Abstract
Peripheral nerve regeneration after injury depends on environmental cues and trophic support. Schwann cells (SCs) secrete trophic factors that promote neuronal survival and help guide axons during regeneration. The addition of SCs to acellular nerve grafts is a promising strategy for enhancing peripheral nerve regeneration; however, inconsistencies in seeding parameters have led to varying results. The current work sought to establish a systematic approach to seeding SCs in cold-preserved acellular nerve grafts. Studies were undertaken to (1) determine the needle gauge for optimal cell survival and minimal epineurial disruption during injection, (2) track the seeded SCs using a commercially available dye, and (3) evaluate the seeding efficiency of SCs in nerve grafts. It was determined that seeding with a 27-gauge needle resulted in the highest viability of SCs with the least damage to the epineurium. In addition, Qtracker® dye, a commercially available quantum dot nanocrystal, was used to label SCs prior to transplantation, which allowed visualization of the seeded SCs in nerve grafts. Finally, stereological methods were used to evaluate the seeding efficiency of SCs in nerve grafts immediately after injection and following a 1- or 3-day in vitro incubation in SC growth media. Using a systematic approach, the best needle gauge and a suitable dye for SC visualization in acellular nerve grafts were identified. Seeding efficiency in these grafts was also determined. The findings will lead to improvements ability to assess injection of cells (including SCs) for use with acellular nerve grafts to promote nerve regeneration.
Keywords: nerve regeneration, cell transplantation, peripheral nerve injury
1. Introduction
Peripheral nerve injury due to complete nerve transection results in a loss of function in the denervated end organ. The peripheral nervous system is capable of limited regeneration when the two severed nerve ends can be rejoined by a direct anastomosis in the case of a small gap (Midha, 2006). However, in the case of longer nerve gaps, excess tension resulting from end-to-end coaptation makes direct anastomosis an undesirable repair strategy (Burnett and Zager, 2004). The gold standard for reconstruction of nerve gaps is the use of a nerve autograft. However, autograft harvest requires a second operative site, results in donor-site sensory loss, and has the potential for neuroma formation resulting in pain. An alternative to nerve autografts is to insert an acellular graft to bridge the gap between the two severed nerve ends (Meek and Coert, 2002). Cold-preserved (CP) nerve grafts have been used as acellular nerve grafts to support growth of regenerating axons from the proximal nerve into the distal stump (Hare et al., 1993; Hare et al., 1995; Levi et al., 1994). Compared with nerve conduits, acellular nerve grafts support superior nerve regeneration because they contain an intact endoneurial microstructure consisting of extracellular matrix (ECM) proteins that support regenerating axons (Whitlock et al., 2009). However, the regenerative capacity of acellular nerve grafts is still inferior to an autograft because they lack Schwann cells (SC). SCs provide regenerative support by secreting the trophic factors and ECM molecules that promote neuronal survival and axonal regeneration (Brenner et al., 2005; Bunge, 1993; Frostick et al., 1998; Nagarajan et al., 2002; Reynolds and Woolf, 1993). To mimic the regenerative capacity of a nerve autograft, acellular grafts can be seeded with host SCs that have been harvested and expanded in culture.
Previously, our laboratory has found mixed results with the uses of SCs to enhance regeneration in CP nerve grafts after peripheral nerve injurry (Brenner et al., 2005; Fox et al., 2005a; Fox et al., 2005b; Hess et al., 2007). These inconsistencies may be due to the different methods used to seed cells into the CP grafts and thus provide the motivation to investigate and determine the factors important for efficient seeding of SCs into CP acellular nerve grafts. In the current study, we systematically evaluated injection of SCs into CP acellular grafts by (1) determining the needle gauge for injection that results in the highest viability of SCs, (2) evaluating whether the seeded SCs are indeed present in the nerve grafts after injection, and (3) evaluating the seeding efficiency by counting the number of SCs present in the grafts.
2. Materials and Methods
2.1 Schwann Cell Culture
The sciatic nerves from 12 Lewis rats were harvested and placed in Leibovitz's L-15 medium (Invitrogen, Carlsbad, CA). Collagenase I (1%) (Fisher, Pittsburgh, PA) and trypsin (2.5%) (Invitrogen) were added to the fascicles and incubated for 30 min at 37°C. After centrifugation at 130 × g for 5 min, the pellet was washed with Dulbecco's modified Eagle medium (DMEM, Invitrogen) supplemented with 10% heat-activated fetal bovine serum (FBS, Sigma-Aldrich, St. Louis, MO) and 1% antibiotic antimycotic (ABAM, Invitrogen). Cells were then plated on poly-L-lysine (pLL) (Sigma-Aldrich)-coated 10 cm tissue culture dishes at 106 cells per dish (BD Falcon, Bedford, MA). Tissue culture plates were prepared by coating with a 10 mL of 0.01% pLL in sterile ddH2O and washing twice with sterile water. On day 2 of culture, 10 μM Cytosine-beta-arabino furanoside hydrochloride (Ara-C) (Sigma-Aldrich), was added to cultures along with the media containing DMEM, FBS and ABAM. On day 6, the fibroblasts were complement-killed using an anti-Thy 1.1 antibody (1:40 dilution in media, Serotec, Raleigh, NC) and guinea pig complement (1:4 dilution in media, Sigma-Aldrich). On subsequent days the culture media was supplemented with 2 μM forskolin (Sigma-Aldrich) and 20 μg/mL pituitary extract (PE) (Biomedical Tech, Inc., Stoughton, MA).
2.2 Preparation of Cells for Injection
Once SCs reached confluence, the cells were washed twice with Hanks' Balanced saline solution (HBSS, Invitrogen). The cells were then incubated with 0.25% trypsin for 3 min at 37°C. After centrifugation for 5 min at 130 × g, the supernatant was removed, and the cells were suspended at the desired concentration in growth media containing DMEM supplemented with 10% FBS and 1% ABAM. A 10nM Qtracker® (Invitrogen) solution was prepared for labeling as recommended by the manufacturer. After mixing 200 μL of fresh media in 1.5 mL of prepared Qtracker® solution, 1 million cells were added to the tube and incubated at 37°C for 60 min. The resulting labeled cells were washed with media twice. The labeled cells were aliquoted and concentrated as needed in D-media.
2.3 Live/Dead Assay and Imaging
Using the desired size of Hamilton™ Syringe (24, 27, 29 and 33-gauge needle), 5 μL of cell suspension were drawn up and injected onto a pLL-coated 24-well plate (Corning, Acton, MA). After incubation at 37°C for 24 hours, allowing the cells to adhere to the surface, a standard LIVE/DEAD® Cell Viability Assay (Invitrogen) was performed. Live cells were identified by the incorporation of the membrane permeable Calcein AM stain within a cell, whereas dead cells were identified by the binding of Ethidium homodomer-1 to the nucleic acids of cells with damaged plasma membranes. Cells were counted from images recorded by a CCD camera (Optronics, Goleta, CA) attached to an Olympus IX70 microscope (Olympus Corporation, Japan) with a 10× objective.
2.4 Nerve Graft Harvest
Lewis rats were anesthetized with a subcutaneous injection of ketamine (75 mg/kg, Ketaset®, Fort Dodge Animal Health, Fort Dodge, IA) and medetomidine (0.5 mg/kg, Dormitor®, Orion Corporation, Espoo, Finland). Under aseptic conditions, both hind limbs were prepped and shaved. Using a no. 15 blade, a 3 cm skin incision was made from the top of the femur towards the knee cap and then the gluteal muscles were separated to expose the sciatic nerve. Using a dissecting microscope, a 30–35 mm sciatic nerve segment was excised bilaterally and used for immediate isograft repair, cold-preservation treatment (preparation of acellular grafts) or SC expansion. The animals were subsequently euthanized with intracardiac injection of Euthasol® (150 mg/kg, Delmarva Laboratories, Des Moines, IA). All animal care was performed according to NIH guidelines and with IUCAC approval.
2.5 Decellularization of Nerve grafts
Sciatic nerve segments from donor Lewis rats (harvested as described above) were immediately transferred into sterile six-well plates with 10 mL of a solution containing UW solution (15 ml; NPBI International BV, Emmer Compascuum, The Netherlands), penicillin G (200,000 U/L), regular insulin (40 U/L), and dexamethasone (16 mg/L). The solution was changed weekly in a sterile hood for seven weeks and stored at 4°C as described previously (Fox et al., 2005a).
2.6 Needle injection and incubation
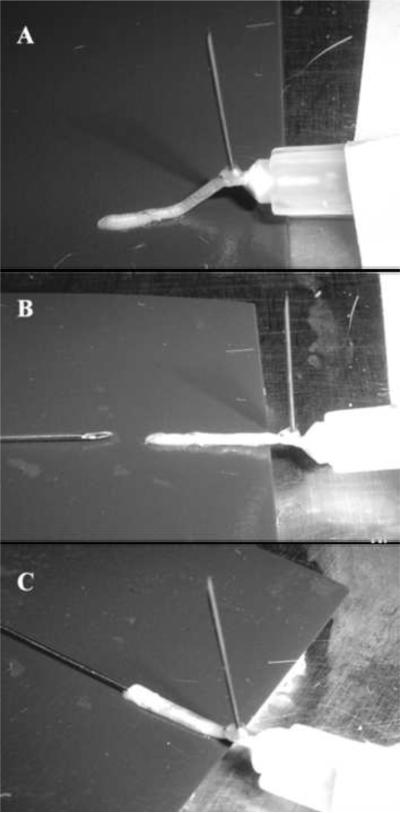
With a 27-gauge Hamilton™ syringe (Hamilton™ Company, Reno, NV) SCs at a concentration of 1 × 105 SCs/5 μL or 1 × 106 SCs/5 μL was injected longitudinally underneath the epineurium of the graft. The proximal stump was first sutured to a sterile syringe bent to form a hook and secured under the operating microscope (Figure 1) to resemble our in vivo model. Following injection of SCs, the distal stump was sutured closed to prevent any leakage of cells or media from the nerve. To confirm that the Schwann cells had been injected into the graft, we imaged the nerve graft under a fluorescence microscope. The grafts were then either fixed and processed for analysis (0 day) or incubated at 37°C in media supplemented with 10% FBS and 1% ABAM for 1 or 3 days prior to fixation.
Figure 1. In vitro set up used to mimic the in vivo model for SC injection into an acellular graft.
The proximal (P) stump of the graft was first sutured to a sterile syringe bent to form a hook and secured under the operating microscope (A). Using a Hamilton™ Syringe, the SCs were injected longitudinally under the epineurium of the distal (D) stump (B, C).
2.7 In vivo Microscopy
Images for in vivo cell visualization in the graft were taken with a fluorescence Olympus MVX10 dissecting microscope (Olympus Corporation, Japan) fitted with a cooled CCD digital camera (Hamamatsu ORCA-ER; Hamamatsu City, Japan) to evaluate changes in SC survival and the possibility of migration within the graft over time (t=0, 1, 3 days). The imaging software used was MetaMorph version 7.0 (Universal Imaging Corporation, PA).
2.8 Stereology
Following injection, all grafts from day 0, 1 or 3, were placed in a phosphate buffered saline (PBS) solution containing 4% paraformaldehyde (Sigma-Aldrich). The grafts were then cryoprotected in a 30% sucrose solution in PBS in preparation for frozen sections, embedded in Tissue-Tek OCT compound Mounting Media (Sakura Finetek, Torence, CA), and cut into 20 μm sagittal sections using a cryostat.
Sagittal sections of the frozen grafts were taken resulting in ~40 frozen serial sections. Every eighth section was then analyzed using stereological techniques resulting in ~5 sections from each graft being analyzed. The sagittal sections were through the longitudinal access of the graft spanning the sub-epineurial space of the nerve. Thus SCs injected under the epineurium were clearly visible in the sections under fluorescence microscopy. Each section was counter stained with DAPI nuclear dye. Co-localization of the Qtracker® quantum dots and DAPI nuclear stain was used to identify a single transplanted cell for stereological counts. All stereological counts were performed using the optical fractionator method as described previously (Keuker et al., 2001), under 40× magnification and verified with a Gunderson coefficient of error less than 0.08.
2.9 Statistical Analysis
Statistical analyses were performed using SigmaStat 3.0 (Systat Software, San Jose, CA) and all data were evaluated with one-way analysis of variance (ANOVA), followed by a Scheffe's F test for comparisons between groups when significance (p<0.05) was present. All results are reported as mean ± standard deviation.
3. Results
3.1 Needle Gauge Effect on SC Survival
This portion of the study assessed the effect of needle gauge on SC survival following injection into CP nerve grafts. In previous studies, a 29-gauge needle was used for injection of SCs into CP nerve grafts at a concentration of 105 SCs/5 μL (Fox et al., 2005b). In the current study we evaluated different needle sizes (24, 27, 29, and 33-gauge) and their effect on SC survival (Table 1). These needle gauge sizes are similar to the disposable needles used in a clinical setting.
Table 1.
Hamilton™ Syringe Needle Gauge Indexa
| Gauge | Nominal O.D.* (mm) | Nominal LD.+ (mm) | Wall Thickness (mm) |
|---|---|---|---|
| 33 | 0.21 | 0.011 | 0.05 |
| 29 | 0.34 | 0.18 | 0.63 |
| 27 | 0.41 | 0.21 | 0.1 |
| 24 | 0.57 | 0.31 | 0.13 |
O.D. = outer diameter
I.D. = inner diameter
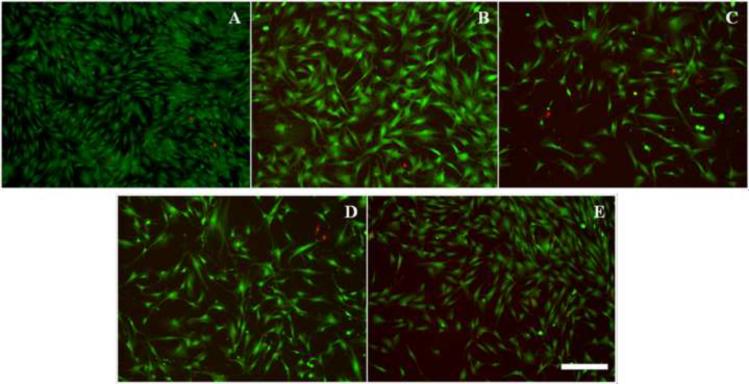
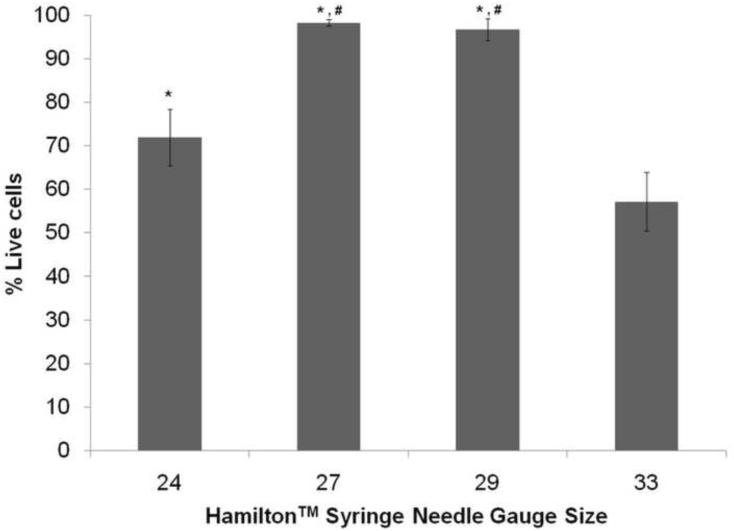
SCs derived from the rat sciatic nerve were cultured and grown to confluence. Once confluent, the SCs were concentrated to 105 SCs/5 μL for the experiment. SCs were drawn up into a Hamilton™ Syringe with the needle sizes to be tested and then plated on 24-well plates coated with pLL. The SCs were then incubated for 24 hours and a standard Live/Dead assay was done to assess their viability (Figure 2). Analysis of the Live/Dead assay showed that SCs plated using the 27 and 29-gauge needles had higher viability than those plated using the 24 and 33-gauge needles (Figure 3). The smallest needle (33-gauge) demonstrated lower viability compared to all other needle sizes.
Figure 2. Live/Dead staining of 105 SCs expelled using different sizes of Hamilton™ Syringe needles.
(A) control (pipette) (B) 24 gauge (C) 27 gauge (D) 29 gauge and (E) 33 gauge. Calcein AM (green) stains the live cells whereas the Ethidium homodimer-1 (red) stains the dead cells. Scale bar = 250 μm.
Figure 3. Cell viability of the SCs after being expelled through Hamilton™ Syringe needle.
The 24, 27 and 29 gauge needles resulted in significantly higher cell viability after expelling the cells into the 24-well plate compare to the 33 gauge needle. The 27 and 29 gauge needles showed an increase in cell viability compared to the 24 gauge needle as well. * denotes p < 0.05 compared to 33gauge needle, # denotes p < 0.05 compared to 24 gauge needle. Error bars represent the standard deviation (n = 5).
3.2 Needle Gauge Effect on CP Nerve Epineurium
Regardless of cell survival, larger-gauge needles are easier to handle technically because they have increased diameter and decreased pliability. 24 and 27-gauge needles were evaluated in order to determine the feasibility of inserting larger-gauge needles under the epineurium. The insertion of the 24-gauge needle longitudinally into a CP nerve resulted in tearing of the epineurium compared to the 27-guage needle (Figure S1). It was discarded from subsequent experiments because its use would allow the seeded SCs to escape the nerve graft. In contrast, the 27-gauge needle provided optimal viability and ease of injection for SC seeding in CP nerve grafts.
3.3 SC Seeding Efficiency
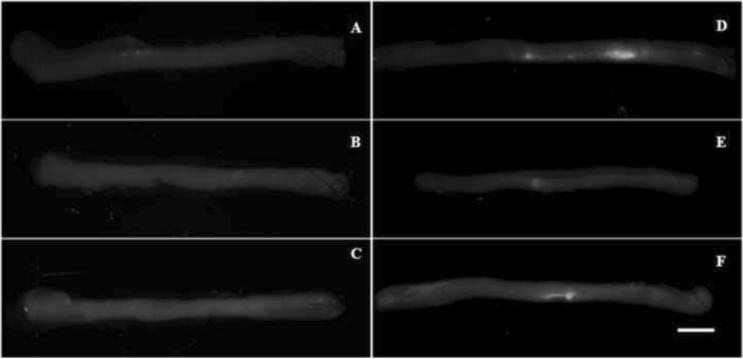
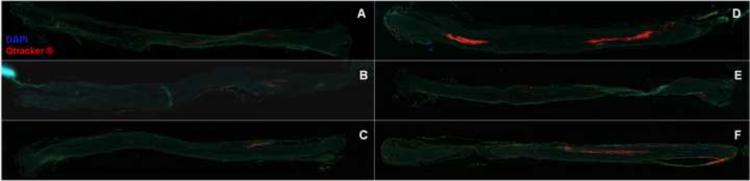
Visualization of fluorescently-labeled SCs under a direct fluorescence microscope was used to verify that SCs remained in the CP nerve grafts after injection. We also evaluated whether the seeded SCs were viable in the grafts after incubation in SC growth media for 1 day or 3 days. Incubation would allow for preservation of the grafts for 1 or more days prior to surgical implantation. SCs were labeled with Qtracker® dye concentrated at 105 and 106 SCs/5 μL. After SC injection, digital images of the nerve grafts under in vivo fluorescence microscopy were taken. No fluorescence was observed in grafts injected with 105 cells on day 0, 1, and 3 of incubation (Figures 4A, B, & C). In contrast, grafts injected with 106 cells had noticeable regions of fluorescently labeled cells even after 1 and 3 days of incubation (Figures 4D, E, and F). Incubation of the fluorescence-labeled SCs after seeding in nerve grafts did not result in qualitative evidence of migration or proliferation. From these images we concluded that Qtracker® can be used to ensure that the SCs are present in the nerve grafts after injection.
Figure 4. Acellular nerve grafts after injection with labeled SCs graft.
Qtracker® was used to label the SCs prior to injection to verify that the transferred cells remained in the graft. The CP nerves injected with 105 cells did not show any fluorescence signal on day 0 (A), day 1 (B) or day 3 (C). The grafts injected with 106 cells did fluoresce on day 0 (D), day 1 (E) or day 3 (F). Scale bar = 1.5 mm.
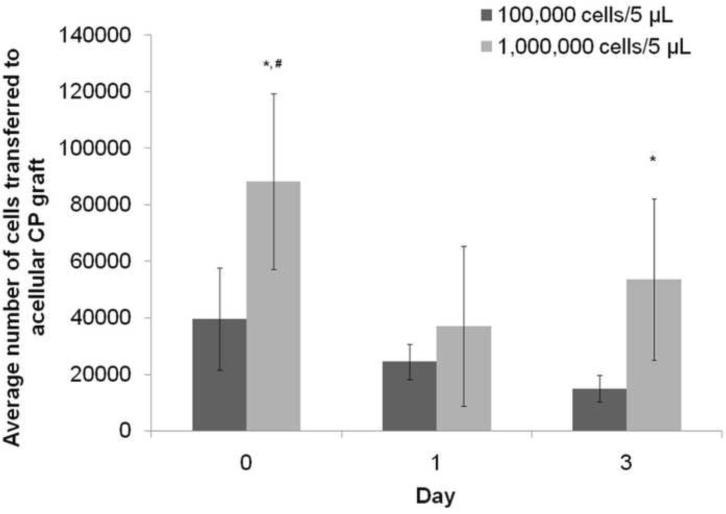
To quantify the number of SCs that were transferred to the nerve grafts after injection, the grafts seeded with labeled SCs from day 0, 1 and 3 of incubation were sectioned, and the number of SCs was counted using stereological analysis, as described previously (Keuker et al., 2001). The analysis revealed that only a fraction of the expected number of labeled SCs were present in the graft after injection (Figures 5 and 6). Of the CP grafts injected with 106 SCs, ~105 cells (or 10% of injected cells) remained in the acellular CP graft. In the CP grafts injected with 105 SCs, ~4×104 cells remained in the CP graft after injection (or 40% of the injected cells). In addition, more cells were present on day 0 compared to day 1 for the 1 million cells group, suggesting that cells were lost during the incubation process.
Figure 5. Stereological analysis of the number of cells transferred into the acellular grafts.
The grafts injected with 106 cells had a higher number of cells than the grafts injected 105 cells for all three groups. Particularly, the groups from day 0 and 3 showed a significantly higher number of cells transferred into the groups than their 105 cell counterparts. * denotes p < 0.05 compared to the grafts injected with 105 cells, # denotes p < 0.05 compared to day 1 with 106 injected cells. Error bars represent the standard deviation (n = 6).
Figure 6. Sagittal sections of the frozen acellular grafts post injection.
The grafts injected with 105 (day 0 (A), day 1 (B), day 3 (C)) and 106 (day 0 (D), day 1 (E), day 3 (F)) cells were frozen and sectioned for stereological counts. The presence of cells was confirmed with co-staining of DAPI (blue) and Qtracker® in the sections. Scale bar = 1.5mm.
4. Discussion
Transplantation of SCs at the site of nerve transection has been previously shown to promote peripheral nerve regeneration, but these studies have not evaluated the ability of syringe injection to successfully transfer SCs. In this study, we systematically evaluated the transplantation of SCs into acellular CP nerve grafts through syringe injection. The current study (1) determined the needle gauge for maximal viability of SCs during injection into nerve grafts, (2) tracked the seeded SCs in vitro, and (3) evaluated the efficiency with which SCs are seeded within acellular nerve grafts using syringe injection.
Needle size is a critical parameter for cell transplantation by injection into nerve grafts. The inner diameter of the needle must be large enough to avoid excessive shear force on the cells, and the outer diameter of the needle must be small enough to avoid tearing of the epineurium. A standard Live/Dead assay was performed to determine the needle gauge would provide the maximal number of viable SCs (after the cells are expelled from the needle). A significant increase in viability was observed for the 24, 27, and 29-gauge needles compared to the 33-gauge needle. The inner diameters of the 24, 27, and 29-gauge needles were large enough to allow the SCs to prevent significant shear forces on the cell membrane. The rate at which the cells are expelled from the needle may introduce mechanical stress, which may compromise the integrity of the cell membrane and eventually lead to cell death (McNeil, 1993). In contrast, the smaller inner diameter of the 33-gauge needle adversely affected the survival of the SCs by likely subjecting the injected cells to intolerable levels of shear force. The outer diameter of the injection needle must also be considered to safeguard against undue damage to the epineurium during the seeding process. Damage to the epineurium would cause the newly seeded SCs to leak from the grafts after injection. Injection with the 24-gauge needle caused damage to the epineurium, whereas the 27-gauge needle allowed successful SC seeding without noticeable damage to the epineurium. Given the combined effects on viability and epineurium integrity, it was determined that the 27-gauge needle was the best option for injecting SCs into acellular nerve grafts.
Injecting under the epineurium does not guarantee successful transfer and seeding of SCs. Developing a method to determine that the injection was successful prior to graft implantation would decrease the probability of experimental error due to low SC numbers within the graft. Previous work in this field failed to track seeded SCs prior to graft implantation (Fox et al., 2005a; Fox et al., 2005b). The inability to monitor injection success could result in implantation of grafts that lack SCs due to injection error and thus contribute to experimental error.
Commercially available fluorescent dyes can be used to label and track cells following injection. One such dye shown to be effective in SCs is the 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE). The advantage of labeling SCs with CFSE is that the transplanted SCs can be distinguished from the host SCs over long periods after transplantation (up to 4 weeks in some studies) due to the tolerance of SCs to CFSE (Li et al., 2003). An alternative method involves the Qtracker® dye (Invitrogen), which uses Qdot nanocrystals to label and track transplanted cells (Chakraborty et al., 2007; Jaiswal et al., 2003; Riegler et al., 2008). In the current study, the Qtracker® was chosen over the CFSE because of the ease of labeling cells: Q-tracker® has been shown to provide an intense fluorescence for cells, can be traced through cell division cycles, and is not transferred to adjacent cells. Studies have used this dye in cell motility, migration, and a number of other functional assays. One distinct feature of Q-tracker® is that it uses a custom targeting peptide to deliver Qdot nanocrystals into the cytoplasm of cells. It is therefore easily adaptable for other cell types (Futaki, 2005; Wender et al., 2002). Also, labeling with Qtracker® requires only a simple incubation for one hour, after which the cells are stable for injection for at least 2 hours, giving us a more flexible time frame for our experiments. In contrast, labeling with CFSE requires incubation with the dye in combination with other chemicals, and the cells must be used immediately after labeling due to instability of the dye.
Qtracker® allowed for qualitative assessment of SCs injection into the graft by fluorescence microscopy. Grafts injected with106 SCs exhibited a higher fluorescence intensity compared to those injected with 105 SCs which exhibited little to no fluorescence. The Qtracker® dye provided the ability to track seeded SCs in CP acellular nerve grafts, which can provide quality control prior to experimental graft implantation in future studies.
Another important factor that affects success or failure of cell transplant therapies is the efficiency with which the therapeutic cells are transferred into the desired tissue. Stereological analysis of grafts after injection revealed that only a fraction of the injected SCs remained in the graft. The failure to successfully transfer all of the cells may be either due to the death of cells post injection, the loss of SCs leaking from grafts post-injection and prior to fixation or the staining efficiency of Qtracker ®. Only 10% of the cells were successfully transferred when 106 SCs were injected into the CP acellular nerve grafts and only 40% were successfully transferred when 105 SCs were injected. Previous studies with CP nerve grafts showed that injection with 105 SCs had little effect on peripheral nerve regeneration (Fox et al., 2005b), while injection with 106 SCs enhanced regeneration over CP grafts alone (Brenner et al., 2005). Based on the current work, we hypothesize that seeding efficiency of cells transferred may have played an important role in the lack of therapeutic outcomes with respect to nerve regeneration in our previous studies. Additionally, the combined results of the current study and our previous studies suggests that there may be a threshold number of SCs necessary to elicit a therapeutic effect in acellular nerve grafts. Mechanical stress may also limit the number of viable cells transferred to the grafts. To reduce the stress on the cells, the injection rate can be optimized to deliver the maximum amount of viable cells to the graft post injection. The number of cells transferred to the grafts after incubation in growth media is much lower than the desired 106 cells, which may have allowed the cells to leak into the media from the graft because they could not successfully adhere to the graft or cell death resulting from insufficient perfusion of the graft in the cell culture media. The staining efficiency (~70% in this study) of the Qtracker can also be determined and increased for future studies to ensure that a sufficient number of cells are labeled prior to injection. To maximize the number of cells transferred to the graft, it is beneficial to use the grafts to repair the injury immediately after the cells are injected, as the number of viable cells decreased with time (Figure 5). A combination of these strategies may increase the seeding efficiency of cells, which may further enhance the effects of transplanted SCs on peripheral nerve regeneration.
From a clinical perspective, we need to consider the source of the SCs used for transplantation as well as the purity of the SCs. The SCs can be derived from the transected nerve itself. A piece of the nerve from the stump can be removed and the SCs can be harvested and cultured as described in the methods. The cell population can be purified by using selection techniques such as immunopanning (Barres et al., 1992) or Dynal magnetic beads (Invitrogen) (Neurauter et al., 2007). SCs express the surface receptor p75NTR (Lemke and Chao, 1988), so SCs can be bound to an anti-p75NTR antibody coated on a Petri dish for immunopanning or on magnetic beads to help purify a pure population of SCs. It is critical to transplant a pure population into a patient to ensure that the transplanted cells will help the injured nerve to regenerate. Our goal in this study was to develop a systematic method to evaluate and determine how to inject cells into acellular grafts for transplantation therapies. We have shown that using these evaluation methods that we are able to successfully re-introduce cells into acellular grafts, which is important to ensure the success of in vivo transplantation studies.
5. Conclusions
In this study, we demonstrated the importance of (1) determining a needle gauge that maximizes the viability of the SCs and limits the damage to the epineurium, (2) tracking SCs after injection using a dye to verify successful injection, and (3) confirming the number of cells that are actually transferred to the graft. We concluded that the best parameters for the injection method of SC seeding in CP acellular nerve grafts is subepineurial injection with a 27-gauge Hamilton™ Syringe, at a concentration of 106 SCs/5 μl labeled with Qtracker®, to provide maximum number of SCs with minimal epineurial damage. This systematic approach can be expanded to include other cell types across different injury modalities to help advance research on peripheral nerve regeneration, as well as other tissues in the future.
Supplementary Material
Acknowledgements
The authors were funded and supported by the NIH RO1 grant 5R01NS033406 and 5R01NS051454-06.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death in the oligodendrocyte lineage. J Neurobiol. 1992;23:1221–30. doi: 10.1002/neu.480230912. [DOI] [PubMed] [Google Scholar]
- Brenner MJ, Lowe JB, 3rd, Fox IK, Mackinnon SE, Hunter DA, Darcy MD, Duncan JR, Wood P, Mohanakumar T. Effects of Schwann cells and donor antigen on long-nerve allograft regeneration. Microsurgery. 2005;25:61–70. doi: 10.1002/micr.20083. [DOI] [PubMed] [Google Scholar]
- Bunge RP. Expanding roles for the Schwann cell: ensheathment, myelination, trophism and regeneration. Current opinion in neurobiology. 1993;3:805–9. doi: 10.1016/0959-4388(93)90157-t. [DOI] [PubMed] [Google Scholar]
- Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16:E1. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- Chakraborty SK, Fitzpatrick JA, Phillippi JA, Andreko S, Waggoner AS, Bruchez MP, Ballou B. Cholera toxin B conjugated quantum dots for live cell labeling. Nano Lett. 2007;7:2618–26. doi: 10.1021/nl0709930. [DOI] [PubMed] [Google Scholar]
- Fox IK, Jaramillo A, Hunter DA, Rickman SR, Mohanakumar T, Mackinnon SE. Prolonged cold-preservation of nerve allografts. Muscle Nerve. 2005a;31:59–69. doi: 10.1002/mus.20231. [DOI] [PubMed] [Google Scholar]
- Fox IK, Schwetye KE, Keune JD, Brenner MJ, Yu JW, Hunter DA, Wood PM, Mackinnon SE. Schwann-cell injection of cold-preserved nerve allografts. Microsurgery. 2005b;25:502–7. doi: 10.1002/micr.20152. [DOI] [PubMed] [Google Scholar]
- Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Futaki S. Membrane-permeable arginine-rich peptides and the translocation mechanisms. Adv Drug Deliv Rev. 2005;57:547–58. doi: 10.1016/j.addr.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Hare GM, Evans PJ, Mackinnon SE, Nakao Y, Midha R, Wade JA, Hunter DA, Hay JB. Effect of cold preservation on lymphocyte migration into peripheral nerve allografts in sheep. Transplantation. 1993;56:154–62. doi: 10.1097/00007890-199307000-00029. [DOI] [PubMed] [Google Scholar]
- Hare GM, Evans PJ, Mackinnon SE, Wade JA, Young AJ, Hay JB. Phenotypic analysis of migrant, efferent lymphocytes after implantation of cold preserved, peripheral nerve allografts. J Neuroimmunol. 1995;56:9–16. doi: 10.1016/0165-5728(94)00120-d. [DOI] [PubMed] [Google Scholar]
- Hess JR, Brenner MJ, Fox IK, Nichols CM, Myckatyn TM, Hunter DA, Rickman SR, Mackinnon SE. Use of cold-preserved allografts seeded with autologous Schwann cells in the treatment of a long-gap peripheral nerve injury. Plastic and reconstructive surgery. 2007;119:246–59. doi: 10.1097/01.prs.0000245341.71666.97. [DOI] [PubMed] [Google Scholar]
- Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat Biotechnol. 2003;21:47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- Keuker JI, Vollmann-Honsdorf GK, Fuchs E. How to use the optical fractionator: an example based on the estimation of neurons in the hippocampal CA1 and CA3 regions of tree shrews. Brain Res Brain Res Protoc. 2001;7:211–21. doi: 10.1016/s1385-299x(01)00064-2. [DOI] [PubMed] [Google Scholar]
- Lemke G, Chao M. Axons regulate Schwann cell expression of the major myelin and NGF receptor genes. Development. 1988;102:499–504. doi: 10.1242/dev.102.3.499. [DOI] [PubMed] [Google Scholar]
- Levi AD, Evans PJ, Mackinnon SE, Bunge RP. Cold storage of peripheral nerves: an in vitro assay of cell viability and function. Glia. 1994;10:121–31. doi: 10.1002/glia.440100206. [DOI] [PubMed] [Google Scholar]
- Li X, Dancausse H, Grijalva I, Oliveira M, Levi AD. Labeling Schwann cells with CFSE-an in vitro and in vivo study. J Neurosci Methods. 2003;125:83–91. doi: 10.1016/s0165-0270(03)00044-x. [DOI] [PubMed] [Google Scholar]
- McNeil PL. Cellular and molecular adaptations to injurious mechanical stress. Trends Cell Biol. 1993;3:302–7. doi: 10.1016/0962-8924(93)90012-p. [DOI] [PubMed] [Google Scholar]
- Meek MF, Coert JH. Clinical use of nerve conduits in peripheral-nerve repair: review of the literature. J Reconstr Microsurg. 2002;18:97–109. doi: 10.1055/s-2002-19889. [DOI] [PubMed] [Google Scholar]
- Midha R. Emerging techniques for nerve repair: nerve transfers and nerve guidance tubes. Clin Neurosurg. 2006;53:185–90. [PubMed] [Google Scholar]
- Nagarajan R, Le N, Mahoney H, Araki T, Milbrandt J. Deciphering peripheral nerve myelination by using Schwann cell expression profiling. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8998–9003. doi: 10.1073/pnas.132080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurauter AA, Bonyhadi M, Lien E, Nokleby L, Ruud E, Camacho S, Aarvak T. Cell isolation and expansion using Dynabeads. Adv Biochem Eng Biotechnol. 2007;106:41–73. doi: 10.1007/10_2007_072. [DOI] [PubMed] [Google Scholar]
- Reynolds ML, Woolf CJ. Reciprocal Schwann cell-axon interactions. Current opinion in neurobiology. 1993;3:683–93. doi: 10.1016/0959-4388(93)90139-p. [DOI] [PubMed] [Google Scholar]
- Riegler J, Ditengou F, Palme K, Nann T. Blue shift of CdSe/ZnS nanocrystal-labels upon DNA-hybridization. J Nanobiotechnology. 2008;6:7. doi: 10.1186/1477-3155-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wender PA, Rothbard JB, Jessop TC, Kreider EL, Wylie BL. Oligocarbamate molecular transporters: design, synthesis, and biological evaluation of a new class of transporters for drug delivery. J Am Chem Soc. 2002;124:13382–3. doi: 10.1021/ja0275109. [DOI] [PubMed] [Google Scholar]
- Whitlock EL, Tuffaha SH, Luciano JP, Yan Y, Hunter DA, Magill CK, Moore AM, Tong AY, Mackinnon SE, Borschel GH. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle & nerve. 2009;39:787–99. doi: 10.1002/mus.21220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.