Abstract
In polycystic kidney disease (PKD), intracellular cAMP promotes cyst enlargement by stimulating mural epithelial cell proliferation and transepithelial fluid secretion. The proliferative effect of cAMP in PKD is unique in that cAMP is anti-mitogenic in normal renal epithelial cells. This phenotypic difference in the proliferative response to cAMP appears to involve cross-talk between cAMP and Ca2+ signaling to B-Raf, a kinase upstream of the MEK/ERK pathway. In normal cells, B-Raf is repressed by Akt (protein kinase B), a Ca2+-dependent kinase, preventing cAMP activation of ERK and cell proliferation. In PKD cells, disruption of intracellular Ca2+ homeostasis due to mutations in the PKD genes relieves Akt inhibition of B-Raf, allowing cAMP stimulation of B-Raf, ERK and cell proliferation. Fluid secretion by cystic cells is driven by cAMP-dependent transepithelial Cl− secretion involving apical cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channels. This review summarizes the current knowledge of cAMP-dependent cyst expansion, focusing on cell proliferation and Cl−-dependent fluid secretion, and discusses potential therapeutic approaches to inhibit renal cAMP production and its downstream effects on cyst enlargement.
Keywords: polycystic kidney disease, cAMP, calcium, cell proliferation, MAP kinase, fluid secretion
1. Introduction
Renal cyst expansion in polycystic kidney disease (PKD) results from aberrant proliferation of the cyst wall epithelial cells and accumulation of fluid within the cavity of the cyst. There is increased extracellular matrix remodeling as the cyst invades the adjacent parenchyma, leading to abnormal matrix deposition and fibrosis. Several signaling pathways have been implicated in the pathogenesis of PKD; however, intracellular 3′, 5′-cyclic adenosine monophosphate (cAMP) has been shown to have a central role in cyst growth by stimulating both epithelial cell proliferation and transepithelial fluid secretion. This review discusses experimental evidence for cAMP-dependent cell proliferation, cAMP-mediated Cl− and fluid secretion, and potential approaches to reduce renal cAMP and its effect on cyst enlargement.
2. Polycystic kidney diseases
Polycystic kidney diseases are a family of hereditary disorders involving the formation and growth of innumerous cysts within the kidneys, often leading to end-stage renal disease (ESRD). Autosomal dominant polycystic kidney disease (ADPKD) is the most common form of PKD with a frequency of 1 in 500 to 1,000 births and accounts for approximately 5–9% of all end-stage renal diseases (ESRD) (reviewed in [1]). The disease is characterized by the formation of benign cysts in ductal organs, chiefly the kidneys and liver, and other extrarenal manifestations such as vascular aneurysms and cardiac valve defects. In ADPKD, the kidneys become grossly enlarged to 4–8 times normal size because of the progressive expansion of fluid-filled cysts that originate predominantly from collecting duct cells [2–6]. There is a high degree of variability in the age of onset and rate of disease progression even within families, but generally there is significant loss of renal function by the fifth to seventh decade of life. Approximately one-half of ADPKD patients progress to chronic renal failure by age 60 and require dialysis or renal replacement therapy.
Autosomal recessive polycystic kidney disease (ARPKD) is a less frequent childhood disease (approximately 1:20,000 live births) and is characterized by cystic fusiform dilations of the renal collecting ducts, accompanied by increased cell proliferation and fluid secretion. In most cases, cysts develop in utero and rapidly progress, causing massive kidney enlargement and renal failure within the first year of life. Congenital hepatic fibrosis is common in ARPKD and can cause significant clinical liver complication. Currently, there is no proven treatment directed at the cellular defect responsible for ADPKD or ARPKD.
2.1. Molecular basis for polycystic kidney disease
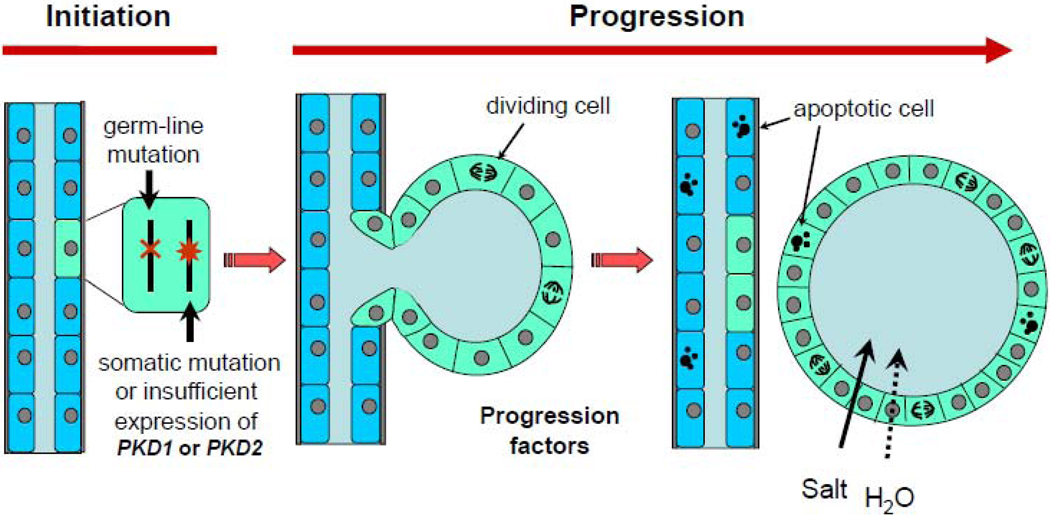
In ADPKD, every cell carries a mutated allele of either PKD1 or PKD2; however, cysts appear in only a small fraction of the nephrons and are thought to originate from clonal growth of single cells within the tubules (Figure 1). A somatic mutation or insufficient expression of the wild-type allele is thought to initiate renal cyst formation. Mutations in PKD1 are responsible for 85% of the cases, and mutations in PKD2 account for the remainder. The PKD1 gene encodes polycystin-1 (PC1), a large protein that contains a large extracellular region, 11 membrane spanning domains and a relatively short intracellular C-tail portion [7, 8]. The extracellular region of PC1 contains protein motifs that are predicted to be involved in cell-cell or cell-matrix interactions, and/or possibly serve as a receptor for extracellular ligands [9]. The intracellular C-terminus has several predicted phosphorylation sites and a conserved G-protein activation sequence [10, 11]. A coiled-coil domain mediates PC1 binding to polycystin-2 (PC2), the gene product of PKD2 [12–14]. PC2, also called TRPP2, is a Ca2+ permeable nonselective cation channel that localizes to different subcellular compartments, including the endoplasmic reticulum (ER), plasma membrane and the primary cilium. PC1 and PC2 may be part of a protein complex that functions as a Ca2+ channel. Clinical and cellular phenotypes of PKD1- and PKD2-initiated diseases are similar, implicating a common signaling pathway for the two proteins [1].
Figure 1. Schematic diagram illustrating the initiation and progression of cyst formation.
In ADPKD, all cells harbor a germ-line mutation in one allele of either PKD1 or PKD2; however, a somatic mutation or insufficient expression of non-mutated allele is thought to initiate cyst formation. Progression factors, including arginine vasopressin and other cAMP agonists stimulate cell proliferation and NaCl and water secretion causing cyst expansion. [Modified from Grantham et al., Kidney Int. 73: 108–116, 2007]
In ADPKD, cyst formation begins in utero in a small fraction of renal cells in which the level of PC1 or PC2 drops below a critical threshold [3]. A somatic “second-hit” mutation, loss-of-heterozygosity or haploinsufficiency may account for the mosaic nature of cyst formation [15, 16]. Cystic epithelial cells are characterized as being incompletely differentiated and persistently proliferative, yet there is an incomplete understanding of the linkage between the mutated polycystins and the resultant abnormal proliferation or cellular differentiation. Aberrant proliferation of tubule epithelial cells is thought to cause the wall of the tubule to expand forming a mural pocket. As the microscopic cyst expands in size, it fills with fluid derived from unreabsorbed glomerular filtrate; however, once cysts expand to approximately 2 mm in diameter, most become detached from the parent tubule and become isolated sacs of fluids, lined by an epithelial cell layer [17]. These isolated cysts continue to expand in size by the combination of mural epithelial cell proliferation and transepithelial fluid secretion (Figure 1). ADPKD kidneys continue to enlarge at a relatively constant rate after birth [18].
ARPKD is caused by genetic mutations in PKHD1, which encodes a large protein, fibrocystin (also called polyductin). The protein is predicted to have a large extracellular domain, a single membrane spanning domain and a short cytoplasmic tail [19–21]. The function of fibrocystin is unknown; however, in common with other proteins associated with PKD pathogenesis [22], fibrocystin localizes to primary cilia of renal and biliary epithelial cells [20]. Cilia are thin microtubule based structures that originate from one of a pair of centrioles in the centrosome and extend from the apical surface into the tubule lumen. Cilia are thought to transduce a Ca2+ signal in response to mechanical stress, such as with fluid flow or chemical stimulation [23]. Fibrocystin interacts with PC2, suggesting that it is part of the same multi-protein complex as PC1 and PC2 to regulate intracellular Ca2+ in response to external stimuli [24–26].
2.2 Dysregulation of intracellular calcium in PKD
The role of PC1, PC2 and fibrocystin in the regulation of intracellular [Ca2+] and the processes by which mutations in their genes cause epithelial cell hyperplasia and cyst formation remain unclear. Converging evidence supports the hypothesis that disruption of intracellular Ca2+ regulation and/or a reduction in steady state Ca2+ levels contribute to cyst formation [27, 28]. Cultured epithelial cells derived from human ADPKD cysts have a basal [Ca2+]i approximately 20 nM lower than normal human kidney (NHK) cells [27]. Interestingly, cells from tubules of non-cystic regions of early-stage ADPKD kidneys have intracellular [Ca2+] similar to NHK cells, suggesting that a germ-line mutation alone is insufficient to cause a decrease in intracellular Ca2+. ARPKD cells have also been shown to have reduced levels of intracellular Ca2+ compared to NHK cells [27]. Furthermore, PKHD1 gene silencing with siRNA leads to a 20 nM decrease in [Ca2+]i [29], comparable to what is seen in human ADPKD cells. Reduction in intracellular Ca2+ appears to be involved in cystogenesis in other organs as well. Cholangiocytes derived from liver cysts of PCK rats, an orthologous model of ARPKD, have reduced intracellular Ca2+ compared to normal biliary epithelial cells [30]. Thus, mutations in both ADPKD and ARPKD genes appear to disrupt intracellular Ca2+ regulation, leading to a reduction in basal intracellular Ca2+ levels, aberrant cell proliferation and cyst formation.
3. Regulation of renal intracellular cAMP
Cyclic AMP is one of the most ubiquitous second messengers and is involved in the regulation of many biological processes including cell proliferation, differentiation, transcription and electrolyte and fluid transport. Several lines of evidence have indicated that factors that elevate renal intracellular cAMP promote cyst growth, kidney enlargement and disease progression.
3.1. Regulation of the cAMP signaling pathway
Levels of intracellular cAMP are regulated by the activities of adenylyl cyclases (ACs), which catalyze the formation of cAMP from ATP, and phophodiesterases (PDEs) which degrade cAMP to AMP. In most cells, basal cAMP levels are approximately 1 µM, whereas a concentration of approximately 10 µM is needed to reach the activation threshold for protein kinase A (PKA), a cAMP-dependent serine/threonine kinase [31]. This threshold for cAMP activation of PKA is achieved when extracellular ligands bind to heterotrimeric G protein-coupled receptors (GPCR) in the plasma membrane, followed by activation of ACs. G-proteins are composed of an α-subunit, which has a high affinity for guanine nucleotides, and a tightly coupled β and γ dimer [32]. When a hormone binds to its receptor, GDP is exchanged for GTP on the α-subunit, resulting in the release of the α-subunit from the β/γ dimer. Both the α-subunit and the β/γ dimer can interact with downstream effectors [33]. GTPase activity intrinsic to the α-subunit hydrolyzes bound GTP to GDP causing reassociation of α and β/γ subunits. Classification of G proteins is determined by the subtype of the α-subunit (Gs, Gi, Gq and G12/13). The traditional view of GPCR regulation of intracellular cAMP involves the regulation of AC activity by stimulatory (Gsα) and inhibitory (Giα) G-proteins; however, the β/γ dimer may also regulate certain AC isoforms [34, 35].
Key features of cAMP signaling are cellular specificity and cellular compartmentalization of the cAMP response [31]. Receptor expression and the unique combination of isoforms of ACs, PDEs and regulatory proteins are important determinants of cell specificity for the cAMP signal. There are nine closely related membrane associated ACs, each with unique tissue distribution and biochemical properties [36]. AC isoforms AC1, AC3, and AC8 are stimulated by Ca2+; whereas, AC5 and AC6 are inhibited by Ca2+. Thus, changes in intracellular Ca2+ levels may affect cAMP production, depending on which AC isoforms are expressed. In the kidney, Ca2+-inhibitable AC5 and AC6, and Ca2+-insensitive AC4 are the predominant isoforms [37]; however, other isoforms are also expressed. AC3, an AC expressed in olfactory neurons, is found in collecting ducts [38]. Recently, knockout of AC3 expression in AC3−/− mice reduced GFR, implicating a role for AC3 in normal renal function [39]. In most cell types, the capacity for cAMP hydrolysis greatly exceeds synthesis, indicating that cAMP levels are generally regulated by the activity of PDEs. The PDE superfamily consists of 11 structurally related gene families with over 60 isoforms [40]. PDE1 and PDE4 are the predominant isoforms in collecting duct cells; PDE1 is positively regulated by Ca2+-calmodulin.
Compartmentalization of the cAMP signal relies on cellular localization of ACs at the plasma membrane and A kinase-anchoring proteins (AKAPs) which hold PKA to specific cellular compartments in close proximity to the receptor, AC and PDE [41, 42]. Detailed understanding of the functional significance of the multiplicity of the cAMP-signaling components has yet to be resolved; however distinct combinations of ACs, PDEs, PKAs and AKAPs are thought to allow precise targeting of the cAMP signal and cross-talk with other signaling systems. The localization of AKAPs close to the target protein may also be necessary for specific and efficient PKA phosphorylation [43]. In addition to PKA, cAMP-dependent guanine nucleotide exchange factors (EPacs) and cyclic nucleotide gated ion channels are downstream effectors of cAMP and provide further diversity in the cellular responses to cAMP stimulation [44–46].
3.2. Vasopressin stimulation of renal cAMP
Arginine vasopressin (AVP) is an important antidiuretic hormone that stimulates cAMP production in collecting ducts and distal nephron, the predominant sites for cyst formation in PKD [5]. AVP binding to vasopressin V2 receptors (V2R) increases intracellular cAMP and PKA phosphorylation of aquaporin-2 (AQP-2) water channels, leading to AQP-2 activation and insertion into the apical membrane. Active AQP-2 channels increase the water permeability of the collecting ducts, allowing water in the glomerular filtrate to be reabsorbed and returned to the circulation. When plasma osmolality is greater than ~285 mOsm per kg, AVP is released from the pituitary gland into the circulation. AVP increases water reabsorption, thus decreasing urine volume and increasing urine osmolality. Shortly after drinking a large volume of fluid, AVP levels drop and urine becomes diluted. In humans, urine osmolality typically exceeds that of plasma, indicating that normal circulating AVP levels maintain cAMP in the collecting duct cells to levels sufficient to concentrate urine.
3.3 Elevated levels of renal cAMP in PKD
Renal cAMP levels are elevated in PKD animals, including pcy mice, jck mice, PCK rats, and Pkd2WS25/− mice [47–50]. A potential explanation for elevated renal cAMP in PKD kidneys is hyperactivation of V2R in the cystic epithelial cells. It is widely accepted that renal cysts in ARPKD originate from collecting ducts, where V2R are predominantly expressed. ADPKD cysts arise in all nephron segments, including the glomerulus; however, microdissection studies of ADPKD kidneys have indicated that collecting duct derived cysts are more numerous and larger [2]. Furthermore, the majority of ADPKD cysts with diameters of 1 mm or greater stain positive for collecting duct markers [5]. In Pkd1 and Pkd2 mouse models, cysts within postnatal kidneys are predominantly of collecting duct origin [3, 4, 6]. Cells cultured from human ADPKD cysts stain positive for collecting duct lectins and express AQP-2 protein [51, 52]. Both ARPKD and ADPKD cells have a greater cAMP response to AVP and 1-deamino-8-D-arginine vasopressin, a selective V2R agonist, than parathyroid hormone (PTH) [53]. PTH receptors are expressed predominantly in proximal convoluted and straight tubules, thick ascending limbs of Henle’s loop and distal convoluted tubules [54]. Thus, ARPKD and ADPKD cell cultures appear to be enriched in cystic cells derived from collecting ducts. Several studies have shown that V2R are overexpressed in cystic kidneys of PKD animals [47, 49, 55, 56], suggesting that the cystic cells may be more responsive to AVP than normal collecting duct cells. Moreover, there are increased levels of circulating AVP in ADPKD and ARPKD patients possibly due to a defect in the concentrating ability of the cystic kidney [57, 58]. The combination of increased V2R expression and increased circulating levels of AVP may give rise to persistent cAMP production in cystic epithelial cells of PKD kidneys.
It has also been suggested that a reduction in intracellular Ca2+, secondary to mutations in the PKD genes, cause increased accumulation of intracellular cAMP. Ca2+ reduction may increase the activity of the Ca2+-inhibitable AC6 and decrease the activity of Ca2+/calmodulin-dependent PDEs [59]. The combination of increased production and decreased degradation of cAMP could raise basal concentrations of cAMP to levels closer to the threshold for PKA activation. Consequently, a higher resting cAMP level could make PKD cells more sensitive to V2R stimulation and/or amplify the cAMP signal.
4. cAMP-dependent cell proliferation in PKD
Important to our understanding of cyst expansion is the discovery that cAMP stimulates the proliferation of cyst epithelial cells derived from human ADPKD kidneys while it inhibits the proliferation of tubule cells from normal human kidneys [60, 61]. cAMP agonists, including AVP, accelerate ADPKD and ARPKD cell proliferation through PKA stimulation of the mitogen-activated protein kinase kinase/extracellular regulated kinase (MEK/ERK) pathway [52, 62]. Virtually all of the stimulatory effect of cAMP is blocked by H-89, a PKA inhibitor, and PD98059, a MEK inhibitor. In contrast, cAMP inhibits ERK and proliferation of normal renal cells, including NHK and M-1 cortical collecting duct cells [60, 61]. Hence, elevated cAMP levels alone are not sufficient to promote renal epithelial cell proliferation. These findings led to a series of studies investigating the relationship between intracellular [Ca2+] and cAMP in the regulation of the MEK/ERK pathway in PKD [27, 56, 63].
4.1. cAMP regulation of the MEK/ERK pathway
The molecular mechanism for the phenotypic difference in the cAMP mitogenic response between normal and PKD cells is linked to the differential regulation of the Raf/MEK/ERK signaling pathway. B-Raf, Raf-1 (also called C-Raf) and A-Raf are a family of serine/threonine kinases that are central intermediates in transmitting extracellular signals, including those from growth factors and hormones, to the MEK/ERK pathway [64, 65]. ERK activation is important for cell proliferation during development and coordinates cell-cycle re-entry during tissue repair. Ras, small GTP-binding proteins (H-Ras, K-Ras, N-Ras), recruit Raf to the plasma membrane which is essential for Raf activation. In addition, Raf kinases are regulated by multiple pathways through phosphorylation of specific serine and threonine residues [66]. The balance between the phosphorylation of stimulatory and inhibitory sites is a major factor in Raf regulation of ERK-mediated cell proliferation. Activated Raf phosphorylates and stimulates MEK1/2, which in turn, phosphorylates and activates ERK1/2. There is translocation of activated ERK into the nucleus where it upregulates the transcriptional activity of a number of genes involved in cell proliferation. The Raf/MEK/ERK pathway exerts its effects on cell proliferation through induction of cell cycle regulatory proteins, including the cyclin-dependent kinases (Cdks), cyclins and p21, and transcription factors such as c-myc and AP-1 [67].
The capacity for cAMP to stimulate or inhibit ERK accounts for many of the cell type-specific cAMP effects on cell proliferation [68, 69]. In astrocytes, smooth muscle cells, fibroblasts and mesangial cells, cAMP inhibits ERK activity and cell proliferation. On the other hand, cAMP stimulates ERK and proliferation of other cell types, including thyroid cells, hepatocytes and PC-12 neuronal cells [70]. Regulation of cAMP signaling to ERK occurs at the level of Raf. While B-Raf and Raf-1 share homology in amino acid sequence; the two kinases are differentially regulated by cAMP. Two activation sites in Raf-1 (T491 and S494) are conserved in B-Raf (T599 and S602) [65], and the phosphorylation of these residues is important for kinase activity. However, unlike B-Raf, S338 and Y341 of Raf-1 must also be phosphorylated for kinase activation. The corresponding serine residue in B-Raf (S446) is constitutively phosphorylated and the tyrosine residue at 341 (Y341) of Raf-1 is replaced in B-Raf with an aspartic acid (D449), which mimics phosphorylated tyrosine. Consequently, fewer phosphorylation events are necessary to activate B-Raf compared to Raf-1. Another important difference is that Raf-1 has three PKA inhibitory phosphorylation sites (S43, S233 and S259), any one of which can block Ras binding to Raf-1 and prevent Raf-1 translocation to the membrane [71, 72]. These PKA phosphorylation sites are not conserved in B-Raf making B-Raf resistant to inhibition by cAMP; instead, PKA phosphorylation stimulates B-Raf activity. In addition, B-Raf has a greater affinity for MEK and produces a stronger MEK stimulation than Raf-1. Thus, B-Raf has higher basal activity compared to Raf-1 [73] and seems to be poised for activation by cAMP.
The effect of cAMP on B-Raf signaling to MEK is cell type and/or content dependent. There are two major isoforms of B-Raf (95 kD and 68 kD) generated by alternative splicing. Vossler et al. had suggested that cAMP stimulates ERK in cells that express the 95 kD isoform of B-Raf and inhibits ERK in cells lacking this isoform [74]. However, in other reports, cAMP was shown to inhibit B-Raf in cells expressing both isoforms, indicating that B-Raf regulation by cAMP may be dependent on cellular conditions. In particular, B-Raf has been shown to be negatively regulated by Akt (also called protein kinase B), a serine-threonine kinase, through a Ca2+-dependent and phosphoinositide 3-kinase (PI3-kinase)-dependent manner [75–77]. Akt phosphorylates B-Raf at S365 and T440, important sites for B-Raf inhibition, and mutations of residues near T440 prevent phosphorylation by Akt, leading to a loss of Akt-mediated B-Raf inhibition. These mutations are associated with activated ERK and increased cell proliferation in certain cancers, including lung small cell carcinoma and malignant melanoma [65, 78].
4.2. Ca2+ regulation of cAMP-dependent ERK activation and cell proliferation
Evidence indicating that PC1, PC2 and fibrocystin normally contribute to the regulation of intracellular Ca2+ led to the hypothesis that a reduction in intracellular Ca2+ in cystic cells may be the basis for cAMP-dependent cell proliferation. To test the role of Ca2+, NHK cells and immortalized mouse collecting duct M-1 cells were treated with Ca2+ channel blockers or EGTA, a Ca2+ chelator, to lower intracellular Ca2+ levels [63]. In these experiments, Ca2+ restriction converted the normal cAMP growth-inhibited phenotype to a cAMP growth-stimulated phenotype, mimicking PKD cells. In these Ca2+-restricted cells, cAMP stimulated B-Raf kinase activity and increased phoshorylated ERK (P-ERK) and cell proliferation. Ca2+ restriction decreased the level of phosphorylated Akt, which normally represses B-Raf. Direct pharmacological inhibition of Akt also caused cAMP-dependent activation ERK and cell proliferation. Moreover, stable overexpression the C-terminal tail of PC1 in M-1 cells, which is thought to act in a dominant negative manner, decreased intracellular Ca2+, and switched the cAMP response, such that cAMP activated B-Raf, ERK and cell proliferation [63]. Interestingly, the Ca2+ switch required several hours, suggesting that additional Ca2+-dependent mechanisms are involved.
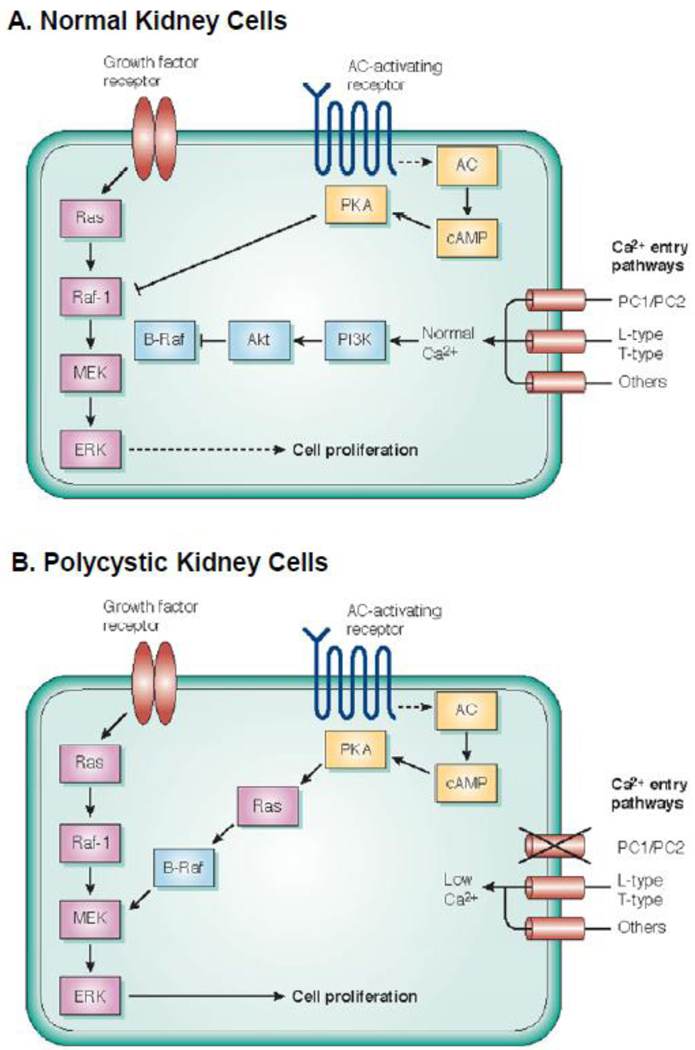
In a reciprocal study, treatment of human ADPKD and ARPKD cells with Bay K8644, a Ca2+ channel activator, or A23187, a Ca2+ ionophore, caused a sustained increase in steady-state Ca2+ levels and completely reversed the mitogenic response to cAMP; thus, rescuing the normal anti-mitogenic response to cAMP [27]. Untreated ADPKD cells had lower basal Akt activity compared to NHK cells and raising intracellular Ca2+ increased Akt activity and suppressed B-Raf, thereby blocking cAMP-dependent ERK activation and cell proliferation. Taken together, these studies support the hypothesis that a reduction in intracellular Ca2+, secondary to mutations in the PKD genes (PKD1, PKD1 or PKHD1), decreases Akt activity, relieving Akt inhibition of B-Raf, and allowing cAMP activation of the B-Raf/MEK/ERK signaling and cell proliferation (Figure 2).
Figure 2. Proposed signal transduction pathways for Ca2+ regulation of cAMP-dependent cell proliferation.
Normally, cell proliferation is controlled by growth factors binding to receptor tyrosine kinase with sequential activation of Ras → Raf-1→ MEK → ERK to induce cell proliferation. There is a phenotypic difference between normal kidney cells and PKD cells in the cAMP effect on proliferation. (A) In normal human kidney (NHK) cells, basal Ca2+ levels, regulated by a variety of Ca2+ entry mechanisms, including the PC1/PC2 complex, maintain the activity of PI3 kinase/Akt pathway which represses B-Raf. B-Raf kinase activity is inhibited by Akt phosphorylation of an inhibitory site. cAMP agonists, i.e. AVP, inhibit Raf-1, ERK and cell proliferation through a PKA-dependent mechanism. Thus, in normal renal cells, the balance of positive and negative signals control Raf-1 signaling to ERK activation and cell proliferation. (B) By contrast, in PKD cells a reduction in intracellular Ca2+ levels due to a loss of PC1/PC2 function relieves Akt inhibition of B-Raf, allowing cAMP to signal through B-Raf to activate MEK and ERK and to stimulate cell proliferation. [Adapted from Cowley, Kidney Int. 73:251–253, 2008; and Yamaguchi et al., J. Am. Soc Nephrol 17: 178–187, 2006]
In further support of this hypothesis, Pkd2 overexpression in collecting duct cells of a transgenic mouse caused formation of typical renal cysts, possibly due to excess PC2 acting in a dominant negative manner or due to an imbalance in the PC1 to PC2 stoichiometry [79]. These cysts had increased levels of P-ERK and cell proliferation that were dependent on B-Raf. Moreover, there were reduced levels of P-Akt in the cystic kidneys, consistent with the human ADPKD cells.
Akt regulation of B-Raf, ERK and cell proliferation in cystic biliary epithelial cells in the PCK rat, an ARPKD model, appears to involve a similar mechanism. Restoration of intracellular Ca2+ levels in cystic cholangiocytes blocked cAMP-dependent activation of B-Raf, ERK and cell proliferation in an Akt-dependent manner [30]. On the other hand, it has been reported that Akt activity is increased in mitotic, but not resting cells, in the proximal tubule-derived cysts of Cy/+ Han:SPRD rats, a non-orthologous model of ADPKD [80]. It is possible that additional pathways, including the Akt/mTOR signaling pathway, are involved in cell proliferation in Cy/+ kidneys [80–82]. Additional studies are needed to delineate the relationships among intracellular Ca2+, Akt, B-Raf and cAMP-dependent activation of the MEK/ERK pathways in PKD.
4.3. Ca2+ channel blockers in PKD animals
Ca2+ channel blockers (CCBs) are widely used for the treatment of hypertension in chronic kidney diseases, including ADPKD. Treatment of ADPKD cells with Ca2+ entry blockers were found to amplify cAMP-dependent ERK activation and cell proliferation, raising the possibility that treatment of ADPKD patients with CCBs might further reduce intracellular Ca2+ and accelerate cyst growth. Three classes of L-type anti-hypertensive CCBs (phenylalkylamines, benzothiazepines and dihydropyridines) have different chemical characteristics, but are generally thought to have similar actions to lower blood pressure. In a preliminary study, Nutahara et al. compared the effects of candesartan, an angiotensin II receptor blocker, and amlodipine, a dihydropyridine L-type CCB, in a small cohort of ADPKD patients [83]. The study suggested that angiotensin II receptor blockers are more effective than CCBs for renal protection in ADPKD patients, independent of the capacity to control hypertension. Blockade of the renin-angiotensin-aldosterone system with angiotensin-converting enzyme inhibitor or angiotensin receptor blockers have been shown to be more beneficial than other agents [84, 85]; although not all studies support this concept [86]. More recently, Nagao et al. examined the effect of CCBs on the growth of cysts and PKD progression by treating Cy/+ rats with verapamil, a phenylalkylamine L-type CCB, twice daily from 5 to 12 weeks [56]. Verapamil treatment increased the renal activity of the B-Raf/MEK/ERK pathway and accelerated renal cyst growth in Cy/+ rats, determined by increases in kidney volume, cystic area, PCNA positive cells and serum urea nitrogen. Despite the potential for Ca2+ restriction to accelerate cyst expansion in PKD, the impact of CCBs on ADPKD progression remains to be tested.
5. cAMP mediated Cl−-dependent fluid secretion
The remarkable appearance of ADPKD kidneys is due to the accumulation of fluid within hundreds or thousands of cysts that grossly enlarge total kidney volume. In the initial stages, cysts fill with fluid derived from glomerular filtrate; however, the majority of the cysts larger than 2 mm in diameter have no connection to the nephron segment from which they originated [17]. Within these isolated cysts, transepithelial fluid secretion is the only means by which solutes and fluid can accumulate. During the past 15 years, several lines of evidence have determined that fluid secretion is driven by active transepithelial Cl− transport stimulated by cAMP [87].
The seminal observation for fluid secretion was made by McAteer et al., where Madin-Darby canine kidney (MDCK) cells were shown to form fluid-filled cysts when seeded in a collagen matrix [88]. Agonists that stimulated the production of cAMP accelerated fluid secretion and in vitro cyst growth [89]. The use of the MDCK cell line has helped to establish experimental procedures that have greatly facilitated research on cAMP-dependent fluid secretion using primary cultures of human ADPKD cells [89–93]. An important study by Ye et al. showed that intact cysts excised from ADPKD kidneys secrete fluid when treated with forskolin, a direct activator of ACs [94]; confirming that intact cyst epithelia secrete fluid by mechanisms regulated by intracellular cAMP.
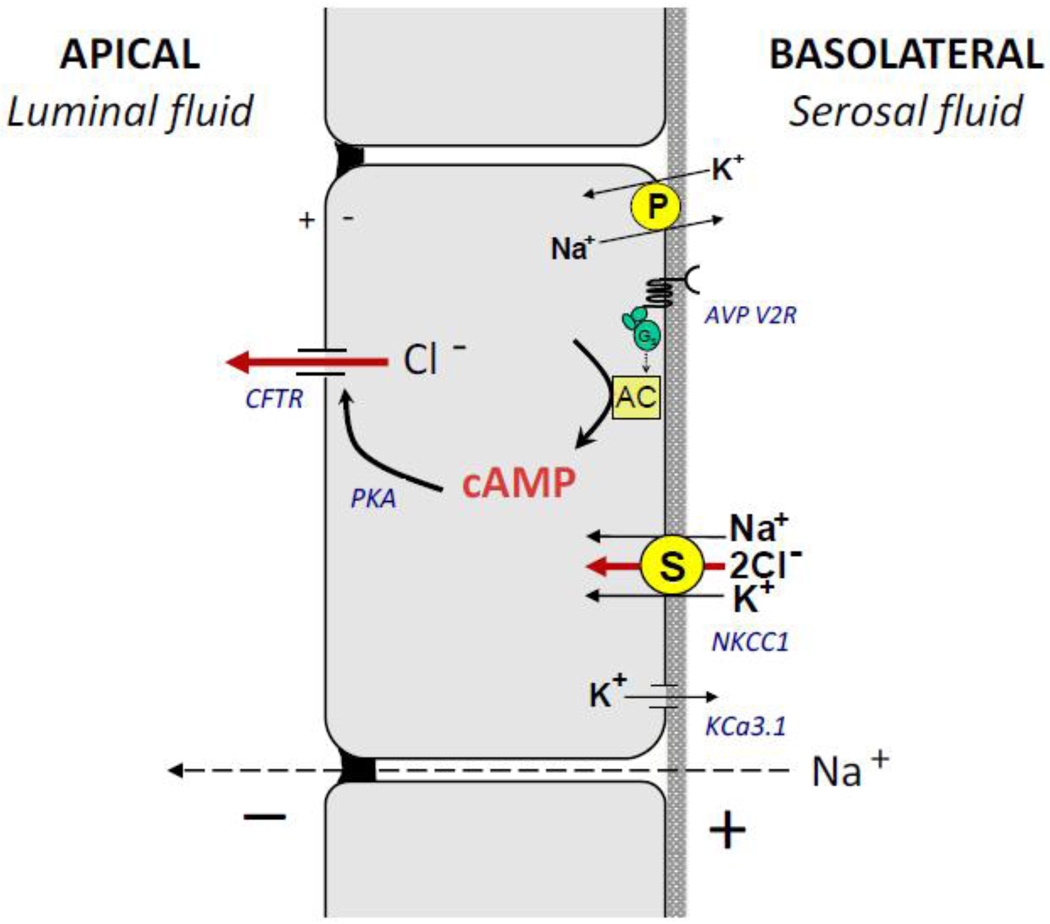
As with other secretory epithelia, fluid secretion by ADPKD cysts is dependent on transporters and ion channels within the apical and basolateral membranes (Figure 3). The Na+, K+ -ATPase, acting in concert with K+ channels in the basolateral membrane, establishes and maintains the chemical and electrical gradients that are utilized by secondary active transporters. Ouabain, an inhibitor of the Na+, K+ -ATPase, blocks cAMP-dependent net fluid secretion by intact cysts [95] and anion secretion by polarized ADPKD cell monolayers [96]. Several K+ channels have been shown to be present in collecting ducts, including inward-rectifying K+ channels (Kir) [97]. These channels are inhibited by intracellular ATP and glibenclamide, a sulfonylurea, and are activated by cAMP. ADPKD and NHK cells were shown to express mRNA for Kir6.2, an ATP-sensitive K+ channel [98]. Basolateral application of glibenclamide potently inhibited anion secretion consistent with inhibition of ATP-sensitive K+ channels. More recently, Albaqumi et al. found that KCa3.1, an intermediate conductance Ca2+-activated K+ channel, is present in ADPKD cells [99]. TRAM-34, a highly specific KCa3.1 blocker, inhibited cAMP-dependent anion secretion and in vitro cyst growth of ADPKD cells. These data demonstrate that Kir6.2 and KCa3.1 participate in cAMP-dependent anion secretion and inhibition of these channels may have therapeutic value in PKD.
Figure 3. Transport mechanisms involved in cAMP-dependent Cl− secretion by ADPKD cyst epithelial cells.
Many of these transporters and channels are involved in Cl− and fluid secretion by a variety of secretory epithelia. The Na+, K+-ATPase, is a primary (P) active transporter that utilizes cell energy to pump 3 Na+ out of the cell in exchange for 2 K+ and is responsible for establishing and maintaining the chemical gradients for Na+ and K+ across the membrane. The bumetanide sensitive NKCC1, a basolateral secondary (S) transporter, utilizes the Na+ gradient to transport Na+, K+, 2 Cl− into the cell, raising the intracellular Cl− concentration above its electrochemical gradient. The V2 receptor (AVP V2R) is a G-protein coupled receptor (GPCR) coupled to Gs. Binding of arginine vasopressin (AVP) to V2R increases the synthesis of cAMP by adenylyl cyclase (AC), causing activation of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel on the apical membrane of the cells. Chloride efflux through apical CFTR channels is the initial step in cAMP-dependent Cl− secretion. Subsequent activation of basolateral K+ conductance, including the KCa3.1 channels, is critical to maintain the negative membrane potential for Cl− transport. The combination of the apical Cl− conductance and the basolateral K+ conductance creates a transepithelial potential driving the paracellular transport of Na+. The net addition of NaCl to the luminal fluid drives the osmotic movement of fluid. [Modified from Sullivan et al. Physiological Rev 78: 1165–1191, 1998]
Several lines of evidence indicate that cAMP-dependent anion secretion is mediated by Cl− transport via apical CFTR Cl− channels [100, 101]. Measurements of intracellular Cl− during cAMP stimulation reveal that there is an initial efflux of Cl−, consistent with activation of apical CFTR channels [102]. Treatment with CFTR inhibitors blocks cAMP-dependent anion secretion by ADPKD cell monolayers [96]. Chloride enters the cell through basolateral NKCC1, an electrically neutral Na+-K+-2Cl− cotransporter, that brings Na+, K+ and Cl− into the cell using the transmembrane Na+ gradient. Thus, intracellular Cl− is maintained above its electrochemical gradient and is poised for rapid Cl− effux across the luminal membrane upon cAMP activation of CFTR. Increases in the apical Cl− conductance and basolateral K+ conductance create a lumen negative transepithelial electrical potential that drives passive Na+ transport through the paracellular pathway. The net addition of Na+ and Cl− into the luminal fluid drives the osmotic movement of water into the cyst cavity [87, 103].
Fluid secretion has been difficult to study in intact PKD kidneys. Recently, Magenheimer et al. showed that cAMP induces the formation of cyst-like dilations in embryonic Pkd1−/− kidneys using metanephric organ cultures [104]. These dilations are eliminated by CFTR and NKCC1 inhibition, and by genetic knock-out of CFTR. Yang et al. showed that treatment with a novel CFTR inhibitor reduces cyst expansion in a PKD mouse model [105]. These in vitro and in vivo studies suggest that ion channels and transporters are potential therapeutic targets to block fluid accumulation in cysts of PKD kidneys.
6. Targeting cAMP-dependent cystic expansion in PKD
The discovery that cAMP has a central role in PKD has led to several preclinical studies in PKD animals testing drugs that target renal cAMP production and/or its downstream effectors. In this section, in vivo studies are discussed, including approaches to reduce renal cAMP production and targets of cAMP-dependent cell proliferation and fluid secretion.
6.1. Blocking the renal effects of vasopressin
OPC-31260, a V2R antagonist, administered to PKD animals orthologous to human disease, including the Pkd2WS25/− mouse (ADPKD), PCK rat (ARPKD) and pcy mouse (nephronophthisis type 3) reduced renal cAMP and inhibited disease progression measured by reductions in kidney volume, cystic area, number of mitotic and apoptotic cells, and blood urea nitrogen (BUN) [47, 49, 106]. There was also a corresponding reduction in the renal activity of the B-Raf/MEK/ERK pathway. Tolvaptan, a potent and highly selective human V2R antagonist, had a similar effect on renal cAMP and PKD progression in ADPKD and ARPKD animal models. Wang et al. confirmed that the effect of these drugs to reduce disease progression was due to inhibition of AVP effects by selectively knocking out AVP in the PCK rat [107]. These animals were generated by crossing PCK rats with Brattleboro (AVP−/−) rats which are unable to express AVP. In the absence of AVP, the PCK mice had reduced renal cAMP accumulation, ERK activity, cell proliferation, and fibrosis and were essentially free of renal cysts. Administration of DDAVP by osmotic minipump restored cystic disease in the AVP-deficient PCK rats providing unequivocal evidence for the roles of AVP and cAMP on cystic disease progression. An alternative approach to decrease plasma AVP levels is to increase water consumption. Increased water intake in PCK rats was shown to be sufficient to reduce renal cAMP and the activity of B-Raf/MEK/ERK signaling. PCK rats on high water intake had reduced renal cell proliferation, cystic area and kidney weight, and improved renal function [55]. These animal studies strongly support the idea that blocking the effects of AVP will provide a protective effect on the kidneys of ADPKD patients. Tolvaptan is currently being evaluated in ADPKD patients in an international clinical trial (TEMPO). Increased water ingestion throughout the day to suppress AVP levels is also being considered as an alternative approach to tolvaptan in ADPKD patients with relatively normal GFR [55, 108, 109].
Somatostatin is a peptide secreted by the neuroendocrine neurons of the hypothalamus and the alpha cells in the Islets of Langerhans in the pancreas. The somatostatin receptor, SSTR2, is a Gi coupled receptor present on renal epithelial cells [110]. Binding of somatostatin to its receptor inhibits AC activity and cAMP production. Low concentrations have been shown to antagonize the effects of AVP in collecting ducts [111]. Octreotide, a stable long-acting somatostatin analogue, was shown to halt kidney and liver cysts in PCK rats [112]. This drug inhibited kidney volume in a small randomized, crossover, placebo-controlled study in ADPKD patients followed for 6 months [113], providing support for ongoing clinical trials.
6.2. Blocking cAMP-dependent MEK/ERK signaling and cell proliferation
Several approaches to target cAMP-dependent proliferation are being considered for inhibition of cyst expansion in ADPKD. One strategy is to restore intracellular Ca2+ in PKD cells to prevent the mitogenic effect of cAMP. Triptolide, a natural medicinal herb that is thought to bind and activate PC2, induces cell cycle arrest in Pkd1−/− cells and retards renal cyst growth in Pkd1−/− kidneys [114, 115]. A similar approach to correct the defect in intracellular Ca2+ is to activate calcium-sensing receptors (CaSR) on the plasma membrane of renal cells. CaSR are coupled to Gq and activate phospholipase C and protein kinase C to increase intracellular Ca2+. Extracellular Ca2+ activates CaSR and calcimimetics bind CaSR to increase the sensitivity of the receptor to external Ca2+. The effect of calcimimetic has been examined in three PKD models with mixed results. Wang et al. found that treatment with a calcimimetic caused a slight reduction in fibrosis, but provided no detectable change in renal cAMP or cystogenesis in the PCK rat and the Pkd2WS25/− mouse [116]. Consistent with this observation, the addition of calcimimetic did not prevent cAMP-dependent proliferation of human ADPKD cells (D. Wallace, unpublished results). Recently, Gattone et al. found the administration of the calcimimetic R-568 in Cy/+ rats had no effect on cyst expansion at 34 wk, but reduced total kidney weight at 38 wk, suggesting that calcimimetics may have a beneficial effect in PKD [117]. Based on the mixed results from these studies, it remains to be determined if calcimimetics are renal protective in PKD. Pharmacological activation of Trpv4, a Ca2+ entry channel, increases Akt activity, and inhibits B-Raf and ERK in cholangiocytes of cystic livers [26]. Treatment with GSK1016790A, a Trpv4 activator, caused a small reduction of liver cysts of PCK rats, but the effect was not statically significant. By contrast, renal cyst area was markedly reduced; indicating that activation of Trpv4 may restore the normal cellular phenotype in PKD cells. Together, these studies suggest that small molecules that activate Ca2+ release from intracellular stores or stimulate Ca2+ entry may have therapeutic value in the treatment of PKD.
Many anti-cancer drugs target the Raf/MEK/ERK pathway and cell proliferation, and may be an attractive option for ADPKD treatment. PD184352, a MEK inhibitor, has been through phase I and II clinical trials for solid tumors with disappointing results [118]. The majority of these patients had tumors caused by a Ras mutation; and it turns out, tumors with B-Raf mutations are extremely sensitive to MEK inhibition in comparison to tumors caused by Ras mutations [119]. Considering that constant cAMP production in cystic cells keeps B-Raf in a constitutively activated state, MEK specific inhibitors may be an effective treatment for ADPKD. Omori et al. showed that treatment of pcy mice for 7 weeks with PD184352 reduced kidney weight and cystic index, increased the renal concentrating ability consistent with improved renal function, and lowered blood pressure [120]. On the other hand, in a model of PKD in which cysts develop rapidly, the delivery of the MEK inhibitor U0126 on postnatal days 4 and 7 inhibited ERK activity, but did not decrease cysts at post-natal day 14 [121]. Since cyst progression in this model occurs during renal development, an array of mitogenic factors may be responsible for the rapid growth of cysts, overriding ERK inhibition.
B-Raf appears to govern the mitogenic response to cAMP agonists in ADPKD and ARPKD cells [27, 52, 63, 79]; by contrast B-Raf is normally inactive in normal kidneys. Thus, there is reason to think that selective B-Raf inhibition would block cAMP-dependent proliferation of cystic epithelial cells without affecting normal renal function. Bay 43–9006, a small molecule Raf inhibitor, blocks cAMP-dependent ERK activation, and inhibits ADPKD cell proliferation [122]. However, treatment of jck mice with Bay 43–9006 did not significantly inhibit disease progression [123]. The reason for the lack of an effect in these PKD animal models remains unclear. Bay 43–9006 is a broad spectrum kinase inhibitor known to inhibit the activity of many kinases. This lack of specificity may complicate the effect on Raf kinases, or alternatively, the drug may lack the bioavailability necessary to inhibit Raf activity in cystic kidneys. Recent evidence demonstrates that the effect of B-Raf inhibition on ERK is not as straightforward as originally thought. B-Raf and Raf-1 can heterodimerize, which impacts the activity of both kinases [124–126]. Interestingly, B-Raf inhibitors have been shown to promote Ras-dependent B-Raf binding to Raf-1, leading to Raf-1 activation and stimulation of MEK/ERK [125, 126]. Kinase-dead B-Raf mimicked the effect of B-Raf inhibitors on ERK activation. These observations indicate that the MEK/ERK signaling is more complex than originally thought and may require a combination of drugs to effectively block ERK activation and cell proliferation. Targeting Src kinase, an intermediate between receptor activation and the Ras/Raf/MEK/ERK pathway, may be an alternative approach. SKI-606, a Src inhibitor, was found to decrease renal and biliary cysts in the PCK rat and bpk mouse models [127], indicating that this may be a pharmacological approach to block receptor kinase activity as well as cAMP activation of the ERK pathway.
In jck mice, a PKD model with elevated renal cAMP and B-Raf/MEK/ERK activity, treatment with roscovitine, a cyclin kinase inhibitor, resulted in long-lasting arrest of cyst growth, inhibition of cystic disease and improved renal function [128]. There was dephosphorylation of Rb and a decrease in cyclin D levels, suggesting a G1/S cell cycle block. In addition, roscovitine decreased renal cAMP levels possibly due to improved epithelial cell differentiation. This study also showed that roscovitine has a long-lasting effect after drug withdrawal and is effective even with intermittent dosing, which may be important for long term treatment of PKD.
6.3. Blocking cAMP-dependent fluid secretion
Cl− dependent fluid secretion is largely responsible for expanding the cyst volume, causing disruption of the normal parenchyma and loss of renal function. It seems reasonable that inhibition of fluid secretion would prevent the expansion of these otherwise benign neoplasms, sparing the normal nephrons. In support of this idea, CFTR inhibitors were shown to slow cyst development in embryonic Pkd1−/− mouse kidney organ cultures and Pkd flox/−; Ksp-Cre mice treated for 3 days [104, 105]. TRAM-34, a KCa3.1 inhibitor, inhibited cAMP-dependent Cl− secretion by ADPKD cell monolayers, and reduced cyst growth of ADPKD cells grown within a collagen matrix [99]. While the effect of TRAM-34 still needs to be demonstrated in a PKD animal model, it is interesting that TRAM-34 attenuates renal fibrosis induced by unilateral ureteral obstruction in mice and rats. Senicapoc (ICA-17043), another KCa3.1inhibitor, is currently in clinical trials for sickle-cell disease and has shown little or no toxicity [129]. Other targets in the secretory pathway include the Na+, K+-ATPase and NKCC1; however, inhibition of these transporters will likely have side effects that would worsen PKD. NKCC1 can be inhibited by furosemide (also known as Lasix), a potent diuretic that also blocks apical NKCC2 in the thick ascending limb of Henle. The loss of body water due to the diuretic effect of furosemide would be expected to increase AVP release from the pituitary gland, and stimulate renal cAMP production. Likewise, concentrations of ouabain necessary to inhibit the Na+, K+-ATPase will also inhibit Na+ absorption by the kidney causing increased water loss. Recently, ouabain, even at normal circulating levels, was shown to bind to a unique site on the Na+, K+ ATPase stimulating the MEK/ERK pathway independent of cAMP or growth factors [51].
7. Concluding remarks
Studies involving human cyst epithelial cells and PKD animal models have uncovered a central role for cAMP in the pathogenesis of PKD. First, a reduction in intracellular Ca2+ due to mutations in the ADPKD and ARPKD genes cause a phenotypic switch in the cellular response to cAMP, such that cAMP stimulates the B-Raf/MEK/ERK pathway, which may be uniquely responsible for unscheduled cell proliferation. Furthermore, activation of ERK by cAMP may lead to secondary events involved in cyst growth, such as stimulation of mTOR pathway [130]. Second, cAMP stimulates transepithelial Cl− and fluid secretion which appears to be necessary for the accumulation of fluid within the cyst. Preclinical studies have indicated that reduction of renal cAMP, elevation in intracellular Ca2+, and inhibition of components of cell proliferation and fluid secretion may have therapeutic potential to reduced cyst growth in PKD.
Acknowledgements
The author apologizes in the event that relevant publications were inadvertently omitted in the review. The author is deeply grateful to Drs. James Calvet and Jared Grantham for their long-standing collaboration, stimulating discussions, and for critically reading this review. Special thanks to the PKD patients who recognize the importance of PKD research and have donated their discarded kidneys for research. It is gratifying to know that the first therapeutic approaches to be tested in ADPKD patients emerged from studies using cells cultured from cystic kidneys donated by the patients. Work from the author's laboratory is supported by grants from the National Institutes of Health (R01DK081579 and P50DK057301) and the PKD Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grantham JJ. 1992 Homer Smith Award. Fluid secretion, cellular proliferation, and the pathogenesis of renal epithelial cysts. J Am Soc Nephrol. 1993;3:1841–1857. doi: 10.1681/ASN.V3121841. [DOI] [PubMed] [Google Scholar]
- 2.Heggo O. A microdissection study of cystic disease of the kidneys in adults. J Pathol Bacteriol. 1966;91:311–315. doi: 10.1002/path.1700910204. [DOI] [PubMed] [Google Scholar]
- 3.Lantinga-van Leeuwen IS, Dauwerse JG, Baelde HJ, Leonhard WN, van de Wal A, Ward CJ, Verbeek S, Deruiter MC, Breuning MH, de Heer E, Peters DJ. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet. 2004;13:3069–3077. doi: 10.1093/hmg/ddh336. [DOI] [PubMed] [Google Scholar]
- 4.Thomson RB, Mentone S, Kim R, Earle K, Delpire E, Somlo S, Aronson PS. Histopathological analysis of renal cystic epithelia in the Pkd2WS25/− mouse model of ADPKD. Am J Physiol Renal Physiol. 2003;285:F870–F880. doi: 10.1152/ajprenal.00153.2003. [DOI] [PubMed] [Google Scholar]
- 5.Verani RR, Silva FG. Histogenesis of the renal cysts in adult (autosomal dominant) polycystic kidney disease: a histochemical study. Mod Pathol. 1988 Jan;:457–463. [PubMed] [Google Scholar]
- 6.Wu G, D'Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell. 1998;93:177–188. doi: 10.1016/s0092-8674(00)81570-6. [DOI] [PubMed] [Google Scholar]
- 7.Delmas P. Polycystins: from mechanosensation to gene regulation. Cell. 2004;118:145–148. doi: 10.1016/j.cell.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Nims N, Vassmer D, Maser RL. Transmembrane domain analysis of polycystin-1, the product of the polycystic kidney disease-1 (PKD1) gene: evidence for 11 membrane-spanning domains. Biochemistry. 2003;42:13035–13048. doi: 10.1021/bi035074c. [DOI] [PubMed] [Google Scholar]
- 9.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, Gamble V, Harris PC. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995;10:151–160. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- 10.Parnell SC, Magenheimer BS, Maser RL, Calvet JP. Identification of the major site of in vitro PKA phosphorylation in the polycystin-1 C-terminal cytosolic domain. Biochem Biophys Res Commun. 1999;259:539–543. doi: 10.1006/bbrc.1999.0810. [DOI] [PubMed] [Google Scholar]
- 11.Parnell SC, Magenheimer BS, Maser RL, Rankin CA, Smine A, Okamoto T, Calvet JP. The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem Biophys Res Commun. 1998;251:625–631. doi: 10.1006/bbrc.1998.9514. [DOI] [PubMed] [Google Scholar]
- 12.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 13.Vandorpe DH, Chernova MN, Jiang L, Sellin LK, Wilhelm S, Stuart-Tilley AK, Walz G, Alper SL. The cytoplasmic C-terminal fragment of polycystin-1 regulates a Ca2+- permeable cation channel. J Biol Chem. 2001;276:4093–4101. doi: 10.1074/jbc.M006252200. [DOI] [PubMed] [Google Scholar]
- 14.Vandorpe DH, Wilhelm S, Jiang L, Ibraghimov-Beskrovnaya O, Chernova MN, Stuart-Tilley AK, Alper SL. Cation channel regulation by COOH-terminal cytoplasmic tail of polycystin-1: mutational and functional analysis. Physiol Genomics. 2002;8:87–98. doi: 10.1152/physiolgenomics.00092.2001. [DOI] [PubMed] [Google Scholar]
- 15.Qian F, Watnick TJ, Onuchic LF, Germino GG. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell. 1996;87:979–987. doi: 10.1016/s0092-8674(00)81793-6. [DOI] [PubMed] [Google Scholar]
- 16.Brasier JL, Henske EP. Loss of the polycystic kidney disease (PKD1) region of chromosome 16p13 in renal cyst cells supports a loss-of-function model for cyst pathogenesis. J Clin Invest. 1997;99:194–199. doi: 10.1172/JCI119147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grantham JJ, Geiser JL, Evan AP. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 1987;31:1145–1152. doi: 10.1038/ki.1987.121. [DOI] [PubMed] [Google Scholar]
- 18.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 19.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 20.Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet. 2003;12:2703–2710. doi: 10.1093/hmg/ddg274. [DOI] [PubMed] [Google Scholar]
- 21.Harris PC. Molecular basis of polycystic kidney disease: PKD1, PKD2 and PKHD1. Curr Opin Nephrol Hypertens. 2002;11:309–314. doi: 10.1097/00041552-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 23.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 24.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol. 2007;27:3241–3252. doi: 10.1128/MCB.00072-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gradilone SA, Masyuk TV, Huang BQ, Banales JM, Lehmann GL, Radtke BN, Stroope A, Masyuk AI, Splinter PL, Larusso NF. Activation of Trpv4 reduces the hyperproliferative phenotype of cystic cholangiocytes from an animal model of ARPKD. Gastroenterology. 2010;139:304–314. doi: 10.1053/j.gastro.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol. 2006;17:178–187. doi: 10.1681/ASN.2005060645. [DOI] [PubMed] [Google Scholar]
- 28.Nauli SM, Rossetti S, Kolb RJ, Alenghat FJ, Consugar MB, Harris PC, Ingber DE, Loghman-Adham M, Zhou J. Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol. 2006;17:1015–1025. doi: 10.1681/ASN.2005080830. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Zhang S, Zhou Q, Guo H, Zhang K, Zheng R, Xiao C. PKHD1 gene silencing may cause cell abnormal proliferation through modulation of intracellular calcium in autosomal recessive polycystic kidney disease. J Biochem Mol Biol. 2007;40:467–474. doi: 10.5483/bmbrep.2007.40.4.467. [DOI] [PubMed] [Google Scholar]
- 30.Banales JM, Masyuk TV, Gradilone SA, Masyuk AI, Medina JF, LaRusso NF. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD) Hepatology. 2009;49:160–174. doi: 10.1002/hep.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houslay MD, Milligan G. Tailoring cAMP-signalling responses through isoform multiplicity. Trends Biochem Sci. 1997;22:217–224. doi: 10.1016/s0968-0004(97)01050-5. [DOI] [PubMed] [Google Scholar]
- 32.Cooper DM, Mons N, Karpen JW. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature. 1995;374:421–424. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- 33.Weiss ER, Kelleher DJ, Woon CW, Soparkar S, Osawa S, Heasley LE, Johnson GL. Receptor activation of G proteins. Faseb J. 1988;2:2841–2848. doi: 10.1096/fasebj.2.13.3139484. [DOI] [PubMed] [Google Scholar]
- 34.Iyengar R. Molecular and functional diversity of mammalian Gs-stimulated adenylyl cyclases. Faseb J. 1993;7:768–775. doi: 10.1096/fasebj.7.9.8330684. [DOI] [PubMed] [Google Scholar]
- 35.Tang WJ, Gilman AG. Adenylyl cyclases. Cell. 1992;70:869–872. doi: 10.1016/0092-8674(92)90236-6. [DOI] [PubMed] [Google Scholar]
- 36.Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- 37.Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol. 2000;279:F400–F416. doi: 10.1152/ajprenal.2000.279.3.F400. [DOI] [PubMed] [Google Scholar]
- 38.Yang B, He B, Abdel-Halim SM, Tibell A, Brendel MD, Bretzel RG, Efendic S, Hillert J. Molecular cloning of a full-length cDNA for human type 3 adenylyl cyclase and its expression in human islets. Biochem Biophys Res Commun. 1999;254:548–551. doi: 10.1006/bbrc.1998.9983. [DOI] [PubMed] [Google Scholar]
- 39.Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, Schnermann J, Caplan MJ. Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci U S A. 2009;106:2059–2064. doi: 10.1073/pnas.0812859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng J, Grande JP. Cyclic nucleotide phosphodiesterase (PDE) inhibitors: novel therapeutic agents for progressive renal disease. Exp Biol Med (Maywood) 2007;232:38–51. [PubMed] [Google Scholar]
- 41.Faux MC, Scott JD. Molecular glue: kinase anchoring and scaffold proteins. Cell. 1996;85:9–12. doi: 10.1016/s0092-8674(00)81075-2. [DOI] [PubMed] [Google Scholar]
- 42.Rubin CS. A kinase anchor proteins and the intracellular targeting of signals carried by cyclic AMP. Biochim Biophys Acta. 1994;1224:467–479. [PubMed] [Google Scholar]
- 43.Klussmann E, Rosenthal W. Role and identification of protein kinase A anchoring proteins in vasopressin-mediated aquaporin-2 translocation. Kidney Int. 2001;60:446–449. doi: 10.1046/j.1523-1755.2001.060002446.x. [DOI] [PubMed] [Google Scholar]
- 44.Roscioni SS, Elzinga CR, Schmidt M. Epac: effectors and biological functions. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:345–357. doi: 10.1007/s00210-007-0246-7. [DOI] [PubMed] [Google Scholar]
- 45.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 46.Frings S, Lynch JW, Lindemann B. Properties of cyclic nucleotide-gated channels mediating olfactory transduction. Activation, selectivity, and blockage. J Gen Physiol. 1992;100:45–67. doi: 10.1085/jgp.100.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gattone VH, 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 48.Smith LA, Bukanov NO, Husson H, Russo RJ, Barry TC, Taylor AL, Beier DR, Ibraghimov-Beskrovnaya O. Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol. 2006;17:2821–2831. doi: 10.1681/ASN.2006020136. [DOI] [PubMed] [Google Scholar]
- 49.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH., 2nd Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med. 2004;10:363–364. doi: 10.1038/nm1004. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi T, Nagao S, Kasahara M, Takahashi H, Grantham JJ. Renal accumulation and excretion of cyclic adenosine monophosphate in a murine model of slowly progressive polycystic kidney disease. Am J Kidney Dis. 1997;30:703–709. doi: 10.1016/s0272-6386(97)90496-0. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen AN, Wallace DP, Blanco G. Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J Am Soc Nephrol. 2007;18:46–57. doi: 10.1681/ASN.2006010086. [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 2003;63:1983–1994. doi: 10.1046/j.1523-1755.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 53.Belibi FA, Reif G, Wallace DP, Yamaguchi T, Olsen L, Li H, Helmkamp GM, Jr, Grantham JJ. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int. 2004;66:964–973. doi: 10.1111/j.1523-1755.2004.00843.x. [DOI] [PubMed] [Google Scholar]
- 54.Lee K, Brown D, Urena P, Ardaillou N, Ardaillou R, Deeds J, Segre GV. Localization of parathyroid hormone/parathyroid hormone-related peptide receptor mRNA in kidney. Am J Physiol. 1996;270:F186–F191. doi: 10.1152/ajprenal.1996.270.1.F186. [DOI] [PubMed] [Google Scholar]
- 55.Nagao S, Nishii K, Katsuyama M, Kurahashi H, Marunouchi T, Takahashi H, Wallace DP. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol. 2006;17:2220–2227. doi: 10.1681/ASN.2006030251. [DOI] [PubMed] [Google Scholar]
- 56.Nagao S, Nishii K, Yoshihara D, Kurahashi H, Nagaoka K, Yamashita T, Takahashi H, Yamaguchi T, Calvet JP, Wallace DP. Calcium channel inhibition accelerates polycystic kidney disease progression in the Cy/+ rat. Kidney Int. 2008;73:269–277. doi: 10.1038/sj.ki.5002629. [DOI] [PubMed] [Google Scholar]
- 57.Danielsen H, Pedersen EB, Nielsen AH, Herlevsen P, Kornerup HJ, Posborg V. Expansion of extracellular volume in early polycystic kidney disease. Acta Med Scand. 1986;219:399–405. doi: 10.1111/j.0954-6820.1986.tb03330.x. [DOI] [PubMed] [Google Scholar]
- 58.Michalski A, Grzeszczak W. [The effect of hypervolemia on electrolyte level and and level of volume regulating hormones in patients with autosomal dominant polycystic kidney disease] Pol Arch Med Wewn. 1996;96:329–343. [PubMed] [Google Scholar]
- 59.Torres VE. Cyclic AMP, at the hub of the cystic cycle. Kidney Int. 2004;66:1283–1285. doi: 10.1111/j.1523-1755.2004.00945.x. [DOI] [PubMed] [Google Scholar]
- 60.Hanaoka K, Guggino WB. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol. 2000;11:1179–1187. doi: 10.1681/ASN.V1171179. [DOI] [PubMed] [Google Scholar]
- 61.Yamaguchi T, Pelling JC, Ramaswamy NT, Eppler JW, Wallace DP, Nagao S, Rome LA, Sullivan LP, Grantham JJ. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int. 2000;57:1460–1471. doi: 10.1046/j.1523-1755.2000.00991.x. [DOI] [PubMed] [Google Scholar]
- 62.Parker E, Newby LJ, Sharpe CC, Rossetti S, Streets AJ, Harris PC, O'Hare MJ, Ong AC. Hyperproliferation of PKD1 cystic cells is induced by insulin-like growth factor-1 activation of the Ras/Raf signalling system. Kidney Int. 2007;72:157–165. doi: 10.1038/sj.ki.5002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem. 2004;279:40419–40430. doi: 10.1074/jbc.M405079200. [DOI] [PubMed] [Google Scholar]
- 64.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 65.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 66.Dumaz N, Marais R. Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the anniversary prize of the Gesellschaft fur Biochemie und Molekularbiologie Lecture delivered on 5 July 2003 at the Special FEBS Meeting in Brussels. Febs J. 2005;272:3491–3504. doi: 10.1111/j.1742-4658.2005.04763.x. [DOI] [PubMed] [Google Scholar]
- 67.Chang F, Steelman LS, Shelton JG, Lee JT, Navolanic PM, Blalock WL, Franklin R, McCubrey JA. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (Review) Int J Oncol. 2003;22:469–480. [PubMed] [Google Scholar]
- 68.Dugan LL, Kim JS, Zhang Y, Bart RD, Sun Y, Holtzman DM, Gutmann DH. Differential effects of cAMP in neurons and astrocytes. Role of B-raf. J Biol Chem. 1999;274:25842–25848. doi: 10.1074/jbc.274.36.25842. [DOI] [PubMed] [Google Scholar]
- 69.Qiu W, Zhuang S, von Lintig FC, Boss GR, Pilz RB. Cell type-specific regulation of B-Raf kinase by cAMP and 14-3-3 proteins. J Biol Chem. 2000;275:31921–31929. doi: 10.1074/jbc.M003327200. [DOI] [PubMed] [Google Scholar]
- 70.Stork PJ, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002;12:258–266. doi: 10.1016/s0962-8924(02)02294-8. [DOI] [PubMed] [Google Scholar]
- 71.Dumaz N, Marais R. Protein kinase A blocks Raf-1 activity by stimulating 14-3-3 binding and blocking Raf-1 interaction with Ras. J Biol Chem. 2003;278:29819–29823. doi: 10.1074/jbc.C300182200. [DOI] [PubMed] [Google Scholar]
- 72.Chong H, Lee J, Guan KL. Positive and negative regulation of Raf kinase activity and function by phosphorylation. Embo J. 2001;20:3716–3727. doi: 10.1093/emboj/20.14.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mason CS, Springer CJ, Cooper RG, Superti-Furga G, Marshall CJ, Marais R. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. Embo J. 1999;18:2137–2148. doi: 10.1093/emboj/18.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 75.Guan KL, Figueroa C, Brtva TR, Zhu T, Taylor J, Barber TD, Vojtek AB. Negative regulation of the serine/threonine kinase B-Raf by Akt. J Biol Chem. 2000;275:27354–27359. doi: 10.1074/jbc.M004371200. [DOI] [PubMed] [Google Scholar]
- 76.Tang X, Batty IH, Downes CP. Muscarinic receptors mediate phospholipase C-dependent activation of protein kinase B via Ca2+, ErbB3, and phosphoinositide 3-kinase in 1321N1 astrocytoma cells. J Biol Chem. 2002;277:338–344. doi: 10.1074/jbc.M108927200. [DOI] [PubMed] [Google Scholar]
- 77.Okuno S, Kitani T, Matsuzaki H, Konishi H, Kikkawa U, Fujisawa H. Studies on the phosphorylation of protein kinase B by Ca(2+)/calmodulin- dependent protein kinases. J Biochem (Tokyo) 2000;127:965–970. doi: 10.1093/oxfordjournals.jbchem.a022712. [DOI] [PubMed] [Google Scholar]
- 78.Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, Einhorn E, Herlyn M, Minna J, Nicholson A, Roth JA, Albelda SM, Davies H, Cox C, Brignell G, Stephens P, Futreal PA, Wooster R, Stratton MR, Weber BL. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- 79.Park EY, Sung YH, Yang MH, Noh JY, Park SY, Lee TY, Yook YJ, Yoo KH, Roh KJ, Kim I, Hwang YH, Oh GT, Seong JK, Ahn C, Lee HW, Park JH. Cyst formation in kidney via B-Raf signaling in the PKD2 transgenic mice. J Biol Chem. 2009;284:7214–7222. doi: 10.1074/jbc.M805890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wahl PR, Le Hir M, Vogetseder A, Arcaro A, Starke A, Waeckerle-Men Y, Serra AL, Wuthrich RP. Mitotic activation of Akt signalling pathway in Han:SPRD rats with polycystic kidney disease. Nephrology (Carlton) 2007;12:357–363. doi: 10.1111/j.1440-1797.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- 81.Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wuthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD) Nephrol Dial Transplant. 2006;21:598–604. doi: 10.1093/ndt/gfi181. [DOI] [PubMed] [Google Scholar]
- 82.Tao Y, Kim J, Schrier RW, Edelstein CL. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol. 2005;16:46–51. doi: 10.1681/ASN.2004080660. [DOI] [PubMed] [Google Scholar]
- 83.Nutahara K, Higashihara E, Horie S, Kamura K, Tsuchiya K, Mochizuki T, Hosoya T, Nakayama T, Yamamoto N, Higaki Y, Shimizu T. Calcium channel blocker versus angiotensin II receptor blocker in autosomal dominant polycystic kidney disease. Nephron Clin Pract. 2005;99:c18–c23. doi: 10.1159/000081790. [DOI] [PubMed] [Google Scholar]
- 84.Cowley BD., Jr Calcium, cyclic AMP, and MAP kinases: dysregulation in polycystic kidney disease. Kidney Int. 2008;73:251–253. doi: 10.1038/sj.ki.5002695. discussion 269. [DOI] [PubMed] [Google Scholar]
- 85.Jafar TH, Stark PC, Schmid CH, Strandgaard S, Kamper AL, Maschio G, Becker G, Perrone RD, Levey AS. The effect of angiotensin-converting-enzyme inhibitors on progression of advanced polycystic kidney disease. Kidney Int. 2005;67:265–271. doi: 10.1111/j.1523-1755.2005.00077.x. [DOI] [PubMed] [Google Scholar]
- 86.Kanno Y, Suzuki H, Okada H, Takenaka T, Saruta T. Calcium channel blockers versus ACE inhibitors as antihypertensives in polycystic kidney disease. Qjm. 1996;89:65–70. doi: 10.1093/oxfordjournals.qjmed.a030139. [DOI] [PubMed] [Google Scholar]
- 87.Sullivan LP, Wallace DP, Grantham JJ. Epithelial transport in polycystic kidney disease. Physiol Rev. 1998;78:1165–1191. doi: 10.1152/physrev.1998.78.4.1165. [DOI] [PubMed] [Google Scholar]
- 88.McAteer JA, Evan AP, Gardner KD. Morphogenetic clonal growth of kidney epithelial cell line MDCK. Anat Rec. 1987;217:229–239. doi: 10.1002/ar.1092170303. [DOI] [PubMed] [Google Scholar]
- 89.Grantham JJ, Uchic M, Cragoe EJ, Jr, Kornhaus J, Grantham JA, Donoso V, Mangoo-Karim R, Evan A, McAteer J. Chemical modification of cell proliferation and fluid secretion in renal cysts. Kidney Int. 1989;35:1379–1389. doi: 10.1038/ki.1989.137. [DOI] [PubMed] [Google Scholar]
- 90.Sullivan LP, Wallace DP, Grantham JJ. Coupling of cell volume and membrane potential changes to fluid secretion in a model of renal cysts. Kidney Int. 1994;45:1369–1380. doi: 10.1038/ki.1994.179. [DOI] [PubMed] [Google Scholar]
- 91.Grant ME, Neufeld TK, Cragoe EJ, Jr, Welling LW, Grantham JJ. Arginine vasopressin stimulates net fluid secretion in a polarized subculture of cyst-forming MDCK cells. J Am Soc Nephrol. 1991;2:219–227. doi: 10.1681/ASN.V22219. [DOI] [PubMed] [Google Scholar]
- 92.Mangoo-Karim R, Uchic ME, Grant M, Shumate WA, Calvet JP, Park CH, Grantham JJ. Renal epithelial fluid secretion and cyst growth: the role of cyclic AMP. Faseb J. 1989;3:2629–2632. doi: 10.1096/fasebj.3.14.2480260. [DOI] [PubMed] [Google Scholar]
- 93.Mangoo-Karim R, Uchic M, Lechene C, Grantham JJ. Renal epithelial cyst formation and enlargement in vitro: dependence on cAMP. Proc Natl Acad Sci U S A. 1989;86:6007–6011. doi: 10.1073/pnas.86.15.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ye M, Grantham JJ. The secretion of fluid by renal cysts from patients with autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329:310–313. doi: 10.1056/NEJM199307293290503. [DOI] [PubMed] [Google Scholar]
- 95.Grantham JJ, Ye M, Gattone VH, 2nd, Sullivan LP. In vitro fluid secretion by epithelium from polycystic kidneys. J Clin Invest. 1995;95:195–202. doi: 10.1172/JCI117638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mangoo-Karim R, Ye M, Wallace DP, Grantham JJ, Sullivan LP. Anion secretion drives fluid secretion by monolayers of cultured human polycystic cells. Am J Physiol. 1995;269:F381–F388. doi: 10.1152/ajprenal.1995.269.3.F381. [DOI] [PubMed] [Google Scholar]
- 97.Welling PA, Ho K. A comprehensive guide to the ROMK potassium channel: form and function in health and disease. Am J Physiol Renal Physiol. 2009;297:F849–F863. doi: 10.1152/ajprenal.00181.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sullivan LP, Wallace DP, Gover T, Welling PA, Yamaguchi T, Maser R, Eppler JW, Grantham JJ. Sulfonylurea-sensitive K(+) transport is involved in Cl(−) secretion and cyst trowth by cultured ADPKD cells. J Am Soc Nephrol. 2002;13:2619–2627. doi: 10.1097/01.asn.0000034220.09301.9c. [DOI] [PubMed] [Google Scholar]
- 99.Albaqumi M, Srivastava S, Li Z, Zhdnova O, Wulff H, Itani O, Wallace DP, Skolnik EY. KCa3.1 potassium channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;74:740–749. doi: 10.1038/ki.2008.246. [DOI] [PubMed] [Google Scholar]
- 100.Brill SR, Ross KE, Davidow CJ, Ye M, Grantham JJ, Caplan MJ. Immunolocalization of ion transport proteins in human autosomal dominant polycystic kidney epithelial cells. Proc Natl Acad Sci U S A. 1996;93:10206–10211. doi: 10.1073/pnas.93.19.10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hanaoka K, Devuyst O, Schwiebert EM, Wilson PD, Guggino WB. A role for CFTR in human autosomal dominant polycystic kidney disease. Am J Physiol. 1996;270:C389–C399. doi: 10.1152/ajpcell.1996.270.1.C389. [DOI] [PubMed] [Google Scholar]
- 102.Wallace DP, Grantham JJ, Sullivan LP. Chloride and fluid secretion by cultured human polycystic kidney cells. Kidney Int. 1996;50:1327–1336. doi: 10.1038/ki.1996.445. [DOI] [PubMed] [Google Scholar]
- 103.Sullivan LP, Wallace DP, Grantham JJ. Chloride and fluid secretion in polycystic kidney disease. J Am Soc Nephrol. 1998;9:903–916. doi: 10.1681/ASN.V95903. [DOI] [PubMed] [Google Scholar]
- 104.Magenheimer BS, St John PL, Isom KS, Abrahamson DR, De Lisle RC, Wallace DP, Maser RL, Grantham JJ, Calvet JP. Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na(+),K(+),2Cl(−) Co-transporter-dependent cystic dilation. J Am Soc Nephrol. 2006;17:3424–3437. doi: 10.1681/ASN.2006030295. [DOI] [PubMed] [Google Scholar]
- 105.Yang B, Sonawane ND, Zhao D, Somlo S, Verkman AS. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19:1300–1310. doi: 10.1681/ASN.2007070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang X, Gattone V, 2nd, Harris PC, Torres VE. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol. 2005;16:846–851. doi: 10.1681/ASN.2004121090. [DOI] [PubMed] [Google Scholar]
- 107.Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19:102–108. doi: 10.1681/ASN.2007060688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Torres VE, Bankir L, Grantham JJ. A case for water in the treatment of polycystic kidney disease. Clin J Am Soc Nephrol. 2009;4:1140–1150. doi: 10.2215/CJN.00790209. [DOI] [PubMed] [Google Scholar]
- 109.Wang CJ, Creed C, Winklhofer FT, Grantham JJ. Water Prescription in Autosomal Dominant Polycystic Kidney Disease: A Pilot Study. Clin J Am Soc Nephrol. 2010 doi: 10.2215/CJN.03950510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bates CM, Kegg H, Grady S. Expression of somatostatin in the adult and developing mouse kidney. Kidney Int. 2004;66:1785–1793. doi: 10.1111/j.1523-1755.2004.00953.x. [DOI] [PubMed] [Google Scholar]
- 111.Ray C, Carney S, Morgan T, Gillies A. Somatostatin as a modulator of distal nephron water permeability. Clin Sci (Lond) 1993;84:455–460. doi: 10.1042/cs0840455. [DOI] [PubMed] [Google Scholar]
- 112.Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3',5'-cyclic monophosphate. Gastroenterology. 2007;132:1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 113.Ruggenenti P, Remuzzi A, Ondei P, Fasolini G, Antiga L, Ene-Iordache B, Remuzzi G, Epstein FH. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68:206–216. doi: 10.1111/j.1523-1755.2005.00395.x. [DOI] [PubMed] [Google Scholar]
- 114.Leuenroth SJ, Bencivenga N, Igarashi P, Somlo S, Crews CM. Triptolide reduces cystogenesis in a model of ADPKD. J Am Soc Nephrol. 2008;19:1659–1662. doi: 10.1681/ASN.2008030259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leuenroth SJ, Okuhara D, Shotwell JD, Markowitz GS, Yu Z, Somlo S, Crews CM. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci U S A. 2007;104:4389–4394. doi: 10.1073/pnas.0700499104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang X, Harris PC, Somlo S, Batlle D, Torres VE. Effect of calcium-sensing receptor activation in models of autosomal recessive or dominant polycystic kidney disease. Nephrol Dial Transplant. 2009;24:526–534. doi: 10.1093/ndt/gfn527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gattone VH, 2nd, Chen NX, Sinders RM, Seifert MF, Duan D, Martin D, Henley C, Moe SM. Calcimimetic inhibits late-stage cyst growth in ADPKD. J Am Soc Nephrol. 2009;20:1527–1532. doi: 10.1681/ASN.2008090927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Calvet JP. MEK inhibition holds promise for polycystic kidney disease. J Am Soc Nephrol. 2006;17:1498–1500. doi: 10.1681/ASN.2006040353. [DOI] [PubMed] [Google Scholar]
- 119.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, Golub TR, Sebolt-Leopold J, Sellers WR, Rosen N. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Omori S, Hida M, Fujita H, Takahashi H, Tanimura S, Kohno M, Awazu M. Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J Am Soc Nephrol. 2006;17:1604–1614. doi: 10.1681/ASN.2004090800. [DOI] [PubMed] [Google Scholar]
- 121.Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG, Igarashi P, Somlo S. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet. 2008;17:1505–1516. doi: 10.1093/hmg/ddn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yamaguchi T, Reif GA, Calvet JP, Wallace DP. Sorafenib inhibits cAMP-dependent ERK activation, cell proliferation, and in vitro cyst growth of human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol. 2010;299:F944–F951. doi: 10.1152/ajprenal.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Natoli TA, Smith LA, Husson H, Russo RJ, Rogers KA, Baldwin AB, Gregory JS, Lillie J, Ibraghimov-Beskrovnaya O. Inhibition of the Raf/MEK/ERK pathway reduces new cyst formation but increases cyst growth in murine PKD. (Abstract) J Am Soc Nephrol. 2009;20:P495A. [Google Scholar]
- 124.Rushworth LK, Hindley AD, O'Neill E, Kolch W. Regulation and role of Raf-1/B-Raf heterodimerization. Mol Cell Biol. 2006;26:2262–2272. doi: 10.1128/MCB.26.6.2262-2272.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer CJ, Pritchard C, Marais R. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor SL, Vigers G, Morales T, Aliagas I, Liu B, Sideris S, Hoeflich KP, Jaiswal BS, Seshagiri S, Koeppen H, Belvin M, Friedman LS, Malek S. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 127.Sweeney WE, Jr, von Vigier RO, Frost P, Avner ED. Src inhibition ameliorates polycystic kidney disease. J Am Soc Nephrol. 2008;19:1331–1341. doi: 10.1681/ASN.2007060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–952. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 129.Ataga KI, Smith WR, De Castro LM, Swerdlow P, Saunthararajah Y, Castro O, Vichinsky E, Kutlar A, Orringer EP, Rigdon GC, Stocker JW. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood. 2008;111:3991–3997. doi: 10.1182/blood-2007-08-110098. [DOI] [PubMed] [Google Scholar]
- 130.Distefano G, Boca M, Rowe I, Wodarczyk C, Ma L, Piontek KB, Germino GG, Pandolfi PP, Boletta A. Polycystin-1 regulates extracellular signal-regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1. Mol Cell Biol. 2009;29:2359–2371. doi: 10.1128/MCB.01259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]