by decapitation at a different time point: before (basal time point, n=18–19/genotype), at the end of (peak time point, n=12–14/genotype) or 120 minutes after (recovery time point, n=16–17/genotype) exposure to a 30 minute restraint stress session. Trunk blood was collected and plasma was isolated by centrifugation. Brains and pituitary glands were rapidly removed from the skull and frozen on powdered dry ice. Both brains and plasma were stored at −80°C until use. Serum and plasma levels of CORT were determined by radioimmunoassay according to protocols provided by the manufacturer (MP Biomedicals, Orangeburg, NY). Samples that fell below the limits of the standard curve were excluded (i.e. less than 5 ng/ml), resulting in the removal of 2 WT and 1 Nrg1Tn tail clip samples in addition to 2 WT and 3 Nrg1Tn trunk samples.
2.6. Open Field Testing and Habituation
For five days prior to testing, adult male WT and Nrg1Tn rats (n=13 per group) were handled for 1–2 minutes each day. On the morning of the third day, animals were moved from the vivarium to the behavioral testing room and allowed to acclimate. Animals were habituated to the open field arena for 10 minutes each, on 2 consecutive days. The arena was a 62 × 62 × 46 cm box with black Plexiglas walls. The floor of the arena was covered with unscented cat litter to allow for easy removal of urine and feces between each animal. Locomotor activity was recorded and analyzed using an ANY-maze video tracking system (version 4.6, Stoelting, Wood Dale, IL).
2.7. Statistical Methods
Statistical analyses were conducted using SPSS statistical software (version 12.0). To analyze CORT data obtained by RIA, multivariate analysis of variance (ANOVA) was used. CORT data from adult and juvenile animals were combined, as age was not found by ANOVA to interact with any other factor. Unpaired t-tests were used to compare maternal behaviors and mean protein expression in Western blots. In the PVN, Welch’s correction was used with an unpaired t-test to account for differences in variance in GR expression. Pituitary samples of animals euthanized at the basal and peak time points were combined for analysis because GR expression was not significantly different between the two time points. For open field habituation, repeated measures ANOVAs were conducted, with trial included as a repeated measures factor. Post hoc tests, conducted using l-matrix contrast statements in SPSS, were used to follow up any significant main effects or interactions.
3. Results
3.1. Nrg1Tn Rat Preparation
The F344-Nrg1Tn(sb-T2/Bart3)2.183Mcwi (Nrg1Tn) strain was identified as previously described [30] as an insertion of the BART3 gene-trap transposon vector in intron 1 of Nrg1 (Fig. 1A). Presence of the insertion was confirmed by sequencing and intercross offspring were genotyped using three primer PCR to distinguish homozygous from heterozygous carriers (see section 2.1). Splicing of the Nrg1 gene is highly complex and produces several isoforms [37], most of which are not yet defined in the rat (see GenBank accessions AF194993-AF194997 for partial expressed sequence tags encoding presumptive rat Type II NRG1 isoforms). The insertion is predicted to intercept splicing from exon 1 and therefore disrupt Type II NRG1 isoforms in tissues where they are expressed. It should be noted that it is not currently known whether Type IV [19,21], identified in human, is transcriptionally conserved in the rat or whether the gene trap insertion would disrupt this isoform.
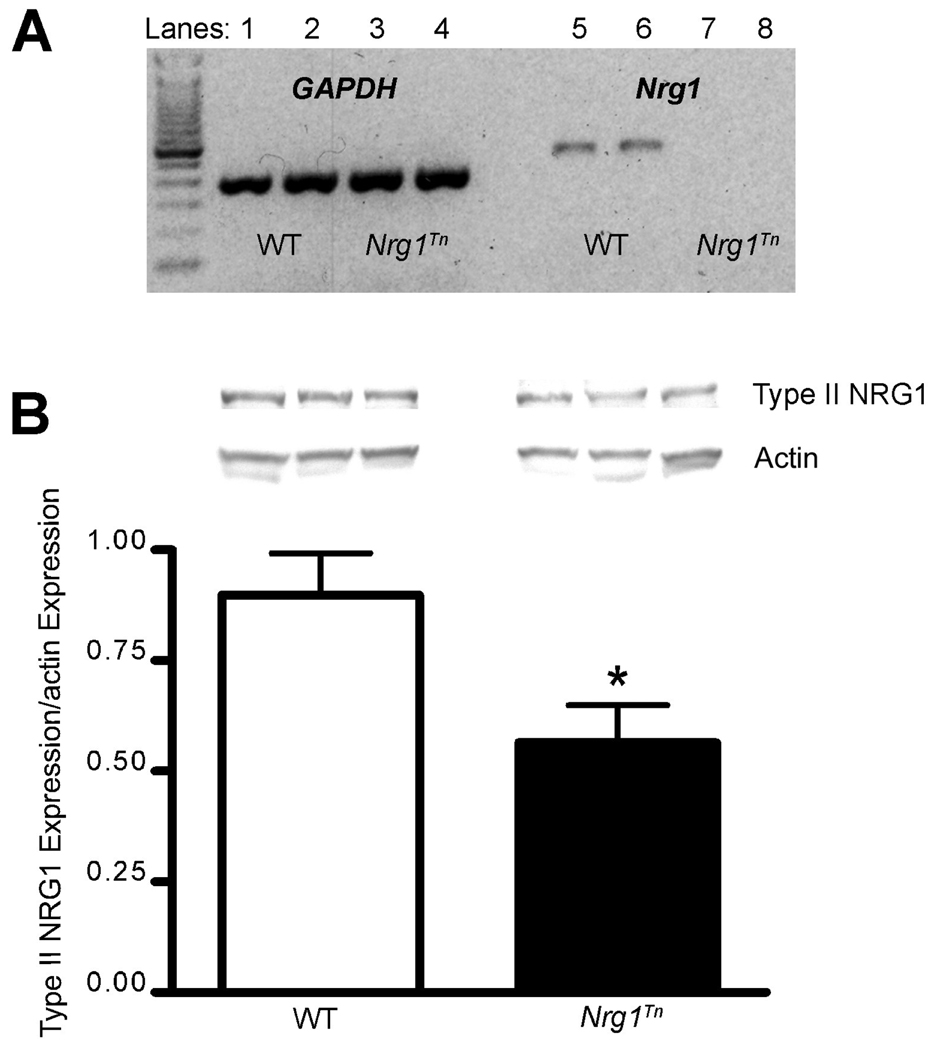
Figure 1.
Expression of Nrg1 mRNA and Type II NRG1 Protein. (A) Representative gel containing RT-PCR products from WT and homozygous Nrg1Tn rats. Lanes 1–2: WT GAPDH, lanes 3–4: Nrg1Tn GAPDH, lanes 5–6: WT exons 1–2 Nrg1 mRNA, and lanes 7–8: Nrg1Tn exons 1–2 Nrg1 mRNA. Expression of the first two exons of Nrg1 in the Nrg1Tn rats was greatly reduced (the original gel showed faint bands that could not be captured in an image), while expression of GAPDH mRNA was unaffected by the insertion of the transposon. (B) Upper panel shows a representative Western blot of Type II NRG1 and the re-blot for actin in WT and Nrg1Tn rats. Image analysis revealed a significant reduction in Type II NRG1 protein in Nrg1Tn rats (*p<0.03). Bars represent mean ± SEM.
3.1.1. Expression of Nrg1 mRNA and Type II NRG1 Protein
Expression of Nrg1 mRNA in the Nrg1Tn rats was determined with RT-PCR, while protein expression of the Type II NRG1 splice variant was assessed by Western blotting. The RT-PCR utilized primers for Nrg1 that corresponded to exons 1 and 2, which encode the Type IV and Type II NRG1 protein splice variants, respectively. Primers for GAPDH, the housekeeping gene, were included as a positive control. RT-PCR revealed that the expression of the first two exons of Nrg1 in the Nrg1Tn rats was greatly reduced, while expression of GAPDH mRNA was unaffected by the insertion of the transposon (Fig. 1A). In addition, expression of Type II NRG1 in tissue samples obtained from the PFC shows that Nrg1Tn rats have reduced expression of Type II NRG1 protein compared to WT rats (p<0.03; Fig. 1B). The Universal Protein Knowledge Base (UniProtKB) was used to identify Type II NRG1 as isoform 9 of NRG1 in humans (http://www.uniprot.org/uniprot/Q02297), which has 79% sequence similarity with the rat Type II NRG1. The predicted molecular weight of this isoform is approximately 86 kDa (http://www.uniprot.org/uniprot/Q9ESA5). The band shown is the most reactive band with a molecular weight of approximately 95 kDa. The reduction but not elimination of immunoreactive NRG1 confirms that the Nrg1Tn rats are hypomorphic for Type II NRG1.
3.1.2. Maternal Behavior
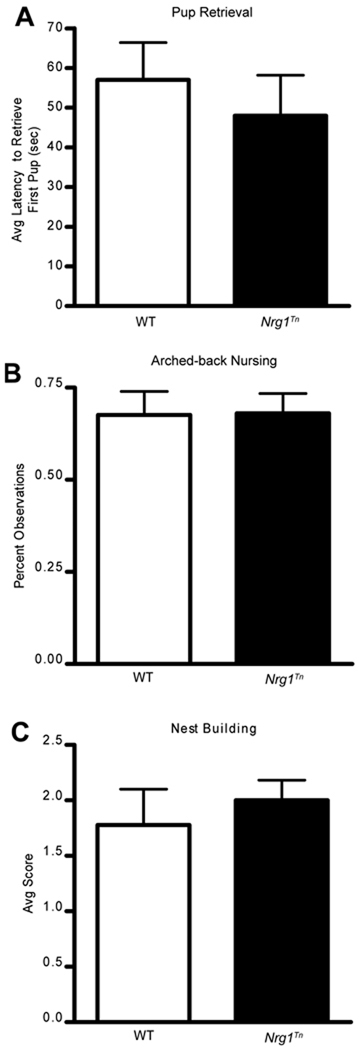
No differences in the examined maternal behaviors were observed. Estimated latency to retrieve the first pup did not differ between WT and Nrg1Tn rats (p=0.5; Fig. 2A). Likewise, percent of observations during which dams were arched-back nursing was not significantly different between the two genotypes (p=0.96; Fig. 2B). Finally, no differences in average scores based on time to incorporate new bedding into nests were detected (p=0.5; Fig. 2C).
Figure 2.
Maternal Behavior. No differences were observed between WT and Nrg1Tn maternal behaviors. (A) Average latency to retrieve first pup did not differ between WT and Nrg1Tn rats (p=0.5). (B) Likewise, percent of observations during which arched-back nursing was observed was not significantly different between the two genotypes (p=0.96). (C) Average scores based on time to incorporate new bedding into nests were not different (p=0.5). Bars represent mean ± SEM.
3.2. Stress Reactivity
3.2.1. NRG1 in the PVN
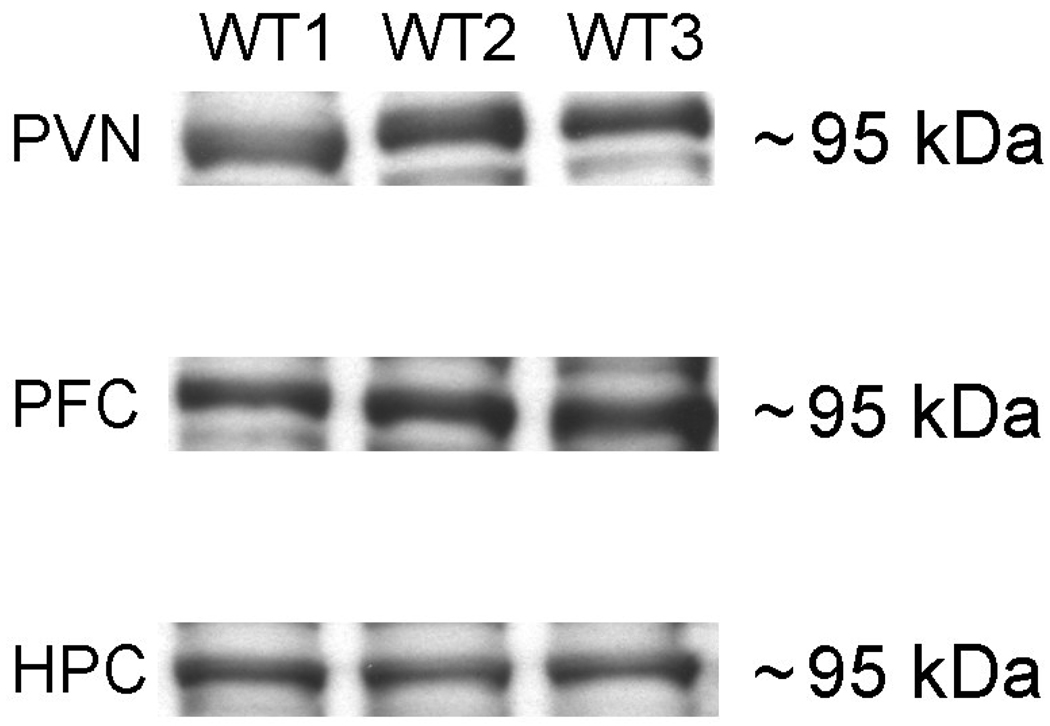
Figure 3 depicts a representative Western blot establishing that protein for the Type II splice variant of NRG1 is expressed in the PVN, HPC and PFC of adult WT animals. Thus Type II NRG1 is expressed in sites that are the primary modulators of the HPA axis in the brain. In the case of the PVN, where surrounding tissue may have contributed to the findings of Type II NRG1 expression, it should be noted that Chen and colleagues [25] found the mRNA signal for Nrg1 to be specific to the PVN, with negligible expression in the surrounding tissue.
Figure 3.
Type II NRG1 protein expression in stress related brain regions. The representative Western blot shows the expression of Type II NRG1 in the PVN (top panel), PFC (middle panel) and HPC (bottom panel) of WT rats.
3.2.2. Acute Restraint Stress
In order to determine if tail clip was a viable method for blood collection in Fischer 344 WT and Nrg1Tn rats, a 2×3×2 ANOVA (Genotype (WT vs. Nrg1Tn) × Time (basal vs. peak vs. recovery) × Method (tail clip vs. trunk blood) was run. This analysis revealed that method significantly interacted with time. Post-hoc analysis revealed a significant difference between methods only at the recovery time point. These analyses showed the tail clip samples had significantly higher CORT levels than trunk blood samples at the recovery time point, indicating that the stress of repeated blood collection may have led to prolonged CORT secretion. In addition, these findings could reflect that Fischer rats may be more sensitive to the additional stressor intensity induced by the tail clip procedure (data not shown). As a consequence, all subsequent analyses were conducted with trunk blood samples from the second acute restraint stress.
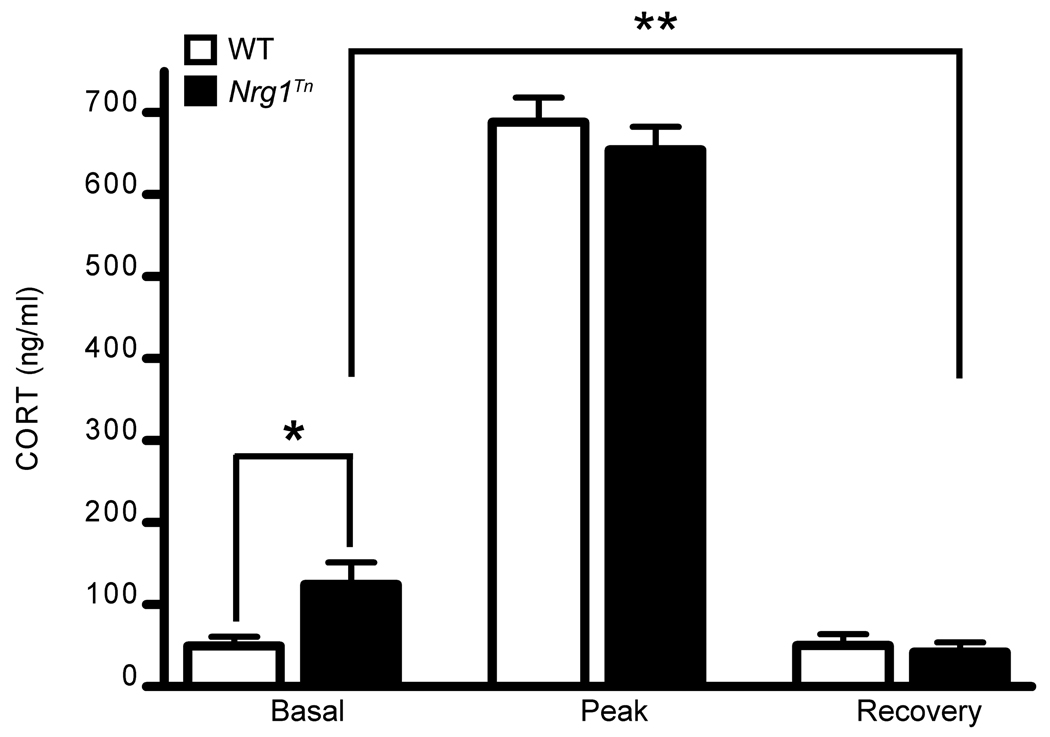
A 2×3 (Genotype (WT vs. Nrg1Tn) × Time (basal vs. peak vs. recovery)) ANOVA revealed a significant effect of time (F2,85=532.312, p<0.001; Fig. 4) on CORT levels obtained from trunk blood samples after a 30 minute restraint stress. Regardless of genotype, all animals mounted a stress response and recovery, with peak CORT levels being significantly greater than both basal (p<0.001) and recovery (p<0.001) CORT levels (Fig. 4). In addition, this effect interacted with genotype (F2,85=3.78, p<0.03; Fig. 4). Post-hoc analyses revealed that Nrg1Tn rats had significantly increased basal CORT concentrations compared to WT rats (p<0.02; Fig. 4) whereas there was no difference between the two genotypes at subsequent time points (Fig. 4). However, at the recovery time point, 120 minutes after the termination of the restraint stress, Nrg1Tn rats showed lower recovery CORT levels relative to their own basal levels (p<0.01), while recovery levels were indistinguishable from basal levels among WT animals (Fig. 4).
Figure 4.
Acute Restraint Stress. Basal, peak and recovery corticosterone (CORT) levels in adult male WT and Nrg1Tn rats following a 30 minute restraint stress. Both genotypes mounted a CORT response to the stress with peak CORT levels being significantly greater than basal (p<0.001) and recovery (p<0.001) CORT levels. Nrg1Tn rats exhibited significantly greater basal CORT concentrations than WT rats (*p<0.02) whereas there was no difference between the two genotypes at subsequent time points. In addition, Nrg1Tn rats showed enhanced suppression of recovery CORT levels relative to their basal levels (**p<0.01) which was not present in WT rats. Bars represent mean ± SEM.
3.2.3. Glucocorticoid and Mineralocorticoid Receptor
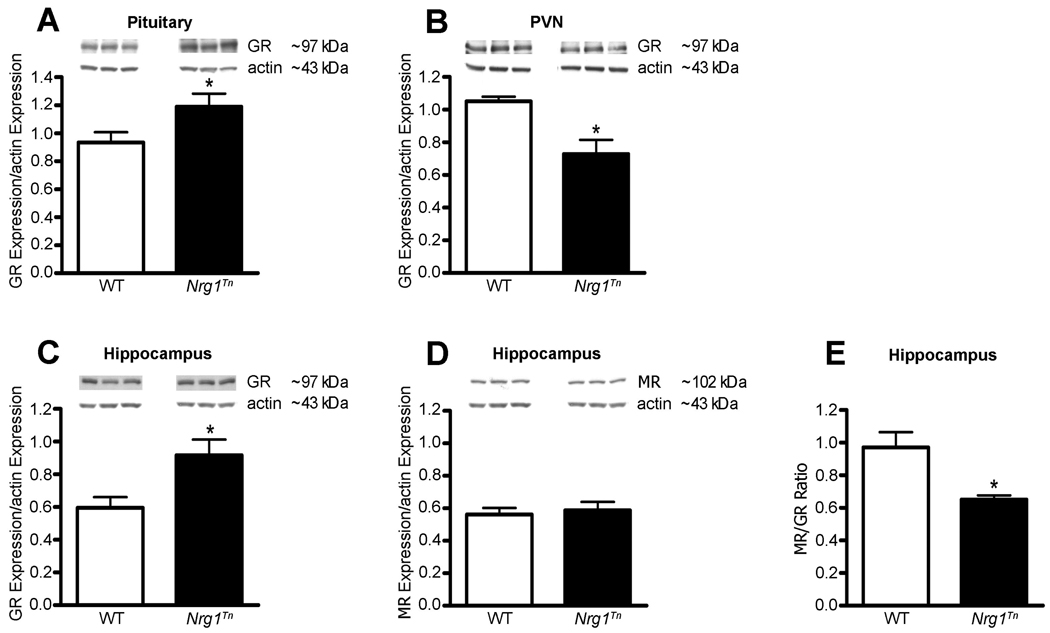
With changes in CORT secretory dynamics during exposure to acute stress, it became relevant to assess whether GR expression was altered in the Nrg1Tn rats. In the pituitary, adult male Nrg1Tn rats showed increased expression of GR compared to WT rats (p<0.05; Fig. 5A). Conversely, adult male Nrg1Tn rats showed decreased expression of GR in the PVN (p<0.05; Fig.5B). The highest concentration of MR in the brain is the HPC, therefore both GR and MR were examined in this structure [6]. In the HPC, GR expression was also significantly increased in Nrg1Tn rats compared to WT rats (p<0.02; Fig. 5C) whereas no difference was found in MR expression between Nrg1Tn and WT rats (Fig. 5D). Consequently, the MR/GR ratio was found to be significantly decreased in the HPC of Nrg1Tn rats (p<0.03; Fig. 5E).
Figure 5.
Glucocorticoid and Mineralocorticoid receptor expression. Upper panels show representative Western blots of GR or MR and actin in WT and Nrg1Tn rats. (A) In the pituitary, adult male Nrg1Tn rats showed increased expression of GR compared to WT rats (*p<0.05). (B) In the PVN, GR expression was decreased in Nrg1Tn rats compared to WT rats (*p<0.05). (C) In the HPC, GR expression was also significantly increased in Nrg1Tn rats compared to WT rats (*p<0.02), (D) whereas no difference was found in MR expression between Nrg1Tn and WT rats. (E) Consequently, the MR/GR ratio was found to be significantly decreased in the HPC of Nrg1Tn rats (*p<0.03). Bars represent mean ± SEM.
3.2.4. Open Field Testing and Habituation
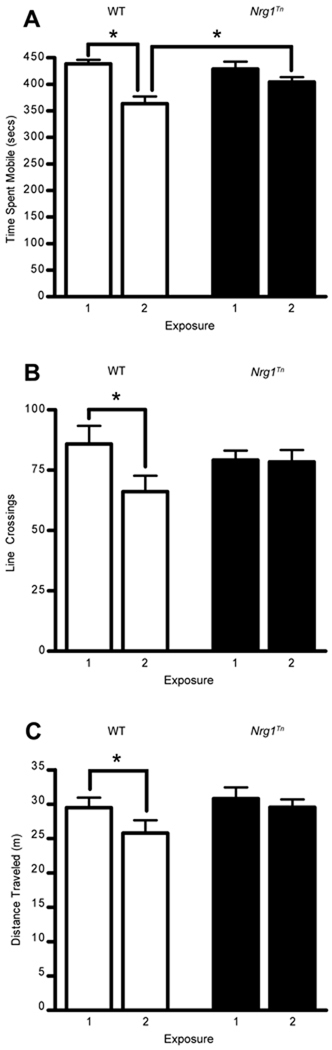
2×2 repeated measures ANOVAs (Genotype (WT vs. Nrg1Tn) × trial (exposure 1 vs. exposure 2)) were conducted on indices of locomotor activity during the open field testing, with trial included as the repeated measure. These analyses revealed that time spent mobile (F1,24=20.12, p<.001; Fig. 6A) and number of line crossings (F1,24=8.5, p<.001; Fig. 6B) both decreased with repeated exposure to the open field arena, however this effect interacted with genotype for both indices (time mobile, F1,24=5.2, p<.05; line crossings, F1,24=7.2, p<.05). Post hoc analyses revealed that the interaction was due to the fact that on the second day of exposure to the open field, WT rats spent significantly less time mobile (p<0.05; Fig. 6A) and made fewer line crossings (p<0.05; Fig. 6B) than they did during their first exposure to the open field. On the other hand, Nrg1Tn rats failed to show a reduction in activity during the second exposure to the open field (Fig. 6 A–C). This pattern resulted in Nrg1Tn rats spending significantly greater time mobile than WT rats on the second day of exposure (p<0.05; Fig. 6A). While there was not a significant interaction between exposure and genotype on distance traveled, planned comparisons within genotypes revealed that, similar to the pattern observed for line crossings, WT animals significantly decreased distance traveled on the second day of exposure (p<0.01; Fig. 6C) while Nrg1Tn animals did not.
Figure 6.
Open Field Testing and Habituation. (A) WT rats spent significantly less time mobile (*p<0.05), (B) made fewer line crossings (*p<0.05) and (C) significantly decreased distance traveled (**p<0.01) upon re-exposure to the open field on the second day of testing compared to their first exposure. However, Nrg1Tn rats failed to show a reduction in (A) time mobile, (B) line crossings and (C) distance traveled during the second exposure to the open field. This pattern resulted in Nrg1Tn rats spending significantly greater time mobile (A) than WT rats on the second day of testing (*p<0.05). Bars represent mean ± SEM.
4. Discussion
4.1. Summary of Findings
Insertion of the Sleeping Beauty transposon into the first intron of the Nrg1 gene reduced expression of both the mRNA and protein corresponding to the Type II NRG1 isoform. These findings establish the Nrg1Tn rat preparation as a functional hypomorph of Type II NRG1. Because we found NRG1 to be expressed in the neurocircuitry involved in regulating HPA axis responses to environmental stimuli, the Nrg1Tn rats were used to test the hypothesis that altered expression of Type II NRG1 disrupts stress regulation and reactivity. In support of this hypothesis, Nrg1Tn rats demonstrated increased basal CORT concentrations and lower CORT concentrations after recovery from a 30 minute restraint stress compared to their own basal levels. Additionally, Nrg1Tn rats showed increased expression of GR in the pituitary and HPC. In the HPC, the change in GR expression resulted in an imbalanced MR/GR ratio. Interestingly, in the PVN, Nrg1Tn rats have decreased GR expression. Maternal behavior is known to alter later life HPA axis function [38–40], but maternal behavior was not affected by the disruption of Nrg1 in these rats. Finally, Nrg1Tn rats sustain reactivity to novel environments, as demonstrated by a failure to habituate to an open field. Together, these findings point to NRG1 as a potential novel regulator of hormonal and behavioral stress reactivity.
4.2. Nrg1 Hypomorphic Rats
The present studies are the first to describe the phenotype of Nrg1Tn rats as hypomorphic for Type II NRG1, as well as to characterize neuroendocrine responses to a bout of restraint stress. The transposon insertion caused a partial loss of Type II Nrg1 mRNA and corresponding protein, which are encoded by exons in the 5’ region of the Nrg1 gene that contains the most risk haplotype associations with schizophrenia and bipolar disorder [13–16]. Both the location of the disruption to Nrg1 and its hypomorphic nature lend a unique translational opportunity to this rat model. Two features of this model are particularly noteworthy. First, the disruption of Nrg1 occurs in a region of the gene that has not previously been disrupted, i.e., within intron 1 in the 5’ region of the gene. In all Nrg1 mutant mouse preparations, mutations have been targeted to 3’ regions of the Nrg1 gene, including the transmembrane domain [13,23,41–43], the EGF domain [44] and immunoglobulin-like domain [45]. These Nrg1 knockout (KO) mouse models have been shown to express several schizophrenia-like phenotypes, but HPA axis function was not tested. In addition, it should be noted that Nrg1 KO models targeting the EGF domain would be expected to disrupt signaling of all types of NRG1, whereas in the Nrg1Tn rats, only one type of NRG1 is expected to be disrupted. A second feature is the lack of lethality associated with the homozygous disruption of Type II NRG1. In all of the mouse models mentioned above, homozygous mutations are embryonically lethal, but homozygous disruption of Type II NRG1 by transposon insertion in rats does not result in embryonic lethality. Furthermore, this is the first non-mouse model of Nrg1 disruption. Thus, this preparation provides the unique opportunity to investigate the neurobiology of a form of NRG1 that has been shown to be reduced in schizophrenia [46]. Moreover, the studies reported here are among the first to specifically examine stress reactivity in any animal model of disrupted NRG1 function.
4.3. Stress Reactivity
4.3.1. NRG1 in the PVN
Stress reactivity is controlled by a neurocircuit that involves the PFC, HPC, amygdala and hypothalamic PVN, with the latter structure representing the final site for integration of brain information regarding environmental stresses [47]. Nrg1 mRNA has been found in many of these brain regions, but this study is the first to demonstrate that Type II NRG1 protein is expressed in the PVN, in addition to the PFC and HPC in WT rats. Detection of Type II NRG1 in brain regions associated with stress reactivity suggested that additional studies on the role of NRG1 in the control of both behavioral and neuroendocrine responses to stress were warranted.
4.3.2. Neuroendocrine Alterations
In order to conserve animals, tail clip procedures were initially utilized to determine HPA axis responses to acute stress as outlined by Vahl and colleagues [35]. These investigators showed that stress hormone profiles were identical in blood samples obtained by tail clipping and decapitation in Sprague-Dawley rats. However, in the present study, we did not find that these procedures resulted in identical hormone profiles. This difference may be due to the use of Fischer 344 rats in our study, which are known to have enhanced HPA axis responses to stress exposure compared to Sprague-Dawley rats [48–50]. Thus, to insure that CORT concentrations were an accurate reflection of the response to acute restraint stress, rather than to the tail clip procedure, only trunk blood data were fully analyzed.
A role for NRG1 in the regulation of the HPA axis has not been previously investigated. Interestingly, we found that Nrg1Tn rats exhibited increased basal CORT compared to WT rats, and a more robust inhibition of CORT after stress exposure relative to their own basal levels. These findings suggest that Nrg1Tn animals have increased basal HPA axis drive. The changes found in GR expression support these findings. Lower expression of GR in the PVN of Nrg1Tn rats would be consistent with reduced inhibitory GC influence over basal CORT levels. In addition, the increased hippocampal GR expression, with no concomitant increase in MR, suggests that the MR/GR balance was disrupted in the Nrg1Tn animals. The equilibrium between MR and GR is known to be crucial for the set point of the HPA axis as well as for HPA axis response to stress [6,11]. A relative reduction in MR activity could lead to increased HPA axis drive. In fact, acute treatment with a selective MR antagonist has been shown to increase morning basal levels of CORT without having an effect on acute stress recovery CORT [51]. While elevated GR expression in both the pituitary and HPC of Nrg1Tn rats may result in increased inhibitory influence over HPA axis activity following exposure to restraint stress, further studies utilizing dexamethasone suppression or a pre-stress GC challenge would be needed to clarify differences in GR mediated negative feedback [6,52,53].
A differential change in GR expression across brain areas in the Nrg1Tn rats was an unexpected finding, which may suggest that there are more complex factors involved in the local regulation of GR gene expression. For example, Uchida and colleagues reported that inhibitory miRNAs are concentrated in the PVN compared to HPC of Fischer rats, which could lead to a selective alteration in PVN GR expression relative to the HPC [54]. In addition, it has been shown that dexamethasone induces NRG1 production and alters expression and dimerization patterns of ErbB receptors in mouse fetal lung cells [55]. Thus a more complex interaction between GC and NRG1-ErbB4 signaling may exist and further experiments will be needed to examine this interaction more thoroughly.
Previous studies by Meaney and colleagues suggest that HPA axis activity can be programmed by maternal care, such that animals exposed to less maternal care show enhanced basal CORT secretion and augmentation of stress responsivity [38–40]. NRG1 has been implicated in the control of pubertal development [24], but to our knowledge there are no data on the role of this gene in maternal behavior. We found that neither pup retrieval, arched-back nursing nor nest building behaviors differ between the WT and Nrg1Tn rats. These findings indicate that the alterations in HPA axis activity observed in the Nrg1Tn rats are not due to the quality or quantity of maternal care provided to the pups.
4.3.3. Behavioral Reactivity
On the first exposure to the open field arena, no differences in indices of locomotor activity were observed between WT and Nrg1Tn rats. However, Nrg1Tn rats failed to habituate to the open field on the second exposure, as demonstrated by persistent mobility, line crossing and distance traveled. In essence, the Nrg1Tn rats appear to find the open field arena just as novel on the second exposure as they do on the first. We have preliminary evidence that the Nrg1Tn rats are competent on object recognition memory in the same environment (unpublished results), so it does not appear that the Nrg1Tn rats have gross memory difficulties in this environment that could account for their failure to habituate to the testing arena. This is an important point as the present findings may indicate that Nrg1Tn rats interpret their environment differently, and sustain reactivity to novel environments for longer than normal.
In Nrg1 KO mouse models, several groups have identified a locomotor or exploration related hyperactive phenotype [41–43,56]. This phenotype is primarily found in the KO models that disrupt expression of the transmembrane and EGF domains of NRG1, but not in the immunoglobulin-like domain KO models [45]. The present studies in Nrg1Tn rats did not identify a hyperactive phenotype, highlighting the importance of different domains of NRG1 and different species in behavioral phenotypes. Fewer studies have examined habituation to an open field/novel environment. In heterozygous KO mice for the EGF domain of NRG1, an improved ability to habituate to a novel environment was found [56]. On the other hand, in heterozygous KO mice for the transmembrane domain of NRG1, a failure to habituate, similar to that reported here, was found [57]. Interestingly, in rats with hippocampal lesions, habituation of exploration is impaired [58]. This may be of relevance given the disruption of normal MR/GR balance found in the HPC of Nrg1Tn rats in the present study. Furthermore, NRG1 coordinates the organization of glutamatergic and GABAergic synapses in the brain [15,17]. Since these neurotransmitters play a critical role in the regulation of behavioral responses, disruptions in behavior might be anticipated due to a slight change in the balance between hippocampal excitation and inhibition. Further studies will be required to examine the importance of changes in these neurotransmitter systems in the Nrg1Tn rats and the relationship of those changes to the behavioral findings of the present study.
4.4. Conclusions
Alterations in HPA axis function are a robust finding in several psychiatric disorders. Patients with schizophrenia frequently experience a disrupted ability to resume normal HPA axis activity following an acute stress [59] and elevated cortisol levels have been reported both during an acute phase of chronic schizophrenia and during the first psychotic episode [60–62]. Additionally, depressed individuals have been reported to show HPA axis dysregulation with decreased negative feedback [63,64]. Many patients and individuals at risk for developing a psychiatric disorder, including depression and psychosis, show HPA axis hyperactivity [65–68]. Conversely, patients with PTSD frequently present with enhanced GR-mediated negative feedback inhibition (see [69] for review). Stress-based animal models of schizophrenia, depression and PTSD confirm the importance of stress and HPA axis dysregulation as etiological factors for these disorders [36,70–76]. Furthermore, the disruption of the MR/GR balance reported here is similar to animals with enhanced fast feedback, found in the single-prolonged stress model of PTSD in rats [77].
The present experiments are among the first to describe a relationship between disrupted NRG1 expression and stress reactivity. Transposon knockdown of Type II NRG1 resulted in increased basal CORT and relatively reduced CORT concentrations 120 minutes after exposure to an acute stressor. In addition, disruption of Type II NRG1 expression in the rat brain caused a decrease in GR expression in the PVN and an increase in GR expression in both the pituitary and HPC, thereby disrupting the critical balance between MR and GR in the HPC. Furthermore, Nrg1Tn rats failed to habituate to an open field, which may be an indication of increased behavioral reactivity related to increased basal HPA axis drive. Importantly, these findings can not be attributed to differences in maternal care. The hypomorphic disruption of NRG1 expression in these rats and the present findings implicating NRG1 in stress regulation may make this model amenable to investigating the effects of gene by environment (i.e. stress) interactions with outcomes potentially relevant to multiple psychiatric disorders.
Research Highlights
Nrg1Tn rats exhibit reduced expression of Type II NRG1 mRNA and protein
Type II NRG1 is expressed in the neurocircuitry that regulates HPA axis responses
Nrg1Tn rats exhibit altered neuroendocrine stress reactivity
Nrg1Tn rats exhibit altered GR expression and disrupted corticosterone secretion
Type II NRG1 plays a role in behavioral habituation to environmental stimuli
Acknowledgements
The presentation of this work at the 2010 Neurobiology of Stress Workshop was supported by a Trainee Travel Award to ST that was funded by a grant from NIMH (R13MH090623). This work was supported by grants and contracts from NIDA (DA59909), NIMH (MH73826, MH82999) and NHLBI (5U01HL066579).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 3.Grandin LA, LG, Abramson LY. Childhood stressful life events and bipolar spectrum disorders. Journal of Social and Clinical Psychology. 2007;26:460–478. [Google Scholar]
- 4.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Koenig JI, Kirkpatrick B, Lee P. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology. 2002;27:309–318. doi: 10.1016/S0893-133X(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 6.de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 7.de Kloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- 8.Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci. 2006;26:1971–1978. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolber BJ, Muglia LJ. Defining brain region-specific glucocorticoid action during stress by conditional gene disruption in mice. Brain Res. 2009;1293:85–90. doi: 10.1016/j.brainres.2009.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci U S A. 2008;105:12004–12009. doi: 10.1073/pnas.0803216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oitzl MS, van Haarst AD, de Kloet ER. Behavioral and neuroendocrine responses controlled by the concerted action of central mineralocorticoid (MRS) and glucocorticoid receptors (GRS) Psychoneuroendocrinology. 1997;22 Suppl 1:S87–S93. doi: 10.1016/s0306-4530(97)00020-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhe D, Fang H, Yuxiu S. Expressions of hippocampal mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) in the single-prolonged stress-rats. Acta Histochem Cytochem. 2008;41:89–95. doi: 10.1267/ahc.08013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E, et al. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet. 2003;72:83–87. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Prata DP, Breen G, Osborne S, Munro J, St Clair D, Collier DA. An association study of the neuregulin 1 gene, bipolar affective disorder and psychosis. Psychiatr Genet. 2009;19:113–116. doi: 10.1097/YPG.0b013e32832a4f69. [DOI] [PubMed] [Google Scholar]
- 17.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 18.Esper RM, Pankonin MS, Loeb JA. Neuregulins: versatile growth and differentiation factors in nervous system development and human disease. Brain Res Rev. 2006;51:161–175. doi: 10.1016/j.brainresrev.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Tan W, Wang Y, Gold B, Chen J, Dean M, Harrison PJ, et al. Molecular cloning of a brain-specific, developmentally regulated neuregulin 1 (NRG1) isoform and identification of a functional promoter variant associated with schizophrenia. J Biol Chem. 2007;282:24343–24351. doi: 10.1074/jbc.M702953200. [DOI] [PubMed] [Google Scholar]
- 20.Shamir A, Buonanno A. Molecular and cellular characterization of Neuregulin-1 type IV isoforms. J Neurochem. 2010;113:1163–1176. doi: 10.1111/j.1471-4159.2010.06677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinthorsdottir V, Stefansson H, Ghosh S, Birgisdottir B, Bjornsdottir S, Fasquel AC, et al. Multiple novel transcription initiation sites for NRG1. Gene. 2004;342:97–105. doi: 10.1016/j.gene.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 22.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean B, Karl T, Pavey G, Boer S, Duffy L, Scarr E. Increased levels of serotonin 2A receptors and serotonin transporter in the CNS of neuregulin 1 hypomorphic/mutant mice. Schizophr Res. 2008;99:341–349. doi: 10.1016/j.schres.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Prevot V, Rio C, Cho GJ, Lomniczi A, Heger S, Neville CM, et al. Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J Neurosci. 2003;23:230–239. doi: 10.1523/JNEUROSCI.23-01-00230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen MS, Bermingham-McDonogh O, Danehy FT, Jr, Nolan C, Scherer SS, Lucas J, et al. Expression of multiple neuregulin transcripts in postnatal rat brains. J Comp Neurol. 1994;349:389–400. doi: 10.1002/cne.903490306. [DOI] [PubMed] [Google Scholar]
- 26.Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol. 2001;433:86–100. doi: 10.1002/cne.1127. [DOI] [PubMed] [Google Scholar]
- 27.Ma YJ, Hill DF, Creswick KE, Costa ME, Cornea A, Lioubin MN, et al. Neuregulins signaling via a glial erbB-2-erbB-4 receptor complex contribute to the neuroendocrine control of mammalian sexual development. J Neurosci. 1999;19:9913–9927. doi: 10.1523/JNEUROSCI.19-22-09913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keri S, Kiss I, Seres I, Kelemen O. A polymorphism of the neuregulin 1 gene (SNP8NRG243177/rs6994992) affects reactivity to expressed emotion in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:418–420. doi: 10.1002/ajmg.b.30812. [DOI] [PubMed] [Google Scholar]
- 29.Hintsanen M, Elovainio M, Puttonen S, Kivimaki M, Raitakari OT, Lehtimaki T, et al. Neuregulin-1 genotype moderates the association between job strain and early atherosclerosis in young men. Ann Behav Med. 2007;33:148–155. doi: 10.1007/BF02879896. [DOI] [PubMed] [Google Scholar]
- 30.Lu B, Geurts AM, Poirier C, Petit DC, Harrison W, Overbeek PA, et al. Generation of rat mutants using a coat color-tagged Sleeping Beauty transposon system. Mamm Genome. 2007;18:338–346. doi: 10.1007/s00335-007-9025-5. [DOI] [PubMed] [Google Scholar]
- 31.Pedrosa E, Locker J, Lachman HM. Survey of schizophrenia and bipolar disorder candidate genes using chromatin immunoprecipitation and tiled microarrays (ChIP-chip) J Neurogenet. 2009;23:341–352. doi: 10.1080/01677060802669766. [DOI] [PubMed] [Google Scholar]
- 32.Silverman JL, Koenig JI. Evidence for the involvement of ERbeta and RGS9-2 in 17-beta estradiol enhancement of amphetamine-induced place preference behavior. Horm Behav. 2007;52:146–155. doi: 10.1016/j.yhbeh.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. Orlando: Academic Press Inc.; 1986. [DOI] [PubMed] [Google Scholar]
- 34.Lonstein JS, Fleming AS. Parental behaviors in rats and mice. Chapter 8. Curr Protoc Neurosci. 2002;(Unit 8):15. doi: 10.1002/0471142301.ns0815s17. [DOI] [PubMed] [Google Scholar]
- 35.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, et al. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289:E823–E828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- 36.Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, et al. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res. 2005;156:251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 37.Falls DL. Neuregulins and the neuromuscular system: 10 years of answers and questions. J Neurocytol. 2003;32:619–647. doi: 10.1023/B:NEUR.0000020614.83883.be. [DOI] [PubMed] [Google Scholar]
- 38.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 39.Levine S. The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Ann N Y Acad Sci. 1994;746:275–288. doi: 10.1111/j.1749-6632.1994.tb39245.x. discussion 89–93. [DOI] [PubMed] [Google Scholar]
- 40.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 41.O'Tuathaigh CM, Babovic D, O'Sullivan GJ, Clifford JJ, Tighe O, Croke DT, et al. Phenotypic characterization of spatial cognition and social behavior in mice with 'knockout' of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007;147:18–27. doi: 10.1016/j.neuroscience.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 42.Karl T, Duffy L, Scimone A, Harvey RP, Schofield PR. Altered motor activity, exploration and anxiety in heterozygous neuregulin 1 mutant mice: implications for understanding schizophrenia. Genes Brain Behav. 2007;6:677–687. doi: 10.1111/j.1601-183X.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- 43.Boucher AA, Arnold JC, Duffy L, Schofield PR, Micheau J, Karl T. Heterozygous neuregulin 1 mice are more sensitive to the behavioural effects of Delta9-tetrahydrocannabinol. Psychopharmacology (Berl) 2007;192:325–336. doi: 10.1007/s00213-007-0721-3. [DOI] [PubMed] [Google Scholar]
- 44.Gerlai R, Pisacane P, Erickson S. Heregulin, but not ErbB2 or ErbB3, heterozygous mutant mice exhibit hyperactivity in multiple behavioral tasks. Behav Brain Res. 2000;109:219–227. doi: 10.1016/s0166-4328(99)00175-8. [DOI] [PubMed] [Google Scholar]
- 45.Rimer M, Barrett DW, Maldonado MA, Vock VM, Gonzalez-Lima F. Neuregulin-1 immunoglobulin-like domain mutant mice: clozapine sensitivity and impaired latent inhibition. Neuroreport. 2005;16:271–275. doi: 10.1097/00001756-200502280-00014. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR. Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry. 2004;9:299–307. doi: 10.1038/sj.mp.4001434. [DOI] [PubMed] [Google Scholar]
- 47.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamopituitary- adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Dhabhar FS, McEwen BS, Spencer RL. Stress response, adrenal steroid receptor levels and corticosteroid-binding globulin levels--a comparison between Sprague-Dawley, Fischer 344 and Lewis rats. Brain Res. 1993;616:89–98. doi: 10.1016/0006-8993(93)90196-t. [DOI] [PubMed] [Google Scholar]
- 49.Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Differential activation of adrenal steroid receptors in neural and immune tissues of Sprague Dawley, Fischer 344, and Lewis rats. J Neuroimmunol. 1995;56:77–90. doi: 10.1016/0165-5728(94)00135-b. [DOI] [PubMed] [Google Scholar]
- 50.Glowa JR, Geyer MA, Gold PW, Sternberg EM. Differential startle amplitude and corticosterone response in rats. Neuroendocrinology. 1992;56:719–723. doi: 10.1159/000126298. [DOI] [PubMed] [Google Scholar]
- 51.Spencer RL, Kim PJ, Kalman BA, Cole MA. Evidence for mineralocorticoid receptor facilitation of glucocorticoid receptor-dependent regulation of hypothalamic-pituitary-adrenal axis activity. Endocrinology. 1998;139:2718–2726. doi: 10.1210/endo.139.6.6029. [DOI] [PubMed] [Google Scholar]
- 52.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 53.de Kloet ER. Brain Corticosteroid Receptor Balance and Homeostatic Control. Front. Neuroendocrinol. 1991;12:95–164. doi: 10.1016/j.yfrne.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Uchida S, Nishida A, Hara K, Kamemoto T, Suetsugi M, Fujimoto M, et al. Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. Eur J Neurosci. 2008;27:2250–2261. doi: 10.1111/j.1460-9568.2008.06218.x. [DOI] [PubMed] [Google Scholar]
- 55.Dammann CE, Nassimi N, Liu W, Nielsen HC. ErbB receptor regulation by dexamethasone in mouse type II epithelial cells. Eur Respir J. 2006;28:1117–1123. doi: 10.1183/09031936.06.00132305. [DOI] [PubMed] [Google Scholar]
- 56.Duffy L, Cappas E, Scimone A, Schofield PR, Karl T. Behavioral profile of a heterozygous mutant mouse model for EGF-like domain neuregulin 1. Behav Neurosci. 2008;122:748–759. doi: 10.1037/0735-7044.122.4.748. [DOI] [PubMed] [Google Scholar]
- 57.O'Tuathaigh CM, O'Sullivan GJ, Kinsella A, Harvey RP, Tighe O, Croke DT, et al. Sexually dimorphic changes in the exploratory and habituation profiles of heterozygous neuregulin-1 knockout mice. Neuroreport. 2006;17:79–83. doi: 10.1097/01.wnr.0000192738.31029.0a. [DOI] [PubMed] [Google Scholar]
- 58.Clark BJ, Hines DJ, Hamilton DA, Whishaw IQ. Movements of exploration intact in rats with hippocampal lesions. Behav Brain Res. 2005;163:91–99. doi: 10.1016/j.bbr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Goldman MB, Blake L, Marks RC, Hedeker D, Luchins DJ. Association of nonsuppression of cortisol on the DST with primary polydipsia in chronic schizophrenia. Am J Psychiatry. 1993;150:653–655. doi: 10.1176/ajp.150.4.653. [DOI] [PubMed] [Google Scholar]
- 60.Ryan MC, Sharifi N, Condren R, Thakore JH. Evidence of basal pituitary-adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology. 2004;29:1065–1070. doi: 10.1016/j.psyneuen.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Tandon R, Mazzara C, DeQuardo J, Craig KA, Meador-Woodruff JH, Goldman R, et al. Dexamethasone suppression test in schizophrenia: relationship to symptomatology, ventricular enlargement, and outcome. Biol Psychiatry. 1991;29:953–964. doi: 10.1016/0006-3223(91)90353-n. [DOI] [PubMed] [Google Scholar]
- 62.Mondelli V, Pariante CM, Navari S, Aas M, D'Albenzio A, Di Forti M, et al. Higher cortisol levels are associated with smaller left hippocampal volume in first-episode psychosis. Schizophr Res. 2010;119:75–78. doi: 10.1016/j.schres.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 64.Young EA, Haskett RF, Murphy-Weinberg V, Watson SJ, Akil H. Loss of glucocorticoid fast feedback in depression. Arch Gen Psychiatry. 1991;48:693–699. doi: 10.1001/archpsyc.1991.01810320017003. [DOI] [PubMed] [Google Scholar]
- 65.Modell S, Lauer CJ, Schreiber W, Huber J, Krieg JC, Holsboer F. Hormonal response pattern in the combined DEX-CRH test is stable over time in subjects at high familial risk for affective disorders. Neuropsychopharmacology. 1998;18:253–262. doi: 10.1016/S0893-133X(97)00144-9. [DOI] [PubMed] [Google Scholar]
- 66.Garner B, Pariante CM, Wood SJ, Velakoulis D, Phillips L, Soulsby B, et al. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol Psychiatry. 2005;58:417–423. doi: 10.1016/j.biopsych.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 67.Mittal VA, Dhruv S, Tessner KD, Walder DJ, Walker EF. The relations among putative biorisk markers in schizotypal adolescents: minor physical anomalies, movement abnormalities, and salivary cortisol. Biol Psychiatry. 2007;61:1179–1186. doi: 10.1016/j.biopsych.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 68.Pariante CM. Risk factors for development of depression and psychosis. Glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Ann N Y Acad Sci. 2009;1179:144–152. doi: 10.1111/j.1749-6632.2009.04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- 70.Willner P. Chronic mild stress (CMS) revisited: consistency and behaviouralneurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 71.Bale TL. Stress sensitivity and the development of affective disorders. Horm Behav. 2006;50:529–533. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 72.Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seckl JR. Glucocorticoids, developmental 'programming' and the risk of affective dysfunction. Prog Brain Res. 2008;167:17–34. doi: 10.1016/S0079-6123(07)67002-2. [DOI] [PubMed] [Google Scholar]
- 74.van Winkel R, Stefanis NC, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr Bull. 2008;34:1095–1105. doi: 10.1093/schbul/sbn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, et al. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009;26:1110–1117. doi: 10.1002/da.20629. [DOI] [PubMed] [Google Scholar]
- 76.Cohen H, Matar MA, Richter-Levin G, Zohar J. The contribution of an animal model toward uncovering biological risk factors for PTSD. Ann N Y Acad Sci. 2006;1071:335–350. doi: 10.1196/annals.1364.026. [DOI] [PubMed] [Google Scholar]
- 77.Liberzon I, Lopez JF, Flagel SB, Vazquez DM, Young EA. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. J Neuroendocrinol. 1999;11:11–17. doi: 10.1046/j.1365-2826.1999.00288.x. [DOI] [PubMed] [Google Scholar]
Web References
- [last accessed November 3, 2010]; http://pga.mcw.edu.
- [last accessed November 3, 2010]; http://www.knockoutrat.com.
- [last accessed November 3, 2010]; http://rgd.mcw.edu/tools/strains/strains_view.cgi?id=2290103.
- [last accessed November 3, 2010]; http://www.uniprot.org/uniprot/Q02297.
- [last accessed November 3, 2010]; http://www.uniprot.org/uniprot/Q9ESA5.