Abstract
Alkynyl- and azido-tagged 3-oxo-C12-acylhomoserine lactone probes have been synthesized to examine their potential utility as probes for discovering the mammalian protein target of the Pseudomonas aeruginosa autoinducer, 3-oxo-C12-acylhomoserine lactone. Although such substitutions are commonly believed to be quite conservative, from these studies, we have uncovered a drastic difference in activity between the alkynyl- and azido-modified compounds, and provide an example where such structural modification has proved to be much less than conservative.
Keywords: Acylhomoserine lactones, Click chemistry, Pseudomonas aeruginosa, Quorum sensing
Quorum sensing is a diffusion-based process whereby bacteria secrete various signaling molecules, or autoinducers, as a population sensor and a means to control gene expression in response to changes in cell number; thus, allowing the bacteria to behave as a multicellular organism.1 Importantly, this process has been correlated with the expression of virulence factors and biofilm formation,1 and a “deafening” of bacteria to these signaling molecules should provide a mechanism by which to target the bacteria without exerting a selective pressure thereby decreasing the possibility of resistance development.2,3 As a result, much effort has been invested in developing small molecule probes,4,5 autoinducer analogues4,5 and protein biologics6–8 for studying quorum sensing.
In particular, quorum sensing in the gram-negative bacteria Pseudomonas aeruginosa has received significant attention due to the species’ role as an opportunistic pathogen in humans.9 Most prominent is the role of P. aeruginosa in patients suffering from cystic fibrosis.10 Affected individuals suffer from frequent bacterial lung infections due to impaired lung defense function, and these infections are often a cause of morbidity.10 With respect to quorum sensing, it has now been established that this signaling process is required for P. aeruginosa to cause disease, including lung infections in cystic fibrosis patients.11–14 More specifically, the ability of the bacteria to grow into sessile, drug-resistant biofilms at high cell densities makes such infections difficult to treat, and resistance is beginning to be observed with currently available antibiotics.15
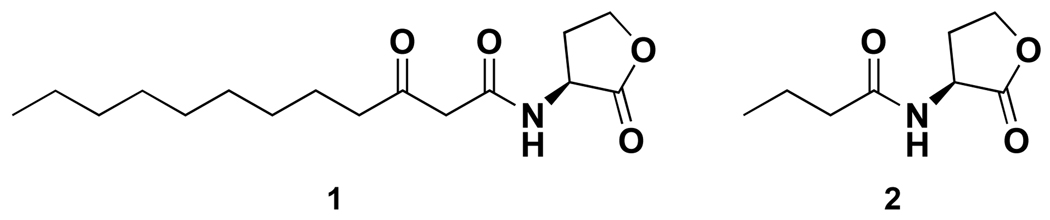
To conduct its quorum sensing-controlled activities, including biofilm formation, P. aeruginosa uses N-acyl L-homoserine lactones (AHLs) as its primary autoinducers (Figure 1).1 AHL 1 activates LasR16 and AHL 2 activates RhlR,17 and activation of both receptor proteins is required for quorum sensing.1 Since LasR activation by AHL 1 is required to initiate the quorum-sensing cascade in this species,1 many studies have been conducted toward deciphering the role of this chemical signal in both quorum sensing and its resulting human pathologies. Recently, our group18,19 and others20,21 have explored the impact of AHL 1 in mammalian cells. For example, AHL 1 inhibits innate immune responses through modulation of NF-κB signaling induced by pro-inflammatory stimuli such as tumor necrosis factor and the Toll-like receptor (TLR) agonists, thereby potentially promoting persistent infection.19 Notably, this molecule directly activates mammalian cells through a mechanism distinct from the TLR and other canonical pathogen-associated receptor pathways.18 Because of the potential significance of these findings, our group and others22 have been actively involved in identifying the biochemical target(s) of this important quorum sensing signaling molecule.
Figure 1.
Autoinducers used by Pseudomonas aeruginosa.
Click chemistry, more specifically CuI-catalyzed azide-alkyne 1,3-dipolar cycloaddition chemistry,23–25 has emerged as a powerful method for bioconjugation.26,27 Coupled with activity-based protein profiling (ABPP), this method grants the ability to probe the molecular interactions of a molecule of choice by modifying the compound with a bioorthogonal azide or alkyne tag.28,29 While this substitution may seem conservative, in fact, this modification can have drastic impact on the activity of the small molecule, although such findings are often not reported. Moreover, as high affinity is a requirement for use of a probe in target identification studies, particularly in cases when the unknown target may be of low abundance,30 choice of this modification may prove critical for such future studies. Herein, we describe our efforts to synthesize clickable AHL probes for target identification studies and demonstrate the critical nature that the choice of an alkyne or azide modification has on AHL activity in mammalian cells.
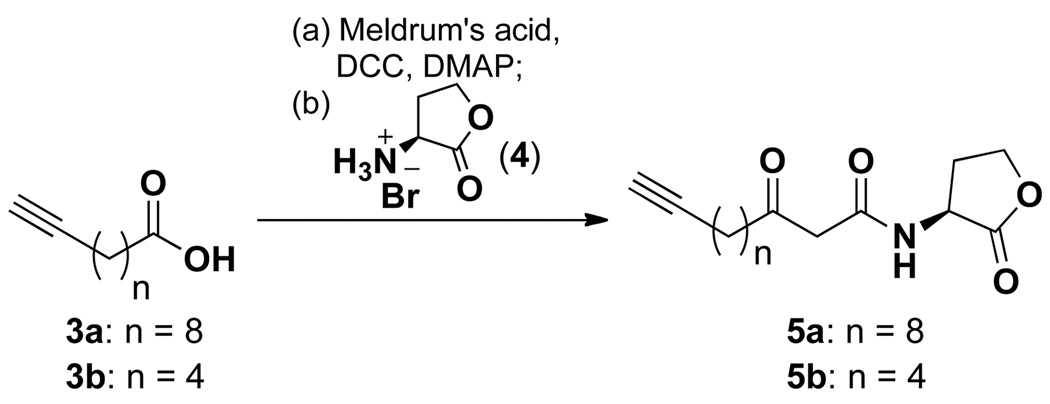
Although much is known about the structural requirements for AHL-mediated activation in P. aeruginosa,4,5 very little has been concluded about the requirements for biological activity in mammalian cells. Previously, our group demonstrated that the L-homoserine lactone, 3-oxo substituent and an alkyl chain of 10–14 carbons were necessary to activate mammalian cells.18 To begin our studies, we first synthesized alkynyl analogues 5a and 5b, as an alkyne seemed to be the most conservative modification since it is composed of only carbons similar to the natural tail of AHL 1. Additionally, previous studies have showed that detection with rhodamine-azide compounds yields lower background signals than detection with the corresponding rhodamine-alkyne tags in click chemistry-mediated ABPP.29 Although a diazirine moiety was used in a previously reported AHL-based probe,22 we hypothesized that the acylhomoserine lactone could act as a covalent tag on its own, particularly since lactone natural products are well known to covalently modify protein targets in vivo.31 The synthesis of alkynyl analogues 5a and 5b is shown in Scheme 1, and proceeded via a straightforward route starting from the commercially available alkynyl fatty acids 3a and 3b. Briefly, alkynyl acids 3 were first activated with DCC followed by nucleophilic displacement of the resultant activated esters with Meldrum’s acid and coupling with L-homoserine lactone 4 to yield alkynyl AHL analogues 5. For carbon chain lengths, we chose to synthesize both a shorter 9 carbon tail and a longer 13 carbon tail for comparison.
Scheme 1.
Synthesis of alkynyl AHL analogues.
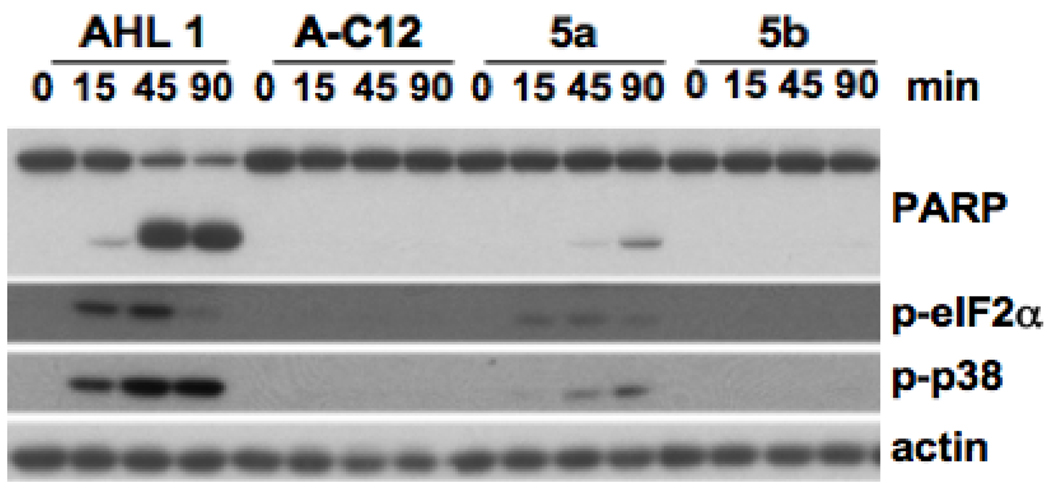
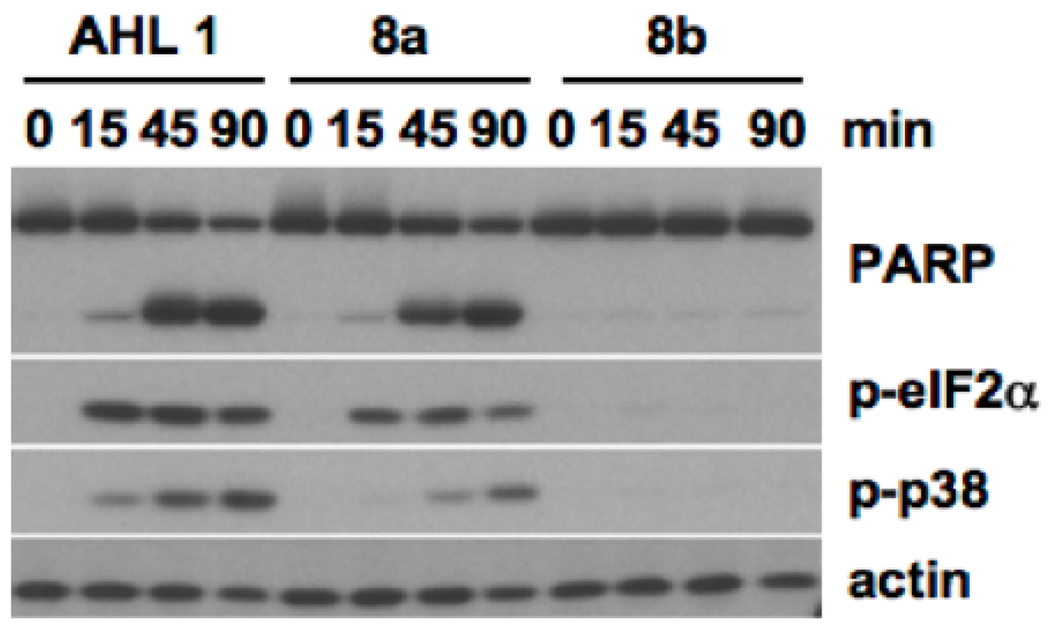
To assess a potential utility of these alkynyl AHL analogues as probes for target identification studies, we tested their biological activities in primary macrophages. In brief, bone marrow-derived macrophages (BMDM) were treated with an alkynyl AHL derivative (50 µM) over a period of 90 minutes, and cellular extracts were examined by Western blot analysis for the biochemical markers indicative of AHL 1-mediated signaling events including the phosphorylation of the protein kinase p38, the phosphorylation of the eukaryotic translation initiation factor 2α(eIF2α) and the cleavage of the poly(ADP)-ribose) polymerase (PARP), as previously described.18 AHL 1 (3-oxo-C12-AHL) and C12-AHL (no 3-oxo group, structure not shown) were used as positive and negative controls, respectively. As Figure 2 shows, only alkynyl analogue 5a exhibited activity; however, its activity was greatly reduced compared to that of the parent compound 1.
Figure 2.
Biological activities of AHL 1, C12-HSL (A-C12; negative control) and its alkynyl analogues 5a and 5b in mammalian cells. BMDM were treated as indicated, and cellular extracts were analyzed by Western blot for the cleavage of PARP, phosphorylated forms of eIF2α(p-eIF2α) or p38 (p-p38) and actin as a loading control.
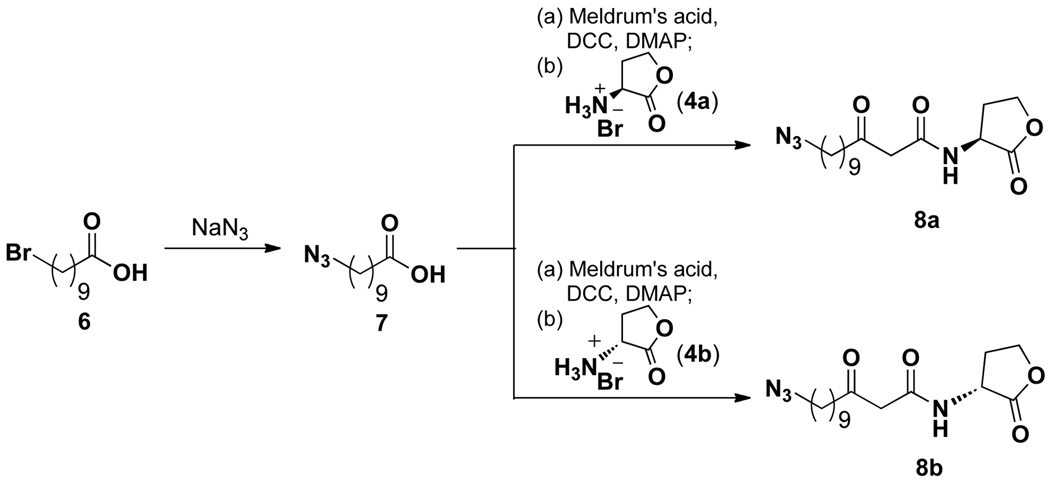
Knowing that the diminished activity of alkynyl analogue 5a and apparent lack of activity in analogue 5b would damper any efforts toward identifying the mammalian target(s) of AHL 1, we next synthesized an azide derivative in hope of identifying a more viable AHL analogue for our desired future studies. Since the shorter alkyne probe showed no activity, we chose to examine only a long alkyl chain. Azido analogue 8a, which contains 12 carbons in its tail, was synthesized in an analogous manner as the alkynyl derivatives (Scheme 2). In this case, however, azido-carboxylic acid 7 was used as a starting material and was obtained via nucleophilic substitution of bromo-acid 6 with sodium azide (Scheme 2). We then tested the activity of this azido derivative in BMDM. Interestingly, unlike the alkynyl derivatives, the azido AHL analogue exhibited activity very similar to that of the parent AHL (Figure 3). Thus, substitution at the tail terminus clearly has a drastic impact on AHL activity. This finding is unanticipated since an azido group, although overall neutral, is polar and carries charged nitrogen atoms, while an alkynyl group is fully neutral and nonpolar similar to the natural AHL tail. Although purely speculative, it is possible that the azido moiety makes additional hydrogen bond or van der Waal contacts32 that aids in its binding of the putative AHL target(s). Importantly, this potential probe exhibits much higher activity in mammalian cells than a previously reported diazirine-modified alkynyl AHL that was used to label and isolate P. aeruginosa LasR.22 As an additional control compound, we also synthesized the corresponding D-homoserine lactone derivative (8b, Scheme 2), and similar to previous experiments with this enantiomer,18 no activity was observed (Figure 3). Thus, 8b could prove useful as a structurally similar negative control for potential target identification strategies.
Scheme 2.
Synthesis of azido AHL analogues.
Figure 3.
Biological activities of azido analogues 8a and 8b in mammalian cells.
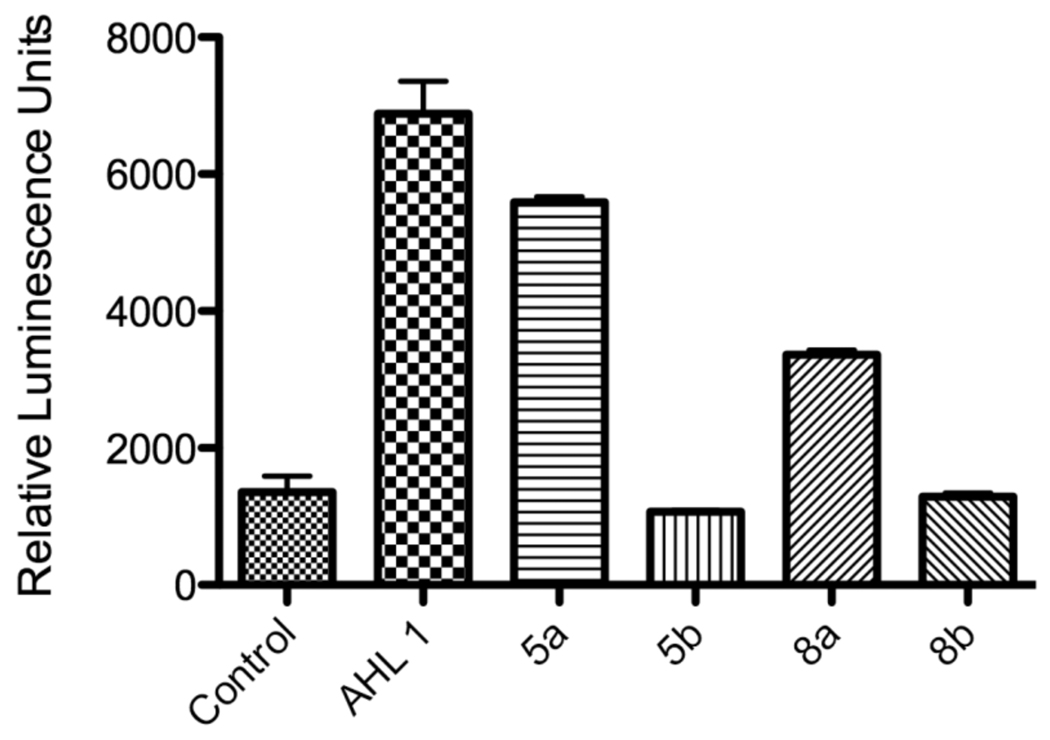
In addition to testing these AHL derivatives in mammalian cells, we also examined their impact as potential autoinducers in P. aeruginosa. Autoinducer assays were conducted using P. aeruginosa luminescence reporter strain PAO-JP2, a PAO1 lasI/rhlI double mutant (PAO-JP2-luxABCDE).33,34 As Figure 4 shows, only alkynyl AHL analogue 5a showed autoinducer activity similar to that of the parent AHL 1. These data indicate that the structural requirements for autoinducer activity and activity in mammalian cells are distinct, and nonpolar, all carbon alkynyl probes may be more useful for identifying bacterial AHL receptors.
Figure 4.
Autoinducer activities of alkynyl- and azido-AHL analogues in P. aeruginosa. Relative luminescence units were normalized with respect to cell viability (OD600).
In conclusion, we have synthesized alkynyl- and azido-tagged 3-oxo-C12-AHL analogues as potential ABPP probes for discovering the mammalian target of this important P. aeruginosa quorum sensing signaling molecule. Of particular significance, we have found a drastic difference in activity between the alkynyl- and azido-tagged AHL derivatives in that only the azide-containing analogue possessed activity similar to that of the natural compound. Thus, this data demonstrates that click chemistry-amenable substitutions may not be as conservative as previously thought, and that no simple guidelines can exist for such click chemistry-mediated ABPP endeavors. Both alkynyl- and azido-modified compounds should be initially examined for activity before examination as potential probes. Additionally, the discovery that polar, yet neutral substituents at the tail are accepted for mammalian cell activity may open up the possibility for the synthesis of new, more potent AHL derivatives. Target identification and additional synthetic studies are currently ongoing and will be reported in due course.
Supplementary Material
Acknowledgments
We thank M. M. Meijler (Ben Gurion University) for kindly providing P. aeruginosa strain PAO-JP2. We gratefully acknowledge the financial support of the NIH (Grants AI079503 and DE018452 to K.D.J., and grant AI085324 to G.F.K.), the Skaggs Institute for Chemical Biology, and a NIH postdoctoral fellowship (F32-DK083179, A.L.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at doi:…
References and notes
- 1.Waters CM, Bassler BL. Annu. Rev. Cell. Dev. Biol. 2005;21:319. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen TB, Givskov M. Int. J. Med. Microbiol. 2006;296:149. doi: 10.1016/j.ijmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Suga H, Smith KM. Curr. Opin. Chem. Biol. 2003;7:586. doi: 10.1016/j.cbpa.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Lowery CA, Salzameda NT, Sawada D, Kaufmann GF, Janda KD. J. Med. Chem. doi: 10.1021/jm901742e. doi:10.1021/jm901742e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattmann ME, Blackwell HE. J. Org. Chem. 2010;75:6737. doi: 10.1021/jo101237e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufmann GF, Sartorio R, Lee SH, Mee JM, Altobell LJ, III, Kujawa DP, Jeffries E, Clapham B, Meijler MM, Janda KD. J. Am. Chem. Soc. 2006;128:2802. doi: 10.1021/ja0578698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Lamo Marin S, Xu Y, Meijler MM, Janda KD. Bioorg. Med. Chem. Lett. 2007;17:1549. doi: 10.1016/j.bmcl.2006.12.118. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann GF, Park J, Mee JM, Ulevitch RJ, Janda KD. Mol. Immunol. 2008;45:2710. doi: 10.1016/j.molimm.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyczak JB, Cannon CL, Pier GB. Microbes Infect. 2000;2:1051. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 10.Lyczak JB, Cannon CL, Pier GB. Clin. Microbiol. Rev. 2002;15:194. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson JP, Feldman M, Iglewski BH, Prince A. Infect. Immun. 2000;68:4331. doi: 10.1128/iai.68.7.4331-4334.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang HB, DiMango E, Bryan R, Gambello M, Iglewski BH, Goldberg JB, Prince A. Infect. Immun. 1996;64:37. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. Science. 1998;280:295. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 14.Calfee MW, Coleman JP, Pesci EC. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11633. doi: 10.1073/pnas.201328498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drenkard E. Microbes Infect. 2003;5:1213. doi: 10.1016/j.micinf.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, Iglewski BH, Greenberg EP. Proc. Natl. Acad. Sci. U. S. A. 1994;91:197. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson JP, Passador L, Iglewski BH, Greenberg EP. Proc. Natl. Acad. Sci. U. S. A. 1995;92:1490. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Wood MR, Brogan AP, Lehmann M, Mee JM, Iwata K, Pan Q, Fearns C, Knaus UG, Meijler MM, Janda KD, Ulevitch RJ. J. Biol. Chem. 2006;281:28822. doi: 10.1074/jbc.M606613200. [DOI] [PubMed] [Google Scholar]
- 19.Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Grauer DC, Lehmann M, Meijler MM, Janda KD, Ulevitch RJ. Science. 2008;321:259. doi: 10.1126/science.1156499. [DOI] [PubMed] [Google Scholar]
- 20.Thomas GL, Bohner CM, Williams HE, Walsh CM, Ladlow M, Welch M, Bryant CE, Spring DR. Mol. Biosyst. 2006;2:132. doi: 10.1039/b517248a. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Hooi D, Chhabra SR, Pritchard D, Shaw PE. Oncogene. 2004;23:4894. doi: 10.1038/sj.onc.1207612. [DOI] [PubMed] [Google Scholar]
- 22.Dubinsky L, Jarosz LM, Amara N, Krief P, Kravchenko VV, Krom BP, Meijler MM. Chem. Commun. 2009:7378. doi: 10.1039/b917507e. [DOI] [PubMed] [Google Scholar]
- 23.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. Int. Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Tornoe CW, Christensen C, Meldal M. J. Org. Chem. 2002;67:3057. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 25.Meldal M, Tornoe CW. Chem. Rev. 2008;108:2952. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J. Am. Chem. Soc. 2003;125:3192. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 27.Best MD. Biochemistry. 2009;48:6571. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]
- 28.Speers AE, Adam GC, Cravatt BF. J. Am. Chem. Soc. 2003;125:4686. doi: 10.1021/ja034490h. [DOI] [PubMed] [Google Scholar]
- 29.Speers AE, Cravatt BF. Chem. Biol. 2004;11:535. doi: 10.1016/j.chembiol.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Sato S, Murata A, Shirakawa T, Uesugi M. Chem. Biol. 2010;17:616. doi: 10.1016/j.chembiol.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Konaklieva MI, Plotkin BJ. Mini-Rev. Med. Chem. 2005;5:73. doi: 10.2174/1389557053402828. [DOI] [PubMed] [Google Scholar]
- 32.Petsalakis EI, Chrysina ED, Tiraidis C, Hadjiloi T, Leonidas DD, Oikonomakos NG, Aich U, Varghese B, Loganathan D. Bioorg. Med. Chem. 2006;14:5316. doi: 10.1016/j.bmc.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 33.Duan K, Surette MG. J. Bacteriol. 2007;189:4827. doi: 10.1128/JB.00043-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amara N, Mashiach R, Amar D, Krief P, Spieser SAH, Bottomley MJ, Aharoni A, Meijler MM. J. Am. Chem. Soc. 2009;131:10610. doi: 10.1021/ja903292v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.