Abstract
We evaluated immunologic predictors of response to HBV vaccine administered in the presence or absence of GM-CSF in HIV infected individuals. We measured peripheral blood hematopoietic progenitor, monocyte and myeloid-derived suppressor cell (MDSC) frequencies, and expression of GMCSF receptor on monocytes and MDSCs, at baseline and 4 weeks after immunization in relation to antibody response. We observed higher baseline progenitor and lower monocyte frequencies among week 16 antibody responders. Week 4 decline in MDSC frequency was associated with week 16 antibody response, while administration of GM-CSF was associated with preservation of these cells. No significant differences in GM-CSF receptor expression were observed in the presence vs. absence of GM-CSF. These findings are consistent with a positive role of progenitor cells and a potential negative role of monocytes in vaccine response. Additionally, GM-CSF augmented the preservation of peripheral blood MDSC, which may contribute to the lack of improved vaccine responses.
Keywords: cellular immunity, vaccine, hepatitis B, monocyte, progenitor cell, human
Introduction
Numerous factors are involved in a successful vaccine response to induce long lasting immune memory[1]. The adaptive arm of the immune system requires adequate antigen uptake and presentation by antigen presenting cells (APC) and adequately responsive T and B lymphocytes capable of forming an effective memory response [2, 3]. Among HIV-infected individuals, vaccine responses, including to hepatitis B (HBV) vaccine, are inadequate [4-8]. Given the increasing incidence of mortality due to hepatic disease among HIV-infected persons, improved HBV vaccine approaches, including alternative adjuvants, are needed to reduce the burden of co-infection among this at risk population[9]. However, the complex milieu of immune defects associated with HIV infection create unique challenges in formulating appropriate vaccine enhancing adjuvants. GM-CSF, when administered as a vaccine adjuvant, has been observed to improve HBV vaccine efficacy in the setting of renal failure [10, 11] and in the setting of HIV infection[12]. In AIDS Clinical Trials Group (ACTG) study A5220, GM-CSF administration was not found to improve HBV vaccine responses[13]. We hypothesized that impairment in antigen presenting cell expansion, differentiation, recruitment, or expansion of a cell population that blocks immune responses in HIV infection underlies the failure of GM-CSF to enhance antibody responses to HBV vaccine.
CD34+ hematopoietic progenitor cells can rapidly differentiate into many cell types, including monocytes and the highly potent APC, dendritic cells [14-16]. Additionally, CD34+ progenitor cells have been described to have T cell suppressive activity that is influenced by a number of factors, including the presence of GMCSF [17]. These myeloid-derived suppressor cells (MDSCs) have been described to a greater degree in the oncology literature [18] as being a heterogeneous population identified as CD34+, CD33+/lineage marker (including CD14-HLADR-) and CD11b+CD14-CD33+[18]. Additionally, another sub-population of MDSCs has been described as CD14+HLADR- [19]. These cells have been regularly identified in cancer patients, have been reported as more prevalent in cancer patient vaccine nonresponders compared to responders, and their numbers are increased after administration of GM-CSF. The CD14 marker is commonly used for identification of monocytes. Monocytes can present antigen themselves and differentiate into dendritic cells in the presence of GM-CSF, but are also capable of contributing to an immune suppressive milieu [20, 21]. Based on these previous findings, we asked if the failure of GM-CSF to augment vaccine responses in A5220 might be related to expansion or induction of suppressive cell populations.
In this exploratory analysis from A5220, we measured the frequency of hematopoietic progenitor cells (CD34+), monocytes (CD14+), and MDSCs (CD14+HLADR-CD11b+, CD34+CD14-HLADR-CD11b+, CD34+CD14-HLADR-), as well as the expression of the GM-CSF receptor (CD116) on monocytes and MDSCs at baseline and week 4 of HBV vaccine protocol. We hypothesized that vaccine response was associated with the baseline prevalence or GM-CSF induced change in frequency of these cells.
Methods
A5220 study and samples for flow cytometric analysis
ACTG trial A5220 was a randomized open-label pilot study to evaluate the effectiveness and safety of GM-CSF adjuvant combined with HBV vaccine. Forty-eight HIV-infected subjects, naïve to HBV vaccine, with CD4 T cell counts ≥200/mm3, and seronegative for HBV and HCV were randomized to administration of 40mcg HBV vaccine at weeks 0, 4 and 12, with or without 250mcg GM-CSF as adjuvant, 24 in each study arm. HBV specific antibody titers (HBsAb) were measured using Vitros Immunodiagnostics Products Anti-HBs Quantitative Assay (Ortho-Clinical Diagnostics, Amersham, Bucks, UK) at weeks 0, 4, 8, 16, 36, and 60 as part of A5220. HBV vaccine response was defined as HBsAb≥10mIU/mL.
PBMC were prepared at each clinical site by standardized ACTG protocol, cryopreserved, then sent to a central storage facility. Samples were prepared at baseline and week 4. Forty seven of the 48 study subjects enrolled to A5220 had samples available for the present analysis which was performed at a single ACTG immunology laboratory.
Flow cytometric analysis of myeloid cell subset peripheral blood frequencies
Cryopreserved PBMC samples were stained with viability stain in pacific blue, anti-CD34 FITC, CD11b PE-Cy5, anti-CD14 PerCP, anti-CD116 PE, and anti-HLA DR-APC or isotype control (BD Biosciences, San Jose, CA) for 10 minutes at room temperature, then washed with 2mL of PBS with 0.01% BSA, then resuspended in 200uL of 1.0% paraformaldehyde solution (Electron Microscopy Science, Hatfield, PA) and 200uL of PBS with 0.01% BSA. Flow cytometric analysis was performed on a LSRII flow cytometer (BD Biosciences, San Jose, CA) with FACS Diva Software (BD Biosciences, San Jose, CA).
Live cells were identified by viability stain and forward and side scatter. Gating was based upon isotype controls. Hematopoietic progenitor cells were identified as CD34+. Monocytes were identified as CD14+. MDSCs were identified as CD14+HLADR-CD11b+, CD34+CD14-HLADR-CD11b+, and CD34+CD14-HLADR-. GM-CSF receptor (CD116) was measured on CD14+ and CD14+HLADR-CD11b+ cells (Figure 1)
Figure 1. Flow Cytometry gating scheme.
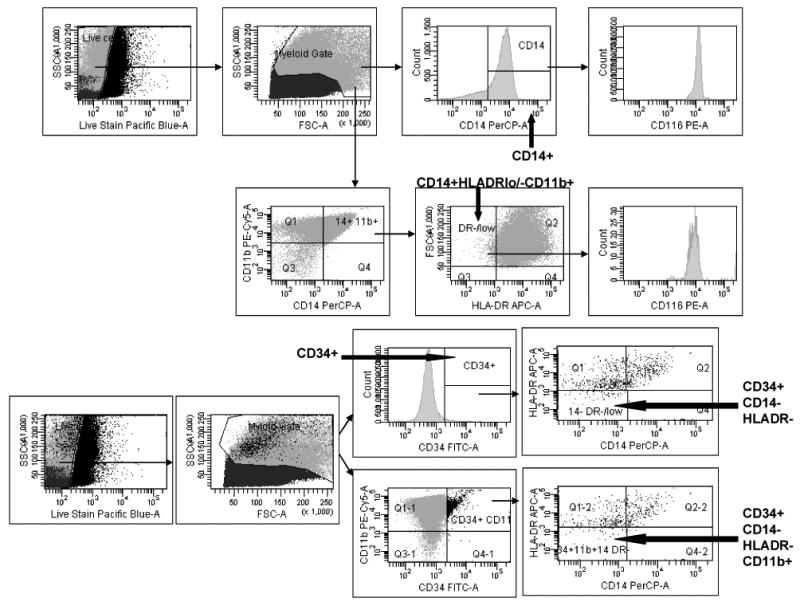
Live cells were identified by viability stain and forward and side scatter. Hematopoietic progenitor cells were identified as CD34+. Monocytes were identified as CD14+. Myeloid lineage suppressor cells were identified as CD14+HLADR-CD11b+, CD34+CD14-HLADR-CD11b+, and CD34+CD14-HLADR-. GMCSF receptor (CD116) was measured on CD14+ and CD14+HLA DR-CD11b+ cells.
Statistical methods
Medians (50% quantile) with Q1 (25% quantile) and Q3 (75% quantile) are provided to summarize immune parameters. Wilcoxon rank-sum tests were conducted to compare immune parameters between two groups (e.g. study arm with vs. without GM-CSF, nonresponders vs. responders). Van Elteren's tests were used for comparisons of vaccine nonresponders and responders stratified by study arm. Changes in immune parameters from baseline at week 4 within a group were assessed using Wilcoxon signed rank tests. Spearman correlation coefficients and tests were used to assess associations between baseline immune parameters and quantitative HBsAb titers at week 16. Two-sided p-values from Fisher's exact tests are reported here as exploratory analyses. P-values <0.05 (two-sided) were considered statistically significant, and no adjustments were made for multiple testing.
Results
Among the 47 subjects with available samples for analysis here, the study subject median age was 47 years; 79% were male; 53% white, 32% black and 15% Hispanic. The median CD4 cell count at entry was 444 cells/mm3, 77% were on antiretroviral treatment and 53% had undetectable HIV-1 RNA (<50 copies/mL).
As recently described [13], there was a modest trend towards a higher proportion of vaccine responders (HBsAb≥10 mIU/mL) in the GM-CSF arm at week 4 (26% vs. 9%, p=0.24), but by week 8 this trend was lost (week 8: 26% vs. 35%, p=0.75; week 16: 52% vs. 65%, p=0.54; week 36: 55% vs. 64%, p=0.76; week 60: 35% vs. 45%, p=0.54).
To identify whether GM-CSF receptor expressing myeloid populations could account for variability in vaccine response we measured peripheral blood hematopoietic progenitor cell (CD34+), monocyte (CD14+), and MDSC. MDSCs are a heterogeneously defined population of cells that have been described as CD34+, CD33+/lineage marker (including CD14-HLADR-) and CD11b+CD14-CD33+[18]. Additionally, another sub-population of MDSCs has been described as CD14+HLADR- [18] [19]. We focused here on CD14+HLADR-CD11b+, CD34+CD14-HLADR-CD11b+, and CD34+CD14-HLADR- cell frequencies at baseline (Figure 2). Comparing week 16 vaccine responders to nonresponders, irrespective of study arm, baseline CD34+ progenitor frequency was higher in subjects with a week 16 antibody response (n=26; median [Q1, Q3]: 0.94% [0.62%, 1.28%]) than those without (n=18; 0.57% [0.47%, 0.68%]; p=0.005) (Figure 2A). When stratified by study arm, this remained statistically significant (p=0.01). Additionally, this relationship held as a positive correlation between CD34+ progenitor cell frequency and week 16 antibody titer (r=0.34, p=0.03, Figure 2B). A subset of CD34+ progenitors with phenotypic markers of MDSCs (CD34+CD14-HLADR-) were also higher in frequency at baseline in week 16 vaccine responders than in nonresponders (0.14% [0.08%, 0.42%] vs. 0.08% [0.03%, 0.19%], p=0.02; p=0.04 when stratified by study arm). In contrast, baseline CD14+ monocyte frequency was lower in week 16 antibody responders than in nonresponders (6.87% [4.15%, 8.29%] vs. 10.17% [7.97%, 12.48%], p=0.02; p=0.06 when stratified by study arm, Figure 2C). This was consistent with an observed negative correlation between baseline CD14+ cell frequency and week 16 antibody titer (r= -0.38, p=0.01, Figure 2D).
Figure 2. Baseline CD34+ progenitor and monocyte frequencies are associated with week 16 vaccine response.
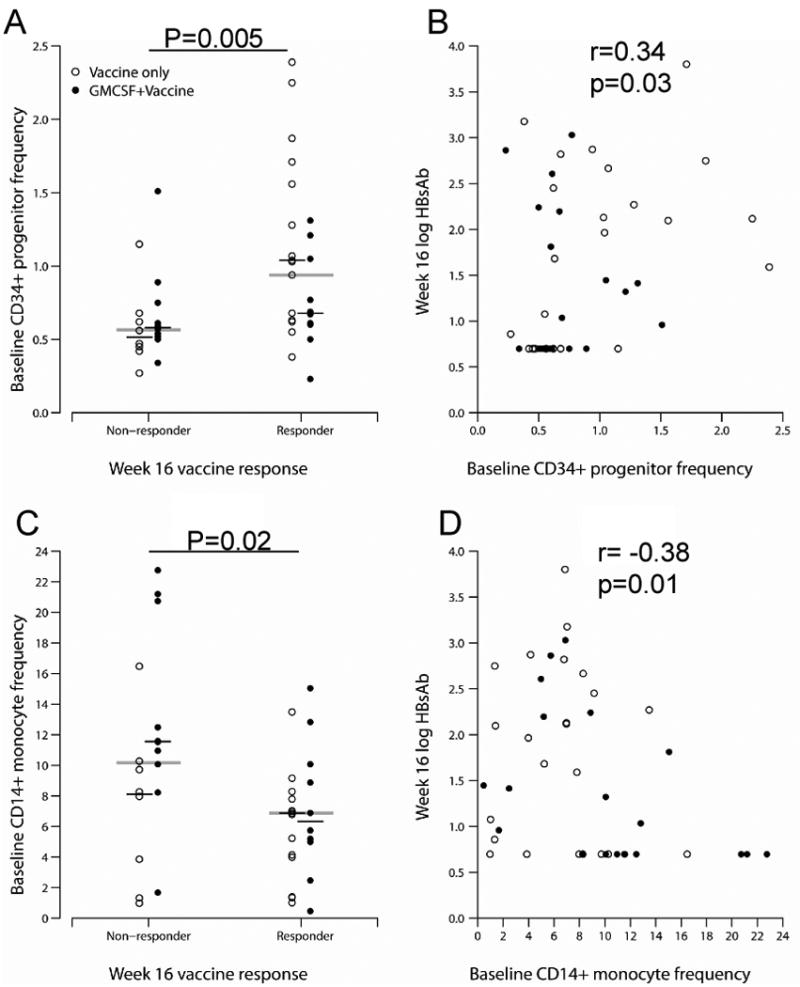
Baseline peripheral blood CD34+ progenitor (Panel A) and CD14+ monocyte (Panel C) frequency were measured by flow cytometric analysis. Correlation between CD34+ progenitor (Panel B) and monocyte (Panel D) frequency and week 16 hepatitis B surface antibody titer are shown. The horizontal lines on Panels A and C represent medians, and p-values are for the comparisons of responders and non-responders, regardless of study arm. P-values on Panels B and D are for the Spearman correlation coefficients.
We next asked if administration of GM-CSF affected the frequencies of these cell populations. At week 4 we observed a decline in CD34+CD14-HLADR- and CD34+CD14-HLADR-CD11b+ cell frequencies in the vaccine arm that did not receive GMCSF (-0.01% [-0.02%, 0%], p=0.02; and 0% [-0.02%, 0%], p=0.01 respectively, Figure 3A and 3B) while there were no such changes observed in the GMCSF arm (p=0.007 and p=0.03 for the comparison of study arms). Notably, 2 subjects in the arm that did not receive GM-CSF had greater frequencies of these cells at baseline (potentially driving this difference). Also, there were more subjects without such measurable cells at baseline in the GM-CSF arm (5 in the GM-CSF arm vs. 4 in the Vaccine only arm and 12 vs. 9 for CD34+CD14-HLADR- and CD34+CD14-HLADR-CD11b+, respectively) who could have only had increased frequency subsequently. However, though overall there were no statistically significant differences in these cell frequencies at baseline. When we evaluated change in cell frequency in relation to week 16 antibody response, vaccine responders had a minimal week 4 decline in CD34+CD14-HLADR- cell frequency (0% [-0.02%, 0%], p=0.02) while non-responders showed no decline (0.01% [-0.01%, 0.01%], p=0.03 for the comparison of responders and nonresponders; p=0.08 when stratified by study arm). In other words, GM-CSF administration may have preserved CD34+CD14-HLADR-(CD11b+/-) cells to a modest extent, and preservation of these cells in circulation was associated with a failure to respond to the vaccine.
Figure 3. GMCSF administration is associated with preservation of CD34+ CD14-HLADR-and CD34+ CD14-HLADR- CD11b+ cells.
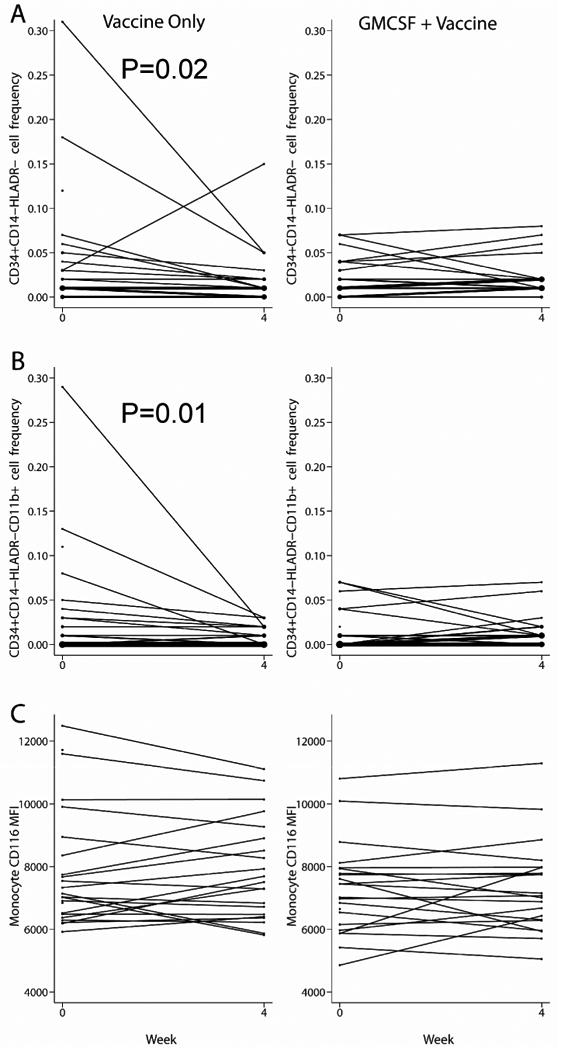
Baseline and week 4 peripheral blood frequencies of CD34+ CD14-HLADR- (panel A) and CD34+ CD14-HLADR- CD11b+ (panel B) cells are shown. GM-CSF receptor (CD116) expression as represented by mean fluorescent intensity (MFI) on monocytes comparing baseline and week 4 for each vaccine arm (panel C). P-values shown are for the change between baseline and week 4 within vaccine only arm. The size of the dot and the thickness of the lines are proportional to the number of subjects.
On the other hand, a decline in the frequency of MDSCs with the CD34+CD14-HLADR-CD11b+ phenotype at week 4 was associated with a week 4 nonresponse to vaccine (p=0.03; p=0.16 when stratified by study arm). While there were few week 4 vaccine responders (8 responders total [13]), it appears that the relation between flux in cell subset frequency and vaccine response is complex and differs depending on time point of response analysis, with a potential delay in effect. Nonetheless, it appears that GM-CSF administration selectively alters peripheral myeloid subset frequencies.
Evaluating frequencies of a different phenotype of MDSCs, CD14+HLADR-CD11b+, we found no statistically significant difference between the study arms, nor between vaccine responders and nonresponders. We also measured GM-CSF receptor (CD116) expression on monocytes and myeloid suppressor cells at baseline and week 4 of the HBV vaccine protocol. Similarly, there was no change in GM-CSF receptor expression from baseline to week 4 in either study arm (Figure 3C for Monocytes: p=0.9 in the Vaccine only arm, p=0.8 in the GM-CSF arm; p=0.9 for the comparison between the study arms), or between week 4 vaccine responders and nonresponders (p=0.9 in both responders and nonresponders; p=0.7 for the comparison between responders and nonresponders, p=0.6 when stratified by study arm; and p>0.1 for all CD14+HLADR-CD11b+ comparisons, not shown).
Discussion
In the setting of HIV infection, vaccination-induced response to neoantigen has been best predicted by baseline naïve CD4+ cell number, decrease in HIV level after initiation of HIV therapy, nadir CD4+ cell numbers, and increased proportions of CD4+ cells expressing CD28[6, 8]. Similarly, in uninfected individuals, younger age and greater frequency of naïve CD4+ cell number are associated with better responses to HBV immunization[22]. The numbers and function of antigen presenting cells and B cells also might predictably determine vaccine responses in HIV infection. Since antigen presenting cells and their precursors are responsive to GM-CSF, we focused on these cells in the present study. We observed week 16 antibody response associations with greater baseline frequencies of peripheral blood CD34+ progenitor cells and lower frequencies of CD14+ monocytes. A decline in the frequency of circulating CD34+CD14-HLADR- MDSCs was also associated with week 16 antibody response, though this relationship was complex and was not supported by the actual numbers of these cells at any time point measured. The latter observation may be due to spurious higher values of such cells at baseline in the vaccine responder group with regression to mean effect, or due to a delayed effect of flux in frequencies of these cells on vaccine response. Analysis of these cells at a later time point such as week 16 may have helped clarify this issue. Finally, we observed no effect on the expression of GM-CSF receptor (CD116) on these cell populations.
While GM-CSF is known to be secreted by activated T and B cells and can mature antigen presenting cells to better drive T cell responses to T cell receptor stimulation, the specific mechanism whereby GM-CSF enhanced responses to HBV immunization in prior studies is not clear. One study that evaluated antigen presenting cell numbers and function in the setting of GM-CSF co-administered with HBV vaccine found decreased circulating dendritic cell numbers to be associated with vaccine response [23]. This finding was postulated to reflect dendritic cell re-distribution and participation in the immune response to vaccine at the site of inflamed tissue.
In the setting of HIV infection, one study with HBV vaccine utilized lower dose GMCSF (20mcg) [12] than that administered in the present A5220 study (250mcg), and GMCSF was observed to enhance vaccine response. In that same study, GMCSF was dosed only once during the HBV vaccine course as compared to with each vaccine dose administered in A5220. Whether the dose of GM-CSF and/or frequency of dosing may account for mixed effectiveness in different clinical trials and a failure to induce the desired effect in A5220 is unclear. In this regard, higher dose GM-CSF may have paradoxical or mixed effects as compared to lower dose GM-CSF[24]. It has been proposed that lower dose and infrequent dosing of GM-CSF results in enhanced DC numbers and function, while higher doses induce MDSCs [24]. It is therefore possible that higher and more frequent GM-CSF dosing in A5220, as compared to the previous study of GMCSF and HBV vaccine in HIV infected individuals, contributed to preservation of a suppressor cell population that inhibited vaccine response. The observation of GM-CSF arm preservation of peripheral CD34+CD14-HLADR- cell frequency could be consistent with such a notion. Alternatively, an induced T cell suppressive phenotype could also be consistent.
While this is an exploratory analysis based on small number of subjects and the results should be interpreted with caution, the findings are consistent with progenitor cells playing a positive role in vaccine response, while monocytes may contribute to a suppressive milieu. The conclusions were consistent using achievement of vaccine response defined as HBsAb≥10mIU/mL and also as quantitative HBsAb titers. Additionally, in the setting of GM-CSF, preservation of myeloid cells with a T cell suppressive phenotype may contribute to blockade of a successful immune response to vaccine. Notably, here we have only studied a select few defined MDSC phenotypes, and have not evaluated myeloid suppressor function. Furthermore, suppressor T cell function has also been implicated in HBV vaccine response failure[25], and we have not evaluated T cell function in the present study. Certainly, frequencies of other phenotypically defined myeloid suppressor cells in conjunction with analysis of CD34+progenitor cells, monocytes, T cells, and cytokines that modulate the function of these cells in relation to vaccine response in the presence and absence of HIV infection is further warranted.
Acknowledgments
The authors thank all of the study participants who have devoted their time and effort to this research.
Grant support: This research was supported in part by the AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases (Grants: AI 68636 and AI 68634), the Case ACTG ISL, NIH R01 DK068361 to DDA, SDAC: AI038855 and AI 068634, NYU: AI069532, UCSF:AI, Wash U:AI069495
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hilleman MR. Overview of the pathogenesis, prophylaxis and therapeusis of viral hepatitis B, with focus on reduction to practical applications. Vaccine. 2001 Feb 28;19(15-16):1837–48. doi: 10.1016/s0264-410x(00)00364-9. [DOI] [PubMed] [Google Scholar]
- 2.Ada G. Overview of vaccines. Mol Biotechnol. 1997 Oct;8(2):123–34. doi: 10.1007/BF02752256. [DOI] [PubMed] [Google Scholar]
- 3.Egea E, Iglesias A, Salazar M, Morimoto C, Kruskall MS, Awdeh Z, et al. The cellular basis for lack of antibody response to hepatitis B vaccine in humans. J Exp Med. 1991 Mar 1;173(3):531–8. doi: 10.1084/jem.173.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess G, Clemens R, Bienzle U, Schonfeld C, Schunck B, Bock HL. Immunogenicity and safety of an inactivated hepatitis A vaccine in anti-HIV positive and negative homosexual men. J Med Virol. 1995 May;46(1):40–2. doi: 10.1002/jmv.1890460109. [DOI] [PubMed] [Google Scholar]
- 5.Kemper CA, Haubrich R, Frank I, Dubin G, Buscarino C, McCutchan JA, et al. Safety and immunogenicity of hepatitis A vaccine in human immunodeficiency virus-infected patients: a double-blind, randomized, placebo-controlled trial. J Infect Dis. 2003 Apr 15;187(8):1327–31. doi: 10.1086/374562. [DOI] [PubMed] [Google Scholar]
- 6.Lange CG, Lederman MM, Medvik K, Asaad R, Wild M, Kalayjian R, et al. Nadir CD4+T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. Aids. 2003 Sep 26;17(14):2015–23. doi: 10.1097/00002030-200309260-00002. [DOI] [PubMed] [Google Scholar]
- 7.Tilzey AJ, Palmer SJ, Harrington C, O'Doherty MJ. Hepatitis A vaccine responses in HIV-positive persons with haemophilia. Vaccine. 1996 Aug;14(11):1039–41. doi: 10.1016/0264-410x(96)00056-4. [DOI] [PubMed] [Google Scholar]
- 8.Valdez H, Smith KY, Landay A, Connick E, Kuritzkes DR, Kessler H, et al. Response to immunization with recall and neoantigens after prolonged administration of an HIV-1 protease inhibitor-containing regimen. ACTG 375 team. AIDS Clinical Trials Group. Aids. 2000 Jan 7;14(1):11–21. doi: 10.1097/00002030-200001070-00002. [DOI] [PubMed] [Google Scholar]
- 9.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006 Aug 14-28;166(15):1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 10.Jha R, Lakhtakia S, Jaleel MA, Narayan G, Hemlatha K. Granulocyte macrophage colony stimulating factor (GM-CSF) induced sero-protection in end stage renal failure patients to hepatitis B in vaccine non-responders. Ren Fail. 2001 Sep;23(5):629–36. doi: 10.1081/jdi-100107359. [DOI] [PubMed] [Google Scholar]
- 11.Kapoor D, Aggarwal SR, Singh NP, Thakur V, Sarin SK. Granulocyte-macrophage colony-stimulating factor enhances the efficacy of hepatitis B virus vaccine in previously unvaccinated haemodialysis patients. J Viral Hepat. 1999 Sep;6(5):405–9. doi: 10.1046/j.1365-2893.1999.00180.x. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki MG, Foccacia R, de Messias-Reason IJ. Efficacy of granulocyte-macrophage colony-stimulating factor (GM-CSF) as a vaccine adjuvant for hepatitis B virus in patients with HIV infection. Vaccine. 2003 Nov 7;21(31):4545–9. doi: 10.1016/s0264-410x(03)00500-0. [DOI] [PubMed] [Google Scholar]
- 13.Overton ET, Kang M, Peters MG, Umbleja T, Alston-Smith BL, Bastow B, et al. Immune response to hepatitis B vaccine in HIV-infected subjects using granulocyte-macrophage colony-stimulating factor (GM-CSF) as a vaccine adjuvant: ACTG study 5220. Vaccine. 2010 Jun 22;28(34):5597–604. doi: 10.1016/j.vaccine.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blom B, Res P, Noteboom E, Weijer K, Spits H. Prethymic CD34+ progenitors capable of developing into T cells are not committed to the T cell lineage. J Immunol. 1997 Apr 15;158(8):3571–7. [PubMed] [Google Scholar]
- 15.Szabolcs P, Avigan D, Gezelter S, Ciocon DH, Moore MA, Steinman RM, et al. Dendritic cells and macrophages can mature independently from a human bone marrow-derived, post-colony-forming unit intermediate. Blood. 1996 Jun 1;87(11):4520–30. [PubMed] [Google Scholar]
- 16.Lathers DM, Lubbers E, Beal NM, Wright MA, Young MR. Cultures derived from peripheral blood CD34+ progenitor cells of head and neck cancer patients and from cord blood are functionally different. Hum Immunol. 1999 Dec;60(12):1207–15. doi: 10.1016/s0198-8859(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 17.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995 Jan;1(1):95–103. [PubMed] [Google Scholar]
- 18.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009 Mar;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007 Jun 20;25(18):2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 20.Lehner T. Special regulatory T cell review: The resurgence of the concept of contrasuppression in immunoregulation. Immunology. 2008 Jan;123(1):40–4. doi: 10.1111/j.1365-2567.2007.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahnke K, Bedke T, Enk AH. Regulatory conversation between antigen presenting cells and regulatory T cells enhance immune suppression. Cell Immunol. 2007 Nov-Dec;250(1-2):1–13. doi: 10.1016/j.cellimm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Looney RJ, Hasan MS, Coffin D, Campbell D, Falsey AR, Kolassa J, et al. Hepatitis B immunization of healthy elderly adults: relationship between naive CD4+ T cells and primary immune response and evaluation of GM-CSF as an adjuvant. J Clin Immunol. 2001 Jan;21(1):30–6. doi: 10.1023/a:1006736931381. [DOI] [PubMed] [Google Scholar]
- 23.Verkade MA, van de Wetering J, Klepper M, Vaessen LM, Weimar W, Betjes MG. Peripheral blood dendritic cells and GM-CSF as an adjuvant for hepatitis B vaccination in hemodialysis patients. Kidney Int. 2004 Aug;66(2):614–21. doi: 10.1111/j.1523-1755.2004.00781.x. [DOI] [PubMed] [Google Scholar]
- 24.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007 Feb;18(2):226–32. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T, Yamauchi K, Kuwata T, Hayashi N. Characterization of hepatitis B virus surface antigen-specific CD4+ T cells in hepatitis B vaccine non-responders. J Gastroenterol Hepatol. 2001 Aug;16(8):898–903. doi: 10.1046/j.1440-1746.2001.02530.x. [DOI] [PubMed] [Google Scholar]


