Abstract
Arterial Calcification due to Deficiency of CD73 (ACDC) results from mutations in the NT5E gene encoding the 5′ exonucleotidase, CD73. We now describe the third familial case of ACDC, including radiological and histopathological details of the arterial calcifications. The medial lesions involve the entire circumference of the elastic lamina, in contrast to the intimal plaque-like disease of atherosclerosis. The demonstration of broken and fragmented elastic fibers leading to generalized vascular calcification suggests an analogy to pseudoxanthoma elasticum (PXE), which exhibits similar histopathology. Classical PXE is caused by deficiency of ABCC6, a C type ABC transporter whose ligand is unknown. Other C type ABC proteins transport nucleotides, so the newly described role of adenosine in inhibiting vascular calcification, along with the similarity of ACDC and PXE with respect to vascular pathology, suggests that adenosine may be the ligand for ABCC6.
Introduction
Calcification of joints and arteries (Arterial Calcification due to Deficiency of CD73 (ACDC); OMIM #211800), as originally described by Magnus-Levy [1] and later by Sharp [2], was recently shown to be caused by deficiency of CD73, encoded by the NT5E gene [3]. CD73 has 5′ exonucleotidase activity that converts AMP to adenosine and inorganic phosphate (Pi) [4]. The enzyme resides on the plasma membrane of vascular cells, supplying adenosine to receptors on the cell surface. Receptor binding of adenosine triggers an intracellular signaling cascade that results in inhibition of tissue nonspecific alkaline phosphatase (TNAP) activity. Thus, CD73 deficiency lowers pericellular adenosine concentrations, increasing alkaline phosphatase and allowing calcification to occur. Clinical findings include calcified large vessels, especially below the waist, and periarticular calcifications in the joints of the hands and feet.
Three families with autosomal recessive CD73 deficiency have been described, with homozygous nonsense, homozygous missense, and compound heterozygous mutations, respectively [3]. In the third family, a single adult patient had extensive lower extremity claudication; a femoral-popliteal bypass was performed, providing vascular tissue for histopathologic studies.
Here we present that case, complete with clinical characterization, radiographic presentations, cell culture findings, and histological appearance of the affected vasculature. Involvement of the elastic lamina in our ACDC patient resembles that in pseudoxanthoma elasticum (PXE), a connective tissue disorder of broken elastic fibers and localized calcification due to deficiency of ABCC6, a membrane transporter whose ligand is unknown [5]. The histopathological similarity between ACDC and PXE suggests that localized adenosine concentrations surrounding small vessels could play a critical role in PXE. Specifically, we suggest that adenosine could be the ligand transported by the ABCC6 transporter deficient in PXE.
Methods
Patient
The patient was admitted to the NIH Undiagnosed Diseases Program and enrolled in clinical protocol 76-HG-0238, approved by the Institutional Review Board of the National Human Genome Research Institute. She gave written, informed consent.
Mutation analysis
Sequencing of the NT5E gene was performed as described [3].
Fibroblast studies
Fibroblasts, obtained from a 4 mm punch skin biopsy, were cultured in DMEM containing 10% FBS and 1% penicillin/streptomycin, as described [6]. Alkaline phosphatase staining was performed using SIGMAFAST| BCIP/NBT tablet (Sigma Aldrich), according to the manufacturer's instructions. Alkaline phosphatase activity was assayed with the StemTAG™ Alkaline Phosphatase Activity Assay Kit (Cell Biolabs, Inc.), using p-nitrophenol phosphate as substrate; the product, p-nitrophenol, was measured by absorption at 405 nm and normalized to ug protein per reaction.
Gene Expression Studies
RNA was isolated from cultured fibroblasts using the RNAeasy® kit (Qiagen) and 1ug of RNA was used to generate cDNA using TaqMan Reverse Transcription reagents (Applied Biosystems). Expression of collagen I α1 (COL1A1) mRNA was measured by quantitative real-time polymerase chain reaction (qPCR) on a Chroma4 Real Time PCR system (Bio-Rad) using iQ SYBR Green Supermix (Bio-Rad). Expression levels were calculated using the 2−ΔCt method, where the cycling threshold (Ct) of the candidate gene was compared to the Ct of 18s rRNA and expressed as a power of two (2(Ct-COL1A2 − Ct-18s)). Primers: COL1A1 sense primer 5′-GCCGTGACCTCAAGATGTG-3′, antisense primer 5′-GCCGAACCAGACATGCCTC-3′; 18S rRNA sense primer 5′-GTAACCCGTTGAACCCCATT-3′ antisense 5′-CCATCCAATCGGTAGTAGCG-3′.
Histology/imaging
Formalin fixed and paraffin-embedded sections of the femoral artery removed during bypass surgery were surface decalcified and sectioned. The H&E stained sections performed at the University of San Francisco were obtained and imaged by oil immersion microscopy using Zeiss Plan NEOFLUOR 10X/0.30(DIC), Plan-APOCHROMAT 20X/0.8, and EC PLAN-NEOFLUAR 40X/1.3 oil objectives at 1.2Mpixels per image (Zeiss Axiovert 200M and Axiocam HRC, using Axiovision software, v 4.5). The UCSF H&E image was reconstructed from the 624 40X images using Photoshop CS4 by overlaying the individual images on a reconstructed 20X composite image that was reconstructed from 230 images formed by overlaying on a composite made from 36 10X images that were automatically assembled using photmerge. The final composite 2.35GB image was cropped to 1.93GB and stored as a PDF file (see supplemental data). Individual sectional frames were assembled into a video tour of the master image to show the total image and the circumferential tract of the internal elastic lamina (see supplemental data). The paraffin block was also sectioned at the NIH and three consecutive sections were stained. The first was stained with a routine H&E stain. The second and third sections were stained for elastin (Verhoeff's Technique of van Gieson staining), and Masson's Trichrome staining, respectively. The last two sections were stained using Dako Artisan kits (Dako North American Inc., Carpinteria, CA 93013). These three serial sections of the artery were imaged and the composites automatically assembled using Zeiss Axiovert 200M with motorized scanning stage, Axiovision software v4.6, a Zeiss MRc5 camera, and the Plan-APOCHROMAT 20X/0.8 objective.
Case report
A 44-year old woman first noted calf pain on exercise at 14 years of age. Over the next 3 years, a calcification on the plantar surface of the right first metatarsal-phalangeal joint caused pain on walking; it was surgically removed at age 16 with a diagnosis of tumoral calcinosis. At age 27, temporal lobe seizures occurred. A benign, calcified oligodendroglioma was removed at age 28; no seizures occurred after the surgery. The patient continued to have joint pain and tender enlargements of the proximal interphalangeal and metacarpophalangeal joints of the hand with radiographic changes described as peri-articular calcifications; pseudogout was diagnosed. She complained of sporadic pain in her enlarged proximal interphalangeal joints, intermittent swelling, pain, and numbness in her hands and wrists (particularly the fourth and fifth digits of her left hand), and lower leg cramping after walking one block. Her joint pain was treated with nonsteroidal anti-inflammatory medications. There were no symptoms of tetany. By 43 years of age, the pain had progressed to definitive lower limb claudication after very brief exercise or a 100-meter walk. There was no pain at rest.
Distal pulses were profoundly diminished. A CT of the lower limbs showed extensive arterial calcifications especially in the distal femoral and proximal tibial arteries. Vascular ultrasound showed extensive calcification and aneurysms with occlusion in the superficial femoral and popliteal arteries bilaterally, and monophasic waveforms in the tibial arteries. There was mild calcification in the left external carotid artery, also involving the aortic arch, bilateral cavernous internal carotid arteries, and distal vertebral arteries bilaterally. The parapharyngeal space contained large amorphic calcific deposits bilaterally. The calcium score on chest CT was 0. Due to progressively worsening claudication, development of foot cramping and burning with exercise and elevation, and a drop in the ankle-brachial index (ABI) from 0.47 to 0.32 over the preceding year, surgery was performed in October of 2009. Specifically, an extensive profunda endarterectomy and reconstruction restored flow, and a right femoral to posterior tibial bypass using the non-reversed ipsilateral greater saphenous vein eradicated symptoms at rest and ambulatory foot pain and burning. The ABI rose from 0.32 to 0.71. At 13 months post-surgery, the bypass and profunda reconstruction are patent, the patient walks 45 minutes at a good pace, and there are no symptoms at rest. Calf claudication occurs on walking uphill. The left leg ABI remains 0.7.
By report, the arterial anastamosis site of the bypass showed “arterial narrowing and calcification of uncertain etiology”. A second opinion noted that “atherosclerosis is unlikely as the basis for the patient's lesions”, and “there is no suggestion of traumatic calcification”. While “the damage to the internal elastic lamina suggests healing giant cell arteritis”, “the overall pattern of calcification militates against healed giant cell arteritis.”
Past medical history revealed no diabetes, chronic renal failure, exposure to bisphosphonates, vitamin D toxicity or hyper- or hypoparathyroidism. The only fracture was a right distal wrist fracture at age 12 during play. The patient is the oldest of three siblings and is the only one affected. There was no family history of claudication, peripheral vascular disease, atherosclerosis, hyperlipidemia/hypercholesterolemia, stroke, arthritis, or pseudogout. The father is English and the mother French. The patient had three children, none of whom had medical problems or complaints of bone pain, peripheral calve pain on exertion, peri-articular pain or enlargement of any small joints.
Upon admission to the NIH Clinical Center, the patient had normal strength and exercise tolerance in the upper limbs, torso and back. Range of motion for all extremities was full without restriction or hypermobility. The external carotids were normal on palpation just posterior to the lateral edge of the sternocleidomastoid muscle below the angle of the mandible bilaterally. The thyroid gland was not enlarged or nodular. The temporal arteries had strong pulses, without swelling or tenderness. Ophthalmic examination revealed no evidence of retinal vessel tortuosity, narrowing or vasculitis.
Laboratory studies, including serum cholesterol, parathyroid hormone, and vitamin D, were normal (Table 1). A metabolic panel, complete blood cell count, serum gamma-glutamyltranspeptidase, prothrombin time, and partial thromboplastin time were normal. The 24-hour urinary excretions of calcium, phosphorus and creatinine were normal. An EKG showed normal sinus bradycardia.
Table 1.
Patient's laboratory values
| Patient | Normal value | |
|---|---|---|
| Cholesterol (mg/dL) | 136 | 100-200 |
| HDL (mg/dL) | 57 | 40-60 |
| LDL mg/dL) | 70 | <100 |
| Triglycerides (mg/dL) | 43 | <150 |
| PTH (pg/mL) | 27 | 16-87 |
| Vitamin D2 (ng/mL) | <4 | Sum of D2+D3 = 10-80 |
| Vitamin D3 (ng/mL) | 32 | |
| Anti ENA (EU) | <20 | <20 |
| RF (IU/mL) | <15 | <15 |
| ESR (mm/s) | 14 | 0-42 |
| CRP (high sensitivity) | 2.33 | <3.0 |
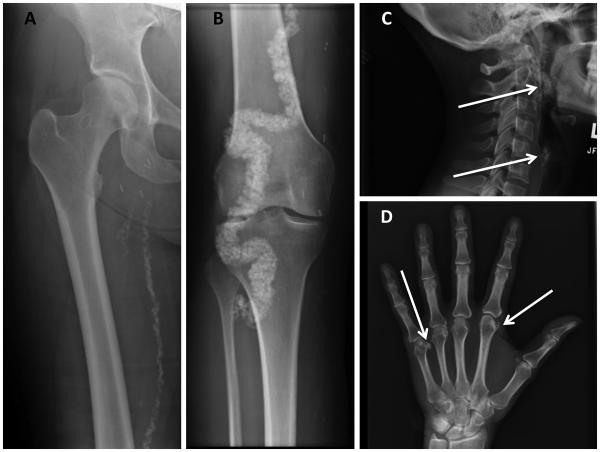
Radiographs showed extensive arterial calcification involving the distal femoral arteries (Fig. 1A) and adjacent sections of the popliteal and posterior tibial arteries (Fig. 1B), as well as the external carotid arteries (Fig. 1C) and the dorsalis pedis and posterior tibial arteries at the ankle (not shown). The internal carotids, aorta and coronary arteries were essentially devoid of calcification, as were the intracerebral vessels. The falx cerebri, the scar at the oligodendroglia excision, the thyroid and crycoid cartilages, and the costochondral cartilages showed premature calcifications; so did the periarticular connective tissue sheaths surrounding the joints of several interphalangeal and metacarpophalangeal joints (Fig. 1D). Coronary calcification was absent; however, the ligamentum arteriosum was calcified (not shown). Total body calcium was illustrated by non-contrast CT, showing calcification and arteriomegaly of the large arteries of the lower extremities (Fig. 2; Supplement 3). The lower extremities had an Agatston calcium score of 194485.
Fig. 1.
Skeletal radiographs of patient.
A. Plain radiograph showing calcification of the femoral artery.
B. Calcification of the popliteal and posterior tibial arteries.
C. Calcification of the internal carotid artery (arrow).
D. Calcification in the metacarpal phalangeal joints (arrows).
Fig. 2.
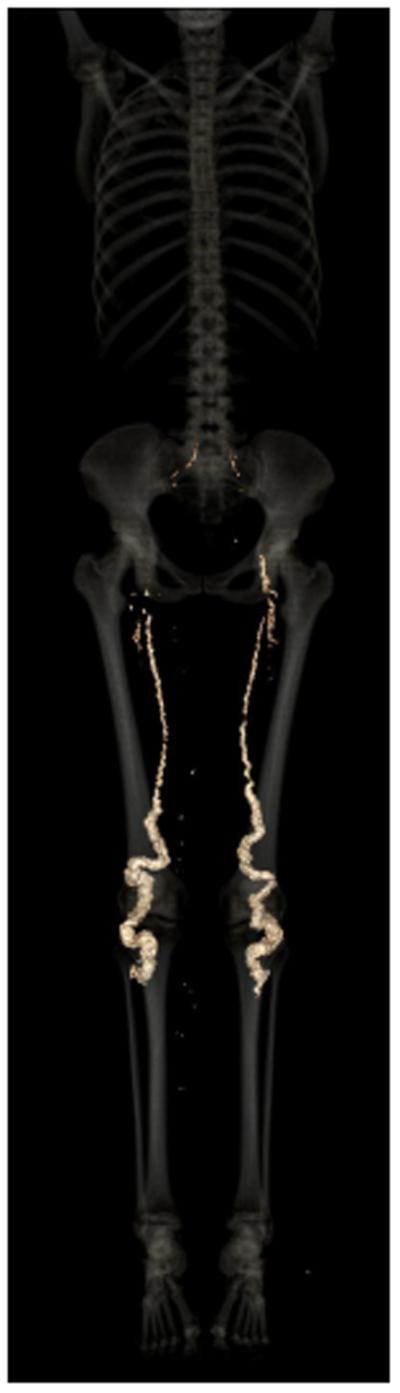
Non-contrast CT for valuation of total body calcium. The arteries of the lower extremities display severe calcification and arteriomegaly.
Results
Mutation analysis
NT5E sequencing, previously described [3], revealed compound heterozygosity for the exon 3, C662A mutation from the paternal allele and the c1609dupA (V537fsX7) from the maternal allele.
In vitro studies
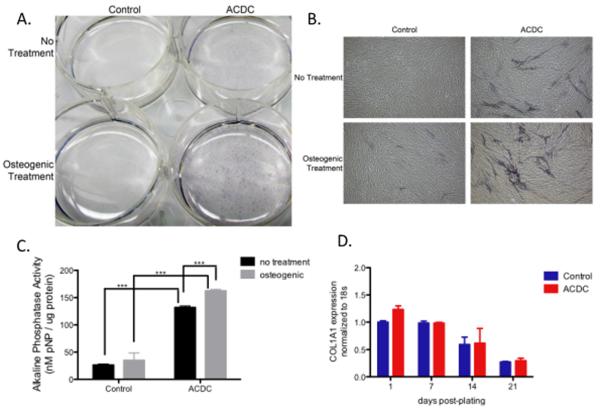
Fibroblasts cultured from the patient's skin biopsy exhibited increased alkaline phosphatase staining even without exposure to osteogenic medium (Fig. 3A, B). Treatment with osteogenic medium increased the amount of alkaline phosphatase staining in both normal and CD73-deficient fibroblasts, although the mutant cells were affected to a much greater extent (Fig. 3A, B). Quantification of alkaline phosphatase enzyme activity measurements confirmed the staining results (Fig. 3C). Cells from patient and controls were isolated after 1, 7, 14, and 21 days in culture and illustrate that CD73-deficient cells do not have an increase in collagen Iα1 mRNA expression at these time-points (Fig. 3D).
Fig. 3.
Alkaline phosphatase staining of control and ACDC (CD73-deficient) fibroblasts.
A. Dishes of confluent fibroblasts from a control and the ACDC patient, stained for alkaline phosphatase. No treatment (top); osteogenic medium (bottom).
B. Microscopic view of cells shown in A.
C. Quantification of alkaline phosphatase enzyme activity, expressed as nM p-nitrophenol produced per μg of fibroblast protein.
D. Gene expression of collagen Iα1 in control and ACDC fibroblasts.
Histopathology
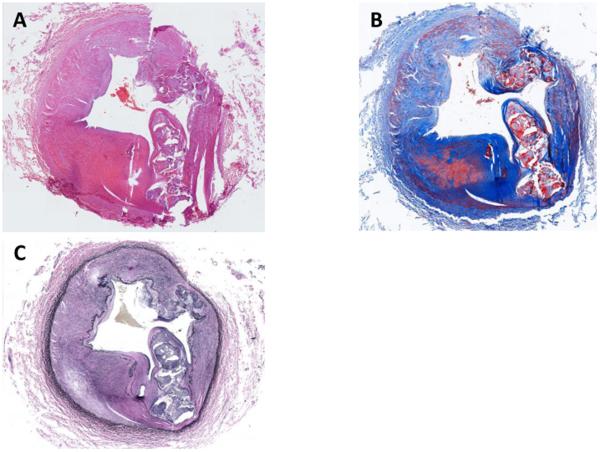
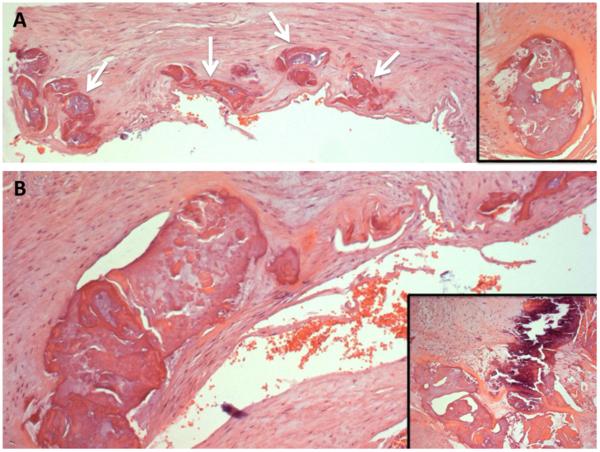
On histology, there was arteriomegaly with luminal stenosis due to a major expansion of the medial elements of the artery, and extensive calcifications (Fig. 4). Von Kossa staining (not shown) was strong for calcium deposition in the major lesion but was not definitive for the minor foci along the internal elastic lamina; the block had been decalcified. Close examination using Masson's trichrome, and elastin staining with the Verhoeff-van Gieson reagent, showed that the entire internal elastic lamina had varying degrees of duplication, along with fragmentation, loss of curvature, and staining of the broken ends (Figs. 5 & 6), resembling the appearance of calcified fibers in PXE. A video survey at high magnification (Supplemental material) showed that the entire circumference was involved; the larger regions of calcification were apparent outgrowths of the elastic lamina. As each smaller nidus of calcification enlarges, differentiating cell types appear, including osteoblast-like cells inside of vacuolar spaces in the calcium-containing regions. Osteoclast-like cell clusters appeared to be remodeling these spaces. The van Gieson stain clearly shows a remaining degenerated fragment of the internal elastic lamina directly centered in the largest calcification focus of that section. Taken as a whole, the pathology of this disorder is consistent with changes in the internal elastic lamina inducing osteogenic activity that becomes circumferential and occludes the lumen.
Fig. 4.
Circumferential views of resected right femoral artery, showing substantial thickening of the media with connective tissue and disrupted elastic fibers.
A. Hematoxylin and eosin stain.
B. Masson's trichrome stain, showing connective tissue.
C. Verhoeff's technique of the Van Gieson reagent stain of elastic fibers.
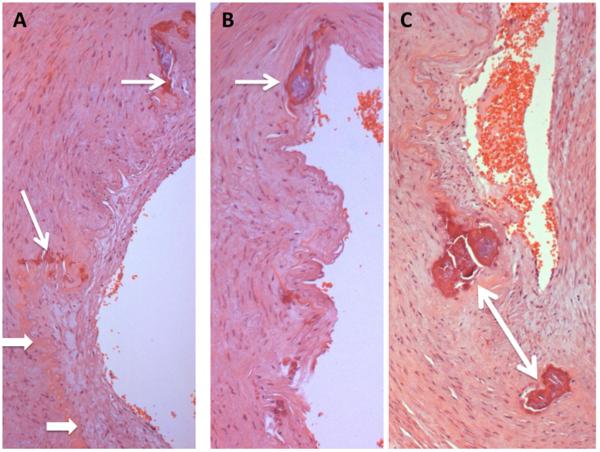
Fig. 5.
Internal elastic lamina of patient's artery (hematoxylin & eosin).
A. Tortuous path of elastic lamina (arrowheads) contiguous with calcified outgrowths (arrows).
B. Larger calcification (arrow) within the path of the internal elastic lamina.
C. Fragmented calcifications (arrow) contiguous with elastic fibers.
Fig. 6.
Calcified regions of the patient's vascular wall (hematoxylin & eosin).
A. Numerous calcifications (arrows) lining the inner wall of the femoral artery, with remnants of elastic lamina. Inset: Enlarged calcification, showing inhomogeneous collection of material.
B. Larger calcified area of vessel.
Inset: Extensive calcium deposition with tissue breakdown.
Discussion
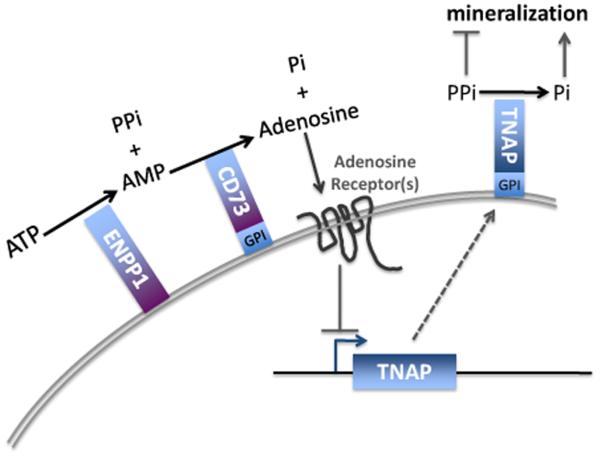
ACDC results from deficiency of CD73, a glycosyl phosphatidylinositol (GPI)-anchored protein that is expressed widely in different tissues such as the colon, kidney, brain, liver, heart and lung. Within the vasculature, CD73 functions in several cell types, including endothelial cells, vascular smooth muscle cells and fibroblasts, by modulating purinergic signaling. Impairment of that signaling results in increased alkaline phosphatase activity within CD73-deficient fibroblasts in vitro [3] (Fig. 3). The increased alkaline phosphatase, which promotes calcification [7] in part by reducing pyrophosphate concentrations (Fig. 7), can be rescued by adenosine, the product of CD73 catalysis [3]. We hypothesize that, in ACDC, the specific vessels (and also the joint capsules) that calcify are determined by their contingent of adenosine receptors; studies in the A2b receptor knockout mouse illustrate that different vascular beds have different A2b adenosine receptor expression patterns [8].
Fig. 7.
A model of the purinergic pathway in vascular cells. ATP is converted to pyrophosphate and AMP by ENPP1. CD73 converts AMP to inorganic phosphate and adenosine, which binds to plasma membrane receptors that induce intracellular signaling. This lowers tissue neutral alkaline phosphatase (TNAP), an extracellular, membrane-bound protein. In the absence of TNAP, extracellular pyrophosphate levels increase, inhibiting mineralization.
We now demonstrate that CD73 deficiency, due to bi-allelic mutations of NT5E, involves calcification of the internal elastic lamina of affected vessels. This previously unrecognized finding has relevance for Marfan syndrome, Loeys-Dietz syndrome, some forms of isolated aortic aneurysms [9], chronic renal failure [10,11] associated with longstanding diabetes, or Monkeberg arteriosclerosis [12], and other disorders [13] in which the genesis and repair of elastic fibers play critical roles.
Calcification of elastic fibers also plays a critical role in the pathology of PXE [14], which involves small vessels and the possibility of a circulating factor [15]. PXE results from bi-allelic mutations affecting the transport protein ABCC6 [16]. ABC membrane transporters of the C class (specifically, ABCC4, ABCC5 and ABCC8) carry nucleotides out of cells in an ATP-dependent fashion [17-19]; the ligand for ABCC6, however, has not been identified. We propose that adenosine or AMP could be the ligand for ABCC6, whose function would involve delivering adenosine to cells of small vessels.
Two lines of evidence support this possibility. First, PXE and ACDC closely resemble each other with respect to the histopathological findings of broken and calcified elastic fibers (Figs. 4-6). Second, on clinical grounds, PXE has been mistaken for another disorder of adenosine production, Generalized Arterial Calcification of Infancy (GACI). This autosomal recessive disorder due to biallelic mutations in ENPP1 (Fig. 7) presents with calcification of large and medium-sized arteries and results in arterial stenosis, myocardial ischemia, and death in early childhood [20]. The mechanism of calcification in GACI is considered to be reduced production of PPi from ATP because of deficient ENPP1. However, in GACI there may also be a paucity of local adenosine because of reduced substrate (AMP) for the downstream enzyme, CD73. The greater severity of clinical and pathological findings in GACI compared with ACDC could be explained by the dual reduction of pyrophosphate concentrations in GACI, i.e., from decreased production by ENPP1 and from reduced adenosine signaling (Fig. 3). In 2010, Le Boulanger et al. described a documented (genetically confirmed) PXE patient whose sibling died in infancy with the clinical diagnosis of GACI [21]. Apparently, PXE can present with such severity that it is mistaken for GACI.
Knowledge of the involvement of adenosine in preventing vascular calcification allows for therapeutic interventions Specifically, the reversal of cellular calcification by adenosine suggests that therapy for arterial medial calcification due to decreased inhibition of alkaline phosphatase might be attempted using adenosine analogs, metabolizable or not. Presumably, the adenosine pathway could also be targeted in cases of diabetic arterial calcification of medium sized vessels, such as Monkeberg arteriosclerosis, and for small vessels, as in PXE.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Programs of the National Human Genome Research Institute and the National Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Von Magnus-Levy A. Ueber ungewöhnliche Verkalkung der Arterien. Deutsche Meidzinische Wochenschrift. 1914;40:1305–1309. [Google Scholar]
- 2.Sharp J J. Heredo-familial vascular and articular calcification. Ann. Rheum. Dis. 1954;13:15–27. doi: 10.1136/ard.13.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilaire C, Ziegler SG, Markello T, Brusco A, Groden C, Gill F, Carlson-Donohoe H, Lederman RJ, Chen MY, Yang D, Siegenthaler MP, Arduino C, Mancini C, Freudenthal B, Stanescu HC, Zdebik AA, Chaganti RK, Nussbaum R, Kleta R, Gahl WA, Boehm M. NT5E mutations and arterial calcifications. N. Engl. J. Med. 2010 doi: 10.1056/NEJMoa0912923. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signaling cascade. Biochim. Biophys. Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Truter S, Rosenbaum-Fiedler J, Sapadin A, Lebwohl M. Calcification of elastic fibers in pseudoxanthoma elasticum. Mt. Sinai J Med. 1996;63:210–215. [PubMed] [Google Scholar]
- 6.Normand J, Karasek MA. A method for the isolation and serial propagation of keratinocytes, endothelial cells, and fibroblasts from a single punch biopsy of human skin. In Vitro Cell. Dev. Biol. Anim. 1995;31:447–55. doi: 10.1007/BF02634257. [DOI] [PubMed] [Google Scholar]
- 7.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. USA. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, Hilaire C, Seldin DC, Toselli P, Lamperti E, Schrieber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J. Clin. Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietz HC. TGF-beta in the pathogenesis and prevention of disease: A matter of aneurysmic proportions. J. Clin. Invest. 2010;120:403–407. doi: 10.1172/JCI42014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moe SM, O'Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, Fineberg N, Kopecky K. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002;61:638–647. doi: 10.1046/j.1523-1755.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 11.Amann K. Medial calcification and intima calcification are distinct entities in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2008;3:1599–1605. doi: 10.2215/CJN.02120508. [DOI] [PubMed] [Google Scholar]
- 12.Mönckeberg JG. Über die reine Mediaverkalkung der Extremitätenarterien und ihr Verhalten zur Ateriosklerose. Virchows Arch. Pathol. Anat. 1903;171:141–167. [Google Scholar]
- 13.Doherty TM, Fitzpatrick LA, Inoue D, Qiao J-H, Fishbein MC, Detrano RC, Shah PK, Rajavashisth TB. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocrine Rev. 2004;25:629–672. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- 14.Katsuaki M, Yuri T, Nobuhiko T, Takehana K, Toshiji I, Tsubura A. An autopsy case of pseudoxanthoma elasticum: histochemical characteristics. Med. Mol. Morphol. 2007;40:172–177. doi: 10.1007/s00795-007-0368-5. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Q, Endo M, Dibra F, Wang K, Uitto J. Pseudoxanthoma elasticum is a metabolic disease. J. Invest. Dermatol. 2009;129:348–354. doi: 10.1038/jid.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, Swart J, Kool M, van Soest S, Bass F, ten Brink JB, de Jong PT PT. Mutations in ABCC6 cause Pseudoxanthoma Elasticum. Nat. Genet. 2000;25:228–231. doi: 10.1038/76109. [DOI] [PubMed] [Google Scholar]
- 17.Sager G, Ravna AW. Cellular efflux of cAMP and cGMP – A question about selectivity. Mini-Rev. Medicinal Chem. 2009;9:1009–1013. doi: 10.2174/138955709788681654. [DOI] [PubMed] [Google Scholar]
- 18.Zhou S-F, Wang L-L, Di Y-M, Xue CC, Duan W, Li CG, Li Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr. Medicinal Chem. 2008;15:1981–2039. doi: 10.2174/092986708785132870. [DOI] [PubMed] [Google Scholar]
- 19.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol. Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 20.Rutsch F, Ruf N, Vaingankar S S, Toliat MR, Suk A, Höhne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill J, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nürnberg P. Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat. Genet. 2003;34:379–81. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- 21.Le Boulanger G, Labreze C, Croue A, Schurgers LJ, Chassaing N, Wittkampf T, Rutsch F, Martin L. An unusual several vascular case of pseudoxanthoma elasticum presenting as generalized arterial calcification of infancy. Am. J. Med. Genet. Part A. 2010;152A:118–123. doi: 10.1002/ajmg.a.33162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.