Abstract
Methylmercury (MeHg) is a ubiquitous environmental contaminant with known neurodevelopmental effects. In humans, prenatal exposures primarily occur through maternal consumption of contaminated fish. In this study, we evaluated the association between prenatal exposure to MeHg and titers of total immunoglobulins (Ig) and specific autoantibodies in both mothers and fetuses by analyzing maternal and cord blood serum samples. We examined multiple immunoglobulin isotypes to determine if these biomarkers could inform as to fetal or maternal responses since IgG but not IgM can cross the placenta. Finally, we evaluated serum cytokine levels to further characterize the immune response to mercury exposure.
The study was conducted using a subset of serum samples (N=61 pairs) collected from individuals enrolled in a population surveillance of MeHg exposures in the Brazilian Amazon during 2000/2001. Serum titers of antinuclear and antinucleolar autoantibodies were measured by indirect immunofluorescence. Serum immunoglobulins were measured by enzyme-linked immunosorbent assay (ELISA) and BioPlex multiplex assay. Serum cytokines were measured by BioPlex multiplex assay.
In this population, the geometric mean mercury level was within the 95th percentile for US populations of women of childbearing age but the upper level of the range was significantly higher. Fetal blood mercury levels were higher (1.35 times) than those in their mothers, but highly correlated (correlation coefficient [r]=0.71; 95% CI: 0.54, 0.89). Total IgG (r=0.40; 95% CI: 0.19, 0.62) and antinuclear autoantibody (odds ratio [OR]=1.05; 95% CI: 1.02, 1.08) levels in paired maternal and fetal samples were also associated; in contrast, other immunoglobulin (IgM, IgE, and IgA) levels were not associated between pairs. Total IgG levels were significantly correlated with both maternal (r=0.60; 95% CI: 0.25, 0.96) and cord blood mercury levels (r=0.61; 95% CI: 0.25, 0.97), but individual isotypes were not. Serum cytokines, interleukin-1β (r=0.37; 95% CI: 0.01, 0.73), interleukin-6 (r=0.34; 95% CI: 0.03, 0.65), and tumor necrosis factor-α (r=0.24; 95% CI: 0.015, 0.47), were positively correlated between maternal and fetal samples. Antinuclear and antinucleolar autoantibody titer and serum cytokine levels, in either maternal or cord blood, were not significantly associated with either maternal or cord blood mercury levels.
These data provide further evidence that there are likely IgG biomarkers of mercury-induced immunotoxicity in this population since IgG levels were elevated with increased, and associated with, mercury exposure. However, unlike previous data from adult males and non-pregnant females, we found no evidence that antinuclear and antinucleolar autoantibody titer is a reliable biomarker of mercury immunotoxicity in this population.
Keywords: Autoimmune, mercury, fetal, immunotoxicity
1. Introduction
Mercury is a ubiquitous pollutant with well-characterized neurodevelopmental toxicity (NRC 2000). Globally, human exposures to mercury are primarily to methylmercury (MeHg) through consumption of contaminated fish (Mahaffey and Mergler 1998; NRC 2000). Exposures during prenatal and perinatal development have been of concern because mercury can cross the placenta (Kajiwara et al. 1996; Yoshida 2002) and is measurable in the breast milk of exposed women (Boischio and Henshel 2000; Grandjean et al. 2003). Low level prenatal exposures have developmental neurological effects in animal models (Newland and Reile 1999; Newland et al. 2008) and in humans (NRC 2000).
A large experimental literature on several mercury compounds indicates that these compounds are also immunotoxic; they can inhibit immune response to infections (Ilback et al. 1996; Silbergeld et al. 2000; Silbergeld et al. 2005) and also induce or exacerbate autoimmunity (Hultman and Hansson-Georgiadis 1999; Pollard et al. 2001; Via et al. 2003). In animal models, prenatal and perinatal exposures to mercury are associated with persistent alterations in the immune response of offspring (Thuvander et al. 1996; Silva et al. 2005a; Silva et al. 2005b).
In humans, there has been less research and thus less evidence associating mercury compounds with immunotoxicity. In adults there are reports of increased circulating levels of autoantibodies (Silva et al. 2004; Alves et al. 2006; Gardner et al. 2010b) and increased risks of autoimmune disease (Cooper et al. 2004). We have also reported on mercury-induced changes in the cytokine profile produced in vitro by stimulated human peripheral blood mononuclear cells (Gardner et al. 2009; Gardner et al. 2010a). Specifically, inorganic and MeHg induced adult peripheral blood mononuclear cells to produce a pro-inflammatory cytokine response without inducing a protective anti-inflammatory response. However, at present very little is known of the immunotoxic effects in fetuses or infants associated with prenatal exposures to low level MeHg. This research has been limited in part by the need to use sensitive and specific biomarkers of immunotoxic effects of mercury that can distinguish between maternal and fetal responses.
Antinuclear and antinucleolar autoantibodies are biomarkers utilized in the diagnosis of certain autoimmune diseases, including systemic lupus erythematosus (Tan et al. 1982). We and others have previously shown that exposure to mercury increases the prevalence and titers of detectable antinuclear/antinucleolar autoantibodies in communities in the Brazilian Amazon (Silva et al. 2004; Alves et al. 2006; Gardner et al. 2010b). In addition, we have recently reported on changes in serum cytokines among persons exposed to mercury compounds in Brazil (Gardner et al. 2010b), specifically pointing to an induction of a pro-inflammatory response. The populations studied previously included adults who eat fish and are, therefore, exposed to MeHg but did not include pregnant females or children. In this study, we examined paired blood samples from a population of mothers and infants recruited from the same region in the Brazilian Amazon as in our previous studies. The mothers were exposed to MeHg through fish consumption in which both rates of fish consumption and levels of MeHg have been measured (Santos et al. 2000) (Santos EC, personal communication). Based on our previous studies, we hypothesize that antinuclear/antinucleolar autoantibody titer may be a biomarker of mercury-induced immune dysfunction and that changes in pro-inflammatory cytokine levels may also be associated with mercury exposures. In this study we measured two main biomarkers of immunotoxicity: immunoglobulin and cytokine level. Within the immunoglobulin component, we measured total immunoglobulin (including individual immunoglobulin isotypes: IgG1, IgG2, IgG3, IgG4, IgM, IgA, and IgE) and antigen-specific immunoglobulin (antinuclear/antinucleolar autoantibodies). We measured total antinuclear/antinucleolar autoantibodies (IgG+IgA+IgM) and antinuclear/antinucleolar IgG to determine whether the immunotoxic effects occur independently in both the mother and the fetus or whether mercury immunotoxicity is induced in the mother and then passed from the mother to the fetus.
2. Materials and methods
2.1. Study population
The study utilized serum samples collected as part of a regional population surveillance program of maternal mercury exposures in the Brazilian Amazon undertaken by the Brazilian National Health Service (Santos et al. 2000) in 2000/2001. The surveillance program included a total of 1510 maternal and fetal pairs, representing about 50% of all births in the three local hospitals of the municipality of Itaituba during the enrollment period. Women entering the maternity clinics at these hospitals in Itaituba, a regional health care center in the mid-Para region of Amazonia, were invited to participate in a study of mercury exposures and maternal-fetal responses to mercury. For this study, a random sample of serum aliquots was analyzed from 61 singleton births and their mothers; these were the only samples made available for shipment to our labs in the US from the parent study. The samples were randomly selected and, based on published data from the full cohort (Santos et al. 2007), are representative of the parent cohort.
Full details of the original study were published by Santos et al (2007). The subjects were enrolled upon entering the maternity hospital and informed consent was obtained in writing (in Portuguese) through a protocol approved by the Fundaçao Nacional de Saúde of Brazil. Subjects were excluded for severe illness of the mother or significant complications at the time of labor (such as emergency surgery), stillbirth, and severe illness of the infant at birth.
2.2. Interview of study participants
Participant interviews were conducted by an epidemiologist in the maternity ward within four days after delivery. The questionnaire, in Portuguese, collected information on sociodemographic variables (age, residence, occupation, education, family size, social strata), medical and reproductive history, recent infections, smoking, alcohol and drug use, and diet. Further information on maternal medical history, maternal health during pregnancy, medications administered during labor and delivery, sex of the infant, birth weight, and infant status at delivery were obtained from the hospital medical records.
2.3. Blood sample collection
Cord and maternal blood samples were collected in the delivery room using procedures developed for field studies (Grandjean et al. 1999; Santos et al. 2000). Blood samples were collected from the peripheral forearm vein from mothers and from umbilical cord using disposable syringes. Serum was isolated from whole blood and stored at −80C until shipment on dry ice to our laboratory for further analyses, as previously described (Silva et al. 2004; Gardner et al. 2010b). All sample identifications for this study were coded and datasets were de-identified; therefore, analyses in our laboratory were masked and performed blinded as to mercury level until statistical analyses were conducted.
2.4. Mercury analyses
Total blood mercury was measured by standard cold vapor atomic absorption spectrophotometry and is reported in ppb (μg/L). Mercury measurements were conducted by a laboratory of the Evandro Chagas Institute that participates in an international quality assurance/quality control program with the Université de Québec à Montréal.
2.5. Immunoglobulin analyses
Antibody analyses were conducted in our laboratory at Johns Hopkins and University of South Carolina. IgG1, IgG2, IgG3, IgG4, IgM, IgE, and IgA were measured by BioPlex multiplex bead-based assay (Bio-Rad) according to manufacturer’s protocols. Total IgG was measured by ELISA (Zeptometrix) according to manufacturer’s instructions. The concentration of serum total immunoglobulins is expressed as mg/ml based on the standard curve.
2.6. Antinuclear and antinucleolar autoantibody analyses
Antinuclear and antinucleolar autoantibody titers were measured by indirect immunofluorescence microscopy using commercially available slides prepared from human epithelial cells (HEp-2) as substrate (INOVA Diagnostics) following the methods of Burek and Rose (Burek and Rose 1995). The slides were stored in the dark at 4C until they were analyzed by a blinded reader. All sera were tested in serial, two-fold dilutions, starting at 1:10. The inverse of the highest dilution at which fluorescence could still be detected was defined as the titer. A negative result at the lowest dilution (1:10) was defined as a titer of zero. Antinuclear and antinucleolar autoantibody data are expressed as the inverse of the highest titer, or the dilution factor, at which fluorescence could still be detected.
2.7. Serum cytokine analyses
Serum cytokines were measured by BioPlex multiplex bead-based assay (Bio-Rad) according to manufacturer’s protocols for the following eight cytokines: interleukin (IL)-1β, interleukin-1 receptor agonist (IL-1ra), interleukin-4, interleukin-6, interleukin-10, interleukin-17, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α. Limits of detection were as follows (in pg/ml): IL-1β= 1.9, IL-1ra= 3.07, IL-4= 0.19, IL-6= 1.52, IL-10= 1.49, IL-17= 1.49, IFN-γ= 1.44, TNF-α= 1.47.
2.7. Statistical analyses
Means for continuous variables (median for variables with skewed distributions) and percents for categorical variables were computed for the descriptive analysis in our data. Mercury, total immunoglobulin, and cytokine levels were log-transformed for statistical analyses as the distributions of these three, as expected, were not normal. Multivariate linear regression modeling was used to evaluate the serum total immunoglobulin and cytokine levels, while controlling for age, education level, and residence. Multivariate logistic regression modeling was used to evaluate the prevalence of antinuclear/antinucleolar autoantibodies (for 1:10 and 1:40 cutoffs), while controlling for age, education level, and residence. These titer cutoffs were chosen on the basis of the clinical literature (Tan et al. 1997). Detection at a dilution or titer of ≥1:40 is considered clinically meaningful in the context of evaluating autoimmunity; however, detection of titers between 1:10 and 1:40 represents an elevation above levels reported in healthy individuals, and may also have health implications. The covariates were included in analyses and are relevant to exposure: age is associated with duration of exposure, education level has been associated with rates of fish consumption in this population, and residence is also associated with exposure to fish as well as to other potential sources of mercury such as gold burning (in towns) or fossil fuel combustion.
3. Results
3.1. Subjects
Sixty-one females with singleton births delivered at three hospitals in Itaituba, Brazil and were selected for this study. The demographic characteristics of these subjects are shown in Table 1. Eighty-four percent of the mothers stated that they lived in Itaituba. The mean age of the mothers was 21 years (range: 13 to 37 years). Mean infant weight at birth was 3.2 kg (range: 2.1 to 4.1). The geometric mean maternal blood mercury level was 6.90 μg/L (range: 0.08 to 55.48) while that of cord blood mercury was 9.63 μg/L (range: 0.08 to 77.80). Maternal and cord blood mercury levels were highly correlated (r=0.71; 95% CI: 0.54, 0.89), but not equivalent, as cord blood levels were significantly higher (1.35 times) than maternal levels (data not shown). Table 1 shows the geometric mean of fetal mercury, immunoglobulin, and autoantibody titers by maternal demographic characteristics.
Table 1.
Fetal mercury, immunoglobulin, and antinuclear autoantibody levels by maternal characteristics.
| Cord blood mercury (log transformed) | Cord blood total IgG | Cord blood total ANA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Maternal characteristic | (n) | GM | 95% CI | (n) | GM | 95% CI | (n) | mean | 95% CI |
| Blood Hg | |||||||||
| 1st tertile | 18 | 0.81 | (0.48, 1.38) | 19 | 6.03 | (2.25, 16.15) | 21 | 14.29 | (−1.97, 30.54) |
| 2nd tertile | 19 | 1.82 | (1.63, 2.05) | 20 | 14.17 | (6.37, 31.53) | 20 | 23.50 | (−10.43, 57.43) |
| 3rd tertile | 20 | 2.32 | (1.96, 2.75) | 19 | 17.55 | (7.09, 43.44) | 20 | 4.50 | (1.29, 7.71) |
| Age | |||||||||
| <18 | 21 | 1.65 | (1.30, 2.10) | 21 | 12.05 | (5.56, 26.12) | 23 | 17.39 | (−11.27, 46.05) |
| 18–24 | 19 | 1.99 | (1.63, 2.43) | 21 | 12.65 | (4.73, 33.86) | 21 | 11.91 | (2.73, 21.08) |
| >25 | 17 | 1.05 | (0.57, 1.90) | 16 | 9.50 | (3.44, 26.23) | 17 | 12.35 | (−7.85, 32.56) |
| Education | |||||||||
| None | 11 | 1.60 | (1.16, 2.20) | 10 | 5.93 | (1.64, 21.35) | 11 | 36.35 | (−27.74, 100.47) |
| Primary | 39 | 1.52 | (1.15, 2.01) | 37 | 14.73 | (8.36, 25.93) | 39 | 10.26 | (1.10, 19.41) |
| Secondary | 11 | 1.54 | (0.80, 2.93) | 11 | 9.09 | (1.60, 51.54) | 11 | 5.46 | (−3.23, 14.14) |
| Child’s sex | |||||||||
| Male | 29 | 1.56 | (1.06, 2.30) | 26 | 13.27 | (5.97, 29.52) | 29 | 21.72 | (−2.95, 46.40) |
| Female | 32 | 1.51 | (1.22, 1.87) | 32 | 10.21 | (5.22, 20.00) | 32 | 7.19 | (1.10, 13.28) |
| Residence | |||||||||
| Itaituba | 23 | 1.74 | (1.29, 2.34) | 21 | 12.00 | (4.56, 31.56) | 23 | 6.96 | (1.67, 12.24) |
| Belem | 13 | 1.50 | (1.08, 2.09) | 13 | 5.43 | (1.50, 19.69) | 13 | 34.62 | (−18.85, 88.08) |
| Other | 18 | 1.84 | (1.39, 2.44) | 18 | 19.88 | (9.72, 40.66) | 5 | 34.00 | (−53.62, 121.62) |
| Sao Luis | 9 | 0.55 | (0.06, 4.81) | 4 | 3.96 | (0.61, 25.75) | 18 | 4.44 | (−1.01, 9.90) |
GM: geometric mean; ANOVA for means comparisons. Total blood Hg levels presented as log transformed ppm; total IgG levels presented as mg/ml; total antinuclear autoantibody (ANA) values presented as inverse titer.
The level of cord blood mercury significantly increased with maternal blood mercury level. Maternal (r=0.54; 95% CI: 0.10, 0.97) but not fetal (r=0.36; 95% CI: −0.07, 0.80) blood mercury levels were positively correlated to the number of fish consumed per day (Table 2). Occupation and area of residence were not associated with elevated maternal blood mercury level and none of the participants listed a current or past involvement in the artisanal gold mining industry (a primary source of direct mercury exposure (either inhalation of vapor or absorption through the skin) in this region of Brazil).
Table 2.
Associations between fetal and maternal biomarkers.
| Unadjusted associations |
||||||
|---|---|---|---|---|---|---|
| Maternal | ||||||
| Fetal | mercury | fish meals per day | Total IgG | Total IgM | Total ANA | ANA IgG |
| mercury | 0.71 (0.54, 0.89) | 0.36 (−0.07, 0.80) | ||||
| maternal fish meals per day | 0.54 (0.10, 0.97) | |||||
| Total IgG | 0.60 (0.25, 0.96) | 0.40a (0.19, 0.62)b | ||||
| Total IgM | 0.001 (−0.05, 0.06) | 0.02 (−0.10, 0.14) | ||||
| Total ANA | 0.70c (0.45, 1.09) | 1.05c (1.02, 1.08) | ||||
| ANA IgG | 0.67c (0.43, 1.05) | 1.04c (1.00, 1.08) | ||||
| Adjusted associations | ||||||
|---|---|---|---|---|---|---|
| Maternal | ||||||
| Fetal | mercury | fish meals per day | Total IgG | Total IgM | Total ANA | ANA IgG |
| mercury | 0.71 (0.53, 0.89) | 0.35 (−0.12, 0.81) | ||||
| maternal fish meals per day | 0.58 (0.11, 1.04) | |||||
| Total IgG | 0.61 (0.21, 1.02) | 0.41 (0.18, 0.64) | ||||
| Total IgM | 0.01 (−0.05, 0.07) | 0.03 (−0.11, 0.16) | ||||
| Total ANA | 0.67c (0.41, 1.10) | 1.05c (1.02, 1.08) | ||||
| ANA IgG | 0.62c (0.37, 1.05) | 1.05c (1.00, 1.09) | ||||
Values reported for adjusted cord blood biomarkers.
r, correlation coefficient;
95% CI;
OR, odds ratio. Lower panel: adjusted for maternal age, education level, and residence. For ease of viewing, significant correlations are included in bolded font and logistic regression analyses are shaded.
3.2. Immunoglobulin levels
We found a positive correlation between maternal and cord blood total IgG (r= 0.40; 95% CI: 0.19, 0.62) (Supplemental figure and Table 2). Total IgG level in cord blood was significantly associated with cord blood (r=0.61; 95% CI: 0.25, 0.97) (Table 2) and maternal mercury level (r=0.60; 95% CI: 0.25, 0.96). Colinearity of the fetal and maternal mercury levels did not allow for including both of these levels in the statistical modeling.
When individual immunoglobulin isotypes (Supplemental figure) were examined, positive correlations were found between maternal and cord blood IgG2 (r=0.50; 95% CI: 0.09, 0.90), IgG3 (r=0.76; 95% CI: 0.60, 0.93), and IgG4 (r=0.69; 95% CI: 0.45, 0.93) but not IgG1. No significant correlation was found between maternal and cord blood IgA, IgE, or IgM.
Table 2 shows the unadjusted correlation coefficient (r) of the indicated cord blood biomarkers by maternal and fetal characteristics (e.g. total fetal IgG by maternal IgG or fetal mercury) in the top panel and correlations adjusted for covariates (age, education, and area of residence) in the bottom panel.
3.3. Autoantibody levels
Antinuclear and antinucleolar autoantibody (total [IgG+IgA+IgM], IgG, and IgM) titers were determined by indirect immunofluorescence. There were no detectable antinuclear IgM levels in cord blood samples. There was only one sample cord blood sample with detectable antinucleolar autoantibody titers (either total [IgG+IgA+IgM] or IgG); therefore further analyses of relationships between antinucleolar autoantibody titers and other factors was not included. We found positive associations between maternal and cord blood total antinuclear autoantibody (OR=1.05; 95% CI: 1.02, 1.08). However, neither total antinuclear autoantibody nor antinuclear IgG titer in either maternal or cord blood was significantly associated with maternal or cord blood mercury levels. Table 2 shows the odds ratio (OR) of cord blood total antinuclear autoantibody and cord blood antinuclear IgG by maternal characteristics and cord blood mercury (both adjusted and unadjusted for covariates) in the shaded sections of the table.
3.4. Cytokine levels
Serum cytokine levels were determined by bead-based multiplex assay. We found a positive correlation between the serum cytokine levels of maternal and cord blood samples (Figure 1) for interleukin-1β (r=0.37; 95% CI: 0.01, 0.73), interleukin-6 (r=0.34; 95% CI: 0.03, 0.65), and tumor necrosis factor-α (r=0.24; 95% CI: 0.02, 0.47). Interleukin-10 and interleukin-17 levels were not detected in the majority of the samples and so statistical analyses were not possible. Interleukin-1ra and interferon-γ were not correlated between maternal and cord blood samples. Neither maternal nor cord blood cytokine levels of any of the cytokines analyzed were significantly associated with either maternal or cord blood mercury levels following multivariate linear regression modeling (data not shown).
Figure 1. Association between cord blood and maternal cytokine levels.
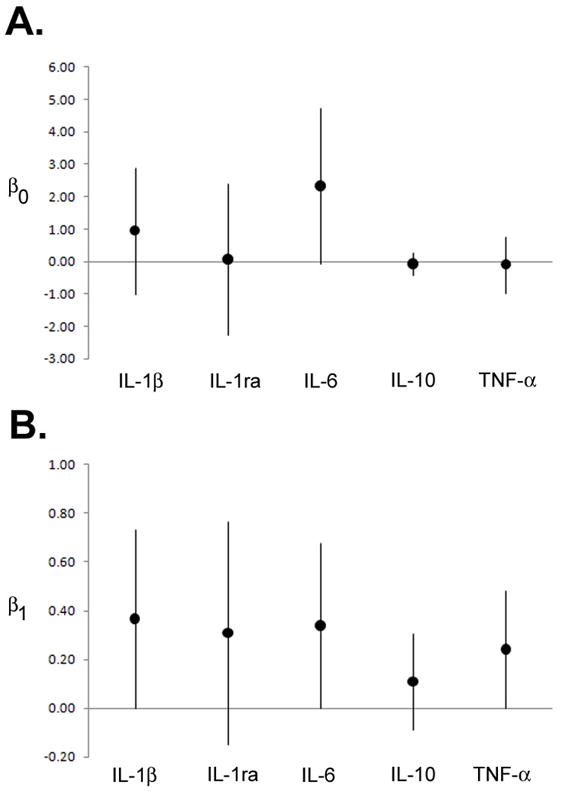
Model estimates [median (95% CI)] for correlation curves derived from log-transformed data for paired maternal and cord blood results for interleukin-1β, interleukin-1ra, interleukin-6, interleukin-10, and tumor necrosis factor-α. (A) β0 is the estimate for the intercept of the predicted line; (B) β1 is the estimate for the slope (log change in pg/ml of cytokine for cord blood per increase in maternal cytokine level). The horizontal axis line indicates zero
4. Discussion
In this study we report on a cross-sectional sample of a prospective mother-infant cohort study conducted in the Brazilian Amazon. This study design allowed for comparison of both exposure and immune biomarkers in both the maternal and fetal compartments. We found a significant correlation between maternal and fetal blood mercury levels, as expected, with fetal mercury levels 1.35 times those of maternal samples. This value is slightly below the mean of 1.86 times reported in a meta-analysis on the topic (Stern and Smith 2003). It is known that mercury can cross the placenta (NRC 2000) and others have reported that blood mercury level is higher in the fetal compartment (Stern and Smith 2003; Sakamoto et al. 2007).
In this study, maternal and cord blood total IgG levels were significantly correlated between paired samples as were isotypes IgG2, IgG3, and IgG4, which is consistent with previous studies that have shown that small immunoglobulins, such as IgG, can pass the placenta (Simister 2003). The finding of associations between elevated blood mercury and increased IgG titers suggests that (1) the maternal immune system is responding to MeHg and that the biomarker of this response, IgG, crosses the placenta to the fetal circulation or (2) that the maternal and fetal immune systems were responding independently and similarly to mercury. We also found, like others, that in the absence of maternal infection (Ben-Hur et al. 2005), larger immunoglobulins, such as IgM, cannot pass the placenta since there was no correlation between total IgM levels in maternal and cord blood. IgM levels were not associated with mercury levels in either maternal or cord blood.
A significant proportion of this population was exposed to increased levels of mercury, indicated by elevated blood mercury levels, primarily through consumption of fish based on questionnaire data (Santos et al 2007). Commonly consumed fish in this region are known to be contaminated by MeHg from gold mining and deforestation activities upstream from Itaituba (Santos et al. 2000; Crompton et al. 2002). These exposures were, for many persons, above the US EPA recommended limit; 36 percent of maternal and 52 percent of cord blood samples were above the US EPA estimated safe level of 5.8 μg/L (NRC 2000). Other studies in this region have reported on neurocognitive effects in children and adults exposed to mercury (Yokoo et al. 2003; Chevrier et al. 2008). This is the first evidence for an effect of these mercury exposures on the immune system of the fetus, as reflected in levels of antibodies in maternal and cord blood. Specifically, total IgG (and some IgG isotypes) levels in cord blood were significantly associated with fetal mercury and maternal mercury level.
We and others have previously reported that antinuclear and antinucleolar autoantibodies may be informative biomarkers of mercury induced immunotoxicity in human populations in Amazonia, including those exposed to MeHg from contaminated fish (Silva et al. 2004; Alves et al. 2006;Gardner et al. 2010b). However, in this study, we did not find an association between antinuclear and antinucleolar autoantibody titers and mercury exposure, as has been reported in studies of adults. This may be due to the differences in exposure or to the responses of pregnant women and fetuses in this study. Alternatively, the lack of association with mercury level may simply be the result of the small sample size. We are planning to undertake further analysis of samples from the larger cohort. However, in our previous studies with similar sample numbers, we observed significant associations between biomarkers of mercury exposures and antinuclear and antinucleolar autoantibody titers. Thus the failure to discern a similar association in this study may reflect differences in the immune responses to mercury among pregnant women and fetuses, but this requires further study. Gestational age is of particular importance for determining the development of the fetal immune system and its capability to respond to antigenic stimuli (Dietert and Zelikoff 2008); unfortunately gestational age of the infants in this study was not included in the database to which we had access. We recognize that this is a major limitation of this study and are attempting to add this parameter to future study designs.
We have also reported that in vitro mercury exposure induces human immune cells, in the presence of stimulation, to produce a pro-inflammatory cytokine response (Gardner et al. 2009; Gardner et al. 2010a). When serum samples from a population involved in small-scale mining in the Brazilian Amazon were analyzed for these same cytokines, similar patterns of pro-inflammatory cytokine levels were observed (Gardner et al. 2010b). We did not observe these same patterns of cytokine levels in the serum from these pregnant women and fetuses in this study. This may be due to differences in the dominant type of mercury involved, inorganic and elemental mercury in the case of miners and MeHg in the case of women in this study. Again, this may also be due to differential immune responses in pregnant women to mercury or to the small sample size of this study.
4.1. Conclusions
The data presented here provide evidence for immunotoxicity of mercury in pregnant women and their fetuses, but suggest that antinuclear and antinucleolar autoantibody titer may not be a specific biomarker for effects in these populations. In order to address this issue, we have begun studies utilizing proteomic technologies to identify biomarkers of mercury exposure for MeHg-exposed populations including this unique cohort of matched maternal and cord blood samples.
Supplementary Material
Scatter plots for paired maternal and cord blood results for (A) IgG1, (B) IgG2, (C) IgG3, (D) IgG4, (E) IgM, and (F) IgA are shown on the natural scale (expressed in mg/ml). The predicted value line fit for each plot (in red) is the overlay with the line expressed as Y=β0 + β1X (p-value) for: (A) Y= 5.16 + 0.345X (p=not significant); (B) Y= 0.234 + 0.233X (p=0.012); (C) Y= 0.276 + 0.384X (p<0.0001); (D) Y= 0.020 + 0.688X (p<0.0001); (E) Y= 0.159 – 0.087X (p=ns); (F) Y= −0.211 + 0.142X (p=ns).
Acknowledgments
The authors would like to thank the research staff of the Evandro Chagas Institute, Belem Brazil, for their work in collecting these samples and in making them available to us; and also Renee M. Gardner, PhD for her advice on the preparation of Figure 1.
Funding sources
This research was supported by award number K99/R00 ES015426 from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health. This research was also supported by the Johns Hopkins Center for a Livable Future.
List of abbreviations
- ANA
antinuclear autoantibody
- ELISA
enzyme-linked immunosorbent assay
- IFN
interferon
- IL
interleukin
- Ig
immunoglobulin
- MeHg
methylmercury
- TNF
tumor necrosis factor
Footnotes
Human subjects research approval
This study was approved and supervised by the institutional review board of Fundaçao Nacional de Saúde, as well as the Committees on Human Research at the Johns Hopkins Bloomberg School of Public Health and University of South Carolina School of Medicine.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Susie B Wang, Email: suswang@jhsph.edu.
Devon L Shirley, Email: devon.landers@uscmed.sc.edu.
Elisabeth O Santos, Email: coehma@amazon.com.br.
Ana Maria Ventura, Email: ana_mariaventura@hotmail.com.
Jose M de Souza, Email: jmsouza@iec.pa.gov.br.
Ellen K Silbergeld, Email: esilberg@jhsph.edu.
References
- Alves MF, Fraiji NA, Barbosa AC, De Lima DS, Souza JR, Dorea JG, Cordeiro GW. Fish consumption, mercury exposure and serum antinuclear antibody in Amazonians. Int J Environ Health Res. 2006;16(4):255–62. doi: 10.1080/09603120600734147. [DOI] [PubMed] [Google Scholar]
- Ben-Hur H, Gurevich P, Elhayany A, Avinoach I, Schneider DF, Zusman I. Transport of maternal immunoglobulins through the human placental barrier in normal pregnancy and during inflammation. Int J Mol Med. 2005;16(3):401–7. [PubMed] [Google Scholar]
- Boischio AA, Henshel DS. Linear regression models of methyl mercury exposure during prenatal and early postnatal life among riverside people along the upper Madeira river, Amazon. Environ Res. 2000;83(2):150–61. doi: 10.1006/enrs.2000.4050. [DOI] [PubMed] [Google Scholar]
- Burek CL, Rose NR. Autoantibodies. In: Colvin R, Bhan A, McCluskey R, editors. Diagnostic Immunopathology. New York: Raven Press; 1995. pp. 207–230. [Google Scholar]
- Chevrier C, Sullivan K, White RF, Comtois C, Cordier S, Grandjean P. Qualitative assessment of visuospatial errors in mercury-exposed Amazonian children. Neurotoxicology. 2008 doi: 10.1016/j.neuro.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Dooley MA. Occupational risk factors for the development of systemic lupus erythematosus. J Rheumatol. 2004;31(10):1928–33. [PubMed] [Google Scholar]
- Crompton P, Ventura AM, de Souza JM, Santos E, Strickland GT, Silbergeld E. Assessment of mercury exposure and malaria in a Brazilian Amazon riverine community. Environ Res. 2002;90(2):69–75. doi: 10.1006/enrs.2002.4358. [DOI] [PubMed] [Google Scholar]
- Dietert RR, Zelikoff JT. Early-life environment, developmental immunotoxicology, and the risk of pediatric allergic disease including asthma. Birth Defects Res B Dev Reprod Toxicol. 2008;83(6):547–60. doi: 10.1002/bdrb.20170. [DOI] [PubMed] [Google Scholar]
- Gardner RM, Nyland JF, Evans SL, Wang SB, Doyle KM, Crainiceanu CM, Silbergeld EK. Mercury induces an unopposed inflammatory response in human peripheral blood mononuclear cells in vitro. Environ Health Perspect. 2009;117(12):1932–8. doi: 10.1289/ehp.0900855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RM, Nyland JF, Silbergeld EK. Differential immunotoxic effects of inorganic and organic mercury species in vitro. Toxicology Letters. 2010a;198(2):182–190. doi: 10.1016/j.toxlet.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RM, Nyland JF, Silva IA, Ventura AM, de Souza JM, Silbergeld EK. Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brasil: a cross-sectional study. Environ Res. 2010b;110(4):345–354. doi: 10.1016/j.envres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jorgensen E, Steuerwald U, Heinzow B, Needham LL, Jorgensen PJ, Weihe P. Attenuated growth of breast-fed children exposed to increased concentrations of methylmercury and polychlorinated biphenyls. Faseb J. 2003;17(6):699–701. doi: 10.1096/fj.02-0661fje. [DOI] [PubMed] [Google Scholar]
- Grandjean P, White RF, Nielsen A, Cleary D, de Oliveira Santos EC. Methylmercury neurotoxicity in Amazonian children downstream from gold mining. Environ Health Perspect. 1999;107(7):587–91. doi: 10.1289/ehp.99107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman P, Hansson-Georgiadis H. Methyl mercury-induced autoimmunity in mice. Toxicol Appl Pharmacol. 1999;154(3):203–11. doi: 10.1006/taap.1998.8576. [DOI] [PubMed] [Google Scholar]
- Ilback NG, Wesslen L, Fohlman J, Friman G. Effects of methyl mercury on cytokines, inflammation and virus clearance in a common infection (coxsackie B3 myocarditis) Toxicol Lett. 1996;89(1):19–28. doi: 10.1016/s0378-4274(96)03777-0. [DOI] [PubMed] [Google Scholar]
- Kajiwara Y, Yasutake A, Adachi T, Hirayama K. Methylmercury transport across the placenta via neutral amino acid carrier. Arch Toxicol. 1996;70(5):310–4. doi: 10.1007/s002040050279. [DOI] [PubMed] [Google Scholar]
- Mahaffey KR, Mergler D. Blood levels of total and organic mercury in residents of the upper St. Lawrence River basin, Quebec: association with age, gender, and fish consumption. Environ Res. 1998;77(2):104–14. doi: 10.1006/enrs.1998.3834. [DOI] [PubMed] [Google Scholar]
- Newland MC, Paletz EM, Reed MN. ‘Methylmercury and nutrition: adult effects of fetal exposure in experimental models. Neurotoxicology. 2008;29(5):783–801. doi: 10.1016/j.neuro.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, Reile PA. Blood and brain mercury levels after chronic gestational exposure to methylmercury in rats. Toxicol Sci. 1999;50(1):106–16. doi: 10.1093/toxsci/50.1.106. [DOI] [PubMed] [Google Scholar]
- NRC. Toxicological effects of methyl mercury. Washington, DC: National Academy Press; 2000. [Google Scholar]
- Pollard KM, Pearson DL, Hultman P, Deane TN, Lindh U, Kono DH. Xenobiotic acceleration of idiopathic systemic autoimmunity in lupus- prone bxsb mice. Environ Health Perspect. 2001;109(1):27–33. doi: 10.1289/ehp.0110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M, Kaneoka T, Murata K, Nakai K, Satoh H, Akagi H. Correlations between mercury concentrations in umbilical cord tissue and other biomarkers of fetal exposure to methylmercury in the Japanese population. Environ Res. 2007;103(1):106–11. doi: 10.1016/j.envres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Santos EC, I, Jesus M, Brabo ES, Loureiro EC, Mascarenhas AF, Weirich J, Camara VM, Cleary D. Mercury exposures in riverside Amazon communities in Para, Brazil. Environ Res. 2000;84(2):100–7. doi: 10.1006/enrs.2000.4088. [DOI] [PubMed] [Google Scholar]
- Santos EO, Jesus IM, Camara Vde M, Brabo Eda S, Jesus MI, Fayal KF, Asmus CI. Correlation between blood mercury levels in mothers and newborns in Itaituba, Para State, Brazil. Cad Saude Publica. 2007;23(Suppl 4):S622–9. doi: 10.1590/s0102-311x2007001600022. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, Sacci JB, Jr, Azad AF. Mercury exposure and murine response to Plasmodium yoelii infection and immunization. Immunopharmacol Immunotoxicol. 2000;22(4):685–95. doi: 10.3109/08923970009016432. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, I, Silva A, Nyland JF. Mercury and autoimmunity: implications for occupational and environmental health. Toxicol Appl Pharmacol. 2005;207(2 Suppl):282–92. doi: 10.1016/j.taap.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Silva IA, El Nabawi M, Hoover D, Silbergeld EK. Prenatal HgCl2 exposure in BALB/c mice: gender-specific effects on the ontogeny of the immune system. Dev Comp Immunol. 2005a;29(2):171–83. doi: 10.1016/j.dci.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Silva IA, Graber J, Nyland JF, Silbergeld EK. In vitro HgCl2 exposure of immune cells at different stages of maturation: effects on phenotype and function. Environ Res. 2005b;98(3):341–8. doi: 10.1016/j.envres.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Silva IA, Nyland JF, Gorman A, Perisse A, Ventura AM, Santos EC, de Souza JM, Burek CL, Rose NR, Silbergeld EK. Mercury exposure, malaria, and serum antinuclear/antinucleolar antibodies in amazon populations in Brazil: a cross-sectional study. Environ Health. 2004;3(1):11–22. doi: 10.1186/1476-069X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21(24):3365–9. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- Stern AH, Smith AE. An assessment of the cord blood: maternal blood methylmercury ratio: implications for risk assessment. Environ Health Perspect. 2003;111(12):1465–70. doi: 10.1289/ehp.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, Gordon T, Hardin JA, Kalden JR, Lahita RG, Maini RN, McDougal JS, Rothfield NF, Smeenk RJ, Takasaki Y, Wiik A, Wilson MR, Koziol JA. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40(9):1601–11. doi: 10.1002/art.1780400909. [DOI] [PubMed] [Google Scholar]
- Thuvander A, Sundberg J, Oskarsson A. Immunomodulating effects after perinatal exposure to methylmercury in mice. Toxicology. 1996;114(2):163–75. doi: 10.1016/s0300-483x(96)03486-5. [DOI] [PubMed] [Google Scholar]
- Via CS, Nguyen P, Niculescu F, Papadimitriou J, Hoover D, Silbergeld EK. Low-dose exposure to inorganic mercury accelerates disease and mortality in acquired murine lupus. Environ Health Perspect. 2003;111(10):1273–7. doi: 10.1289/ehp.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoo EM, Valente JG, Grattan L, Schmidt SL, Platt I, Silbergeld EK. Low level methylmercury exposure affects neuropsychological function in adults. Environ Health. 2003;2(1):8. doi: 10.1186/1476-069X-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M. Placental to fetal transfer of mercury and fetotoxicity. Tohoku J Exp Med. 2002;196(2):79–88. doi: 10.1620/tjem.196.79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatter plots for paired maternal and cord blood results for (A) IgG1, (B) IgG2, (C) IgG3, (D) IgG4, (E) IgM, and (F) IgA are shown on the natural scale (expressed in mg/ml). The predicted value line fit for each plot (in red) is the overlay with the line expressed as Y=β0 + β1X (p-value) for: (A) Y= 5.16 + 0.345X (p=not significant); (B) Y= 0.234 + 0.233X (p=0.012); (C) Y= 0.276 + 0.384X (p<0.0001); (D) Y= 0.020 + 0.688X (p<0.0001); (E) Y= 0.159 – 0.087X (p=ns); (F) Y= −0.211 + 0.142X (p=ns).


