Abstract
BACKGROUND & AIMS
Fibrosis is an abnormal extension of the wound healing process that follows tissue damage; it is involved in pathogenesis in a variety of chronic diseases. The formation of extracellular matrix is an essential response in wound healing. Although it has been proposed that collagen organization and assembly depend on the fibronectin matrix in culture, the contribution of fibronectin to these processes remains to be defined in vivo.
METHODS
We generated a conditional, fibronectin-deficient mouse model of liver injury and explored whether fibronectin would be a suitable target for preventing extensive collagen deposits and scar formation that could lead to liver fibrosis.
RESULTS
The lack of fibronectin did not interfere with reconstruction of collagen fibril organization in response to liver injury. Signaling by transforming growth factor (TGF)-β and type V collagen were required for collagen fibrillogenesis during remodeling of adult liver tissue.
CONCLUSIONS
TGF-β and type V collagen are targets for regulating the initial fibrogenic response to liver damage.
Keywords: liver disease, hepatic stellate cells, mouse model of fibrosis, conditional knockout, ECM
Introduction
Wound-healing is a crucial response to maintain organ structure and integrity following tissue damage. If injuries persist, they underlie the cause of pathogenesis in a variety of chronic fibrotic diseases such as liver fibrosis1–2. The essential response in wound healing is the deposition of extracellular matrices (ECMs). The key players in ECM production and remodeling in the liver are hepatic stellate cells (HSCs), which undergo activation and transdifferentiate into myofibroblast-like cells in response to liver damage3–4. A paradigm of ECMs in adult liver remodeling is that, like other tissues, the initial deposition of plasma type fibronectin and fibrinogen exudated from plasma to form provisional matrix can stabilize wounded areas and induces the local production of cellular type fibronectin in activated HSCs5. In vitro data suggest that the subsequent formation of an extensive collagen network requires the presence of fibronectin6. It is also believed that the profibrogenic cytokine transforming growth factor (TGF)-β is responsible for upregulating ECM production in activated HSCs7.
Fibronectin is a dimeric glycoprotein and exists as two types. Plasma fibronectin is a soluble form produced solely by hepatocytes, while cellular fibronectin is an insoluble form produced by a variety of cells and incorporated into tissue ECM. Both isoforms are generated from a single gene by alternative splicing8. Although considerable in vitro functional studies have indicated that fibronectin plays a key role in cell differentiation, proliferation, migration, and survival9–10, knowledge of the functional identity of each fibronectin isoform in adult tissue-remodeling remains loosely defined due to the complexity and the lack of model systems.
A recent study suggests a mechanism by which fibronectin plays a role in TGF-β signaling. Fibronectin participates in the initial incorporation of latent TGF-β-binding protein (LTBP)-1 into the ECM in vitro11. A cytokine TGF-β is secreted in a biologically inactive (latent) form in a complex with TGF-β latency associated protein (LAP) and LTBPs. The tissue concentration of these latent complexes is maintained at a constant level12. In response to injury, local latent TGF-β complexes are converted into active TGF-β. There are several mechanisms for activation, such as via chaotropic agents, proteases, integrins (αvβ6, αvβ8), and thrombospondin (TSP)-1, all of which are likely to be tissue specific13–14. However, fibronectin/TGF-β interdependence on the initial fibrogenic response to liver damage is not yet addressed in vivo and it remains unknown whether the collagen fibrillogenesis could be prevented or attenuated by interfering with fibronectin during the response process. Here, using our mouse model lacking both fibronectin types in the adult liver, we investigated whether fibronectin is a suitable molecular target for ameliorating the initial fibrogenic response to liver injury.
Materials and Methods
Generation of mutant mice and animal studies
Mice carrying a fibronectin-floxed gene (Fn[fl/fl]) were generated (Supplementary Fig. 1). Mice null both plasma and cellular fibronectin in the liver were established by mating Fn(fl/fl) mice with mice expressing Cre recombinase under the control of the interferon- and pI-pC-inducible Mx promoter (Mx-Cre) described by Sakai et al.15. It was confirmed that fibronectin gene was deleted from parenchymal as well as non-parenchymal cells (‘Liver fibronectin-null mice’) (Supplementary Methods; Supplementary Figs. 1 and 2). β6 integrin- and TSP-1-knockout mice were kindly provided by Dr. Dean Sheppard (University of California, San Francisco) and Dr. Jack Lawler (Beth Israel Deaconess Medical Center), respectively16–17. Liver injury was induced by carbon tetrachloride (CCl4)1,18.
Isolation of primary HSCs and generation of adult HSC lines
Primary HSCs were isolated principally according to the method described by Schafer et al.19. Adult control and fibronectin-null HSC lines were generated from HSCs of mice on p53- and p21-null genetic background20: HSCs from adult livers of Fn(fl/fl)/p53(−/−) and Fn(fl/fl)/p21(−/−) mice were isolated and treated with a Cre-transducing adenovirus to delete the fibronectin-floxed genes (Supplementary Methods; Supplementary Fig. 3).
Data presentation and statistical analysis
All experiments were performed in triplicate as a minimum on separate occasions and the data shown were chosen as representative of results consistently observed. Results are presented as means ± standard deviation. Differences between groups were analyzed using the two-sided Student’s t test on raw data. A P value of < .05 was considered significant.
For more detailed descriptions of the Materials and Methods used, please see Supplementary Materials and Methods.
Results
Generation of liver fibronectin-null mice
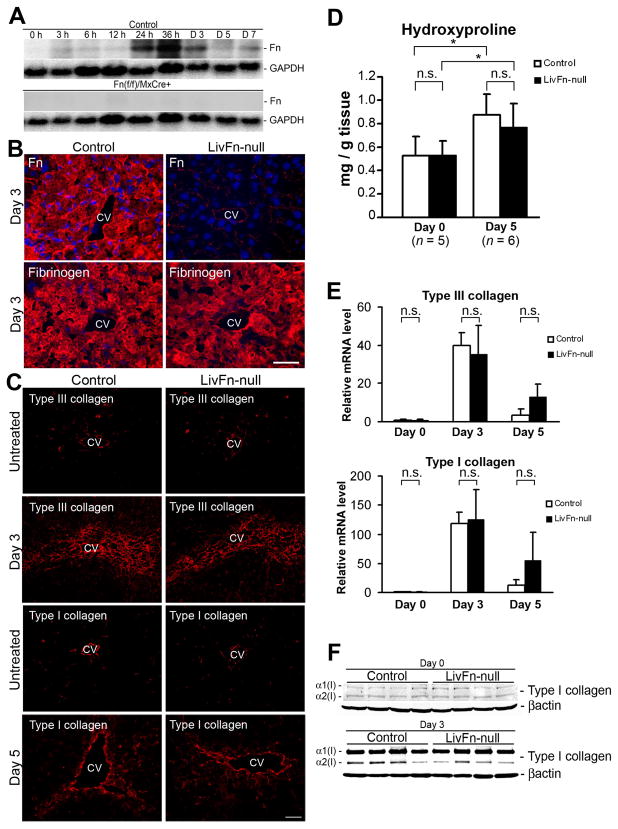
To address the role of Fn in remodeling of adult tissue ECMs, we established (liver) fibronectin-null (mutant) mice (Fn(fl/fl)/Mx-Cre+) and verified deletion efficiency (Supplementary Figs. 1 and 2). Acute liver injury was induced with a well-established model1,18 of a single treatment with the liver-damaging agent CCl4 (1 ml/kg body weight). Fibronectin mRNA was undetectable in Fn(fl/fl)/Mx-Cre+ liver throughout the acute injury-period (baseline time 0 through day 7 after injection). In contrast, considerable induction of fibronectin mRNA was noted in control liver (Fig. 1A). No newly deposited fibronectin protein was obvious in mutant livers throughout the regeneration process, except for a small remnant amount in the sinusoidal ECM as detected with immunohistochemistry (Fig. 1B; data not shown). These results demonstrate that we successfully established an adult mouse model lacking both fibronectin types in the liver during tissue-remodeling. Deposition of fibrin(ogen), a counterpart of the provisional matrix with plasma fibronectin, showed no marked differences by immunohistochemistry between control and mutant mice throughout the injury (Fig. 1B).
Fig. 1. Effect of fibronectin-deficiency on hepatic collagen production and assembly following acute liver injury.
(A) Time course of fibronectin mRNA expression. As a loading control, the blot was rehybridized with a GAPDH probe. Note that there is no induction of fibronectin (FN) mRNA in pI-pC-injected Fn(fl/fl)/Mx-Cre+ liver throughout the time course.
(B) Depositions of fibronectin and fibrinogen in control and mutant livers at 72 hrs after injury by immunofluorescent staining (fibronectin and fibrinogen in red; DAPI [cell nuclei] in blue). CV, central vein. Bar = 25 μm.
(C) Deposition and assembly of type III (untreated and at day 3) and type I (untreated and at day 5) collagen fibrils by immunofluorescent staining. Bar = 50 μm.
(D) Hepatic hydroxyproline content at day 0 (untreated) and day 5 (n = 5 at day 0 and n = 6 at day 5 for each group). Note that there are no significant differences between control and mutant livers at day 0 and 5, whereas the amounts in both livers at day 5 are significantly higher compared to day 0. *, P< .05.
(E) Real-time PCR analysis of Col3a1 and Col1a1 mRNAs. Relative mRNA expression levels are shown relative to the control value of 1.0 at day 0 (n = 5 for each group). Note that the expression levels in each time point (days 0, 3 and 5) are not significantly different between control and mutant livers.
(F) Western blot analysis of collagen α1(I) and α2(I) chains at day 0 (untreated) and day 3 after injury. Note that the control and mutant livers show identical expression levels.
Fibronectin-deficiency does not attenuate collagen network formation in acute liver injury
Collagen assembly upon tissue damage occurs as a multi-step process. The expression level of collagen mRNAs, processing during collagen protein secretion, and the level of degradation contribute to collagen assembly and maintenance21. Since a preformed fibronectin matrix is indispensable for type III and type I collagen-containing fibril formation in culture6,22–23, it was hypothesized that the absence of fibronectin could attenuate collagen fibrillogenesis following acute liver injury. However, any difference was not detected between control and fibronectin-null livers in (i) the deposition and assembly of type III and type I collagens; (ii) hepatic hydroxyproline content as a measure of net collagen production; (iii) expression levels of Col3a1 and Col1a1 mRNAs and α1(I) and α2(I) protein chains; and (iv) expression levels of collagen-related matrix metalloproteinase (MMP), MMP8 and MMP13 mRNAs (Fig. 1C-F; data not shown). These findings indicate that there is an alternative in vivo mechanism for collagen fibrillogenesis in the absence of fibronectin.
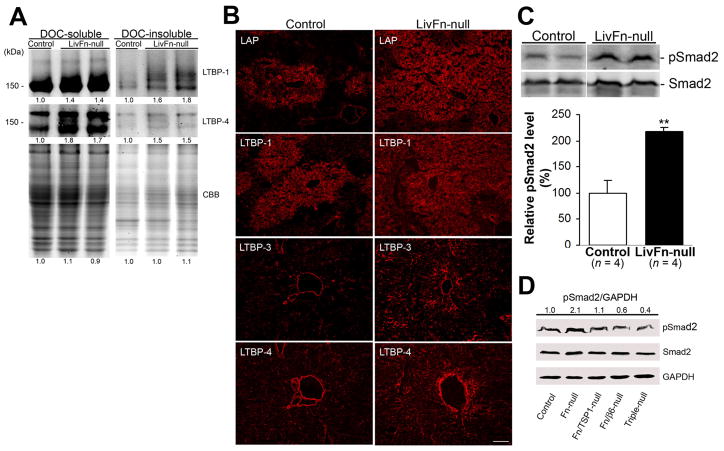
Liver lacking fibronectin elevates the production of TGF-β latent complexes and local TGF-β bioavailability in response to injury
Since fibronectin is associated with latent TGF-β complexes to incorporate into ECM7,12,14, we next determined whether lacking fibronectin affected the production of latent complexes of TGF-β following liver injury. The expression of both 2% deoxycholate-soluble and -insoluble fractions (defined as immaturely and maturely assembled matrices, respectively24) of LTBP-1 and -4 was higher in fibronectin-null livers post injury (Fig. 2A). The substantial depositions of LAP and of LTBP-1, −3 and −4 in the ECM of the mutant liver post injury were confirmed with immunohistochemistry (Fig. 2B). While LTBPs are associated with ECM components such as fibronectin and fibrillin12,14, they showed fibrillar structures even without fibronectin.
Fig. 2. Effect of fibronectin-deficiency on latent TGF-β production and TGF-β activation at 40 hrs after injury.
(A) Increased expression of both 2% deoxycholate-soluble and -insoluble LTBP-1 and LTBP-4 in fibronectin-null livers by Western blot analysis under non-reducing conditions. The intensity of the bands was measured by densitometry, and the intensities of each control sample were set to 1.0. The same samples were stained with Coomassie brilliant blue (CBB) to confirm that comparable amounts of protein were loaded. The positions of the molecular weight markers are indicated.
(B) Increased depositions of LAP and LTBP-1, −3 and −4 in fibronectin-null livers by immunofluorescent staining. Bar = 100 μm.
(C) Upper panels: Representative Western blot analysis of pSmad2 (C-terminal serine 465/467) and total Smad2 expression. Lower panel: Analysis of pSmad2 intensities. The pSmad2 expression levels are shown relative to the control value of 100 (percent of control) (n = 4 for each group). Note that pSmad2 expression level in fibronectin-null livers is significantly higher compared to controls. **, P< .01.
(D) Western blot analysis of pSmad2, total Smad2 and GAPDH (loading control) expression in control, fibronectin-null, fibronectin/TSP1-double null, fibronectin/β6 integrin-double null, and fibronectin /β6 integrin/TSP1-triple null (Triple-null) livers. Pooled samples from 4 mouse livers from each strain were used for the analysis. The intensity of the bands was measured by densitometry, and each pSmad2 intensity was normalized to GAPDH, then the intensity of the control was set to 1.0. Each pSmad2 intensity is shown relative to the control value.
Latent TGF-β that is activated in response to injury binds to the TGF-β type I receptor, which then phosphorylates the C-terminal regions of Smad 2/3 transcription factors. Activated Smads translocate to the nucleus where they are involved in the regulation of gene expression25–26. Therefore, it was next examined whether the elevated production of latent TGF-β complexes in fibronectin-null livers altered the local activation of TGF-β following injury. Phosphorylation of Smad2 (pSmad2) in fibronectin-null livers at 40 hrs after injury was 2.2-fold higher (P < .01) than in controls (Fig. 2C). To determine the molecular mechanisms underlying elevated TGF-β activity in fibronectin-null livers post injury, we generated fibronectin/TSP-1- and fibronectin/β6 integrin-double and fibronectin/β6 integrin/TSP-1-triple knockout mice16–17. The induction level of pSmad2 was downregulated to 52% in fibronectin/TSP-1-null, 29% in fibronectin/β6 integrin-null, and 19% in fibronectin/β6 integrin/TSP-1-null livers in comparison with fibronectin-null livers (Fig. 2D). These results demonstrated that although both β6 integrin and TSP-1 participate in local TGF-β activation in acute liver injury, β6 integrin plays a more significant role in its activation than does TSP-1.
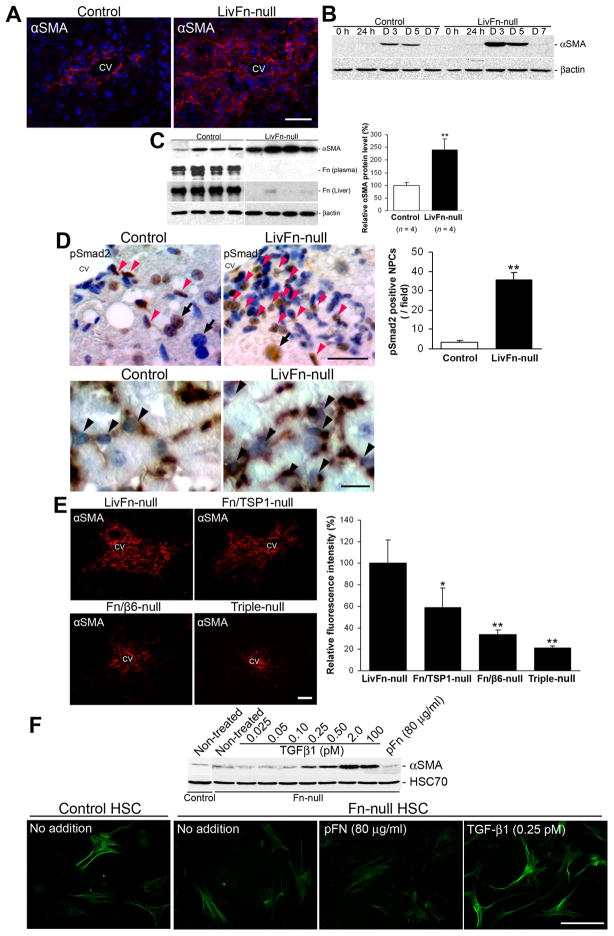
Lack of fibronectin results in significantly increased HSC activation following acute liver injury
The key event for ECM remodeling in response to liver injury is the activation of HSCs3. Both TGF-β and cellular fibronectin extradomain A (EDA) segment promote the initiation of HSC activation in vitro, accelerating the induction of the activated HSC marker, α-smooth muscle cell actin (αSMA)27–28. Since the absence of fibronectin elevates local TGF-β activity post injury (Fig. 2C), we next addressed the functional relationship of fibronectin and TGF-β with HSC phenotypes both in vivo and in vitro. We found that post acute injury fibronectin-null livers had higher αSMA levels than controls, especially in the areas of the central vein (Fig. 3A–C). The number of αSMA-positive cells at day 3 were 173.8 ± 17.1 cells/field in mutant (field = 0.24 mm2, n = 8) vs. 69.4 ± 12.4 cells/field in control (n = 8; P < .01). Fibronectin-null livers also showed a significantly increased number of nuclear pSmad2-positive nonparenchymal cells post injury (35.1 ± 3.8 cells/field in mutant; field = 0.24 mm2 [n = 10] vs. 3.5 ± 1.1 cells/field in control [n = 10], P < .01) and those pSmad2-positive cells expressed αSMA (Fig. 3D). The αSMA induction was diminished in fibronectin/β6 integrin- and fibronectin/TSP-1-double-knockout livers and even further reduced in fibronectin/β6 integrin/TSP-1-triple knockout livers (Fig. 3E). To further determine the extent, if at all, that TGF-β can activate HSCs in the absence of fibronectin, we prepared primary HSCs isolated from adult control and fibronectin-null mice. TGF-β1 stimulation induced αSMA production at concentrations as low as 0.25 pM (6.25 pg/ml). The production surpassed that of non-treated control and cells treated with 80 μg/ml plasma fibronectin. TGF-β1 addition (0.25 pM) also induced characteristic stellate shapes with long cytoplasmic processes (Fig. 3F). Taken together our results indicate that TGF-β1 alone sufficiently initiates HSC activation after liver injury.
Fig. 3. The lack of fibronectin elevates activation of HSCs after acute liver injury.
(A) Pronounced induction of hepatic αSMA in fibronectin-null livers at 72 hrs after injury by immunofluorescent staining (αSMA in red; DAPI in blue). CV, central vein. Bar = 25 μm.
(B) Western blot analysis of the time course of αSMA induction following injury. Note that a higher induction level of αSMA is indicated in fibronectin-null livers from 3 to 5 days post injury.
(C) Left panel: Western blot analysis of αSMA, and plasma and liver fibronectin protein levels at 72 hrs after injury. Right panel: Analysis of αSMA intensities. The αSMA expression levels are shown relative to the control value of 100 (percent of control) (n = 4 for each group). **, P< .01.
(D) Upper left panels: Immunostaining for pSmad2 at 40 hrs after injury. Sections were counterstained with hematoxylin. Bar = 25 μm. Upper right panel: Analysis of pSmad2-positive cells. Note that the number of nuclear pSmad2-positive non-parenchymal cells (NPCs in brown; red arrowheads) is significantly higher in fibronectin-null livers. For comparison, hepatocyte nuclei are indicated (black arrows). **, P< .01. Lower panels: Double-immunostaining for pSmad2 (in purplish-blue) and αSMA(in brown). Note that the nuclear pSmad2-positive cells express activated HSC marker αSMA in their cytoplasms (black arrowheads). Bar = 25 μm.
(E) Left panels: Induction of αSMA in fibronectin-null, fibronectin/TSP-1- and fibronectin/β6 integrin-double, and fibronectin/β6 integrin/TSP-1-triple knockout livers at 72 hrs after injury by immunofluorescent staining. Bar = 50 μm. Right panel: Analysis of intensity in assembled collagen fibrils. Relative fluorescence intensities are shown relative to the control value of 100 (LivFn-null). *, P< .05; **, P< .01.
(F) TGF-β1 effectively induces activation of primary HSCs in vitro. Upper panel: Western blot analysis of αSMA at 72 hrs after the isolation of primary HSCs from adult control and fibronectin-null intact livers. Primary HSCs were incubated in DMEM containing 2% Fn-depleted FBS with indicated supplements. HSC70, loading control. Lower panels: Immunofluorescent staining for αSMA (in green) at 72 hrs after the isolation of primary HSCs. Note that TGF-β1 (0.25 pM) induces characteristic stellate shapes with long cytoplasmic processes in addition to intense αSMA induction in fibronectin-null HSCs. Bar = 100 μm.
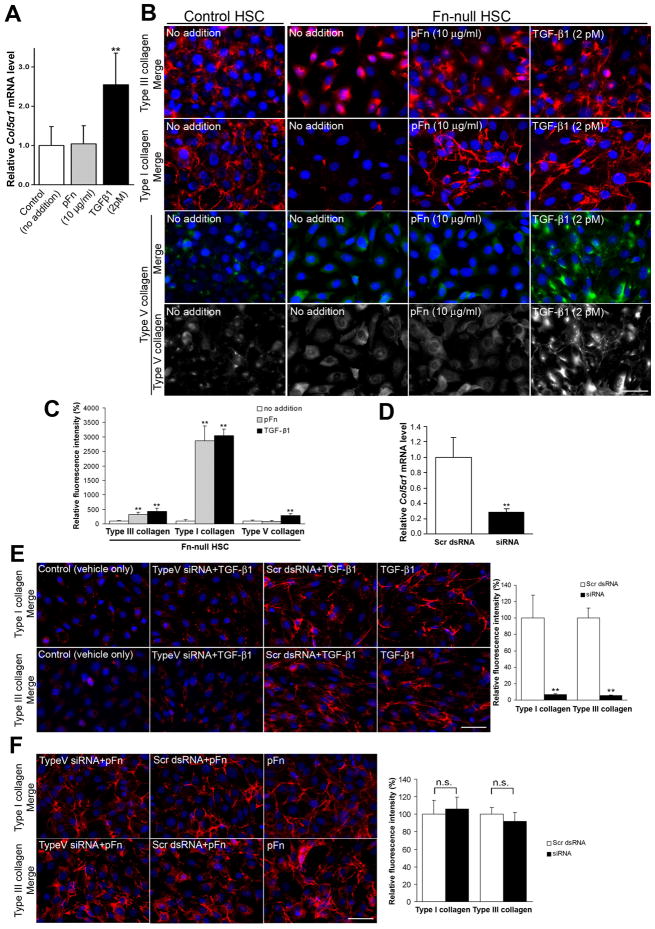
TGF-β-induced collagen V generates type III/I collagen networks in the absence of fibronectin both in vitro and in vivo
To determine whether TGF-β is involved in type III/I collagen network formation in the absence of fibronectin, adult control and Fn-null HSC lines were generated from HSCs of mice on p53- and p21-null genetic background20 (Supplementary Fig. 3). Since all fibronectin-null cells, including adult fibronectin-null HSCs, do not assemble collagen fibrils in normal cultures6, 22–23, it was examined which of those factors that regulate activated HSC phenotypes were involved in collagen fibrillogenesis. We found that TGF-β1, which is activated and secreted in response to liver injury7 and showed elevated activity in fibronectin-null livers (Fig. 2C), was the most potent factor. The addition of TGF-β1 to fibronectin-null HSCs in culture at concentrations as low as 2 pM (50 pg/ml) induced type I collagen fibril network formation. Other cytokines/growth factors such as platelet-derived growth factor (PDGF)-AA (mitogenic factor; up to 800 pM = 20 ng/ml) and amphiregulin (injury-protective factor; up to 3,500 pM = 40 ng/ml) had no effect on collagen fibril assembly (Supplementary Fig. 4).
There is evidence that TGF-β1 upregulates Col5a1 chain mRNA level during osteogenesis29. Of the 20 type I/III collagen assembly-related molecules examined (Supplementary Table 1), Col5a1 had the strongest response to TGF-β1: TGF-β1 addition (2 pM) to the media of fibronectin-null HSCs significantly increased Col5a1 mRNA levels (>2.5-fold) in comparison with controls (P < .01; Fig. 4A). The upregulation of Col5a1 mRNA levels was observed at concentrations of TGF-β1 as low as 0.1 pM (data not shown). TGF-β1 (2 pM) was able to induce type V collagen assembly in fibronectin-null HSCs, and underwent type III and type I collagen fibril network formation. In contrast, plasma fibronectin (10 μg/ml) clearly organized type III and type I fibrils but did not induce type V collagen assembly (Fig. 4B,C). When fibronectin-null HSCs were exposed to both TGF-β1 and plasma fibronectin, there was a synergistic effect on type I collagen organization (supplementary Fig. 5), suggesting that TGF-β1 and fibronectin mediate at least in part different pathways to induce collagen assembly. Untreated fibronectin-null and its parental HSC cells expressed similar levels of Col3a1 or Col1a1 mRNAs by real-time PCR and the type I collagen secretion by pulse chase analysis (Supplementary Fig. 3D,E). Thus, these findings suggest that the fibronectin-null HSCs have a defect in the process of fibril assembly or their attachment to the cells as they form the matrices. The addition of exogenous pepsin-treated type V collagen alone in fibronectin-null HSCs could form short and thin type I collagen fibrils but at a lesser extent than TGF-β1 (Supplementary Fig. 6), suggesting that the intracellular process may play an important role in type V collagen-mediated type I collagen fibril network formation30. Matrix metalloproteinase (MMP) inhibitor GM6001 at a concentration up to 25 μM did not support type III and I collagen assembly in fibronectin-null HSCs (data not shown).
Fig. 4. Fibronectin-null HSCs form type V collagen-nucleated type III and type I collagen fibril networks even in the absence of a fibronectin matrix in vitro.
(A) Real-time PCR analysis of Col5a1 mRNAs in fibronectin-null HSCs. Cells were incubated for 12 hrs with plasma fibronectin (pFn, 10 μg/ml) or TGF-β1 (2 pM). mRNA expression levels are shown relative to the control value of 1.0 (no addition) (n = 4 for each group). Note that TGF-β1 significantly upregulates Col5a1 mRNA levels, whereas plasma fibronectin does not have any remarkable effect. **, P< .01.
(B and C) TGF-β1 induces type III, type I, and type V collagen assembly, whereas plasma fibronectin induces assembly of type III and type I but not type V collagen in fibronectin-null HSCs.
(B) Double immunofluoresent staining for type III (in red), type I (in red), or type V collagen (in green) and DAPI (in blue) in control and fibronectin-null HSCs. Cells were incubated for 18 hrs with plasma fibronectin (pFn, 10 μg/ml) or TGF-β1 (2 pM). For type V collagen stainings, the same images for type V single staining (black and white) are also shown. Bar = 50 μm.
(C) Analysis of intensity in assembled collagen fibrils in fibronectin-null HSCs shown in Fig. 4B. Relative fluorescence intensities are shown relative to each control value of 100 (no addition). **, P< .01.
(D–F) Effect of Col5a1 siRNA on fibronectin-null HSC phenotypes.
(D) Real-time PCR analysis of Col5a1 mRNA levels in fibronectin-null HSCs at 27 hrs after transfection. Relative mRNA expression levels are shown relative to the control value of 1.0 (scrambled dsRNA transfectants) (n = 3 for each group). Note that siRNA leads to a significant knockdown (~75%) of Col5a1 mRNA levels in fibronectin-null HSCs. **, P< .01.
(E and F) Effect of Col5a1 siRNA on TGF-β1 (2 pM)-induced (E) or plasma fibronectin (10 μg/ml)-mediated (F) type I and type III collagen assembly. Left panels: Deposition and assembly of collagen fibrils (type I and type III collagen in red; DAPI in blue) in fibronectin-null HSCs at 45 hrs after transfection by immunofluorescent staining. Note that Col5a1 siRNA almost completely inhibits TGF-β1-induced collagen network formation, whereas it does not remarkably affect plasma fibronectin-mediated collagen network formation. Control (vehicle only), treated with Lipofectamine only; Scr dsRNA, scrambled double-stranded RNA transfectant. Bar = 50 μm. Right panels: Analysis of intensity in assembled collagen fibrils. Relative fluorescence intensities are shown relative to each control value of 100 (cells transfected with scrambled dsRNA and TGF-β1 [2 pM] added [E]; cells transfected with scrambled dsRNA and plasma fibronectin [10 μg/ml] added [F]). The background fluorescence intensity measured in control (vehicle only) is subtracted from each value. **, P< .01.
It was further examined whether type V collagen is directly involved in de novo type I and type III collagen network formation. Transfection of Col5a1 siRNA into fibronectin-null HSCs led to the efficient knockdown of Col5a1 mRNA levels at 27 hrs (~75%; P < .01) and maintained ~55% reduction 45 hrs later compared to scrambled dsRNA transfectants (Fig. 4D). Accordingly, the siRNA treatment of fibronectin-null HSCs resulted in the reduction of TGF-β-induced Col5a1 mRNA levels (~50%) and significant inhibition of type I and type III collagen assembly. No obviously assembled fibril networks were detected with immunocytochemistry (~93% inhibition of type I and ~95% inhibition of type III fibril formation, respectively, P < .01; Fig. 4E). In contrast, Col5a1 siRNA did not affect plasma fibronectin-mediated type III and I collagen assembly even though it reduced Col5a1 mRNA level to the same extent (~50%) (Fig. 4F). Scrambled dsRNA had no affect on these phenotypes. Thus, these findings demonstrate that type V collagen functions to generate type III/I collagen fibril assembly even in the absence of a fibronectin matrix. Interestingly, this phenotype seems to be specific for adult HSCs. When fibronectin-null embryonic fibroblasts were exposed to plasma fibronectin in culture, type III and type I collagen fibril networks were clearly organized in response to plasma Fn without a type V collagen network formation. In contrast, TGF-β1 at concentrations from 2 – 500 pM supported neither type V collagen nor type III and type I collagen fibril assembly in this cell type (Supplementary Fig. 7).
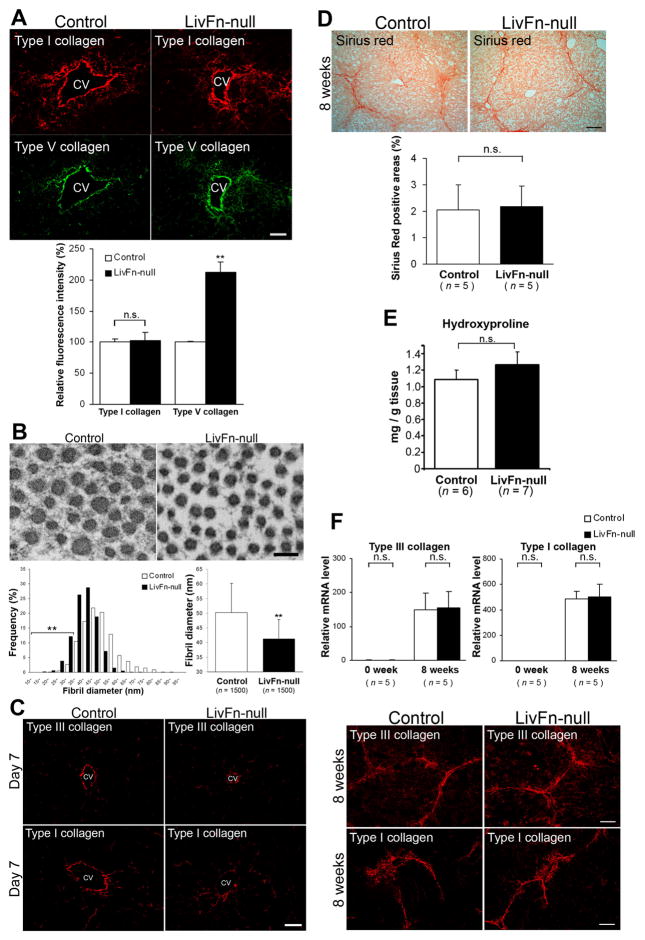
In vivo analysis after acute liver injury revealed significantly more extensive deposition and assembly of type V collagen in fibronectin-null livers at day 5 in comparison with the controls (P < .01; Fig. 5A). The localization of assembled type V collagen fibrils in fibronectin-null livers often overlapped with type I fibrils. Subsequent analysis of collagen fibril ultrastructure demonstrated that the thinner fibril subpopulation (<35 nm diameter) was significantly increased in the fibronectin-null livers at day 5 after injury (16.8% in mutant vs. 3.2% in control: 5.3-fold, P < .01; Fig. 5B). As a consequence, the average diameter of fibrils in fibronectin-null livers was smaller than control livers (50.2 ± 10.0 nm in control vs. 41.2 ± 6.7 nm in mutant, P < .01; Fig. 5B). Newly organized collagen networks were nearly completely cleared from both control and mutant livers by day 7 (Fig. 5C).
Fig. 5. Collagen fibril organization in control and fibronectin-null livers in vivo.
(A–C) In response to acute liver injury.
(A) Upper panels: Deposition and assembly of type I (in red) and type V (in green) collagens at day 5 after injury by immunofluorescent staining. CV, central vein. Bar = 50 μm. Lower panel: Analysis of intensity in assembled collagen fibrils. Relative fluorescence intensities are shown relative to each control value of 100 (control liver). **, P< .01.
(B) Ultrastructural analysis of collagen fibrils at day 5 after injury. Upper panels: Transmission electron micrographs of transverse sections. Bar = 100 nm. Lower left panel: Morphometric analysis of collagen fibril diameter in control and fibronectin-null livers at day 5 after injury (1,500 fibrils for each group). Note that there is a significantly increased thinner fibril subpopulation (<35 nm diameter) in fibronectin-null livers. **, P< .01. Lower right panel: The average diameter of collagen fibrils in control and fibronectin-null livers (1,500 fibrils for each group). Note that the fibrils in fibronectin-null livers (41.2 ± 6.7 nm) show significantly smaller diameter than controls (50.2 ± 10.0 nm). **, P< .01.
(C) Deposition and assembly of type III and type I collagen fibrils at day 7 by immunofluorescent staining. Bar = 50 μm.
(D–F) The initial fibrogenic response to chronic liver injury.
(D) Upper panels: Sirius-Red staining. Bar = 100 μm. Lower panel: Quantification of fibrotic areas (n = 5 for each group).
(E) No significant differences in hepatic hydroxyproline content between control and fibronectin-null livers (n = 6 for control; n = 7 for mutant).
(F) Upper panels: Real-time PCR analysis of Col3a1 and Col1a1 mRNAs. Relative mRNA expression levels are shown relative to the control value of 1.0 at 0 week (untreated) (n = 5 for each group). Lower panels: Deposition and assembly of type III and type I collagen fibrils by immunofluorescent staining. Bar = 50 μm.
Finally, the initial fibrogenic response to chronic liver injury induced by CCl4 was examined18. It was confirmed that there was no apparent fibronectin protein induction in fibronectin-null livers after injury by Western analysis (Supplementary Fig. 8). Histologically, the bridging-fibrosis formation was found in both livers with the same extents and there were no obvious differences in fibrotic areas by Sirius-Red staining (Fig. 5D). Serum alanine aminotransferase (ALT) and total bilirubin levels, and albumin/globulin ratio were not significantly different between control and mutant livers after CCl4 treatment (Supplementary Fig. 9)31. Furthermore, any difference was not detected between control and fibronectin-null livers in hepatic hydroxyproline content (Fig. 5E), Col3a1 and Col1a1 mRNA levels, and deposition and assembly of type III and type I collagens (Fig. 5F).
Discussion
Based on the experimental evidence showing that the preformed fibronectin matrix is required for in vitro collagen network formation6,22–23, we investigated in the present study whether fibronectin was a suitable molecular target for preventing the initial fibrogenic response to liver damage. We found that fibronectin-deficiency resulted in elevated local TGF-β activity and formed collagen fibril networks like control livers following injury.
A number of genetic studies suggest that the absence of or a mutation in LTBP-binding ECMs such as fibrillin-1 results in increased TGF-β activity and Smad signaling, whereas the absence of or mutation in LTBPs results in decreased activity32–36. These results support the hypothesis that the LTBPs and their binding molecules such as fibronectin determine the spatial localization of LTBPs in tissues, thereby regulating the activating mechanisms of TGF-β. We have identified two different mechanisms of latent TGF-β activation in response to liver injury: one mediated by TSP-1 and the other, more dominant one, mediated by β6 integrin. Our findings imply that fibronectin regulates the balance of the active and inactive (latent) TGF-β, which in turn modulates ECM production and remodeling, and consequently maintains adult liver homeostasis. Indeed, mouse models of TGF-β1 overexpression exhibit dominant phenotypes such as advanced liver fibrosis37–38.
Our present study provides compelling evidence that collagen fibrillogenesis in adult tissues in response to damage is mediated by both fibronectin and type V collagen. Type V collagen-null mouse embryos display defects in collagen fibril formation in the mesenchyme30. These results support the hypothesis that both fibronectin and type V collagen play a critical role in collagen fibrillogenesis in embryonic development and adult tissue remodeling, the extent according to the temporal and spatial expression pattern of each molecule. Interestingly, type V collagen-mediated type III/I collagen fibril assembly in response to liver injury seems to be specific for adult HSCs since TGF-β1 supported neither type V collagen nor type III/I collagen fibril assembly in fibronectin-null embryonic fibroblasts. The diameter of formed type I collagen fibrils is inversely proportional to the type V/type I collagen ratio, and adult Col5a1(+/−) mouse dermis contains a larger and abnormal population of collagen fibrils30,39–40. Indeed, our ultrastructural analysis of collagen fibrils provides evidence that type V-mediated collagen fibrils in fibronectin-null livers contain a significantly increased number of the thinner subpopulation in response to acute liver injury. Taken together, these findings suggest that the excess type V collagen could result in a heterotypic type I collagen assembly. The contribution of type V collagen-nucleated collagen fibrillogenesis in adult tissues is largely undetermined. It remains to be elucidated clinically whether newly reconstructed ECMs induced by initial type V collagen deposition contribute to the critical turning point from normal to abnormal healing. Our current study, therefore, has identified two players, TGF-β and type V collagen that regulate the initial fibrogenic response to liver damage.
Although a prominent expression of fibronectin is observed during tissue repair, the contribution of fibronectin to an adult ECM remodeling, particularly to collagen fibrillogenesis, has been remained as an unsolved question5,9–10,41. Our results have wiped out the long-standing concept that collagen fibril organization requires the prior assembly of fibronectin matrix6,22–23, and the further interpretation that fibronectin matrix is probably serving as a scaffold for collagen fibril organization. Our present study has demonstrated that fibronectin scaffold is not always essential for tissue remodeling and a certain cell type can assemble collagen fibril networks in the complete absence of fibronectin in vivo.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by National Institutes of Health research grant DK074538 and The Cleveland Clinic (to T.S.).
We are grateful to Dr. Claudio Fiocchi for critical reading of the manuscript and valuable suggestions. We thank Dr. Klaus Rajewsky for Mx-Cre mice, Dr. Dean Sheppard for β6 integrin-knockout mice, Dr. Jack Lawler for TSP-1-knockout mice, Dr. Dusko Ilic for p53- and p21-knockout mice, Dr. Deane Mosher for fibronectin-null embryonic fibroblasts, and Drs. Koichi Matsuzaki and Lynn Sakai for antibodies. We also thank Dr. Peter Yurchenco for initial electron microscopic investigations, and Dr. Shin Hasegawa for support. Some preliminary studies were initiated in the laboratory of Reinhard Fässler, at the Department of Experimental Pathology, Lund University, Sweden. This work was generated as an independent new project and supported by National Institutes of Health research and The Cleveland Clinic (to T.S.).
Abbreviations
- αSMA
α-Smooth muscle cell actin
- CBB
coomassie brilliant blue
- CCl4
carbon tetrachloride
- DAPI
4′6-diamidino-2-phenylindole
- DMEM
Dulbecco’s modified Eagle’s medium
- ECM
extracellular matrix
- ES cells
embryonic stem cells
- FBS
fetal bovine serum
- FIAU
1-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)-5-iodouracil
- FITC
fluorescein isothiocyanate
- Fn[fl/fl]
floxed fibronectin gene
- GAPDH
glyceraldehyde 3-phosphate dehydorgenas
- HSC
hepatic stellate cell
- LTBP
latent TGF-β-binding protein
- MMP
matrix metalloproteinase
- mAb
monoclonal antibody
- PCR
polymerase chain reaction
- PDGF
platelet-derived growth factor
- pAb
polyclonal antibody
- pI-pC
polyinosinic-polycytidic acid
- PVDF
polyvinylidene fluoride
- TGF-β
transforming growth factor-β
- TSP-1
thrombospondin-1
Footnotes
Conflict of interest: The authors disclose no conflicts.
Author contributions:
Takao Sakai conceived ideas, designed experiments, and supervised the project. Kazuhisa Honda, Keiko Sakai, Hiroshi Kamisoyama and Takao Sakai generated fibronectin-floxed mice. Kei Moriya, Eunnyung Bae, Keiko Sakai, Takehisa Sakaguchi, and Ikuko Tsujimoto performed experiments. Douglas Keene carried out electron microscopic studies. Takako Sasaki generated anti-type I, III and V collagen, LAP, and LTBP-3 and 4 antibodies. Kei Moriya and Takao Sakai analyzed the data. Takao Sakai wrote and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–9. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 4.Bedossa P, Paradis V. Liver extracellular matrix in health and disease. J Pathol. 2003;200:504–15. doi: 10.1002/path.1397. [DOI] [PubMed] [Google Scholar]
- 5.Clark RAF. The molecular and cellular biology of wound repair. Plenum Press; 1996. [Google Scholar]
- 6.Velling T, Risteli J, Wennerberg K, et al. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277:37377–81. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- 7.Gressner AM, Weiskirchen R, Breitkopf K, et al. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793–807. doi: 10.2741/A812. [DOI] [PubMed] [Google Scholar]
- 8.Schwarzbauer JE. Fibronectin: from gene to protein. Curr Opin Cell Biol. 1991;3:786–91. doi: 10.1016/0955-0674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- 9.Mosher DF. Fibronectin. Academic Press; 1989. [Google Scholar]
- 10.Hynes RO. Fibronectins. Springer-Verlag; 1990. [Google Scholar]
- 11.Dallas SL, Sivakumar P, Jones CJ, et al. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J Biol Chem. 2005;280:18871–80. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- 12.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–12. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 13.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–24. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 14.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakai T, Johnson KJ, Murozono M, et al. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med. 2001;7:324–30. doi: 10.1038/85471. [DOI] [PubMed] [Google Scholar]
- 16.Lawler J, Sunday M, Thibert V, et al. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101:982–92. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang XZ, Wu JF, Cass D, et al. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–8. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Constandinou C, Henderson N, Iredale JP. Modeling liver fibrosis in rodents. Methods Mol Med. 2005;117:237–50. doi: 10.1385/1-59259-940-0:237. [DOI] [PubMed] [Google Scholar]
- 19.Schafer S, Zerbe O, Gressner AM. The synthesis of proteoglycans in fat-storing cells of rat liver. Hepatology. 1987;7:680–7. doi: 10.1002/hep.1840070411. [DOI] [PubMed] [Google Scholar]
- 20.Howerton K, Schlaepfer DD, Ilic D. Establishment of cell lines from mouse embryos with early embryonic lethality. Cell Commun Adhes. 2008;15:379–83. doi: 10.1080/15419060802440054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–53. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- 22.Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell. 2002;13:3546–59. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sottile J, Shi F, Rublyevska I, et al. Fibronectin-dependent collagen I deposition modulates the cell response to fibronectin. Am J Physiol Cell Physiol. 2007;293:C1934–46. doi: 10.1152/ajpcell.00130.2007. [DOI] [PubMed] [Google Scholar]
- 24.Sechler JL, Takada Y, Schwarzbauer JE. Altered rate of fibronectin matrix assembly by deletion of the first type III repeats. J Cell Biol. 1996;134:573–83. doi: 10.1083/jcb.134.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 26.Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- 27.Jarnagin WR, Rockey DC, Koteliansky VE, et al. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–48. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachem MG, Meyer D, Melchior R, et al. Activation of rat liver perisinusoidal lipocytes by transforming growth factors derived from myofibroblastlike cells. A potential mechanism of self perpetuation in liver fibrogenesis. J Clin Invest. 1992;89:19–27. doi: 10.1172/JCI115561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahai S, Vary CP, Gao Y, et al. Collagen, type V, alpha1 (COL5A1) is regulated by TGF-beta in osteoblasts. Matrix Biol. 2004;23:445–55. doi: 10.1016/j.matbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Wenstrup RJ, Florer JB, Brunskill EW, et al. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–7. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- 31.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–51. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neptune ER, Frischmeyer PA, Arking DE, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–11. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 33.Koli K, Wempe F, Sterner-Kock A, et al. Disruption of LTBP-4 function reduces TGF-beta activation and enhances BMP-4 signaling in the lung. J Cell Biol. 2004;167:123–33. doi: 10.1083/jcb.200403067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshinaga K, Obata H, Jurukovski V, et al. Perturbation of transforming growth factor (TGF)-beta1 association with latent TGF-beta binding protein yields inflammation and tumors. Proc Natl Acad Sci U S A. 2008;105:18758–63. doi: 10.1073/pnas.0805411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loeys BL, Gerber EE, Riegert-Johnson D, et al. Mutations in fibrillin-1 cause congenital scleroderma: stiff skin syndrome. Sci Transl Med. 2010;2:23ra20. doi: 10.1126/scitranslmed.3000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazzieri R, Jurukovski V, Obata H, et al. Expression of truncated latent TGF-beta-binding protein modulates TGF-beta signaling. J Cell Sci. 2005;118:2177–87. doi: 10.1242/jcs.02352. [DOI] [PubMed] [Google Scholar]
- 37.Sanderson N, Factor V, Nagy P, et al. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci U S A. 1995;92:2572–6. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueberham E, Low R, Ueberham U, et al. Conditional tetracycline-regulated expression of TGF-beta1 in liver of transgenic mice leads to reversible intermediary fibrosis. Hepatology. 2003;37:1067–78. doi: 10.1053/jhep.2003.50196. [DOI] [PubMed] [Google Scholar]
- 39.Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32:223–37. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 40.Adachi E, Hayashi T. In vitro formation of hybrid fibrils of type V collagen and type I collagen. Limited growth of type I collagen into thick fibrils by type V collagen. Connect Tissue Res. 1986;14:257–66. doi: 10.3109/03008208609017469. [DOI] [PubMed] [Google Scholar]
- 41.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.