Abstract
Background
Cellular changes associated with diabetic and idiopathic gastroparesis are not well described.
Aim
Describe histologic abnormalities in gastroparesis and compare findings in idiopathic versus diabetic gastroparesis.
Methods
Full thickness gastric body biopsies were obtained from 40 gastroparetics (20 diabetic) and matched controls. Sections were stained for H&E and trichrome, and immunolabeled with antibodies against PGP 9.5, nNOS, VIP, substance P and tyrosine hydroxylase to quantify nerves, S100β for glia, Kit for interstitial cells of Cajal (ICC), CD45 and CD68, for immune cells and smoothelin for smooth muscle cells. Tissue was also examined by transmission electron microscopy (TEM).
Results
Histological abnormalities were found in 83% of patients. Most common defects were loss of ICC with remaining ICC showing injury, an abnormal immune infiltrate containing macrophages, and decreased nerve fibers. On light microscopy, no significant differences were found between diabetic and idiopathic gastroparesis with the exception of nNOS expression which was decreased in more idiopathic gastroparetics (40%) compared to diabetic (20%) patients by visual grading. On electron microscopy, a markedly increased connective tissue stroma was present in both disorders.
Conclusion
This study suggests that on full thickness biopsies, cellular abnormalities are found in the majority of patients with gastroparesis. Most common findings were loss of Kit expression suggesting loss of ICC and an increase in CD45 and CD68 immunoreactivity. These findings suggest that examination of tissue can lead to valuable insights into the pathophysiology of these disorders and offers hope that new therapeutic targets can be found.
Keywords: gastric emptying, smooth muscle, interstitial cells of Cajal, enteric nerves
Introduction
Gastroparesis is a chronic disorder defined as delayed gastric emptying of solids and liquids in the absence of obstruction. Symptoms include early satiety, nausea, vomiting, bloating, and pain.1–5 Gastroparesis is most commonly associated with diabetes (both type I and type II) 6 or is of unknown cause (idiopathic). Less common causes include postsurgical and medication related gastroparesis.1–5
Gastroparesis is increasingly being recognized as a cause of significant morbidity. Accurate prevalence numbers are hard to come by and world wide estimates of prevalence are not available. Recent data from Olmsted County in the United States show an age adjusted prevalence of gastroparesis of 9.6 for men and 37.8 for women per 100,000 persons. Young women are most commonly afflicted with a female: male ratio of 4:1 and a mean age of onset of 44 years.7 A significant limitation to developing targeted therapy for gastroparesis is lack of understanding of the pathological and cellular etiology. Normal gastric emptying requires coordinated correct function of several cell types, including the extrinsic innervation to the stomach, enteric nerves, glia, smooth muscle cells, interstitial cell of Cajal (ICC) and immune cells. Most of our understanding of the cellular basis for gastroparesis has come from animal studies focused on diabetic gastroparesis. Although several cellular abnormalities have been described,5, 8, 9 the two most common abnormalities noted have been loss of expression of neuronal nitric oxide synthase (nNOS) and loss of ICC.8, 10–14 In the limited human studies, the most common findings have also been loss of ICC in diabetic and/or in idiopathic gastroparesis15–23 and loss of nNOS.17–19, 23 Other studies have shown decreases in nerve fibers and neurons,17, 18, 22, 23 inflammatory infiltrate17, 23, 24 and fibrosis.22, 23, 25 Human studies are significantly limited by lack of access to prospectively collected tissue. Also, the distribution of the key cell types that control gastric motility is non-uniform26 making prospective collection of tissue from carefully mapped sites essential. Given these limitations, the National Institute of Health established a Gastroparesis Clinical Research Consortium (GpCRC). As part of that consortium we have collected site-matched, full thickness gastric body biopsies from patients with diabetic and idiopathic gastroparesis and age and sex matched controls. The aim of this study was to study the cellular abnormalities in gastroparesis and to compare findings in idiopathic versus diabetic gastroparesis.
Methods
Specimens
Sixty, full-thickness gastric biopsies were collected from the anterior aspect of the stomach, midway between the greater and lesser curvatures where the gastroepiploic vessels meet. The anatomy of individual stomachs varies but, in general, the region where the gastroepiploic arteries meet is about 9 cm proximal to the pylorus. Tissue was obtained from 20 idiopathic and 20 diabetic gastroparetic patients undergoing surgery for placement of a Gastric Stimulator and from 20 age and sex matched patients undergoing duodenal switch gastric bypass surgery following IRB approved protocols. The controls were all obtained from surgeries at Mayo Clinic and were screened to ensure they did not have diabetes or gastrointestinal diseases. The tissue appeared normal on H and E prior to being included in the study. The tissue was collected from the same site as the gastroparetics and was obtained and processed in identical fashion using an agreed upon identical protocols. None of these patients have been previously reported on. When the gastric stimulator wire insertion point overlapped with the preferred location, the biopsy was obtained above the insertion point. Each collected piece was approximately 1.5cm by 1.5cm. Tissue was collected from Temple University, University of Mississippi, California Pacific Medical Center and Mayo Clinic. Gastric specimens were directly placed in Ham F12 media (Invitrogen, Carlsbad, CA). A 5 mm piece of tissue was placed in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) in 1X phosphate buffer and shipped overnight to Mayo Clinic with ice-packs.
To determine if the 20 controls used (duodenal switch gastric bypass surgery for obesity) were different from non-obese controls, thus causing any significant histological changes, we prospectively collected full thickness gastric biopsy specimens from four non-obese, non gastroparetic subjects (GE junction cancer with no previous weight loss [<5 lbs], and no previous chemotherapy or radiation therapy) and compared histological changes with the above control group. No significant differences were observed in ICC numbers (5.3 ± 0.2 in obese vs. 4.9 ± 0.7 in non-obese, p=0.58), PGP expression (44.3 ± 2.3 in obese vs. 46.8 ± 6.6 in non-obese, p=0.67), and CD 45 positive bodies in circular muscle (14.3 ± 0.7 in obese vs. 12.3 ± 0.5 in non-obese, p=0.24) or myenteric plexus (20.3 ± 1.1 in obese vs 23.6 ± 3.1 in non-obese, p=0.23). Therefore obesity is not a confounding factor in histological changes observed between the control and gastroparetic groups.
Light microscopy
Antibodies to PGP9.5 were used to examine the enteric nervous system. Antibodies to nNOS and vasoactive intestinal peptide (VIP) were used to examine the inhibitory component of the enteric nervous system and to substance P (SP) to assess the excitatory component. Antibodies to tyrosine hydroxylase (TH) were used to assess extrinsic motor innervation. Antibodies to S100β were used to assess glia. ICC networks were assessed using an antibody to Kit. Smooth muscle morphology was assessed on H&E staining and by use of an anti-smoothelin antibody. A trichrome stain was used to quantify fibrosis. A general marker for immune cells27 (CD45) was used and markers for T lymphocytes28, 29 (CD3, CD4, CD8), B lymphocytes30 (CD79) and macrophages31 (CD68) used to characterize the immune cells. Further details on staining and quantification methodology and antibodies used (supplementary table 1) are in supplementary methods.
Electron microscopy
A subset of specimens (5 patients with diabetic gastroparesis, ages 19–66 yr, 4 females and 5 patients with idiopathic gastroparesis, ages 19–55 yr, 4 females) and five controls were also examined by electron microscopy (see supplementary methods). At least four blocks were sectioned for each patient and at least three grids were examined for each block and each grid contains 5–10 sections resulting in a minimum of 60 sections/patient.
Statistical Analysis
Data for c-Kit, PGP, nNOS, VIP, SP, tyrosine hydroxylase and CD 45 positive cell bodies are represented as mean ± SEM. Statistical significance was determined using Kruskal-Wallis one way ANOVA with Newman-Keuls Multiple Comparison test. A P-value of <0.05 was considered statistically significant.
Results
The mean age for the controls was 43 yrs (70% female), 45 yrs for diabetic gastroparesis (65% female) and 39 yrs for idiopathic gastroparesis (75% female). Further demographic and clinical details on gastroparetic patients are provided in table 1. On light microscopy histological changes were found in 16/20 diabetic and 17/20 idiopathic gastroparetics, that is in 83% of tissues examined (supplementary table 2). On quantification (table 2) significant changes were seen for c-Kit (ICC), PGP9.5 (nerves) and CD 45 (immune cells).
Table 1.
Clinical characteristics of subjects with diabetic and idiopathic gastroparesis
| Diabetic | Idiopathic | |
|---|---|---|
| Age (median, range) | 45 (20–68 years) | 39 (19–75 years) |
| Sex (% females) | 65% | 75% |
| BMI (mean ± SEM) | 28.5 ± 1.6 | 24.6 ± 0.9 |
| Diabetes | - | |
| - Type | Type I-65%, Type 2–35% | |
| - Duration (Mean ± SEM) | 17.3 ± 2.8 years | |
| - Neuropathy | 66% | |
| Gastric Emptying (% Retention at 4 hrs) (Mean ± SEM) | 35.47 ± 5.33 | 22.5 ± 4.06 |
| Predominant Clinical | ||
| Symptoms | ||
| -Nausea | 31% | 47% |
| -Fullness | 62% | 37% |
| -Bloating | 25% | 31% |
| Weight loss | 56% | 85% |
| Presumed Post- Infectious | 5% | 25% |
| EGD Findings | ||
| - Retained food | 10% | 10% |
| - Bezoar | 5% | 0% |
| Feeding Tube | 5% | 0% |
Table 2.
Quantitative immunolabeling data for controls, diabetic and idiopathic gastroparetics
| Immuno Staining | Diabetic Gastroparesis (Mean/HPF ± SEM) | Idiopathic Gastroparesis (Mean/HPF ± SEM) | Controls (Mean/HPF ± SEM) | ANOVA |
|---|---|---|---|---|
| c-Kit (Muscle) | 2.78 ± 0.41 | 3.24 ± 0.40 | 5.31 ± 0.22 | P<0.0001* |
| CD45 (Muscle) | 16.88 ± 0.84 | 16.47 ± 0.81 | 14.32 ± 0.74 | P=0.06 |
| CD 45 (Myenteric plexus) | 25.48 ± 1.55 | 26.47 ± 1.19 | 20.28 ± 1.07 | P=0.0025* |
| PGP (Muscle) | 36.49 ± 1.85 | 38.05 ± 1.52 | 44.26 ± 2.27 | P=0.0134* |
| Substance P (Muscle) | 16.32 ± 1.19 | 16.48 ± 1.35 | 20.18 ± 1.29 | P=0.06 |
| VIP (Muscle) | 32.70 ± 2.46 | 35.38 ± 2.59 | 30.64 ± 1.85 | P=0.35 |
| n NOS (Myenteric plexus) | 1.99 ± 0.18 | 2.17 ± 0.21 | 2.25 ± 0.19 | P=0.62 |
| n NOS (Muscle) | 20.9 ± 1.75 | 23.59 ± 1.64 | 24.24 ± 1.70 | P=0.34 |
| Tyrosine Hydroxylase (Myenteric plexus) | 52.91 ± 5.22 | 63.17 ± 5.07 | 56.64 ± 3.39 | P=0.29 |
mean ± SEM,
<0.05 considered significant
Enteric nervous system
On visual grading, PGP9.5 immunolabeling was normal in all patients with diabetic gastroparesis, suggesting that the number of enteric nerves was not markedly altered in diabetic gastroparesis. Three of 20 patients with idiopathic gastroparesis had abnormal PGP 9.5 immunolabeling. All 3 had a <25% decrease in the number of PGP 9.5 positive nerve fibers in the muscle layers and one had also evidence of neuronal drop-out in the myenteric plexus (figure 1). On quantification, idiopathic and diabetic gastroparetics were found to have 14–18% decrease in PGP9.5 expression in the muscle layer when compared with controls (36.5±1.8 fibers per field for diabetic and 38.0±1.5 fibers per field for idiopathic vs 44.3±2.3 fibers per field for controls, P<0.05) (Table 2). There were no significant differences between the diabetic and idiopathic gastroparesis groups (supplementary figure 1).
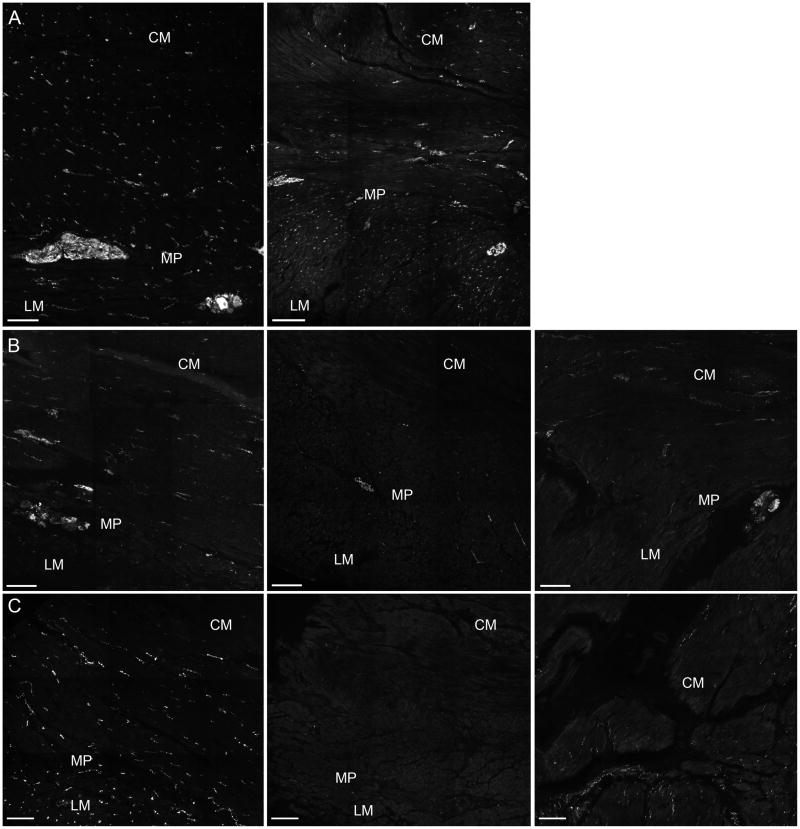
Figure 1.
Representative images for PGP9.5 immunoreactivity (PGP9.5-IR) used as a general neuronal marker, nNOS immunoreactivity (nNOS-IR) and VIP immunoreactivity (VIP-IR). Panel A: PGP9.5-IR. Left image is control and right image is from a patient with idiopathic gastroparesis with <25% decrease of PGP 9.5-IR in the circular muscle (CM) and longitudinal muscle (LM) and myenteric plexus (MP) region. Panel B: nNOS-IR. Left image is control, middle image and right image are from patients with diabetic gastroparesis and idiopathic gastroparesis respectively showing decreased nNOS-IR in the CM and LM and MP regions. Panel C: VIP-IR. Left image is control; middle image and right image are from patients with diabetic gastroparesis and idiopathic gastroparesis respectively showing decreased VIP-IR in the CM and LM and MP regions. Scale bar = 100μm.
On visual grading, nNOS was decreased in 4/20 patients with diabetic gastroparesis (3 <25% loss and 1 25–75% loss) and in 8/20 with idiopathic gastroparesis (3 patchy decrease, 1 <25% loss, 3 25–75% loss and 1 with decreased nerve bundles in the myenteric plexus but normal nerve fiber innervation of the muscle layers, figure 1). All 3 patients with idiopathic gastroparesis and decreased PGP 9.5 immunolabeling had decreased nNOS. The decrease in nNOS expression in these 3 patients was uniform in 2 and patchy in 1 with areas of severe decrease (<75% loss) and areas which appeared normal. On overall quantification, there were no statistically significant differences in the nNOS expression in myenteric plexus (1.9±0.2 neurons per field for diabetic, 2.2±0.2 neurons per field for idiopathic and 2.2±0.2 neurons per field for controls) or in the muscle layer (20.9±1.7 fibers per field for diabetic, 23.6±1.6 fibers per field for idiopathic and 24.2±1.7 fibers per field for controls) in the three groups (Table 2 and supplementary figures 2a and b). VIP expression was decreased in 4/20 patients with diabetic gastroparesis (1 with patchy loss, 2 with <25% loss and 1 with 25–75% loss) on visual grading. Of these 4, only one had loss of nNOS expression (figure 1). In the patients with idiopathic gastroparesis 3 patients had decreased VIP immunolabeling (2 with patchy decrease, 1 with 25–75% loss) and of these 3, 2 had decreased nNOS expression. On overall quantification, the three groups did not differ in VIP expression in the muscle layer (32.7±2.5 fibers per field for diabetic, 35.4±2.6 fibers per field for idiopathic and 30.6±1.8 fibers per field for controls) (Table 2 and supplementary figure 3).
Substance P immunolabeling was increased in 1 patient with diabetic gastroparesis and decreased in 4 (3 with <25% loss, 1 with 25–75% loss) on visual grading. In the idiopathic gastroparesis patients, substance P was decreased in 2 patients (1 with <25% loss, 1 with 25–75% loss) and increased in 2 patients (supplementary figure 4). There were no significant differences amongst three groups in the VIP expression on quantification (16.3±1.2 fibers per field for diabetic, 16.5±1.3 fibers per field for idiopathic and 20.2±1.3 for controls) (Table 2 and supplementary figure 5). Glial cells were examined using an antibody against S100β. S100β expression was decreased in 2 patients (<25% loss) with diabetic gastroparesis. Three patients with idiopathic gastroparesis had decreased S100β (1 with patchy decrease, 1 with <25% loss and 1 with 25–75% loss, supplementary figure 6). PGP9.5 immunolabeling was normal in the two patients with diabetic gastroparesis and decreased S100β expression and decreased in 2 of the 3 patients with idiopathic gastroparesis and decreased S100β expression.
Extrinsic sympathetic motor innervation
Tyrosine hydroxylase immunolabeling was decreased in 2 patients with diabetic gastroparesis (1 with <25% loss, 1 with 25–75% loss) and in 4 patients with idiopathic gastroparesis (2 with <25% loss, 2 with 25–75% loss, supplementary figure 6). On quantification, the three groups did not differ in TH expression (52.9 ± 5.2 immunofluorescent bodies per field for diabetic, 63.2 ± 5.1 immunofluorescent bodies per field for idiopathic and 56.6 ± 3.4 immunofluorescent bodies per field for controls) (Table 2 and supplementary figure 7).
Interstitial cells of Cajal
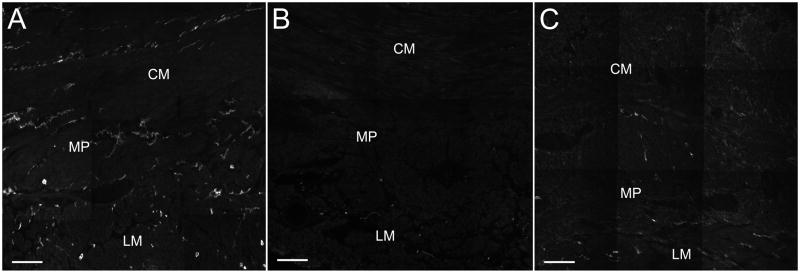
The most common histological abnormality found was a decrease in ICC as identified by Kit immunolabeling. Ten patients with diabetic and 10 with idiopathic gastroparesis had greater than 25% decrease in Kit expression (figure 2). These numbers were confirmed by counting ICC cell bodies. A well defined myenteric plexus network of ICC was not seen. Rather ICC were present throughout the muscle layers with a discontinuous and irregular accumulation of cells between the muscle layers. All patients graded as normal also had a normal ICC body count that was similar to the control patients (P>0.05). In contrast, the mean number of ICC bodies for patients graded as having decreased expression of Kit was different from controls (1.1±0.1 cell bodies per field for diabetic and 1.6±0.2 cell bodies per field for idiopathic vs 5.3±0.2 cell bodies per field for controls, P<0.05). The difference in cell bodies between controls and gastroparetics was still present when all gastroparetic tissues were grouped together (2.8±0.4 cell bodies per field for diabetic and 3.2±0.4 cell bodies per field for idiopathic vs 5.3±0.2 cell bodies per field for controls, P<0.05) (Table 2 and supplementary figure 8). There was no difference in ICC loss between diabetic and idiopathic gastroparesis.
Figure 2.
Representative images for Kit immunoreactivity as a marker for interstitial cells of Cajal (ICC). Panel A: Control. Panel B: Diabetic gastroparesis with decreased ICC. Panel C: Idiopathic gastroparesis with decreased ICC. CM: circular muscle layer; LM: longitudinal muscle layer; MP: myenteric plexus region. Scale bar = 100μm.
Smooth muscle cells
Hematoxylin and eosin staining was graded as normal in all 20 patients with diabetic gastroparesis and in 19 patients with idiopathic gastroparesis. Smoothelin immunolabeling was decreased in 3 patients with diabetic gastroparesis and in 6 with idiopathic gastroparesis (figure 3).
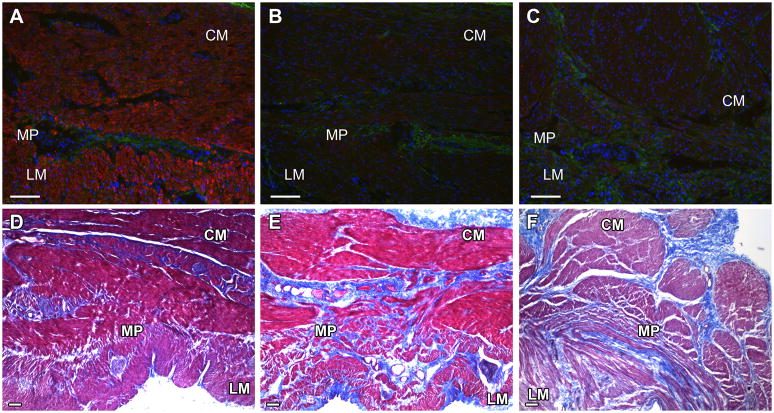
Figure 3.
Representative images for smoothelin immunoreactivity as a marker for smooth muscle cells and trichrome stained sections as a marker for fibrosis. Panels A and D: Controls. Panel B: Diabetic gastroparesis with decreased smoothelin immunoreactivity. Panel C: Idiopathic gastroparesis with decreased smoothelin immunoreactivity. Red signal represents smoothelin IR, blue signal is DAPI counterstain. Panel E: Diabetic gastroparesis with fibrosis. Panel F: Idiopathic gastroparesis with fibrosis CM: circular muscle layer; LM: longitudinal muscle layer; MP: myenteric plexus region. Scale bar = 100μm.
Fibrosis
Presence of fibrosis as determined by Trichrome staining was present in 1 patient with diabetic and 2 patients with idiopathic gastroparesis (figure 3). All other patients had normal Trichrome staining, including 8 of the 9 patients with abnormal smoothelin immunolabeling.
Immune cell infiltrate
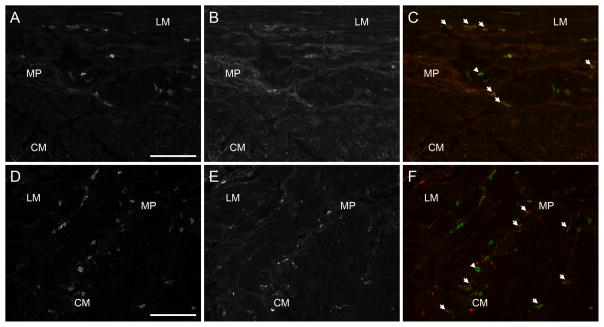
The second most common abnormality noted was altered immune cells in the muscularis propria. As determined by immunolabeling against CD45, immune cells were abnormal in 9 patients with diabetic gastroparesis and 8 patients with idiopathic gastroparesis on visual grading. Of these, 8 had altered cellular morphology (increased processes or elongated shape versus mainly round cells in the controls), 1 had increased number of cells in the muscle layers, and 8 had an infiltrate in the myenteric plexus region (figure 4). On quantification, there was a significant increase in the CD 45 immunofluorescence in the myenteric plexus in both gastroparesis groups (25.5±1.5 positive cell bodies per field for diabetic and 26.5±1.2 positive cell bodies per field for idiopathic vs 20.3±1.1 positive cell bodies per field for controls, P<0.05). There was no difference between diabetic and idiopathic gastroparesis groups. Also, there was a trend towards increased population of CD 45 immunofluorescence in the muscle layers but this did not reach statistical significance (16.9±0.84 positive cell bodies per field for diabetic, 16.5±0.8 positive cell bodies per field for idiopathic and 14.3±0.7 positive cell bodies per field for controls, P = 0.06) (Table 2 and supplementary figures 9 a and b). The nature of the infiltrate was further determined using antibodies to CD79 for B cells, to CD3 for T cells, to CD4 for T-helper cells, and to CD8 for T-suppressor cells. No differences were noted in the immunolabeling for all markers between patients and controls suggesting the infiltrate was not due to B or T cells (data not shown). In contrast, antibodies against CD68, a marker expressed on macrophages, showed increased macrophages (figure 4)
Figure 4.
Images of CD45 and CD68 immunoreactivity. Panel A: CD45 immunoreactivity in a control. Panel B: CD68 immunoreactivity from the same section as panel A. Panel C: CD45 and CD68 merged. Panel D: CD45 immunoreactivity from diabetic gastroparesis with increased number of CD45 positive cells. Panel E: CD68 immunoreactivity from the same section as panel D. Panel F: CD45 and CD68 immunoreactivity. Arrow heads point to CD45 positive, CD68 negative IR. Arrows point to co-localized immunoreactivity. CM: circular muscle layer; LM: longitudinal muscle layer; MP: myenteric plexus region. Scale bar = 100μm.
Electron microscopy
Transmission electron microscopy confirmed the reduction of ICC and the presence of altered nerve structures as showed by immunohistochemistry. TEM also showed that an abnormal connective stroma was present in all the patients examined. All patients examined by TEM had at least one abnormality on light microscopy.
In tissues from patients with diabetic gastroparesis, some of the residual ICC had normal features but the others showed intracytoplasmatic vacuoles and swollen mitochondria (figure 5A); none of these cells were in contact with nerve endings and rarely with smooth muscle cells or with other ICC. The smooth muscle cells were surrounded by fibrotic connective tissue and spaced apart from each other compared to controls but still connected by gap junctions (figure 5B). In most of the patients there was a thick basal lamina around ICC and smooth muscle cells (figure 5A–C). Nerve endings were very large and often empty (figure 5C).
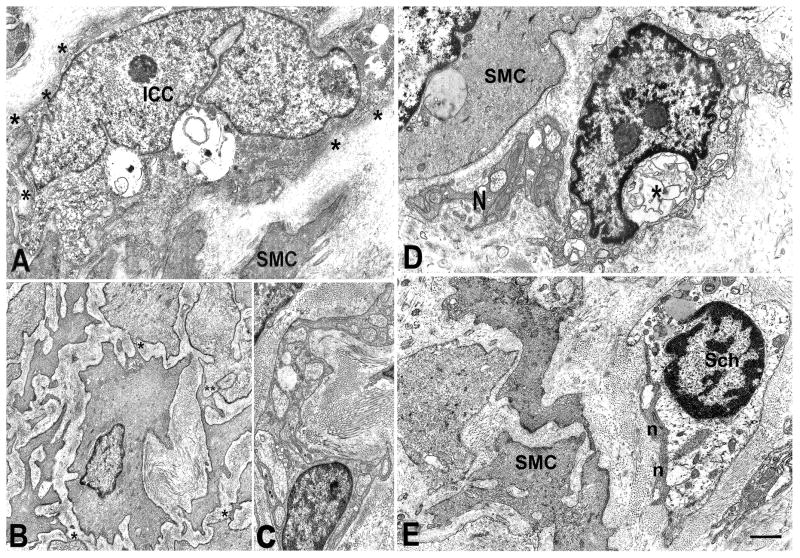
Figure 5.
Diabetic gastroparesis and idiopathic gastroparesis. Panels A–C are from diabetic gastroparesis and D–E from idiopathic gastroparesis. Panel A: An interstitial cell of Cajal (ICC) with large vacuoles in the cytoplasm and a discontinuous, thick basal lamina (asterisks). SMC: smooth muscle cell. Panel B: Smooth muscle cells immersed in a fibrotic stroma and separated, except for small junctional areas, most of which are gap junctions (asterisks). Panel C: Nerve bundle surrounded by a very thick basal lamina and numerous collagen fibers. The nerve endings are empty. Panel D: A presumptive ICC with swollen mitochondria and intracytoplasmatic lamellar bodies (asterisk) near a small nerve bundle (N). SMC: smooth muscle cell. Panel E: Nerve bundle endowed in a fibrotic capsule. The nerve endings (n) are filled with filaments and do not contain synaptic vesicles. SMC immersed in a fibrotic stroma and far away to each other. Sch: Schwann (glial) cell with a clear cytoplasm and several lipofuscinic bodies. Bar: A, B, D, E =1 μm, C=0.8 μm. Please refer to the supplementary figure 10 for EM controls.
In patients with idiopathic gastroparesis most of the nerve structures showed markedly altered morphology (figures 5D and E). The nerve endings were either empty or containing filaments, sequestered synaptic vesicles and lamellar bodies. Glial cells were also altered, with the cytoplasm filled with lysosomes and lipofuscinic bodies (figure 5E). Cells with normal ICC features were practically absent and the residual ICC showed vacuoles, lamellar bodies, lipofuscinic bodies and swollen mitochondria (figure 5D). In contrast to diabetic gastroparesis, the basal lamina of the smooth muscle cells had a normal thickness (figure 5D and E). The connective stroma showed fibrosis, which was particularly noted around the nerve structures (figure 5E).
Discussion
Idiopathic and diabetic gastroparesis are disorders associated with considerable morbidity. While progress has been made in the use of animal models to understand the cellular changes that underlie these disorders8, 10–12, 14, human data are limited.15–20, 22, 23, 25, 32 In this large and comprehensive study, we prospectively collected gastric body tissue from patients with diabetic and idiopathic gastroparesis from a well defined region of the stomach. Prospective collection of tissue allowed us to fix, store and process the tissue appropriately allowing optimal study of a large number of cell markers. A major finding of the study was that the majority of patients with gastroparesis, 83%, had defined gastric wall cellular abnormalities underlying the disease.
Idiopathic and diabetic gastroparesis are assumed to have very different causation. Diabetes is associated with changes to the autonomic nervous system,33–38 increased oxidative stress10, 39, 40 and microangiopathy.41, 42 Despite the apparent different initiating factors, there was no individual or set of markers that differentiated diabetic from idiopathic gastroparesis at the light microscopy level. In both diseases, the most common abnormality found was a decrease in ICC, confirmed by a quantitative assessment of ICC bodies. Interestingly, there appeared to be two groups of patients, ones with a normal number of ICC and the other with a marked decrease in ICC numbers. The clear separation of these two groups suggests that this reflects a true finding. However, it is also possible that loss of ICC may have been limited to a different region of the stomach in the ones with a normal distribution of ICC in the body. In animal models of diabetes a difference between ICC also in the antrum and body has been shown.12, 14 ICC loss may be patchy even within a defined region of the stomach. An underestimate of ICC abnormalities due to patchiness of loss is less likely in this study as care was taken to examine sufficient tissue from non-adjacent sections. We also first established the number of fields that needed to be examined in order to get an accurate representation of ICC. Furthermore, when we have had the opportunity to study larger portions of the stomach from patients with total gastrectomies we did not see the degree of patchiness seen in mouse models (unpublished observations). Under TEM, there were differences in ICC ultrastructure between diabetic and idiopathic gastroparesis with more diffuse and greater damage to ICC being noted in the patients with idiopathic gastroparesis.
The second most common abnormality noted was the presence of either altered immune cell morphology or an increase in the number of immune cells in about 43% of patients, as documented by anti-CD45 antibodies. CD45 is a general hematopoietic cell marker that labels all hematopoietic derived cells, with the exception of mature red cells.27 As in the case of ICC loss, there was no difference in the prevalence of this finding between idiopathic and diabetic gastroparesis. On overall quantification, this infiltrate of CD45 cells was more prominent in myenteric plexus as compared to muscle layers. Interestingly, these immune cells did not appear to be lymphocytes as pan-T and B cell markers did not show a difference between patients and controls. In contrast, anti-CD68 antibodies showed increased immunoreactivity suggesting an increase in macrophages. CD68 is classically used as a selective marker for macrophages although recent data show that it also labels some fibroblasts. The finding of increased CD68 immunoreactivity is of interest given the recent finding that M1 (classically activated, proinflammatory) and M2 (alternatively activated, antiinflammatory) macrophages may play opposing roles in the pathophysiology of diabetic gastroparesis.10, 43 Macrophage activation of the M1 type has been shown to play a role in rat post-operative ileus. It has been hypothesized that intestinal manipulation activates resident macrophages present in the intestinal muscularis externa which leads to cytokine release and leucocyte influx perpetuating a late inflammatory phase of post operative ileus.44
In this study we carefully assessed the enteric nervous system, including both excitatory and inhibitory neurons and glia. Whole mounts have been traditionally used for studying the enteric nervous system in animal models; however, a recent consensus statement in the 2009 neuromuscular international working group45 states that for thick human specimens sectioning made parallel to muscle layer is appropriate. There is a 14–18% decrease in the expression of neuronal marker PGP9.5 suggesting decrease in nerve fibers in gastroparesis. Neuronal cell cultures,46 animal models8, 47 and the limited human data17, 22 have implicated changes in enteric nerves in gastroparesis. By visual grading there was loss of nNOS expression in a subset of patients with diabetic and idiopathic gastroparesis but on quantification, overall there was no change in nNOS expression among the three groups. In contrast to the findings on ICC and on immune cells, there appeared to be a difference between diabetic and idiopathic gastroparesis as on visual grading in idiopathic gastroparesis 40% of patients had decreased nNOS expression and 20% in diabetic gastroparesis. This difference between diabetic and idiopathic gastroparesis was confirmed on TEM. Unlike in diabetic gastroparesis, TEM showed markedly altered morphology in all of the nerve endings in the idiopathic gastroparesis. Interestingly, in both disorders most of the nerve endings were empty. Empty nerve endings have been described in other pathological conditions such as achalasia.48 Given that nNOS is not sequestered in vesicles, this suggests that other neurotransmitters may be lost in gastroparesis.
An autonomic neuropathy has been well documented in multiple studies on patients with diabetes and in diabetic gastroparesis.33–38 In the current study abnormalities in expression of TH were seen in only 2 patients with diabetic gastroparesis and in 4 patients with idiopathic gastroparesis and there were no differences in overall quantification, suggesting that a sympathetic denervation of the stomach is present, but is not universal, in gastroparesis. The prevalence of a parasympathetic denervation could not be assessed by histology as there is no marker that allows separation of parasympathetic extrinsic nerves and intrinsic nerves that express acetylcholine.
The TEM findings of a markedly increased connective tissue stroma in both diabetic and idiopathic gastroparesis and a thickened basal lamina in diabetic gastroparesis suggest that it is not only the presence or absence of a cell type or protein that may cause disease. The separation of nerves from ICC, and ICC and nerves from smooth muscle, as seen by TEM, suggest that there may be functional consequences with impaired neurotransmission and transmission of the ICC electrical signal to smooth muscle even in the absence of loss of the cell type. Future electrophysiological studies on fresh tissue and/or careful cutaneous electrogastrography or preferably mucosa or serosal electrogastrography will be needed to assess the functional implications of these TEM findings.
There is no established marker to assess gastrointestinal smooth muscle. A study of different markers for gastrointestinal smooth muscle in a variety of motility disorders found that smooth muscle actin is a poor marker of smooth muscle dysfunction.49 The same study found that antibodies to smoothelin49 had the highest sensitivity in picking up smooth muscle abnormalities. Smoothelin-A is a cytoskeletal protein specific for smooth muscle cell50 and the smoothelin deficient mouse shows impaired contraction of intestinal smooth muscle.51 In the current study, abnormalities in smoothelin-A immunolabeling were seen approx. 23% of patients. The specificity of this finding is however unclear, as TEM did not show marked smooth muscle cell abnormalities in both diabetic and idiopathic gastroparesis.
On light microscopy there did not appear to be significant fibrosis present in the gastric wall for both diabetic and idiopathic gastroparesis. The presence of fibrosis in human gastroparesis has been controversial with both presence25 and abscence32 reported. In the non-obese mouse model of type 1 diabetes significant smooth muscle changes were seen12 and linked to loss of ICC through decreased secretion of stem cell factor.11 The increased thickness of the connective tissue stroma seen on TEM may decrease the availability of stem cell factor and other factors secreted by smooth muscle even if the smooth muscle cell may appear to be intact. A study of other markers for gastrointestinal smooth muscle such as smooth muscle myosin heavy chain49 and histone deacetylase 849 is needed to determine the optimal smooth muscle marker to use in gastroparesis.
In summary, we report on the first comprehensive study of patients with diabetic and idiopathic gastroparesis. Several findings bear highlighting, including the finding that 83% of patients with gastroparesis, when carefully studied, have a cellular abnormality. Data suggest that examination of adequate tissue can lead to valuable insights into the pathophysiology of these disorders and offers hope that new therapeutic targets can be found. The two most common findings, loss of ICC and an immune infiltrate offer future avenues of study to better understand how to prevent or reversing these cellular changes. Other major findings are the heterogenous nature of these two disorders and the lack of a clear separation of the cellular findings between diabetic and idiopathic gastroparesis. The common factor underlying the cellular changes in both disorders is currently unknown. Many other questions remain unanswered, including the association between particular cellular defects and symptoms, what other markers, including hormonal changes, are altered in both disorders, given that 17% of patients had no abnormalities found, despite targeting most of the known cell types, and the functional consequences of the cellular changes found. Electrophysiological, genomic and proteomic studies in both humans and in animal models coupled with detailed clinical information, careful histological studies and advances in nonsurgical approaches to obtaining full thickness biopsies should continue to shed light on these disorders that still offer a significant clinical challenge to manage and treat.
Supplementary Material
Acknowledgments
We give thanks to Megan Garrity-Park, Shelly Gray, Valerie McNair, Eve Pillor, Gary Stoltz, Peter Strege and Kristy Zodrow for excellent technical and secretarial assistance and to Daniele Guasti for his excellent technical support for the electron microscopy studies.
Abbreviations not mentioned in the style guide
- PGP9.5
Protein Gene Product 9.5
- nNOS
neuronal Nitric Oxide Synthase
- VIP
Vasoactive Intestinal Peptide
- ICC
interstitial cells of Cajal
- TEM
transmission electron, microscopy
- GpCRC
Gastroparesis Clinical Research Consortium
- TH
tyrosine hydroxylase
- DAPI
4′,6-diamidino-2-phenylindole
- SEM
standard error of the mean
List of Authors and their Roles
Henry Parkman, Thomas Abell, William Snape, Pankaj J. Pasricha, Madhusudan Grover, Gianrico Farrugia: Acquisition of tissue and drafting of manuscript, critical review of the manuscript for important intellectual content, study concept and design, analysis and interpretation of data, critical revision of the data for important intellectual content, obtained funding.
Matthew Lurken, Cheryl Bernard, Maria Simonetta Faussone-Pellegrini, Thomas Smyrk: Study concept and design, analysis and interpretation of data, critical revision of the data for important intellectual content.
William Hasler, Aynur Unalp-Arida, Linda Nguyen, Kenneth Koch, Jorges Calles, Linda Lee, James Tonascia, Frank Hamilton: Analysis, interpretation of data, critical revision of the manuscript for important intellectual content.
Members of the Gastroparesis Clinical Research Consortium as of October 2010
Clinical Centers
Stanford University, Stanford, CA: Pankaj Jay Pasricha, MD (Principal Investigator); Linda Nguyen, MD; Nighat Ullah, MD
California Pacific Medical Center, San Francisco, CA: William Snape, MD (Principal Investigator); Robin Bishop (2008); Nata DeVole, RN; Mary Greene, MS; Sue Louiseau; Amy Marincek, RN, BSN (2008); Shelly Parker, RN MSN ANP-C ANP; Eve Pillor, RN MSN FNP; Courtney Ponsetto, RN; Katerina Shetler, MD
Mayo Clinic College of Medicine, Rochester, MN: Gianrico Farrugia, MD; Madhusudan Grover, MD; Cheryl Bernard; Matt Lurken (2007–2009) K. Robert Shen MD; Michael Sarr, MD; Michael Kendrick, MD
Temple University, Philadelphia, PA: Henry P. Parkman, MD (Principal Investigator); Siva Doma, MD (2006–2007); Javier Gomez, MD (2008–2009); Steven Kantor; Vanessa Lytes, CRNP; Amiya Palit, MD; Zeeshan Ramzan, MD (2007–2008); Priyanka Sachdeva, MD; Kellie Simmons, RN; Sean Harbison, MD
Texas Tech University Health Sciences Center: Richard W. McCallum, MD (Principal Investigator); Reza Hejazi, MD; Irene Sarosiek, MD; Denise Vasquez; Natalia Vega
University of Michigan, Ann Arbor, MI: William Hasler, MD (Principal Investigator); Michelle Atkinson, CSC; Radoslav Coleski, MD (2007–2008)
University of Mississippi Medical Center, Jackson, MS: Thomas Abell, MD (Principal Investigator); JoAnne Fordham; Olivia Henry, RD; Archana Kedar, MD; Valerie McNair, LPN II; Susanne Pruett, RN (2007–2008); Margaret Smith, RN; Danielle Spree, CNP
Wake Forest University, Winston-Salem, NC: Kenneth Koch, MD (Principal Investigator); Lynn Baxter; Jorge Calles, MD; Samantha Culler; Judy Hooker, RN; Paula Stuart, PA
Resource Centers
National Institute of Diabetes, Digestive and Kidney Diseases, Bethesda, MD: Frank Hamilton, MD, MPH (Project Scientist); Steven James, MD; Rebecca Torrance, RN, MSN; Rebekah Van Raaphorst, MPH
Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD: James Tonascia, PhD (Principal Investigator); Patricia Belt; Ryan Colvin, MPH; Michele Donithan, MHS; Mika Green, MA; Milana Isaacson; Wana Kim; Linda Lee, MD; Alison Lydecker, MPH (2006–2008); Pamela Mann, MPH (2008–2009); Laura Miriel; Alice Sternberg, ScM; Aynur Ünalp-Arida, MD, PhD; Mark Van Natta, MHS; Ivana Vaughn, MPH; Laura Wilson, ScM; Katherine Yates, ScM
Footnotes
Disclosures: none to declare for all authors
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Park MI, Camilleri M. Gastroparesis: clinical update. Am J Gastroenterol. 2006;101:1129–39. doi: 10.1111/j.1572-0241.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- 2.Parkman HP, Hasler WL, Fisher RS, et al. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 3.Pasricha PJ. The riddle, mystery, and enigma of gastroparesis. J Support Oncol. 2007;5:368–70. [PubMed] [Google Scholar]
- 4.Patrick A, Epstein O. Review article: gastroparesis. Aliment Pharmacol Ther. 2008;27:724–40. doi: 10.1111/j.1365-2036.2008.03637.x. [DOI] [PubMed] [Google Scholar]
- 5.Vittal H, Farrugia G, Gomez G, et al. Mechanisms of disease: the pathological basis of gastroparesis--a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol. 2007;4:336–46. doi: 10.1038/ncpgasthep0838. [DOI] [PubMed] [Google Scholar]
- 6.Farrell FJ, Keeffe EB. Diabetic gastroparesis. Dig Dis. 1995;13:291–300. doi: 10.1159/000171509. [DOI] [PubMed] [Google Scholar]
- 7.Jung HK, Choung RS, Locke GR, 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–33. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spangeus A, El-Salhy M. Myenteric plexus of obese diabetic mice (an animal model of human type 2 diabetes) Histol Histopathol. 2001;16:159–65. doi: 10.14670/HH-16.159. [DOI] [PubMed] [Google Scholar]
- 9.Spangeus A, Suhr O, El-Salhy M. Diabetic state affects the innervation of gut in an animal model of human type 1 diabetes. Histol Histopathol. 2000;15:739–44. doi: 10.14670/HH-15.739. [DOI] [PubMed] [Google Scholar]
- 10.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–64. 2064, e1–2. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvath VJ, Vittal H, Lorincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–70. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Ordog T, Takayama I, Cheung WK, et al. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–9. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 13.Wang XY, Huizinga JD, Diamond J, et al. Loss of intramuscular and submuscular interstitial cells of Cajal and associated enteric nerves is related to decreased gastric emptying in streptozotocin-induced diabetes. Neurogastroenterol Motil. 2009;21:1095–e92. doi: 10.1111/j.1365-2982.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T, Nakamura K, Itoh H, et al. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology. 1997;113:1535–44. doi: 10.1053/gast.1997.v113.pm9352855. [DOI] [PubMed] [Google Scholar]
- 15.Battaglia E, Bassotti G, Bellone G, et al. Loss of interstitial cells of Cajal network in severe idiopathic gastroparesis. World J Gastroenterol. 2006;12:6172–7. doi: 10.3748/wjg.v12.i38.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forster J, Damjanov I, Lin Z, et al. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102–8. doi: 10.1016/j.gassur.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Harberson J, Thomas RM, Harbison SP, et al. Gastric Neuromuscular Pathology in Gastroparesis: Analysis of Full-Thickness Antral Biopsies. Dig Dis Sci. 2010;10:359–70. doi: 10.1007/s10620-009-1071-2. [DOI] [PubMed] [Google Scholar]
- 18.He CL, Soffer EE, Ferris CD, et al. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–34. doi: 10.1053/gast.2001.26264. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki H, Kajimura M, Osawa S, et al. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41:1076–87. doi: 10.1007/s00535-006-1909-8. [DOI] [PubMed] [Google Scholar]
- 20.Lin Z, Sarosiek I, Forster J, et al. Association of the status of interstitial cells of Cajal and electrogastrogram parameters, gastric emptying and symptoms in patients with gastroparesis. Neurogastroenterol Motil. 2010;22:56–61. e10. doi: 10.1111/j.1365-2982.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- 21.Miller SM, Narasimhan RA, Schmalz PF, et al. Distribution of interstitial cells of Cajal and nitrergic neurons in normal and diabetic human appendix. Neurogastroenterol Motil. 2008;20:349–57. doi: 10.1111/j.1365-2982.2007.01040.x. [DOI] [PubMed] [Google Scholar]
- 22.Pasricha PJ, Pehlivanov ND, Gomez G, et al. Changes in the gastric enteric nervous system and muscle: a case report on two patients with diabetic gastroparesis. BMC Gastroenterol. 2008;8:21. doi: 10.1186/1471-230X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarate N, Mearin F, Wang XY, et al. Severe idiopathic gastroparesis due to neuronal and interstitial cells of Cajal degeneration: pathological findings and management. Gut. 2003;52:966–70. doi: 10.1136/gut.52.7.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sokol H, Lavergne-Slove A, Mikol J, et al. Severe isolated myopathic gastroparesis: a case report with pathological findings. Gut. 2006;55:1662. doi: 10.1136/gut.2006.105742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ejskjaer NT, Bradley JL, Buxton-Thomas MS, et al. Novel surgical treatment and gastric pathology in diabetic gastroparesis. Diabet Med. 1999;16:488–95. doi: 10.1046/j.1464-5491.1999.00086.x. [DOI] [PubMed] [Google Scholar]
- 26.Choi KM, Gibbons SJ, Roeder JL, et al. Regulation of interstitial cells of Cajal in the mouse gastric body by neuronal nitric oxide. Neurogastroenterol Motil. 2007;19:585–95. doi: 10.1111/j.1365-2982.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 27.Trowbridge IS. CD45. A prototype for transmembrane protein tyrosine phosphatases. J Biol Chem. 1991;266:23517–20. [PubMed] [Google Scholar]
- 28.Jones M, Cordell JL, Beyers AD, et al. Detection of T and B cells in many animal species using cross-reactive anti-peptide antibodies. J Immunol. 1993;150:5429–35. [PubMed] [Google Scholar]
- 29.Mason DY, Cordell JL, Gaulard P, et al. Immunohistological detection of human cytotoxic/suppressor T cells using antibodies to a CD8 peptide sequence. J Clin Pathol. 1992;45:1084–8. doi: 10.1136/jcp.45.12.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satoh K, Yoshida N, Imaizumi K, et al. Reversible methotrexate-associated lymphoproliferative disorder resembling advanced gastric cancer in a patient with rheumatoid arthritis. Am J Med Sci. 2009;338:334–5. doi: 10.1097/MAJ.0b013e3181acbb49. [DOI] [PubMed] [Google Scholar]
- 31.Horny HP, Ruck P, Xiao JC, et al. Immunoreactivity of normal and neoplastic human tissue mast cells with macrophage-associated antibodies, with special reference to the recently developed monoclonal antibody PG-M1. Hum Pathol. 1993;24:355–8. doi: 10.1016/0046-8177(93)90081-q. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida MM, Schuffler MD, Sumi SM. There are no morphologic abnormalities of the gastric wall or abdominal vagus in patients with diabetic gastroparesis. Gastroenterology. 1988;94:907–14. doi: 10.1016/0016-5085(88)90546-x. [DOI] [PubMed] [Google Scholar]
- 33.Bernstein G. The diabetic stomach: management strategies for clinicians and patients. Diabetes Spectrum. 2000;13:11–21. [Google Scholar]
- 34.Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820–9. doi: 10.1056/NEJMcp062614. [DOI] [PubMed] [Google Scholar]
- 35.Camilleri M, Malagelada JR. Abnormal intestinal motility in diabetics with the gastroparesis syndrome. Eur J Clin Invest. 1984;14:420–7. doi: 10.1111/j.1365-2362.1984.tb01206.x. [DOI] [PubMed] [Google Scholar]
- 36.Carnethon MR, Prineas RJ, Temprosa M, et al. The association among autonomic nervous system function, incident diabetes, and intervention arm in the Diabetes Prevention Program. Diabetes Care. 2006;29:914–9. doi: 10.2337/diacare.29.04.06.dc05-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guy RJ, Dawson JL, Garrett JR, et al. Diabetic gastroparesis from autonomic neuropathy: surgical considerations and changes in vagus nerve morphology. J Neurol Neurosurg Psychiatry. 1984;47:686–91. doi: 10.1136/jnnp.47.7.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zochodne DW. Diabetes mellitus and the peripheral nervous system: manifestations and mechanisms. Muscle Nerve. 2007;36:144–66. doi: 10.1002/mus.20785. [DOI] [PubMed] [Google Scholar]
- 39.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 40.Varvarovska J, Racek J, Stetina R, et al. Aspects of oxidative stress in children with type 1 diabetes mellitus. Biomed Pharmacother. 2004;58:539–45. doi: 10.1016/j.biopha.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Kihara S, Mori K, Akagi M. Electron microscopic observation of gastric mucosal capillaries in diabetics--relationship between diabetic microangiopathy and complications following gastrectomy. Gastroenterol Jpn. 1983;18:181–96. doi: 10.1007/BF02774959. [DOI] [PubMed] [Google Scholar]
- 42.Said G. Diabetic neuropathy--a review. Nat Clin Pract Neurol. 2007;3:331–40. doi: 10.1038/ncpneuro0504. [DOI] [PubMed] [Google Scholar]
- 43.Choi KM, Kashyap PC, Dutta N, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138:2399–409. 2409, e1. doi: 10.1053/j.gastro.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. 2009;58:1300–11. doi: 10.1136/gut.2008.169250. [DOI] [PubMed] [Google Scholar]
- 45.Knowles CH, De Giorgio R, Kapur RP, et al. Gastrointestinal neuromuscular pathology: guidelines for histological techniques and reporting on behalf of the Gastro 2009 International Working Group. Acta Neuropathol. 2009;118:271–301. doi: 10.1007/s00401-009-0527-y. [DOI] [PubMed] [Google Scholar]
- 46.Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–56. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watkins CC, Sawa A, Jaffrey S, et al. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest. 2000;106:373–84. doi: 10.1172/JCI8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faussone-Pellegrini MS, Cortesini C. The muscle coat of the lower esophageal sphincter in patients with achalasia and hypertensive sphincter. An electron microscopic study. J Submicrosc Cytol. 1985;17:673–85. [PubMed] [Google Scholar]
- 49.Wedel T, Van Eys GJ, Waltregny D, et al. Novel smooth muscle markers reveal abnormalities of the intestinal musculature in severe colorectal motility disorders. Neurogastroenterol Motil. 2006;18:526–38. doi: 10.1111/j.1365-2982.2006.00781.x. [DOI] [PubMed] [Google Scholar]
- 50.van der Loop FT, Schaart G, Timmer ED, et al. Smoothelin, a novel cytoskeletal protein specific for smooth muscle cells. J Cell Biol. 1996;134:401–11. doi: 10.1083/jcb.134.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niessen P, Rensen S, van Deursen J, et al. Smoothelin-a is essential for functional intestinal smooth muscle contractility in mice. Gastroenterology. 2005;129:1592–601. doi: 10.1053/j.gastro.2005.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.