Abstract
Background & Aims
Children who receive cancer therapy experience numerous acute gastrointestinal (GI) toxicities. However, the long-term GI consequences have not been extensively studied. We evaluated the incidence of adverse long-term GI outcomes and identified treatment-related risk factors.
Methods
Upper GI, hepatic, and lower GI adverse outcomes were assessed in cases randomly selected from participants in the Childhood Cancer Survivor Study, a study of 14,358 survivors of childhood cancer who were diagnosed between 1970 and 1986; data were compared with those from siblings. The median age at cancer diagnosis was 6.8 years (0–21.0 years), the median age at outcome assessment was 23.2 years (5.6–48.9 years) for survivors and 26.6 years (1.8–56.2 years) for siblings. Rates of self-reported, late complications of the GI tract (occurred 5 or more years after cancer diagnosis) were determined and associated with patient characteristics and cancer treatments, adjusting for age, sex, and race; data were compared with those from siblings.
Results
Compared with siblings, survivors had increased risk for late-onset complications of the upper GI tract (relative risk [RR]=1.8; 95% confidence interval [CI], 1.6–2.0), liver (RR=2.1; 95% CI, 1.8–2.5), and lower GI tract (RR=1.9; 1.7–2.2). The RR for requiring colostomy, ileostomy, or liver biopsy, or for developing liver cirrhosis were 5.6 (95% CI, 2.4–13.1), 24.1 (95% CI, 7.5–77.8), and 8.9 (95% CI, 2.0–40.0), respectively. Older age at diagnosis, intensified therapy, abdominal radiation, and abdominal surgery increased the risk of certain GI complications.
Conclusions
Individuals who received therapy for cancer during childhood have an increased risk of developing GI complications later in life.
Keywords: tumor, chemotherapy, side effect, toxicity, pediatric
Introduction
While various disease-specific combinations of chemotherapy, radiation, and surgery have dramatically improved survival, these treatment modalities have the potential to cause significant gastrointestinal (GI) complications. 1–3 Abdominal radiation often results in several acute toxicities, including enteritis and abnormal motility of the intestinal tract. Chemotherapy is associated with many acute GI toxicities, including nausea, vomiting, diarrhea, constipation, and increased susceptibility to gastrointestinal infections. Intra-abdominal surgery and subsequent GI complications are also contributors to GI toxicity of cancer therapy. However, there is a paucity of data regarding the long-term GI complications in childhood cancer survivors.
The Childhood Cancer Survivor Study (CCSS) is a large cohort study of over 14,000 survivors of childhood cancer that offers a unique opportunity to study GI late-effects. The goals of this analysis were to describe the incidence of self-reported adverse GI conditions occurring at least five years after diagnosis and to evaluate the effect of different treatment-associated factors on the risk of developing these GI events.
Patients and Methods
Inclusion Criteria
The CCSS is a retrospective cohort of survivors of childhood leukemias, brain tumors, lymphomas, Wilms’ tumor, neuroblastoma, sarcomas, and bone tumors diagnosed between 1970 and 1986 at one of 26 collaborating institutions in the US and Canada (Supplement 1).4 Eligibility for the CCSS includes age less than 21 years at diagnosis and survival of at least 5 years from diagnosis, independent of disease status.
Approval for the study was obtained from the human subjects committee at each collaborating institution. Consent was obtained from patients (or their proxy) to participate in the study and to release information from their medical records.
Collaborating institutions identified 20,691 5-year survivors who met eligibility criteria. Of these, 14,358 completed a questionnaire or telephone interview, 3,204 declined to participate, 3,058 were lost to follow-up, 6 are currently being recruited having been recently located, and 65 patients were unable to participate due to a language barrier. We previously compared demographic and cancer-related characteristics among participants, non-participants and those lost to follow-up, and found that these 3 groups were similar.5, 6 A group of 5,857 closest-aged siblings of randomly-selected CCSS survivor participants were invited to participate in the survey. Of these, 4,023 siblings completed the survey and serve as the comparison group in this analysis. Siblings were not matched to cases. We did account for potential within-family correlation in the analysis.
Data Collection
At the time of enrollment, a comprehensive “baseline questionnaire” was completed by the participant (if age ≥18 years) or his/her parent (if age <18 years). Surveys were distributed by mail or administered by phone using trained interviewers. Survey questions regarding various GI conditions began with the phrase, “have you ever been told by a doctor or other health care professional that you have or have had. . . ?” If a participant gave a “yes” response to a specific GI condition, they were then asked the age of first diagnosis of the GI condition.
Three types of GI outcomes were considered: (1) upper GI complications, (2) liver conditions, and (3) lower GI complications. Upper GI complications included: ulcer, esophageal disease, frequent indigestion or heartburn, nausea/vomiting, or other upper GI trouble. Liver conditions included: gallstone or other gall bladder issues, liver cirrhosis, jaundice, liver biopsy, or other liver trouble. Lower GI complications included intestinal polyps or diverticular disease, colitis, frequent constipation, chronic diarrhea, fisutula or stricture, colostomy or ileostomy, or other lower intestinal trouble. A response indicating a problem to any component of an aggregated variable was considered a “yes” for that GI outcome.
Cancer treatment information was abstracted from medical records at the participating institutions. Information on cancer therapy included in this analysis is that of the initial therapy and treatment for any relapse within 5 years of diagnosis. Data regarding exposure to 42 chemotherapeutic agents (either yes or no) were abstracted and cumulative doses were calculated for selected agents. The total exposure to alkylating agents (carmustine, busulfan, lomustine, chlorambucil, cyclophosphamide, ifosfamide, melphalan, nitrogen mustards, procarbazine, and thiotepa) was measured by calculating an alkylating agent score. The total dose in milligrams per square meter was calculated for each subject. The distribution of the doses received by all subjects in the CCSS cohort was determined for each alkylating agent, and an overall alkylating agent score of 0, 1, 2, or 3 was assigned.7 Surgeries performed for cancer treatment, site of tumor(s), and fields of radiation therapy were recorded. Abdominal surgeries included those considered intra-abdominal by ICD-9 codes reported on the medical records abstraction form. Survivors treated with abdominal radiation or total body irradiation (TBI) were identified after central review at the Radiation Physics Center at MD Anderson Cancer Center. The baseline questionnaire and medical-record abstraction form are available at http://ccss.stjude.org.
Statistical Analysis
Demographics and treatment characteristics of the 5-year childhood cancer survivors were tabulated. Incidence rates of each GI outcome following the 5-year survival were estimated by dividing the observed count of the GI outcome (only first events were counted for the composite-event outcomes) by the person years at risk for the first occurrence of the GI outcome. Subjects were followed from the 5th anniversary of the original diagnosis and censored at the time of the survey completion, or at death, which ever occurred first. Starting at five years, cumulative incidence was calculated individually for upper GI complications, liver conditions, lower GI complications, or at least one GI condition/complication, including those which occurred within the first 5 years from the diagnosis as prevalent cases. An adjusted rate ratio (RR) for developing each late GI event (at least 5 years after diagnosis), comparing survivors to siblings, was estimated by Poisson regression, adjusting for age at the time of the study, gender, and race. Potential within-family correlation was accounted for by the Generalized Estimating Equation.8
Unadjusted and adjusted Poisson regression analysis was performed for each late-onset GI outcome to evaluate effect of various factors including age at diagnosis, therapy factors, relapse within 5 years of the original cancer, and TBI. The factors included in the multivariable models were selected by the backward selection among a pre-specified set of factors that included age at diagnosis, abdominal radiation, alkylating agents score, vincristine exposure (yes/no), anthracycline dose, abdominal surgery, recurrence, and TBI. We checked a-priori hypothesized two-way interactions between TBI, abdominal surgery, abdominal radiation, alkylating agent score, and anthracycline exposure: no interaction was statistically significant. All treatment exposures within five years from original diagnosis of cancer were included. Because 19% and 16% of dates of reported GI events were missing among survivors and siblings, respectively, multiple imputations under the assumption of “missing at random” 9 were used to impute age at first occurrence of a GI outcome if a “yes” response was recorded without an age at first diagnosis.10 We used multiple-imputation methodology for event-time imputations using the method of Taylor et al. 11 with slight modifications. This imputation was repeated ten times creating ten complete datasets without missing values of age. Each analysis was conducted ten times using the ten datasets and the results were summarized by standard method for combining multiple-imputation analyses.12
Results
Study population characteristics
General demographic information for patients and siblings, in addition to basic treatment information for patients, is shown in Table 1. The median age at diagnosis of initial cancer was 6.8 years (0–21.0). The median age at outcome assessment was 23.2 years (5.6–48.9) for survivors and 26.6 years (1.8–56.2) for siblings. The median follow-up time from cancer diagnosis to death or survey was 14.8 years (range = 5.0 – 31.1 years). In general, survivors were slightly younger, more likely to be male, and more often reported being non-Caucasian than siblings .
Table 1.
Characteristics of Childhood Cancer Survivors and the Sibling Comparison Group
| Characteristics | Survivors (N=14358) No. (%) |
Siblings (N=3899) No. (%) |
|---|---|---|
| Age at interview (years) | ||
| <20 | 5237 (36.5%) | 1092 (27.1%) |
| 20–29 | 5885 (41.0%) | 1418 (35.2%) |
| 30–39 | 2904 (20.2%) | 1170 (29.1%) |
| 40+ | 332 (2.3%) | 343 (8.5%) |
| Sex | ||
| Male | 7713 (53.7%) | 1937 (48.1%) |
| Female | 6645 (46.3%) | 2086 (51.9%) |
| Race/Ethnicity | ||
| White, NH* | 11943 (83.1%) | 3509 (87.2%) |
| Black, NH | 713 (5.0%) | 113 (2.8%) |
| Hispanic/Latino | 715 (5.0%) | 148 (3.7%) |
| Other | 987 (6.9%) | 253 (6.3%) |
| Vital status at time of interview | ||
| Alive | 13173 (91.7%) | |
| Dead | 1185 (8.3%) | |
| Age at diagnosis (years) | ||
| <3 | 3346 (23.3%) | |
| 3–9 | 5610 (39.1%) | |
| 10+ | 5402 (37.6%) | |
| Diagnosis | ||
| Acute Lymphoblastic Leukemia | 4329 (30.2%) | |
| Acute Myelogenous Leukemia | 356 (2.5%) | |
| Other Leukemia | 145 (1.0%) | |
| Astrocytoma | 1182 (8.2%) | |
| Medulloblastoma/PNET | 381 (2.7%) | |
| Other CNS Tumor | 314 (2.2%) | |
| Hodgkin’s Disease | 1927 (13.4%) | |
| Non-Hodgkin’s Lymphoma | 1080 (7.5%) | |
| Kidney Tumor | 1256 (8.7%) | |
| Neuroblastoma | 954 (6.6%) | |
| Soft Tissue Sarcoma | 1246 (8.7%) | |
| Ewing Sarcoma | 403 (2.8%) | |
| Osteosarcoma | 733 (5.1%) | |
| Other Bone Tumor | 52 (0.4%) | |
| Recurrent Disease | ||
| yes | 1563 (10.9%) | |
| no | 12795 (89.1%) | |
| Total Body Irradiation* | ||
| yes | 184 (1.5%) | |
| no | 12375 (98.5%) | |
| Abdominal Primary Tumor | ||
| yes | 1747 (12.2%) | |
| no | 12611 (87.8%) | |
| Abdominal Radiation * | ||
| yes | 3786 (30.1%) | |
| no | 8773 (69.9%) | |
| Chemotherapy* | ||
| yes | 10119 (80.5%) | |
| no | 2455 (19.5%) | |
| Alkylating Agents Score* | ||
| None (AA Score=0) | 5814 (52.4%) | |
| Low dose (AA Score=1) | 2509 (22.6%) | |
| Medium dose (AA Score=2) | 1657 (14.9%) | |
| High dose (AA Score=3) | 1125 (10.1%) | |
| Vincristine* | ||
| yes | 9026 (71.8%) | |
| no | 3548 (28.2%) | |
| Anthracycline* | ||
| None | 7595 (63.1%) | |
| ≤100 | 480 (4.0%) | |
| 101–200 | 951 (7.9%) | |
| 201–300 | 913 (7.6%) | |
| >300 | 2106 (17.5%) | |
| Abdominal Surgery* | ||
| yes | 3802 (30.3%) | |
| no | 8754 (69.7%) |
Note: some variables had missing values (such as race and treatment data), the numbers and percentages are based on available data only
Most (62.4%) survivors were less than 10 years of age at cancer diagnosis. Almost all survivors (91.7%) were alive at the time of interview. Primary diagnostic groups included: leukemia/lymphoma (54.6%), bone or soft-tissue sarcoma (16%), brain tumor (13.1%), kidney tumor (8.7%), and neuroblastoma(6.6%).
Of the three main modalities of therapy relevant to this analysis (i.e., chemotherapy, abdominal radiation, and abdominal surgery), 49.4% of the patients were treated with a single modality, 23.2% with 2 modalities, 15.0% with 3.modalities, and 12.4% received none. The majority of patients (80.5%) were treated with chemotherapy. Among patients who received chemotherapy, 89.2% received vincristine, 52.3% received alkylating agents, and 44.0% were treated with anthracyclines. Abdominal radiation was utilized in 30.1%. Only 1.5% underwent TBI.
The overall cumulative incidence of any GI condition was 37.6% at 20 years from the childhood cancer diagnosis (Figure 1). Upper GI complications were most common with a cumulative incidence of 25.8% at 20 years, followed by lower GI complications (15.5%% at 20 years), and liver conditions (9.4% at 20 years).
Figure 1.
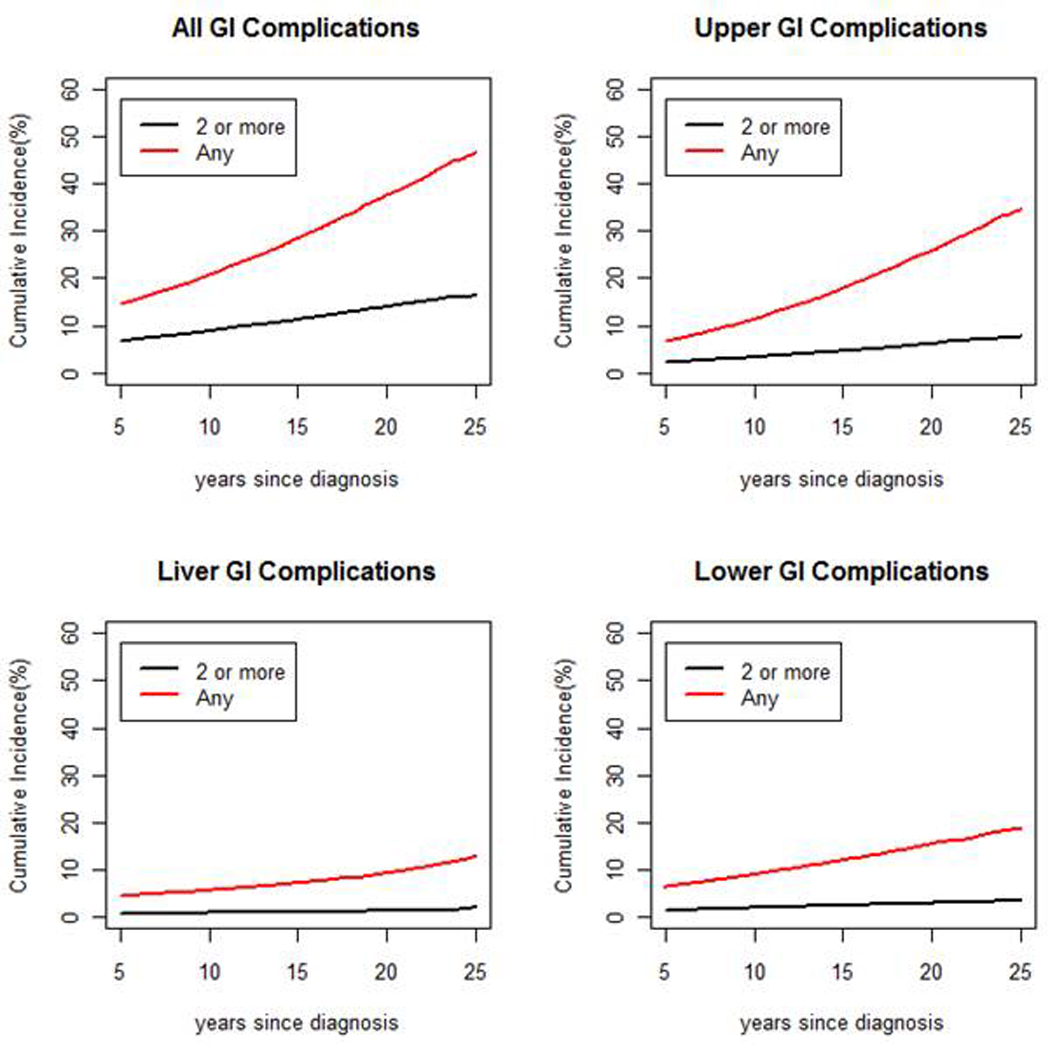
Cumulative incidence of GI conditions (Any = red, 2 or more = black) among 5 year survivors, with cumulative incidence [%] on the vertical axis and years since diagnosis on the horizontal axis.
Upper GI Complications
A total of 3,811 upper GI complications were reported (Table 2) by 2,884 patients. Of the upper GI complications, 2,642 complications (69.3%) developed late (at least 5 years after diagnosis). Late onset of frequent heartburn and indigestion were more frequent (11.2 per 1,000 person-years) than that of other upper GI complications (3.0 per 1,000 person-year). Relative to siblings, survivors were at elevated risk for all late onset upper GI complication categories with rate ratios ranging from 1.5 for ulcers to 3.3 for frequent nausea and vomiting. Among the 2,884 patients who reported any upper GI complication, only 24.7% reported 2 or more upper GI complications.
Table 2.
Occurrence of Late Adverse Gastrointestinal Outcomes by Time Period
| Outcome Occurred After Cancer Diagnosis |
Outcome Occurred 5+ Years from Cancer Diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|
| Condition | Sur yes # |
Sibs Yes # |
Yes | Rate‡ | 95% CI | RR§ | 95% CI | |
| Upper GI Complications | Ulcer | 618 | 141 | 401 | 2.8 | 2.5 – 3.1 | 1.5 | 1.3 – 1.9 |
| Esophageal Disease | 289 | 47 | 190 | 1.3 | 1.1 – 1.5 | 2.6 | 1.8 – 3.5 | |
| Indigestion/ Heartburn | 2087 | 495 | 1524 | 11.2 | 10.6 – 11.8 | 1.8 | 1.6 – 1.9 | |
| Nausea/ Vomiting | 192 | 21 | 97 | 0.7 | 0.5 – 0.8 | 3.3 | 2.0 – 5.5 | |
| Other Upper GI Trouble | 625 | 107 | 430 | 3.0 | 2.7 – 3.3 | 2.6 | 2.1 – 3.3 | |
| Liver Conditions | Gallstones & Other Gall Bladder Issues | 338 | 84 | 285 | 1.9 | 1.7 – 2.2 | 2.0 | 1.6 – 2.6 |
| Liver Cirrhosis | 42 | 2 | 27 | 0.2 | 0.1 – 0.3 | 8.9 | 2.0 – 40.0 | |
| Jaundice | 444 | 158 | 84 | 0.6 | 0.5 – 0.7 | 1.3 | 1.0 – 1.8 | |
| Liver Biopsy | 170 | 3 | 103 | 0.7 | 0.6 – 0.9 | 24.1 | 7.5 – 77.8 | |
| Other Liver Trouble | 364 | 11 | 148 | 1.0 | 0.9 – 1.2 | 13 | 6.8 – 24.9 | |
| Lower GI Complications | Intestinal Polyps/Diverticular Disease | 96 | 20 | 75 | 0.5 | 0.4 – 0.6 | 2.2 | 1.3 – 3.7 |
| Colitis | 119 | 51 | 87 | 0.6 | 0.5 – 0.7 | 0.9 | 0.7 – 1.4 | |
| Constipation | 921 | 180 | 407 | 2.9 | 2.6 – 3.2 | 1.8 | 1.5 – 2.2 | |
| Diarrhea | 664 | 91 | 351 | 2.4 | 2.2 – 2.7 | 2.7 | 2.1 – 3.5 | |
| Rectal/Anal Fistula/Stricture/Other Obstruction Surgery | 165 | 41 | 98 | 0.7 | 0.5 – 0.8 | 1.5 | 1.0 – 2.2 | |
| Colostomy/ Ileostomy | 121 | 7 | 43 | 0.3 | 0.2 – 0.4 | 5.6 | 2.4 – 13.1 | |
| Other Lower Intestinal Trouble | 247 | 62 | 185 | 1.3 | 1.1 – 1.5 | 1.8 | 1.4 – 2.5 | |
Abbreviation: RR, rate ratio;
Excludes conditions prior to cancer diagnosis.
Includes “not sure” and missing responses.
Rate per 1,000 person-years.
RR, adjusted for age, sex and race; relative to siblings.
Poisson regression analysis (Tables 3 and 4) revealed several factors that influenced risk of upper GI complications after 5 years from cancer diagnosis. Patients who were treated with abdominal radiation and anthracyclines were at higher risk for upper GI complications. Survivors who were older at cancer diagnosis had higher risk of upper GI complications compared to children treated under 3 years of age, adjusting for abdominal radiation and anthracyclines.
Table 3.
Unadjusted Poisson Regression Analysis of Late-onset GI outcomes
| Upper GI Complication | Liver Complications | Lower GI Complication | ||||
|---|---|---|---|---|---|---|
| RR (95% CI) |
P-value | RR (95% CI) |
P-value | RR (95% CI) |
P-value | |
| Age at Diagnosis | ||||||
| <3 (Referent) | 1.0 | |||||
| 3–9 | 1.2(1.1 – 1.4) | 0.001 | 1.9(1.4 – 2.6) | <.001 | 1.1(0.9 – 1.4) | 0.16 |
| 10+ | 1.5(1.3 – 1.7) | <.001 | 2.6(2.0 – 3.5) | <.001 | 1.4(1.1 – 1.6) | <.001 |
| Abdominal Primary Tumor | ||||||
| No(Ref) | ||||||
| Yes | 0.9(0.8 – 1.0) | 0.18 | 0.8(0.6 – 1.1) | 0.13 | 0.9(0.7 – 1.1) | 0.25 |
| Abdominal Radiation | ||||||
| No(Ref) | ||||||
| Yes | 1.3(1.2 – 1.5) | <.001 | 1.4(1.1 – 1.7) | 0.002 | 1.4(1.2 – 1.6) | <.001 |
| Chemotherapy | ||||||
| No(Ref) | ||||||
| Yes | 1.2(1.0 – 1.3) | 0.03 | 1.9(1.4 – 2.5) | <.001 | 1.1(0.9 – 1.4) | 0.18 |
| Alkylating Agents Score | ||||||
| None(Ref) (AA Score=0) | ||||||
| Low dose(AA Score=1) | 1.1(1.0 – 1.3) | 0.1 | 1.2(0.9 – 1.7) | 0.22 | 1.1(0.9 – 1.4) | 0.2 |
| Medium dose(AA Score=2) | 1.2(1.0 – 1.4) | 0.04 | 1.4(1.0 – 1.9) | 0.05 | 1.2(0.9 – 1.5) | 0.13 |
| High dose(AA Score=3) | 1.3(1.1 – 1.5) | 0.006 | 2.3(1.8 – 3.1) | <.001 | 1.6(1.3 – 2.0) | <.001 |
| Vincristine | ||||||
| None(Ref) | ||||||
| Yes | 1.1(1.0 – 1.2) | 0.21 | 1.4(1.1 – 1.8) | 0.002 | 1.3(1.1 – 1.5) | 0.007 |
| Anthracycline | ||||||
| None(Ref) | ||||||
| ≤100 | 1.1(0.8 – 1.5) | 0.55 | 1.3(0.7 – 2.3) | 0.39 | 1.1(0.7 – 1.7) | 0.63 |
| 101–200 | 1.3(1.1 – 1.6) | 0.003 | 1.2(0.8 – 1.8) | 0.48 | 1.1(0.8 – 1.5) | 0.56 |
| 201–300 | 1.2(1.0 – 1.4) | 0.14 | 2.1(1.6 – 3.0) | <.001 | 1.4(1.1 – 1.9) | 0.01 |
| >300 | 1.2(1.1 – 1.4) | 0.002 | 1.5(1.1 – 1.9) | 0.003 | 1.1(0.9 – 1.3) | 0.44 |
| Abdominal Surgery | ||||||
| No(Ref) | ||||||
| Yes | 1.2(1.1 – 1.3) | 0.002 | 1.3(1.0 – 1.5) | 0.02 | 1.3(1.1 – 1.5) | 0.003 |
| Abdominal Therapy | ||||||
| None(Ref) | ||||||
| Radiation Only | 1.3(0.8 – 1.9) | 0.28 | 1.7(0.7 – 4.2) | 0.25 | 1.4(0.8 – 2.4) | 0.18 |
| Surgery only | 1.4(0.9 – 2.0) | 0.09 | 1.9(0.8 – 4.5) | 0.14 | 0.7(0.3 – 1.4) | 0.33 |
| Chemotherapy only | 1.3(1.1 – 1.6) | 0.001 | 2.2(1.4 – 3.4) | <.001 | 1.0(0.8 – 1.2) | 0.73 |
| Rad+chemo | 1.6(1.3 – 2.0) | <.001 | 2.7(1.7 – 4.4) | <.001 | 1.3(0.9 – 1.7) | 0.13 |
| Rad+Surg | 1.8(1.4 – 2.3) | <.001 | 1.7(0.9 – 3.1) | 0.1 | 0.9(0.6 – 1.4) | 0.8 |
| Surg+Chemo | 1.2(0.9 – 1.5) | 0.2 | 2.3(1.4 – 3.8) | 0.002 | 1.1(0.8 – 1.6) | 0.43 |
| all treatment | 1.7(1.4 – 2.0) | <.001 | 3.1(2.0 – 4.9) | <.001 | 1.5(1.2 – 2.0) | 0.001 |
| Recurrence | ||||||
| No(Ref) | ||||||
| Yes | 1.3(1.1 – 1.5) | <.001 | 1.8(1.4 – 2.3) | <.001 | 1.3(1.0 –1.6) | 0.02 |
| Total Body Irradiation | ||||||
| No(Ref) | ||||||
| Yes | 1.3(0.8 – 2.1) | 0.3 | 3.9(2.3 – 6.8) | <.001 | 1.5(0.7 – 2.9) | 0.27 |
| Diagnose | ||||||
| Leukemia (Ref) | ||||||
| CNS | 0.9(0.8 – 1.1) | 0.27 | 0.5(0.4 – 0.8) | 0.001 | 1.4(1.1 – 1.7) | 0.007 |
| Hodgkin Disease | 1.5(1.3 – 1.8) | <.001 | 1.4(1.1 – 1.8) | 0.005 | 1.6(1.3 – 2.0) | <.001 |
| Non-Hodgkin Lymphoma | 1.2(1.0 – 1.4) | 0.06 | 1.0(0.7 – 1.4) | 0.87 | 1.0(0.8 – 1.4) | 0.84 |
| Kidney (Wilms) | 0.9(0.8 – 1.1) | 0.4 | 0.8(0.5 – 1.1) | 0.14 | 1.0(0.8 – 1.4) | 0.75 |
| Neuroblastoma | 0.7(0.6 – 0.9) | 0.007 | 0.5(0.3 – 0.9) | 0.01 | 0.8(0.6 – 1.2) | 0.27 |
| Soft tissue sarcoma | 1.0(0.9 – 1.2) | 0.73 | 0.9(0.7 – 1.3) | 0.65 | 1.5(1.2 – 1.9) | 0.001 |
| Bone cancer | 1.2(1.0 – 1.5) | 0.01 | 1.1(0.8 – 1.5) | 0.54 | 1.2(0.9 – 1.5) | 0.28 |
Table 4.
Multivariable Poisson Regression Analysis of Late-onset GI outcomes
| Upper GI Complication | Liver Complications | Lower GI Complication | ||||
|---|---|---|---|---|---|---|
| RR (95% CI) |
P-value | RR (95% CI) |
P-value | RR (95% CI) |
P-value | |
| Age at Diagnosis | ||||||
| <3 (Ref) | ||||||
| 3–9 | 1.3(1.1 – 1.5) | <.001 | 2.2(1.5 – 3.2) | <.001 | 1.2(1.0 – 1.5) | 0.09 |
| 10+ | 1.5(1.3 – 1.7) | <.001 | 2.6(1.8 – 3.8) | <.001 | 1.3(1.0 – 1.6) | 0.03 |
| Abdominal Radiation | ||||||
| No(Ref) | ||||||
| Yes | 1.3(1.2 – 1.4) | <.001 | 1.3(1.1 – 1.5) | 0.005 | ||
| Alkylating Agents Score | ||||||
| None(Ref) (AA Score=0) | ||||||
| Low dose(AA Score=1) | 1.0(0.7 – 1.4) | 0.95 | 1.2(0.9 – 1.4) | 0.17 | ||
| Medium dose(AA Score=2) | 1.2(0.9 – 1.6) | 0.32 | 1.2(0.9 – 1.4) | 0.22 | ||
| High dose(AA Score=3) | 1.8(1.3 – 2.4) | <.001 | 1.5(1.1 – 1.8) | 0.002 | ||
| Anthracycline | ||||||
| None(Ref) | ||||||
| ≤100 | 1.1(0.9 – 1.5) | 0.38 | 1.4(0.7 – 2.6) | 0.30 | ||
| 101–200 | 1.3(1.1 – 1.6) | 0.002 | 1.1(0.6 – 1.8) | 0.80 | ||
| 201–300 | 1.1(0.9 – 1.4) | 0.25 | 2.1(1.5 – 3.0) | <.001 | ||
| >300 | 1.2(1.1 – 1.4) | 0.007 | 1.3(1.0 – 1.8) | 0.05 | ||
| Abdominal Surgery | ||||||
| No(Ref) | ||||||
| Yes | 1.3(1.1 – 1.7) | 0.02 | ||||
| Total Body Irradiation | ||||||
| No(Ref) | ||||||
| Yes | 3.8(2.0 – 7.2) | <.001 | ||||
Liver Conditions
Overall 1,358 liver conditions were reported (Table 2) by 1,117 patients. Of these liver conditions, 647 complications (47.6%) developed late (more than 5 years after diagnosis). Late gallbladder disease occurred at the highest rate (1.9 per 1,000 person years) and liver cirrhosis had the lowest rate (0.2 per 1,000 person years). Relative to siblings, patients were at elevated risk for gallbladder disease (RR=2.0; 95% CI 1.6–2.6), liver cirrhosis (RR=8.9,; 95%CI 2.0–40.0) and liver biopsy (RR=24.1,; 95 CI 7.5–77.8). Also, 15.2% patients of the 1,117 who had a liver complication reported 2 or more liver conditions. Older age at diagnosis, increasing alkylating agent exposure, anthracycline use, abdominal surgery, and TBI were all independent risk factors for liver conditions (Table 4).
Lower GI Complications
A total of 2,333 lower GI complications (Table 2) were reported by 1,823 patients. More than half (53.4%) of the lower GI complications were a late onset problem. Among lower GI complaint (Table 2), late onset of frequent constipation was the most common (2.9 per 1,000 person-years). Relative to siblings, survivors were at elevated risk for all late onset lower GI complication categories except for colitis and fistulas/strictures. Survivors were at increased risk for developing any late lower GI complications when compared to siblings (RR=1.9; 95% CI 1.7–2.2). Older age at diagnosis, abdominal radiation, and high dose alkylating agents were treatment-associated factors that increased the risk of lower GI complications (Table 4).
Multiple GI Conditions
To assess whether findings were driven by a smaller group of survivors with multiple abnormalities or a larger group with only one abnormality, we evaluated how many survivors reported 2 or more GI issues. The majority (61.9%) reported only one problem in the 3 GI categories. The relative risk of reporting any GI complication was 1.7 and the relative risk of reporting 2 or more GI complications was similar at 1.6. There was a substantially higher risk of reporting 2 or more liver problems compared to sibling controls (RR 12.2; 4.8–30.7).
Discussion
As more children diagnosed with cancer are surviving long-term, it is increasingly important to recognize the long-term consequence of their cancer and its therapy. This analysis demonstrates that survivors of childhood cancer have a higher incidence of self-reported GI complaints compared with their siblings. Risks for colostomy/ileostomy, cirrhosis or liver biopsy were highest. Older age at diagnosis, exposure to abdominal radiation, and certain chemotherapy treatments increase that risk.
Over 40% of childhood cancer survivors reported a late GI complication by 20 years after cancer therapy. Studies of adult onset cancer survivors support that late GI complications are relatively common. This is particularly true in patients with abdominal or pelvic carcinomas treated with radiation. These GI conditions can have a significant impact on quality of life.13–15 Up to 50% of adult onset cancer patients treated with pelvic radiation report that quality of life is impacted by GI symptoms. Information regarding late GI consequences in childhood cancer is limited. In a study of females treated for pelvic rhabdomyosarcoma, 41 GI late-effects were identified in 18 of 26 patients studied.16 Intestinal complications included strictures, gastritis, incontinence, enteritis, perforation, constipation, and liver conditions. Thus, the CCSS data and other data indicate that late GI complications are a relatively common, and perhaps under-investigated, problem.
The probability of experiencing a late GI consequence was greater in survivors compared to siblings in 16 out the 17 categories evaluated. Cancer therapy has well known acute GI toxicities, including nausea, vomiting, esophagitis, mucositis, enteritis, diarrhea, and liver dysfunction. The survivor’s prior experience may increase their sensitivity to GI related symptoms, but cancer treatments may cause direct damage to the GI organ system. Late GI toxicity may result from repeated injury and scarring of the tissue from chemotherapy or gastrointestinal infections related to chemotherapy-related myelosuppression. Blood products needed during therapy increase the risk of hepatitis.17, 18 Radiation can cause vascular injury and result in intestinal ischemia and/or fibrosis.19, 20 The incidence of intestinal fibrosis is dose dependent, 5% at 40Gy and up to 40% at 60Gy.20 Radiation can also damage abdominal blood vessels and cause clinically significant vascular compromise including impaired growth and stenosis of the abdominal aorta.21 Radiation can also cause long-standing liver damage that is dose and volume dependent.22 Thus, it is not surprising that survivors of childhood cancer have long-term GI complications.
Some of the factors that influenced risk for late GI toxicity were expected and others were not. As expected, exposure to abdominal radiation increased the risk of upper and lower GI complications. However, abdominal radiation did not increase the risk of liver complications. This may reflect the fact that most patients needing abdominal radiation (i.e, neuroblastoma, Wilms tumor) may have had the liver spared from direct and/or high doses of radiation. The liver seems to tolerate relatively smaller doses (<20 Gy) to larger areas of the liver or higher doses to smaller areas of the liver. 23 Patients undergoing BMT with TBI did have a high rate of liver conditions. This may reflect the overall intensity of the therapy and associated increased risk of VOD, infectious complications (including viral hepatitis), and/or GVHD.
Chemotherapy, like radiation, causes toxicity in organs with rapid cell turnover. Alkylators are mutagenic and may cause long-term genetic changes in tissues of organs like the gastrointestinal tract.24 Anthracyclines have a well known association with early and late cardiac damage.25 They also cause significant acute GI toxicities.26 Therefore, it is understandable why alkylators and anthracyclines influenced the risk of late hepatic and lower GI consequences. Consistent with this finding, concurrent chemotherapy in adult patients with cervical carcinoma have a higher risk of bowel damage and late GI toxicity.27
Abdominal surgery may predispose survivors to the development of adhesions and late GI complications. Based on anecdotal long-term reports, small bowel obstruction can be a late sequlae of abdominal surgery.23 However, on multivariate analysis in this study, abdominal surgery was only associated with late hepatic consequences.
The association between older age at diagnosis and increased risk of developing late GI complications was unanticipated. The increasing incidence with increasing age may reflect less resiliency of the GI tissue with age. Alternatively, older patients develop different cancers (e.g., Hodgkin disease, bone sarcoma) that may employ treatments with greater potential for GI toxicity or a greater tendency to use radiation in older children.
The large size of the CCSS cohort and the high participation rates are strengths of our study. Several limitations should also be discussed. The first limitation relates to the self-reported nature of the data and the potential for under- or over-reporting outcomes. While CCSS investigators have had excellent success in validating selected outcomes, such as second malignancies, our success in obtaining complete medical records on a high proportion of survivors for validation of other major outcomes has been limited.28 Previous reports have demonstrated that survivors of stem cell transplantation have the ability to recall many medical outcomes with a relatively good level of sensitivity and specificity 29. Additionally, using siblings as a control could introduce bias. Siblings might be predicted to have more GI complaints than the general populations; if so this would make the results somewhat conservative. Another limitation of this study is that some reported late-effects could be related to recurrence or progression of their cancer. Patients who were alive, but had active disease at 5-years after diagnosis, were included. However, when we added an indicator variable of recurrence to the multivariable model, we found that there were no significant differences in the three outcomes’ rates by recurrence (data not shown). Although this was likely a small number of patients, the methods of data capture do not allow separate analysis of disease state at time of entry. Lastly, it is not possible to distinguish between the direct effects of the cancer treatment and indirect effects (such as hepatitis C infection from transfusion on the liver).
In conclusion, survivors are at elevated risk for ongoing GI complications after therapy. As these GI complications may impact quality of life, health care providers should be aware of potential GI problems in this population as they evaluate acute complaints and plan ongoing follow-up care. Longitudinal follow-up survivors of childhood cancer will help determine the effects of aging on GI symptoms and disease. In addition, the risks of late GI complications may change, as therapy for childhood and adolescent cancer continues to evolve, and will require studies of more recently treated patients.
Supplementary Material
Acknowledgements
We thank Dr. Arthur Ablin for his input, support and guidance.
Financial Support: CCSS is supported by a grant from the National Cancer Institute (U24 CA55727, L.L. Robison, Principal Investigator) and the American Lebanese Syrian Associated Charities (St. Jude Children’s Research Hospital). Supported in part by funds from the Campini Foundation, Mount Zion Foundation, and Swim Across America.
Study concept and design (RG, KD, MS, NK-L, KF, MS, KO, CS, GTA, LLR, LD); acquisition of data(SR, LL, YC, YY, LLR); analysis and interpretation of data (RG, YC, YY, LLR, LD); drafting of the manuscript (RG, YC, SR, LL, KD, MS, NK-L, FK, YY, MS, KO, CS, GTA, LLR, LD); critical revision of the manuscript for important intellectual content (RG,YC, SR, LL, KD,MS,NK-L, FK, YY, MS, KO, CS, GTA,LLR, LD); statistical analysis (YC, YY); obtained funding (RG, LLR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Di Fiore F, Van Cutsem E. Acute and long-term gastrointestinal consequences of chemotherapy. Best Pract Res Clin Gastroenterol. 2009;23:113–124. doi: 10.1016/j.bpg.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Shafi MA, Bresalier RS. The gastrointestinal complications of oncologic therapy. Gastroenterol Clin North Am. 39:629–647. doi: 10.1016/j.gtc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Robison LL, Green DM, Hudson M, Meadows AT, Mertens AC, Packer RJ, Sklar CA, Strong LC, Yasui Y, Zeltzer LK. Long-term outcomes of adult survivors of childhood cancer. Cancer. 2005;104:2557–2564. doi: 10.1002/cncr.21249. [DOI] [PubMed] [Google Scholar]
- 5.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, Li FP, Meadows AT, Mulvihill JJ, Neglia JP, Nesbit ME, Packer RJ, Potter JD, Sklar CA, Smith MA, Stovall M, Strong LC, Yasui Y, Zeltzer LK. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 6.Mertens AC, Walls RS, Taylor L, Mitby PA, Whitton J, Inskip PD, Potter JD, Robison LL. Characteristics of childhood cancer survivors predicted their successful tracing. J Clin Epidemiol. 2004;57:933–944. doi: 10.1016/j.jclinepi.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Chemaitilly W, Mertens AC, Mitby P, Whitton J, Stovall M, Yasui Y, Robison LL, Sklar CA. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab. 2006;91:1723–1728. doi: 10.1210/jc.2006-0020. [DOI] [PubMed] [Google Scholar]
- 8.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 9.Little RJA, Rubin DB. Statistical Analysis with Missing Data. John Wiley & Sons; 2002. [Google Scholar]
- 10.Rubin D. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; 1987. [Google Scholar]
- 11.Taylor JM, Munoz A, Bass SM, Saah AJ, Chmiel JS, Kingsley LA. Estimating the distribution of times from HIV seroconversion to AIDS using multiple imputation. Multicentre AIDS Cohort Study. Stat Med. 1990;9:505–514. doi: 10.1002/sim.4780090504. [DOI] [PubMed] [Google Scholar]
- 12.Little RJA, Rubin DB. Statistical Analysis with Missing Data. John Wiley & Sons; 1987. [Google Scholar]
- 13.Andreyev HJ, Vlavianos P, Blake P, Dearnaley D, Norman AR, Tait D. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist? Int J Radiat Oncol Biol Phys. 2005;62:1464–1471. doi: 10.1016/j.ijrobp.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 14.Andreyev HJ. Gastrointestinal problems after pelvic radiotherapy: the past, the present and the future. Clin Oncol (R Coll Radiol) 2007;19:790–799. doi: 10.1016/j.clon.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Hauer-Jensen M, Wang J, Denham JW. Bowel injury: current and evolving management strategies. Semin Radiat Oncol. 2003;13:357–371. doi: 10.1016/s1053-4296(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 16.Spunt SL, Sweeney TA, Hudson MM, Billups CA, Krasin MJ, Hester AL. Late effects of pelvic rhabdomyosarcoma and its treatment in female survivors. J Clin Oncol. 2005;23:7143–7151. doi: 10.1200/JCO.2005.12.096. [DOI] [PubMed] [Google Scholar]
- 17.Lansdale M, Castellino S, Marina N, Goodman P, Hudson MM, Mertens AC, Smith SM, Leisenring W, Robison LL, Oeffinger KC. Knowledge of hepatitis C virus screening in long-term pediatric cancer survivors: a report from the Childhood Cancer Survivor Study. Cancer. 116:974–982. doi: 10.1002/cncr.24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strickland DK, Riely CA, Patrick CC, Jones-Wallace D, Boyett JM, Waters B, Fleckenstein JF, Dean PJ, Davila R, Caver TE, Hudson MM. Hepatitis C infection among survivors of childhood cancer. Blood. 2000;95:3065–3070. [PubMed] [Google Scholar]
- 19.Coia LR, Myerson RJ, Tepper JE. Late effects of radiation therapy on the gastrointestinal tract. Int J Radiat Oncol Biol Phys. 1995;31:1213–1236. doi: 10.1016/0360-3016(94)00419-L. [DOI] [PubMed] [Google Scholar]
- 20.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 21.Sutton EJ, Tong RT, Gillis AM, Henning TD, Weinberg VA, Boddington S, Haas-Kogan DA, Matthay K, Sha V, Gooding C, Coakley FV, Daldrup-Link H. Decreased aortic growth and middle aortic syndrome in patients with neuroblastoma after radiation therapy. Pediatr Radiol. 2009;39:1194–1202. doi: 10.1007/s00247-009-1351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 23.Bolling T, Willich N, Ernst I. Late effects of abdominal irradiation in children: a review of the literature. Anticancer Res. 2010;30:227–231. [PubMed] [Google Scholar]
- 24.Phillips TL, Fu KK. Acute and late effects of multimodal therapy on normal tissues. Cancer. 1977;40:489–494. doi: 10.1002/1097-0142(197707)40:1+<489::aid-cncr2820400714>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 25.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 26.Wong KY, Lampkin BC. Anthracycline toxicity. Am J Pediatr Hematol Oncol. 1983;5:93–97. [PubMed] [Google Scholar]
- 27.King M, McConkey C, Latief TN, Hartley A, Fernando I. Improved survival after concurrent weekly cisplatin and radiotherapy for cervical carcinoma with assessment of acute and late side-effects. Clin Oncol (R Coll Radiol) 2006;18:38–45. doi: 10.1016/j.clon.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Leisenring WM, Mertens AC, Armstrong GT, Stovall MA, Neglia JP, Lanctot JQ, Boice JD, Jr, Whitton JA, Yasui Y. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louie AD, Robison LL, Bogue M, Hyde S, Forman SJ, Bhatia S. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25:1191–1196. doi: 10.1038/sj.bmt.1702419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


